1. Tumour suppressor genes in cancer
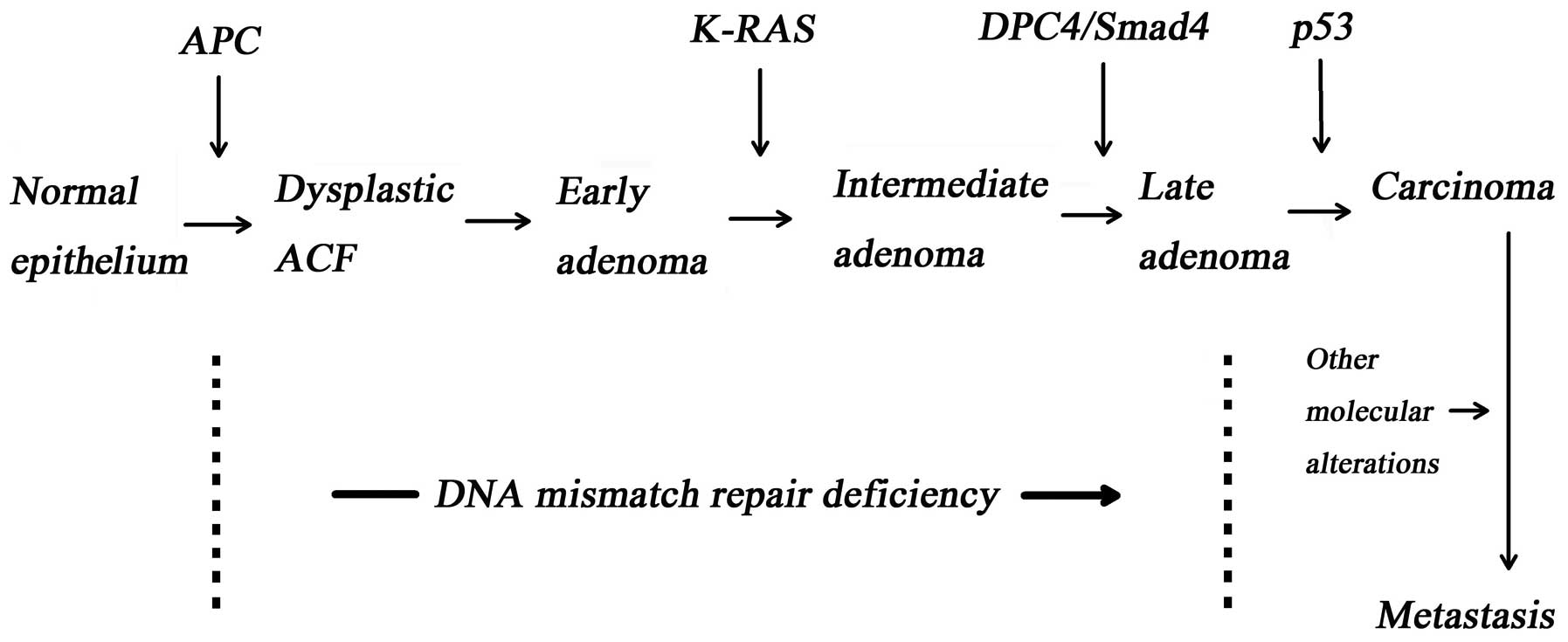
The accumulation of intracellular gene mutation
results in neoplastic transformation. The normal or abnormal
phenotype of a cell is dependent on the cell genotype and
intracellular epigenetic alternations. Gene mutations, which
include tumour suppressor gene (1), oncogene (e.g., K-ras,
C-myc and src) and the DNA mismatch repair gene, are
the basis of cancerisation (Fig.
1) (2–4).
Tumour suppressor genes maintain cell stability by
regulating cell proliferation and differentiation. Therefore,
uncontrollable cell proliferation and the formation of tumours may
be a result of inactivated tumour suppressor genes caused by
somatic mutation or heredity. Adenomatous polyposis coli
(APC) (5) located at
chromosome 5q.22.2 and the p53 gene located at chromosome 17p are
possibly the most extensively investigated tumour suppressor genes,
both of which are involved in the regulation of the cell cycle,
apoptosis, cellular senescence and cellular functions of intestinal
cells (e.g., proliferation, differentiation, migration and
polarity) (6). Another protein
complex that may have tumour and tumour metastasis suppressing
functions is Kiss-1 and the Kiss-1 receptor (Kiss-1R).
2. Kiss-1
Lee et al (7) introduced the full length of
chromosome 6 into the C8161 human metastatic melanoma cell line
using a microcell-mediated transferring technique and then the same
study group found that the introduction of chromosome 6 suppressed
metastasis without affecting tumourigenicity and tumour invasion
(8). Two years later, the human
Kiss-1 gene was isolated and identified from the melanoma
cells by Lee et al (7) and
Lee and Welch (9). The premature
coding product of the Kiss-1 gene is a protein with 145
amino acids. The protein is subsequently cleaved into a family of
Kisspeptins, including Kisspeptin-10, Kisspeptin-13, Kisspeptin-14
and Kisspeptin-54 (2,10,11). The Kiss-1 gene mapped to
chromosome 1q32 was identified as a human melanoma metastasis
suppressor gene through the analysis of subtractive hybridisation
in highly metastatic cell lines as compared with non-metastatic
cell lines (8). These early
findings suggested that there was a regulatory gene existing on
chromosome 6, which regulates the expression of Kiss-1 gene.
Subsequent research revealed that the regulatory region of Kiss-1
was in a locus of a 40-cM region between 6q16.3 and q23 of
chromosome 6 (12). A
complementary analysis of 51 melanoma patients by Shirasaki et
al (13) revealed that the
loss of 6q16.3–q23 was observed in 14 melanoma patients (51%),
which had a significant correlation (P=0.03) with the loss of
Kiss-1 expression (44%). In addition, Goldberg et al
(14) found the high expression
of thioredoxin interacting protein (TXNIP, also known as vitamin D
upregulated protein 1, thioredoxin binding protein 2 or VDUP1) in
non-metastatic melanomas, which was mapped to chromosome 1q and
also expressed in the neo6/C8161 melanoma cell line. In subsequent
studies, the authors reported that increased TXNIP expression
inhibited the metastasis of melanoma cells via an upregulation of
Kiss-1. Moreover, PCR karyotyping revealed that the expression of
Kiss-1 and TXNIP was upregulated in the cells transfected with
CRSP3 (vitamin D receptor interacting protein), which was mapped to
chromosome 6 and led to a suppression of metastasis (14). The decreased Kiss-1 expression and
increased metastasis have been shown to correlate with the loss of
CRSP3 (14).
