Introduction
Osteoarthritis (OA), the most common age-related
cartilage and joint disorder (1),
is a slowly progressive degenerative disease characterized by the
degradation of the extracellular matrix (ECM) and cell death,
resulting in a gradual loss of articular cartilage integrity
(2,3). The balance of catabolism and
anabolism within chondrocytes helps to maintain the structural and
functional integrity of the ECM. During the early stages of OA
development, cartilage tissues show self-repair activity, the
volume of chondrocytes increases and proteoglycan synthesis is
accelerated. During the late stages of OA, this balance is broken
and the damaging effects of inflammation become more evident.
Chondrocytes rapidly respond to changes in the microenvironment of
the joint and regulate the dynamic equilibrium between the
degradation and synthesis of the ECM, which plays a crucial role in
the maintenance of cartilage function (4–7).
In a word, the functional changes in chondrocytes play an important
role in the degeneration of joint cartilage and chondrocyte
proliferation is one of the important factors contributing to the
maintenance of cellular function.
The cell cycle plays an important role in the
effects on chondrocyte function, which takes place in chondrocytes,
leading to division and duplication. Cell cycle control is a highly
regulated process that involves a complex cascade of events, in
which regulation can be realized through a complex network of
cyclins, cyclin-dependent kinases (CDKs) and cyclin-dependent
kinase inhibitors (CDKIs). The G1/S checkpoint that exists at the
end of the G1 stage is the key point of intracellular and
extracellular signaling which integrates into the nucleus, then
stimulates S phase cells to begin a new round of proliferation,
differentiation and death, or into the G0 phase (8,9).
Briefly, extracellular signals induce the expression of cyclin D1
in cells entering the cell cycle and this binds to and activates
CDK4 and CDK6 (10–12). The ensuing complexes in turn lead
to the phosphorylation of retinoblastoma protein (Rb), resulting in
its dissociation from the transcription factors, predominantly
members of the E2F family, which then activate the many
genes required for the progression of the cell cycle to the S phase
(10). P16, also known as
p16INK4a, a member of the INK4 family of CDKIs, inhibits
CDK4 and CDK6, maintaining Rb in its unphosphorylated
E2F-associated state and thereby preventing the
progression from the G1 to the S phase (13–14). In conclusion, the G1/S progression
is highly regulated by cyclin D1, CDK4, CDK6, Rb and p16.
Chinese herbal medicine, a major modality in
Traditional Chinese Medicine (TCM) and practiced for thousands of
years in China and other Asian countries, is used for the treatment
of arthritis and related disorders or ‘Bi syndrome’ (15,16). Herbal formulas are the common form
of administration in Chinese herbal practice and herbal formulas
have been well documented in ancient and modern literature
(17). According to Chinese
herbal theory, interactions among the different herbs in a formula
exert a synergistic effect and neutralize the potential toxic and
side-effects of the individual constituents (15,18). However, there is as yet a lack of
rigorous scientific evaluation of such formulas.
The classical formula, Duhuo Jisheng Decoction
(DHJSD), first documented by Sun (19), consists of 15 component herbs, Du
Huo (Angelica pubescens), Fang Feng (Saposhnikovia
divaricata), Chuan Xiong (Ligusticum chuanxiong), Niu Xi
(Achyranthes bidentata), Shang Ji Sheng (Loranthus
parasiticus), Qin Jiao (Gentiana macrophylla), Du Zhong
(Eucommia ulmoides), Dang Gui (Angelica sinensis), Fu
Ling (Poria cocos), Dang Shen (Codonopsis pilosula),
Shu Di Huang (Radix rehmanniae preparata), Bai Shao
(Radix paeoniae alba), Xi Xin (Asarum sieboldii), Gan
Cao (Glycyrrhiza uralensis) and Rou Gui (Cinnamomum
cassia). As a well known traditional Chinese folk medicine, it
is used for eliminating wind and dampness, relieving pain due to Bi
syndrome, tonifying the liver and kidneys and tonifying the qi and
blood; it is commonly used for the treatment of various diseases,
including OA. Previous studies have proven that DHJSD has drug- and
lead-like compounds with potential synergy and polypharmacology
against OA (20). However, to our
knowledge, the effects of DHJSD on the proliferation of
chondrocytes have not yet been reported. To further elucidate the
precise mechanisms behind the therapeutic effects of DHJSD in OA,
in the present study, we investigated the effects of DHJSD on the
proliferation of chondrocytes. We found that DHJSD promoted
chondrocyte G1/S transition, which was accompanied by the
upregulation of the expression of cyclin D1, CDK4, Rb and CDK6 and
the downregulation of p16.