These findings indicate that CRSP3 mapped to
chromosome 6 is an upstream regulator of TXNIP, which subsequently
regulates the expression of Kiss-1. In other words, a loss of CRSP3
expression, which is caused by the structural abnormality of
chromosome 6, impairs the expression of Kiss-1 and TXNIP.
3. Kiss-1 receptor, discovery and
structure
Kiss-1R, also known as G protein-coupled receptor 54
(GPR54), hOT7T175, AXOR12 and metastin receptor, was first
discovered and cloned from a rat brain in 1999 (15). In humans it was mapped to
chromosome 19p13.3 and contains 5 exons and 4 introns and encodes
398 amino acids (75 kDa) and shares 81% protein homology with the
preceding cloned rat orthologue (11,16).
The tissue distribution of Kiss-1 and its receptor
are often concordant. For example, Kiss-1 and Kiss-1R are highly
expressed in placental tissue (2,7,16).
Moreover, Kiss-1 and its receptor are widely distributed throughout
the central nervous system (16).
High levels of Kiss-1R have been observed in the cerebral vortex,
cerebellum, thalamus and pons-medulla (16). By contrast, its cognate ligand
precursor, Kiss-1, is highly expressed in the hypothalamus and
pituitary gland (11). In
addition to placental tissue, the expression of Kiss-1R is also
high in the pancreas, whilst it is expressed at low levels in
adipose tissue, the lymph nodes, peripheral blood lymphocytes, the
pituitary gland and spleen (2,16).
4. Kiss-1, Kiss-1 receptor and downstream
pathways
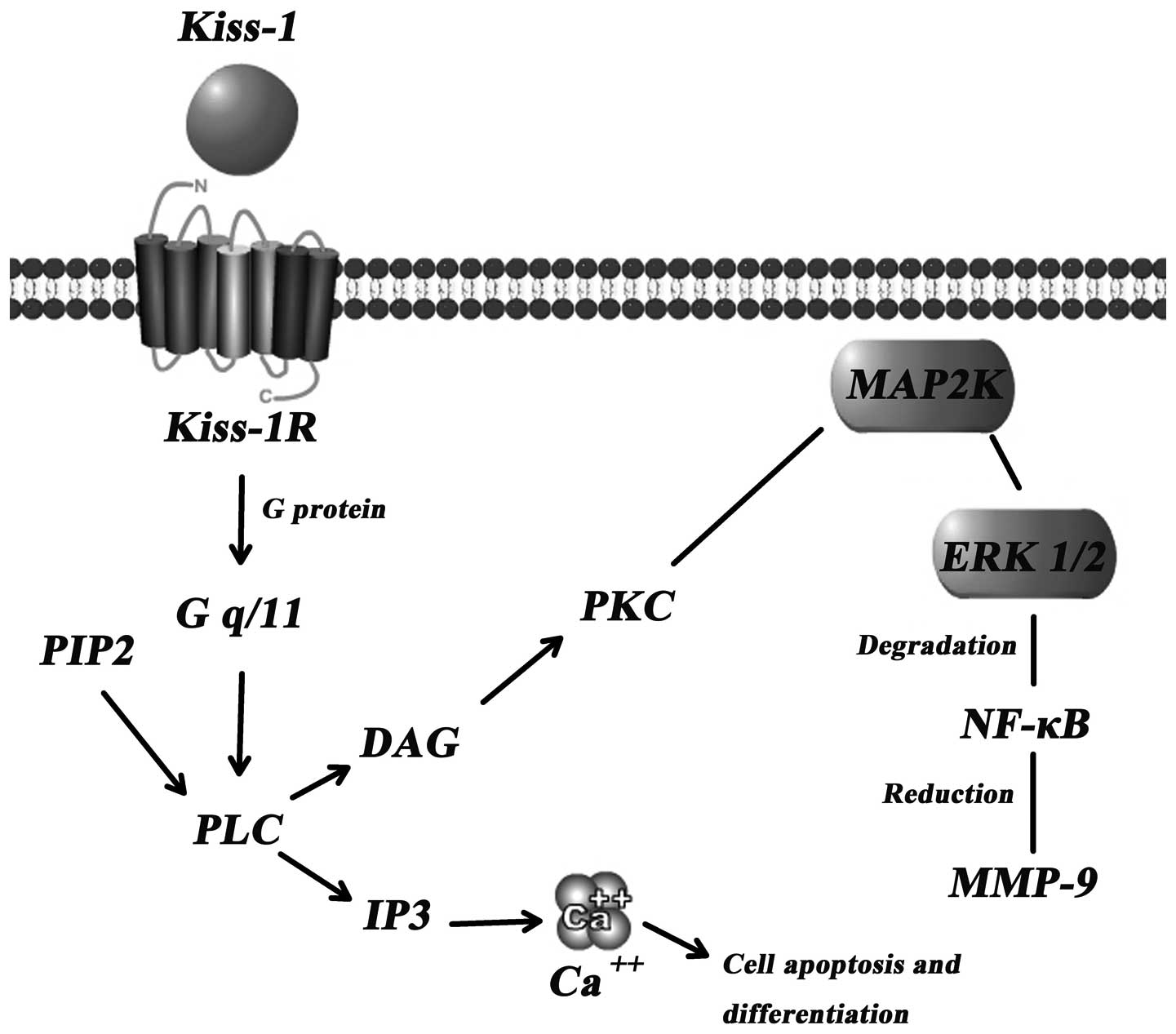
The concrete mechanism(s) through which Kiss-1
suppresses tumour metastasis are yet to be established. However, a
few signal transduction pathways have been indicated as the key
downstream events of the Kiss-1/Kiss-1R complex (Fig. 2).
Calcium mobilisation through Gq
activation
Intracellular Ca2+ levels have been shown
to significantly increase following transfection with Kiss-1R
followed by treatment with Kiss-1 or Kisspeptins in B16-BL6
melanoma (2), CHO-K1 (11) and HEK293 cells (16). Hence, the mobilisation of calcium
in cells, when treated with Kiss-1, manifests the important role of
Kiss-1R. Kiss-1R is likely coupled to G-proteins of the Gαq/11
subfamily rather than to the Gi or Gs subfamily (16). Through the Gαq/11-mediated
signaling pathway, phospholipase Cβ (PLCβ) is activated, which
consequently generates 2 types of second messengers in cells
(inositol triphosphate 3 and diacylglycerol), leading to
intracellular Ca2+ release and protein kinase C
activation, respectively (17).
Increased intracellular Ca2+ levels markedly suppress
tumour metastasis and induce the differentiation and apoptosis of
human cancer cells (17). In
addition, the capacity of colony formation of breast cancer cells
(MDA-MB-435) in hard agar medium has been shown to negatively
correlate with the expression of Kiss-1 (18).
Matrix metalloproteinases (MMPs)
MMPs can degrade the extracellular matrix and play
dominating roles in the process of tumour metastasis. MMP-9, known
as the most important protease related with tumour metastasis, is
capable of degrading the primary structure of the extracellular
matrix and basement membrane (collagen, laminin, fibronectin), and
thereby promotes tumour metastasis (19). Yan et al (20) showed that there was a marked
reduction in in vitro invasion through
Matrigel®-coated porous filters by HT-1080 cells which
had reduced transcription and activity of MMP-9 following the
induced overexpression of Kiss-1. This reduction is credited in
part to the diminished p65 and p50 NF-κB proteins which interact
with the promoter of MMP (17).
Consequently, the reduced synthesis of MMP-9 induces certain
inhibitory effects on the mobility and invasion of cancer cells
(2,17). In addition, activated focal
adhesion kinase (FAK) and paxillin, which play a crucial role in
the process of the formation of focal adhesion, exhibit certain
links with Kiss-1 and its receptor (21). In ARO thyroid cancer cells
transfected with Kiss-1R, Kiss-1 treatment has been shown to elicit
a strong and sustained phosphorylation of ERK1/2, and at the same
time, a weak phosphorylation of p38 or pAKT (21). A similar concentration-dependent
release of arachidonic acid induced by Kisspeptin-10 was observed
in CHO-K1/MR cells, in which Kisspeptin-10 markedly inhibited cell
proliferation (11). Activated
ERK1/2 significantly induces the formation of stress fibres, focal
adhesion and activates RHO, via the phosphorylation of FAK and
paxillin (2,21).
5. Kiss-1 and Kiss-1R in cancer
Breast cancer
Lee and Welch (9)
hypothesised that Kiss-1 suppresses metastasis in other tumour
types, in addition to melanomas, in which Kiss-1 was discovered.
This was on the basis of the map location of the Kiss-1 gene
(chromosome 1 bands q32–41), where its 1q alternations are peculiar
in a majority of human tumours but not in breast carcinomas. To
examine this hypothesis, the authors first transfected full-length
Kiss-1 cDNA into a human breast cancer cell line (MDA-MB-435) and
then implanted the cells into athymic nude mice with paired control
cells. The results demonstrated that the metastatic ability of the
transefected cells markedly increased, although tumourigenecity was
suppressed (18). To further
examine the hypothesis, using RT-PCR, Mitchell et al
(22) discovered that the loss of
Kiss-1 gene expression was associated directly with the
expression levels of activator protein-2a and specificity
protein-1, 2 known transcription factors expressed in highly
metastatic breast cancer cell lines (22). In addition, Marot et al
(23) found low levels of Kiss-1
mRNA in estrogen receptor (ER)α-negative MDA-MB-231 cells, whereas
ERα-positive MCF7 and T47D cells exhibited higher expression levels
of Kiss-1 and its receptor. Of note, data from post-menpopausal
women with breast cancer showed an opposite trend (23). An analysis of Kiss-1 mRNA in
paraffin-embedded stage II or III lymph node-positive breast
adenocarcinomas demonstrated that Kiss-1 gene expression was
silenced. These observations also support the anti-metastatic
potential of Kiss-1 in certain breast cancers (24). Likewise, another study
investigated the metastasis-suppressor gene profiles in breast
cancer using frozen tissue samples, and analysed Kiss-1 expression
at the mRNA and protein levels, using RT-PCR and
immunohistochemical staining, respectively (25). The study showed that Kiss-1 mRNA
expression was suppressed in samples of brain metastases from
breast cancer. In contrast to the studies supporting the
anti-metastatic potential of Kiss-1 in breast cancer, Martin et
al (26) found that the
expression of Kiss-1 was markedly increased in primary tumours
compared with normal mammary tissues. The increased expression of
Kiss-1 in primary tumours also correlated with metastasis to lymph
nodes. Likewise, the expression of Kiss-1 increased in relation to
tumour grade and increased TMN status. By contrast, its receptor
(Kiss-1R) was significantly decreased in patients with poor
survival. Moreover, the invasiveness of breast cancer cells was
impaired by the knockdown of Kiss-1 (26).
Gastric cancer
Dhar et al (27) found that reduced Kiss-1 expression
in gastric cancer correlated with an increased risk of distant
metastases and tumour recurrence. The study analysed the expression
of Kiss-1 in frozen tissue samples from 40 gastric adenocarcinomas
using RNase protection assays, and suggested that Kiss-1 was a
possible independent predictor of patient survival compared with
the conventional routine prognostic predictors of gastric cancer
(27). Likewise, an investigation
of 2 metastasis suppressor genes, Kiss-1 and KAI-1 in 49 gastric
adenocarcinoma tissues together with 20 pre-cancerous tissues,
showed that Kiss-1 mRNA leves were significantly lower in cancer
tissues compared with pre-cancerous tissues. This indicates the
possible predictive value of Kiss-1 in the prognosis of patients
with gastric cancer. Similar results were obtained in the study by
Yao et al (28),
demonstrating reduced Kiss-1 expression in moderate-to-severe
hyperplasia compared with normal-to-moderate hyperplasia, as well
as in T3/T4 tumours with lymph node involvement and distant
metastases compared with tumours at earlier stages, i.e., T1-2
without any metastasis (28).