Materials and methods
Materials and reagents
Cyclin D1, CDK4, CDK6, Rb and p16 antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
DNA primers were synthesized by Sangon Biotech (Shanghai, China).
The VECTASTAIN® Elite ABC kit (Rabbit IgG) and
VECTASTAIN Elite ABC kit (Mouse IgG) were provided by Vector
Laboratories, Inc. (Burlingame, CA, USA). The DAB Substrate kit was
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Papain was obtained from Sigma Chemical Co. (St. Louis, MO,
USA).
Herbal preparation
The herbs used in DHJSD, Angelica pubescens,
Saposhnikovia divaricata, Ligusticum chuanxiong, Achyranthes
bidentata, Loranthus parasiticus, Gentiana macrophylla, Eucommia
ulmoides, Angelica sinensis, Poria cocos, Codonopsis pilosula,
Radix rehmanniae preparata, Radix paeoniae alba, Asarum sieboldii,
Glycyrrhiza uralensis and Cinnamomum cassia, were prepared with
traditional methods following harvest (15) and purchased from Tong Ren Tang
Pharmaceutical Co. (Beijing, China). The 15 herbs were ground into
a powder. DHJSD was formulated by mixing herbal powders in relative
proportions according to the Chinese pharmacopoeia (19) (Table
I). Stock solutions of DHJSD were prepared by decocting the
DHJSD powder in water to a concentration of 3 g/ml and stored at
−20°C.
 | Table IComposition of Duhuo Jisheng
Decoction (DHJSD) formula. |
Table I
Composition of Duhuo Jisheng
Decoction (DHJSD) formula.
| Herb name | Relative
proportion |
|---|
| Angelica
pubescens | 3 |
| Saposhnikovia
divaricata | 2 |
| Ligusticum
chuanxiong | 2 |
| Achyranthes
bidentata | 2 |
| Loranthus
parasiticus | 2 |
| Gentiana
macrophylla | 2 |
| Eucommia
ulmoides | 2 |
| Angelica
sinensis | 2 |
| Poria
cocos | 2 |
| Codonopsis
pilosula | 2 |
| Radix rehmanniae
preparata | 2 |
| Radix paeoniae
alba | 2 |
| Asarum
sieboldii | 2 |
| Cinnamomum
cassia | 2 |
| Glycyrrhiza
uralensis | 2 |
Animals
Healthy and clean, 27 2-month-old male Sprague
Dawley rats were purchased from SLAC Laboratory Animal Co. Ltd.
(Shanghai, China) [Laboratory Animal Use Certificate no.
SCXK(SH)2007-0005] and raised in a sterile environment. The
Experimental Animal Center of Fujian University of TCM offers
sterile environment facilities, qualified number SYXK(Min)
2009-0001. All experiments involving the animals complied with the
Guidance Suggestions for the Care and Use of Laboratory Animals
(2006) administered by the Ministry of Science and Technology of
China (21).
Experimental design
Papain-induced rat OA was performed as previously
described (22), with slight
modifications. Briefly, after 1 week of acclimation, OA was induced
in the right knees of 18 rats by an injection of 0.2 ml of 4%
papain solution with 0.1 ml of 0.03 M cystein as an activator on
days 1, 4 and 7. Two weeks after papain-induced OA, the animals
were randomly divided into 3 groups: the control group (no
papain-induced OA; received an equivalent amount of saline only),
the model group (papain-induced OA; received an equivalent amount
of saline only) and the DHJSD group [papain-induced OA; received a
clinical oral dose of DHJSD (9.3 g/kg/day)]. All groups were
treated once a day for 8 consecutive weeks, following which the
animals were sacrificed and tibial plateau cartilage specimens were
obtained. The morphological changes were observed under an optical
microscope following staining with hematoxylin and eosin (H&E)
and by transmission electron microscopy (TEM). The mRNA and protein
levels of cyclin D1, CDK4, CDK6, Rb and p16 were measured by RT-PCR
and immunohistochemistry, respectively.
Optical microscopy analyses
The rats were sacrificed after being treated
consecutively with or without DHJSD for 8 consecutive weeks. The
rat tibial plateau cartilage (inside) specimens, were cut into
5×4×3 mm sections, fixed in 4% paraformaldehyde for 2 days,
decalcified in 10% EDTA for 8 weeks, dehydrated in gradient ethanol
and subsequently embedded in paraffin. The 4-μm-thick paraffin
sections were cut using a microtome, dewaxed in xylene and stained
with H&E. The changes in cartilage morphological were observed
under a microscope (Olympus, Tokyo, Japan) and images were acquired
at a magnification of ×200.