Oesophageal carcinoma
Oesophageal squamous cell carcinoma (ESCC) is one of
the malignancies for which limited treatment means are available. A
number of reasons may contribute to the difficulties in treating
this type of tumour, including late diagnosis and advanced stages
of the disease at the time of the first visit, commonly ocurring
lymph node metastasis in most patients, the anatomical reasons that
the organ is closely surrounded by certain key organs and tissues
and its rich lymphatic drainage. The latter is of particular
interest: even when diagnosed at an early stage, the majority of
ESCC patients have already developed lymphatic metastases or lymph
node metastasis soon after surgery (29). Therefore, lymph node metastasis is
suggested as the most important predictor of prognosis in ESCC.
Ikeguchi et al (30)
assessed the expression levels of Kiss-1 and Kiss-1R in ESCC
tumours and non-cancerous tissues of the oesophagus of 71 patients
with ESCC. Using real-time PCR, the authors found that the loss of
expression of Kiss-1 and its receptor was detected in 86–100% of
primary tumours with lymph node metastases, suggesting the
relevance to lymphatic metastasis and unfavorable prognosis,
independent of the depth of tumour invasion (30). Furthermore, the loss of expression
of Kiss-1 and its receptor has been observed in primary tumours
with invasion to the adventitia compared with the control groups
without invasion and has been associated with lymph node metastasis
(2). These findings strongly
suggest that the loss of expression of one or both genes (Kiss-1
and Kiss-1R) is vital to lymph node metastasis; thus, Kiss-1 and
Kiss-1R can be considered as prognostic factors in ESCC (2).
Hepatocellular carcinoma (HCC)
A small number of studies on the expression of
Kiss-1 are available in the literature regarding tumour thrombus
caused by the invasion of HCC to the portal vein as the most vital
prognostic factor of orthotopic liver transplantation for HCC
(20–70%) (31). Hou et al
(32) reported a correlation
between the expression of Kiss-1 and MMP-9 in the formation of
portal vein tumour thrombus (PVTT) in HCC by analysing 50 specimens
of HCC (31 with PVTT and 19 without PVTT). The expression of Kiss-1
was lower in HCC with PVTT than that in HCC without PVTT. It is
noteworthy that a contrasting expression pattern of MMP-9 was
observed in HCC with and without PVTT. Hou et al (32) thus suggested that Kiss-1 may
possibly prevent PVTT by upregulating MMP-9 expression. Of note, a
higher expression of Kiss-1 and Kiss-1R genes was detected in more
advanced tumours by Ikeguchi et al (33). This indicates that the expression
levels of Kiss-1 and its receptor genes may promote tumour
progression rather than suppressing metastasis (33). Similarly, the strong positive
immunoreactivity of Kiss-1 and Kiss-1R was also observed in HCC
samples which showed higher Kiss-1 expression in HCC cases with
poor prognosis (34).
Pancreatic cancer
In pancreatic cancer, the loss of 6q, 8q, 9q, 17q
and 18q has been observed and this loss is associated with lymph
node and distant metastases, suggesting that there is a vital
suppressor gene existing in these regions for pancreatic cancer
(35,36). Due to the presence of the
Kiss-1 gene in the region, it was suggested that Kiss-1
expression may be downregulated in pancreatic cancer. In a
subsequent study employing a number of pancreatic cancer cell lines
(AsPC-1, BxPC-3, Capan-2, CFPAC-1, PANC-1 and SUIT-2), Masui et
al (37) found that Kiss-1
mRNA levels were reduced in pancreatic cancer, whereas Kiss-1R mRNA
expression was higher. In 2007, Liang et al (38) obtained similar results after
detecting Kiss-1 expression in pancreatic tumors induced in
Sprague-Dawley rats by in situ hybridisation. Lower levels
of Kiss-1 mRNA were observed in the pancreatic cancer tissues
compared with normal pancreatic tissues, suggesting that Kiss-1 may
be an inhibitor of pancreatic cancer metastasis (38).
Thyroid cancer
Distant metastasis is also a crucial factor for the
shortened survival of patients suffering from thyroid cancer.
Patients who develop distant metastases from thyroid cancer have a
5-year survival rate of approximately 50% (39). Previous studies have demonstrated
the importance of certain receptors involved in the metastatic
capacity of thyroid cancer cells. Through the analysis of 36
thyroid tissue samples (13 papillary carcinomas, 10 follicular
carcinomas, 2 benign follicular adenomas and 11 normal samples)
using real-time PCR, Ringel et al (21) reported that higher levels of
expression of Kiss-1 and its receptor were detected in papillary
and follicular cancers compared with normal tissue. The increased
expression of both gene products was associated with the greater
possibility of developing distant metastases (21). Furthermore, the overexpression of
Kiss-1R has been shown to result in decreased growth and migration
capabilities of thyroid cancer cells following exposure to
Kisspeptin-54 (40). The authors
found that Kisspeptin-54 increased the protein levels of
myocyte-enriched calcineurin interacting protein 1 (MCIP1), which
is known as a calcineurin inhibitor. MCIP1 expression has been
shown to be increased during the early stages of thyroid tumours
and then to be reduced or absent from lymph node metastases
(40).
Ovarian cancer
Ovarian cancer is one of the leading female cancers
globally. Data have demonstrated that 24,400 women were newly
diagnosed with ovarian cancer and almost 58% of them died from
ovarian cancer in the United States in 2003 (41). Jiang et al (42) measured 7 ovarian cancer cell lines
using real-time RT-PCR and reported that the overexpression of
Kiss-1 reduced metastatic colony formation by inhibiting cell
migration. Similar data were reported by Gao et al (43), who, using immunohistochemical
staining, analysed the expression of Kiss-1 protein in 100 primary
ovarian epithelial tumours (10 normal tissues, 20 benign adenomas,
20 borderline tumours and 50 malignant tissues). The study
demonstrated that there was a significant increase in Kiss-1
expression levels in cancerous tissues compared with benign and
normal tissue, wherease increased Kiss-1 expression in cancer cell
lines suppressed the expression of MMP-9 and NF-κB (43). Furthermore, Hata et al
(44) found that the lower
expression of Kisspeptin-54 and Kiss-1R correlated with more
deteriorative tumours and worse prognosis in 76 epithelial ovarian
cancers.
Gestational trophoblastic neoplasia
(GTN)
All forms of GTN secrete human chorionic
gonadotrophin (hCG), which is a helpful marker in the diagnosis,
staging and subsequent assessment of the therapeutic response of
malignant GTN by monitoring the serum levels of hCG. hCG levels
increase and reach a plateau in patients who are developing
malignant changes. It is important to detect the serum hCG levels
in patients with GTN. However, similar to the problems associated
with parallel assays, issues exist regarding false positives caused
by heterophile antibodies in commercially available tests (45). The research community remains
highly interested, searching for new biomarkers for tumour types.
Horikoshi et al (46)
showed that there was a significant increase in the concentration
of plasma Kiss-1 receptor (Kisspeptin-54) in pregnant females.
Dhillo et al (48)
assessed the levels of Kisspeptin-54 in GTN by determining plasma
Kisspeptin-immunoreactive (Kisspeptin IR) using chromatographic
analysis. They found that there was a fluctuation in plasma
Kisspeptin-54 levels following chemotherapy. Moreover, they
continued to determine the concentration of plasma Kisspeptin IR in
female volunteers. Together with hCG, progesterone and estradiol
levels were determined in 11 healthy non-pregnant females, 5
healthy females who had previously been pregnant, 13 healthy
females in the first trimester of pregnancy, 38 volunteers at 38
weeks of pregnancy and the same females 15 days post-partum and 11
females diagnosed with invasive GTN using radioimmunoassays and
chemiluminescent microparticle immunoassays (47). The result revealed that there was
a significant positive correlation between plasma Kisspeptin IR and
circulating levels of progesterone and oestradiol. This suggests
that plasma Kisspeptin levels may be considered as a tumour marker
in patients with malignant GTN (48).
Bladder cancer
As the fourth most common malignancy among men,
bladder cancer can be classified based on the depth of invasion.
Non-muscle-invasion (pTis, pTa and pT1) occupies 75% of
transitional cell carcinomas (TCCs), muscle infiltration (pT2–pT4)
occupies 20% and the remainder TCCs are metastatic at the time of
diagnosis (49). The 5-year
survival rate is >90% for patients presenting with localised
TCC, whilst it is only 50 and 10% for patients diagnosed with
regional and distant metastatic disease spread, respectively
(49). Similar to the effect of
suppressing cancer metastasis by Kiss-1 observed in other solid
tumours, the loss of Kiss-1 was observed in advanced bladder
tumours (3). Moreover, low levels
of Kiss-1 expression are associated with increased
histopathological stage, poor tumour cell differentiation and a
poor survival rate (3,50).
Prostate cancer
Prostate cancer is the most common malignancy and
the second leading cause of cancer-related mortality among men
older than 40 years. There are no curative therapies for advanced
prostate cancer (PCa) (51). Even
if surgical resection is successful, there is a high probability
that months or even year later, the majority of patients will
develop local recurrence or distant metastases, which are
responsible for the majority of PCa-related deaths (52,53). Due to the lack of timeliness of
the analysis of the primary tumour size and histology, the
information provided by identification and characterisation of
molecular signatures is particularly crucial for the diagnosis of
PCa (53). The downregulation of
Kiss-1 has been shown to correlate with decreased Kiss-1R
expression, which is inversely associated with clinical stage and
tumour grade. The enforced expression of Kiss-1 has been shown to
increase cell sensitisation to anoikis and chemotherapeutic drugs.
Kiss-1 suppresses the migration and invasion of prostate cancer
cells. Kiss-1 has therefore been suggested as a potential factor
for the risk assessment of PCa progression (4).
Endometrial cancer
Uterine corpus cancer is the main cause of malignant
gynaecological disease with >42,000 cases diagnosed, and is
still stably increasing each year in the United States. Cancer cell
invasion followed by metastasis profoundly affects patient
prognosis and is considered as a vital issue for the improvement of
prognosis for females diagnosed with endometrial cancer (54). Kang et al (55) assessed Kiss-1 and its receptor
expression in 92 adenocarcinomas of endometrial cancer using IHC
staining and real-time PCR. The results revealed that the low
expression of Kiss-1 and its receptor was significantly associated
with certain well known poor prognostic factors of distant
metastasis (invasion into lymphovascular space and deep myometrial
invasion) (54,55). Furthermore, based on the study of
subcutaneous xenografts established by inoculating Ishikawa cells
into female nude mice, they reported that the decreased number of
lymph node metastases of Kiss-1R + Ishikawa cells was observed in
metastin-10-treated mice, while there was no significant difference
in the tumour size between the metastin treated and non-treated
groups. This indicates that Kiss-1 affects the metastatic potential
of cancer cells rather than directly inhibiting tumour growth
(55).
The expression of Kiss-1 and Kiss-1R manifests
relative differences compared with normal tissue in a variety of
tumours, as shown by the data in Table I. As these data were collected
from different studies, it is difficult to quantitatively analyse
differences in the expression of Kiss-1 and its receptor among
different types of cancer. However, the differences in expression
between Kiss-1 and Kiss-1R in comparison with normal tissues can be
studied qualitatively in each group of cancers. The expression of
Kiss-1 and Kiss-1R in these cancers can be generally divided into 2
categories; one expresses Kiss-1 and Kiss-1R in accord compared
with the control group, but not the other. The upregulation and
downregulation of Kiss-1 and Kiss-1R expression have been indicated
in various malignancies. The reduced expression of Kiss-1 and
Kiss-1R has been detected in gastric cancer (without data on
Kiss-1R), oesophageal carcinoma, prostate cancer and endometrial
cancer (without data on Kiss-1). By contrast, the increased
expression of Kiss-1 and its receptor has also been observed in
certain solid tumours, including HCC, thyroid cancer and GTN. Of
note, several studies have presented evidence of the opposite
expression of Kiss-1 and Kiss-1R in various types of cancer (e.g.,
breast cancer and gestational trophoblastneoplasia). Additionally,
the opposite expression of Kiss-1 compared with that of Kiss-1R has
been reported in pancreatic, ovarian and bladder cancer. In
addition, Table II demonstrates
the negative regulatory effects of Kisspeptin-10 on the motility of
several cancer cell types compared with control cells (e.g.,
breast, pancreatic, ovarian, prostate and endometrial cancer)
(2,4,18,37,42,55).
 | Table IExpression of Kiss-1 and Kiss-1R in
cancer tissue compared with corresponding normal or background
tissue. |
Table I
Expression of Kiss-1 and Kiss-1R in
cancer tissue compared with corresponding normal or background
tissue.
| Tumour type | Kiss-1 | Kiss-1R | Refs. |
|---|
| Breast cancer | ↓/↑ | ↓/↑ | (18,26) |
| Gastric cancer | ↓ | | (27,28) |
| Esophageal
carcinoma | ↓ | ↓ | (30) |
| Hepatocellular
carcinoma | ↑ | ↑ ↑ | (33) |
| Pancreatic
cancer | ↓ | ↑ | (37) |
| Thyroid cancer | ↑ | ↑ | (21) |
| Ovarian cancer | ↓ | ↑ | (2) |
| Gestational
trophoblastic neoplasia | ↓/ ↑ | ↑/↓ | (48,60,61) |
| Bladder cancer | ↓ | ↑ | (3) |
| Prostate
cancer | ↓ | ↓ | (4) |
| Endometrial
cancer | | ↓ | (55,62) |
 | Table IINegative regulatory effects of
Kisspeptin-10 on the motility of several cancer cell types compared
with control cells. |
Table II
Negative regulatory effects of
Kisspeptin-10 on the motility of several cancer cell types compared
with control cells.
| Cell/tumour
types | Migration | Invasion | Proliferaiton | Refs. |
|---|
| Breast cancer | | ↓ | None | (2,18) |
| Pancreatic
cancer | ↓ | None | None | (37) |
| Ovarian cancer | ↓ | | | (42) |
| Prostate
cancer | ↓ | ↓ | | (4) |
| Endometrial
cancer | ↓ | | None | (55) |
6. Conclusion
In conclusion, although it remains controversial to
some degree, Kiss-1 has been demonstrated as a suppressor of
metastasis in the majority of cancers, including gastric cancer,
oesophageal carcinoma, pancreatic, ovarian, bladder and prostate
cancer. Correlations between the reduced expression of Kiss-1 and
poor clinical outcomes have been evident in the majority of
malignancies that have been investigated. A possible explanation
for the role played by Kiss-1 in cancer biology can be extrapolated
from the correlation between Kiss-1 and MMPs, particularly MMP-9
and MMP-2, whose significance in tumour invasion and metastasis
formation is well known (56).
Kiss-1 has been reported to negatively regulate MMP-9 expression in
ovarian tumours (43). Gao et
al reported that Kiss-1 protein formed a steady complex with
pro-MMP-2 and pro-MMP-9, which possibly had an effect on the
proteolytic processing of Kiss-1 rather than the pro-MMP processing
(43). However, there are several
reports suggesting that Kiss-1 plays contrasting roles in certain
types of cancer (e.g., HCC and breast cancer) (26,33). In these cases it is believed that
the relative expression of Kiss-1 and its receptor may be the
determining factor. The reason for the contrasting effect in the
expression of Kiss-1 and its receptor on the progression of HCC and
breast cancer in comparison with other malignant tumours remains
unknown, whereas the existence of a correlation between Kiss-1
expression and the hormonal environment has been suggested. Gottsch
et al (57) reported that
the role played by the protein product of Kiss-1 and Kiss-1R was a
vital part of the regulation of gonadotropin releasing hormone
(GnRH) secretion. Neurons expressing Kiss-1 can regulate the
release of GnRH via Kiss-1R in an autocrine or paracrine manner,
thus regulating the release of luteinizing hormone
(LH)/follicle-stimulating hormone (FSH) by effecting the pituitary
gland; the expression of Kiss-1 increases through the feedback
regulation of high estrogen levels (57). Hence, patients with HCC suffering
from liver cirrhosis, which leads to a disturbed hormonal balance,
generally show high estrogen levels, and the hyperestrogenic state
activate ERα, which binds to the Kiss-1 gene promoter, in turn
elevating Kiss-1 expression (58). The possible correlation between
Kiss-1 expression and estrogen levels may also be relevant to
breast cancer, since blocking the ER pathway has been suggested as
one of the most significant systemic therapies in breast cancer
(34). A role played by Kiss-1
and Kiss-1R in other types of cancer regulated by hormones has also
been suggested. The increasing expression of Kiss-1 has been
reported to suppress the metastasis of SKOV3 ovarian cancer cells
through the reverse effects caused by protein kinase Cα (PKCα)
(42). Consistently, this effect
has also been observed in melanoma cells (59). Taken together, the data presented
in this review demonstrate that the Kiss-1/Kiss1R complex is an
interesting molecular complex that has significant prognostic and
potential therapeutic value in cancer. The Kiss-1/Kiss-1R complex
is also an intriguing complex in cells and cell signaling; its
precise mechanisms of action warrant further investigation.
Acknowledgements
The authors wish to thank the Albert Hung Foundation
and Cancer Research Wales for supporting their study. Dr K.J. is a
recipient of the China Medical Scholarship of Cardiff
University.
References
|
1
|
McKay JA, Williams EA and Mathers JC:
Effect of maternal and post-weaning folate supply on gene-specific
DNA methylation in the small intestine of weaning and adult apc and
wild type mice. Front Genet. 2:232011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtaki T, Shintani Y, Honda S, et al:
Metastasis suppressor gene Kiss-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanchez-Carbayo M, Schwarz K,
Charytonowicz E, Cordon-Cardo C and Mundel P: Tumor suppressor role
for myopodin in bladder cancer: loss of nuclear expression of
myopodin is cell-cycle dependent and predicts clinical outcome.
Oncogene. 22:5298–5305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Jones J, Turner T, et al: Clinical
and biological significance of KISS1 expression in prostate cancer.
Am J Pathol. 180:1170–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cutait R, Calache JE, Borges JL, et al:
The value of colonoscopy in low digestive hemorrhages of
unexplained cause: analysis of 132 patients. Rev Paul Med.
96:66–68. 1980.(In Portuguese).
|
|
6
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Miele ME, Hicks DJ, et al: KiSS-1,
a novel human malignant melanoma metastasis-suppressor gene. J Natl
Cancer Inst. 88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welch DR, Chen P, Miele ME, et al:
Microcell-mediated transfer of chromosome 6 into metastatic human
C8161 melanoma cells suppresses metastasis but does not inhibit
tumorigenicity. Oncogene. 9:255–262. 1994.PubMed/NCBI
|
|
9
|
Lee JH and Welch DR: Identification of
highly expressed genes in metastasis-suppressed chromosome 6/human
malignant melanoma hybrid cells using subtractive hybridization and
differential display. Int J Cancer. 71:1035–1044. 1997. View Article : Google Scholar
|
|
10
|
Bilban M, Ghaffari-Tabrizi N, Hintermann
E, et al: Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is
a physiological invasion inhibitor of primary human trophoblasts. J
Cell Sci. 117:1319–1328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kotani M, Detheux M, Vandenbogaerde A, et
al: The metastasis suppressor gene KiSS-1 encodes kisspeptins, the
natural ligands of the orphan G protein-coupled receptor GPR54. J
Biol Chem. 276:34631–34636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miele ME, Jewett MD, Goldberg SF, et al: A
human melanoma metastasis-suppressor locus maps to 6q16.3–q23. Int
J Cancer. 86:524–528. 2000.PubMed/NCBI
|
|
13
|
Shirasaki F, Takata M, Hatta N and
Takehara K: Loss of expression of the metastasis suppressor gene
KiSS1 during melanoma progression and its association with LOH of
chromosome 6q16.3–q23. Cancer Res. 61:7422–7425. 2001.PubMed/NCBI
|
|
14
|
Goldberg SF, Miele ME, Hatta N, et al:
Melanoma metastasis suppression by chromosome 6: evidence for a
pathway regulated by CRSP3 and TXNIP. Cancer Res. 63:432–440.
2003.PubMed/NCBI
|
|
15
|
Lee DK, Nguyen T, O’Neill GP, et al:
Discovery of a receptor related to the galanin receptors. FEBS
Lett. 446:103–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muir AI, Chamberlain L, Elshourbagy NA, et
al: AXOR12, a novel human G protein-coupled receptor, activated by
the peptide KiSS-1. J Biol Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hori A, Honda S, Asada M, et al: Metastin
suppresses the motility and growth of CHO cells transfected with
its receptor. Biochem Biophys Res Commun. 286:958–963. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JH and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
19
|
Lee KH and Kim JR: Kiss-1 suppresses MMP-9
expression by activating p38 MAP kinase in human stomach cancer.
Oncol Res. 18:107–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan C, Wang H and Boyd DD: KiSS-1
represses 92-kDa type IV collagenase expression by down-regulating
NF-kappa B binding to the promoter as a consequence of Ikappa
Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem.
276:1164–1172. 2001. View Article : Google Scholar
|
|
21
|
Ringel MD, Hardy E, Bernet VJ, et al:
Metastin receptor is overexpressed in papillary thyroid cancer and
activates MAP kinase in thyroid cancer cells. J Clin Endocrinol
Metab. 87:23992002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitchell DC, Stafford LJ, Li D, Bar-Eli M
and Liu M: Transcriptional regulation of KiSS-1 gene expression in
metastatic melanoma by specificity protein-1 and its coactivator
DRIP-130. Oncogene. 26:1739–1747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marot D, Bieche I, Aumas C, et al: High
tumoral levels of Kiss1 and G-protein-coupled receptor 54
expression are correlated with poor prognosis of estrogen
receptor-positive breast tumors. Endocr Relat Cancer. 14:691–702.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kostadima L, Pentheroudakis G and Pavlidis
N: The missing kiss of life: transcriptional activity of the
metastasis suppressor gene KiSS1 in early breast cancer. Anticancer
Res. 27:2499–2504. 2007.PubMed/NCBI
|
|
25
|
Stark AM, Tongers K, Maass N, Mehdorn HM
and Held-Feindt J: Reduced metastasis-suppressor gene
mRNA-expression in breast cancer brain metastases. J Cancer Res
Clin Oncol. 131:191–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin TA, Watkins G and Jiang WG: KiSS-1
expression in human breast cancer. Clin Exp Metastasis. 22:503–511.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhar DK, Naora H, Kubota H, et al:
Downregulation of KiSS-1 expression is responsible for tumor
invasion and worse prognosis in gastric carcinoma. Int J Cancer.
111:868–872. 2004. View Article : Google Scholar
|
|
28
|
Yao HL, Yang ZL, Li YG and Liu GW: In situ
hybridization study on the expression of Kiss-1 and KAI-1
metastasis suppressor genes in gastric cancer. Zhonghua Wei Chang
Wai Ke Za Zhi. 10:274–277. 2007.(In Chinese).
|
|
29
|
Tachibana M, Kinugasa S, Dhar DK, et al:
Prognostic factors in T1 and T2 squamous cell carcinoma of the
thoracic esophagus. Arch Surg. 134:50–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikeguchi M, Yamaguchi K and Kaibara N:
Clinical significance of the loss of KiSS-1 and orphan
G-protein-coupled receptor (hOT7T175) gene expression in esophageal
squamous cell carcinoma. Clin Cancer Res. 10:1379–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cedrone A, Rapaccini GL, Pompili M, et al:
Portal vein thrombosis complicating hepatocellular carcinoma. Value
of ultrasound-guided fine-needle biopsy of the thrombus in the
therapeutic management. Liver. 16:94–98. 1996. View Article : Google Scholar
|
|
32
|
Hou YK, Wang Y, Cong WM and Wu MC:
Expression of tumor metastasis-suppressor gene KiSS-1 and matrix
metalloproteinase-9 in portal vein tumor thrombus of hepatocellular
carcinoma. Ai Zheng. 26:591–595. 2007.(In Chinese).
|
|
33
|
Ikeguchi M, Hirooka Y and Kaibara N:
Quantitative reverse transcriptase polymerase chain reaction
analysis for KiSS-1 and orphan G-protein-coupled receptor
(hOT7T175) gene expression in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 129:531–535. 2003. View Article : Google Scholar
|
|
34
|
Schmid K, Wang X, Haitel A, et al: KiSS-1
overexpression as an independent prognostic marker in
hepatocellular carcinoma: an immunohistochemical study. Virchows
Arch. 450:143–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rigaud G, Moore PS, Zamboni G, et al:
Allelotype of pancreatic acinar cell carcinoma. Int J Cancer.
88:772–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yatsuoka T, Sunamura M, Furukawa T, et al:
Association of poor prognosis with loss of 12q, 17p, and 18q, and
concordant loss of 6q/17p and 12q/18q in human pancreatic ductal
adenocarcinoma. Am J Gastroenterol. 95:2080–2085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masui T, Doi R, Mori T, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang S and Yang ZL: Expression of
KiSS-1mRNA in pancreatic ductal adenocarcinoma and non-cancerous
pancreatic tissues in SD rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
32:109–113. 2007.(In Chinese).
|
|
39
|
Singer PA, Cooper DS, Daniels GH, et al:
Treatment guidelines for patients with thyroid nodules and
well-differentiated thyroid cancer. American Thyroid Association.
Arch Intern Med. 156:2165–2172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stathatos N, Bourdeau I, Espinosa AV, et
al: KiSS-1/G protein-coupled receptor 54 metastasis suppressor
pathway increases myocyte-enriched calcineurin interacting protein
1 expression and chronically inhibits calcineurin activity. J Clin
Endocrinol Metab. 90:5432–5440. 2005. View Article : Google Scholar
|
|
41
|
Jemal A, Murray T, Samuels A, Ghafoor A,
Ward E and Thun MJ: Cancer statistics, 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar
|
|
42
|
Jiang Y, Berk M, Singh LS, et al: KiSS1
suppresses metastasis in human ovarian cancer via inhibition of
protein kinase C alpha. Clin Exp Metastasis. 22:369–376. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao GL, Liu LD, Zou XS and Chen WX:
Expression of KiSS-1, matrix metalloproteinase-9, nuclear
factor-kappaBp65 in ovarian tumour. Zhonghua Fu Chan Ke Za Zhi.
42:34–38. 2007.(In Chinese).
|
|
44
|
Hata K, Dhar DK, Watanabe Y, Nakai H and
Hoshiai H: Expression of metastin and a G-protein-coupled receptor
(AXOR12) in epithelial ovarian cancer. Eur J Cancer. 43:1452–1459.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cole LA: Phantom hCG and phantom
choriocarcinoma. Gynecol Oncol. 71:325–329. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Horikoshi Y, Matsumoto H, Takatsu Y, et
al: Dramatic elevation of plasma metastin concentrations in human
pregnancy: metastin as a novel placenta-derived hormone in humans.
J Clin Endocrinol Metab. 88:914–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cole LA and Sutton JM: Selecting an
appropriate hCG test for managing gestational trophoblastic disease
and cancer. J Reprod Med. 49:545–553. 2004.PubMed/NCBI
|
|
48
|
Dhillo WS, Savage P, Murphy KG, et al:
Plasma kisspeptin is raised in patients with gestational
trophoblastic neoplasia and falls during treatment. Am J Physiol
Endocrinol Metab. 291:E878–E884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
50
|
Nicolle G, Comperat E, Nicolaïew N,
Cancel-Tassin G and Cussenot O: Metastin (KISS-1) and
metastin-coupled receptor (GPR54) expression in transitional cell
carcinoma of the bladder. Ann Oncol. 18:605–607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Buchan NC and Goldenberg SL: Intermittent
androgen suppression for prostate cancer. Nat Rev Urol. 7:552–560.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramiah V, George DJ and Armstrong AJ:
Clinical endpoints for drug development in prostate cancer. Curr
Opin Urol. 18:303–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Plantade A, Massard C, de Crevoisier R and
Fizazi K: Locally advanced prostate cancer: definition, prognosis
and treatment. Bull Cancer. 94:F50–F61. 2007.(In French).
|
|
54
|
Creasman WT, Odicino F, Maisonneuve P, et
al: Carcinoma of the corpus uteri. FIGO 26th Annual Report on the
Results of Treatment in Gynecological Cancer. Int J Gynaecol
Obstet. 95(Suppl 1): S105–S143. 2006.PubMed/NCBI
|
|
55
|
Kang HS, Baba T, Mandai M, et al: GPR54 is
a target for suppression of metastasis in endometrial cancer. Mol
Cancer Ther. 10:580–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nomura H, Sato H, Seiki M, Mai M and Okada
Y: Expression of membrane-type matrix metalloproteinase in human
gastric carcinomas. Cancer Res. 55:3263–3266. 1995.PubMed/NCBI
|
|
57
|
Gottsch ML, Clifton DK and Steiner RA:
Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive
axis. Mol Cell Endocrinol. 254–255:91–96. 2006.PubMed/NCBI
|
|
58
|
Li D, Mitchell D, Luo J, et al: Estrogen
regulates KiSS1 gene expression through estrogen receptor alpha and
SP protein complexes. Endocrinology. 148:4821–4828. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dissanayake SK, Wade M, Johnson CE, et al:
The Wnt5A/protein kinase C pathway mediates motility in melanoma
cells via the inhibition of metastasis suppressors and initiation
of an epithelial to mesenchymal transition. J Biol Chem.
282:17259–17271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ohta S, Lai EW, Pang AL, et al:
Downregulation of metastasis suppressor genes in malignant
pheochromocytoma. Int J Cancer. 114:139–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Janneau JL, Maldonado-Estrada J, Tachdjian
G, et al: Transcriptional expression of genes involved in cell
invasion and migration by normal and tumoral trophoblast cells. J
Clin Endocrinol Metab. 87:5336–5339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang T, Zhang SL, Lin B, Meng LR and Gao
H: Expression and clinical significance of KISS-1 and GPR54 mRNA in
endometrial carcinoma. Zhonghua Zhong Liu Za Zhi. 27:229–231.
2005.(In Chinese).
|