TEM
The rats were sacrificed after being treated
consecutively with or without DHJSD for 8 weeks. The tibial plateau
cartilage (outside) specimens, were cut into 1×1×2 mm sections,
pre-fixed in 4% glutaraldehyde and 1.5% paraformaldehyde solution
(pH 7.3) at 4°C for 3 days, post-fixed with 1% osmium tetroxide at
4°C for 2 h after being decalcified in 5.5% EDTA for 12 weeks at
4°C. The specimens were dehydrated with graded alcohol-acetone and
embedded in Epon-618 resin. After the 1-μm-thick resin semi-thin
sections were cut using a microtome and stained with azur-methylene
blue, the structure of the cartilage was observed and the
ultra-thin sections were observed under an optic microscope. The
70-nm ultrathin sections were cut using a Leica ultramicrotome,
stained with 2% aqueous uranyl acetate and counterstained with 0.3%
lead citrate. The ultrastructure of the articular cartilage was
observed under a transmission electron microscope (Hitachi H7650;
Shangai, China).
RNA extraction and RT-PCR analysis
Following treatment with or without DHJSD for 8
consecutive weeks, the rats were sacrificed and tibial plateau
cartilage specimens were obtained from each group. Total RNA from
the tibial plateau cartilage specimens was isolated using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) after
being grinded in liquid nitrogen using a mortar. Oligo(dT)-primed
RNA (1 μg) was reverse-transcribed using SuperScript II reverse
transcriptase (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. The obtained cDNA was used to
determine the mRNA levels of cyclin D1, CDK4, CDK6, Rb and p16 by
PCR suing Taq DNA polymerase (Fermentas/Thermo Fisher Scientific,
Pittsburgh PA, USA). β-actin was used as an internal control. The
primers and the annealing temperature (°C) used for the
amplification of cyclin D1, CDK4, CDK6, Rb, p16 and β-actin
transcripts were as follows: cyclin D1 sense, 5′-GAC ACC AAT CTC
CTC AAC GAC-3′ and antisense, 5′-AGA CAA GAA ACG GTC CAG GTA G-3′
(216 bp, 55°C); CDK4 sense, 5′-CCT ACG GAC ATA CCT GGA CAA-3′ and
antisense, 5′-GAG GCA ATC CAA TGA GAT CAA-3′ (404 bp, 55°C); CDK6
sense, 5′-GTT TCA GCT TCT CCG AGG TCT-3′ and antisense, 5′-CGT CAA
GCA TTT CAG AAG GAG-3′ (469 bp, 55°C); Rb sense, 5′-CTT TAT TGG CCT
GTG CTC TTG-3′ and antisense, 5′-ATT CCA TGA TTC GAT GCT CAC-3′
(225 bp, 55°C); p16 sense, 5′-GCT CTC CTG CTC TCC TAT GGT-3′ and
antisense, 5′-AGA AGT TAT GCC TGT CGG TGA-3′ (268 bp, 55°C);
β-actin sense, 5′-GGG AAG TGC TGG ATA G-3′ and antisense, 5′-GTG
ATG TTT CGG ATG G-3′ (453 bp, 55°C).
Immunohistochemical analysis
Following treatment with or without DHJSD for 8
consecutive weeks, the rats were sacrificed and the rat tibial
plateau cartilage (inside) specimens from each group, were cut into
5×4×3 mm sections, fixed in 4% paraformaldehyde for 2 days,
decalcified in 10% EDTA for 8 weeks, dehydrated in gradient ethanol
and embedded in paraffin. The 5-μm-thick paraffin sections were cut
using a microtome, dewaxed in xylene and analyzed by
immunohistochemistry. The sections were then incubated with sodium
citrate buffer (10 mM sodium citrate, pH 6.0) in a microwave for
antigen retrieval. Endogenous peroxidase activity of the sections
was quenched by incubating in PBS containing 0.3%
H2O2 and 0.3% Triton X-100 for 30 min
following repeated washing in PBS. Immunohistochemical staining was
performed using the VECTASTAIN elite ABC kit according to the
manufacturer’s instructions. Briefly, after blocking with normal
serum in PBS, the sections were treated with an optimal dilution of
primary antibody overnight at 4°C (cyclin D1, 1:100; CDK4, 1:150;
CDK6, 1:100; Rb, 1:80 and p16, 1:100). The sections were incubated
with a biotinylated anti-rabbit or mouse IgG antibody for 60 min
and then treated with ABC reagent for 60 min. They were finally
treated with DAB for 5 min. Subsequently, they were dehydrated with
ascending concentrations of ethanol solutions, cleared with xylene
and mounted on a coverslip using neutral gum. After staining, 5
random fields (x100) were randomly selected in each slide and the
average proportion of positive cells in each field was counted
using the true color multi-functional cell image analysis
management system. To rule out any non-specific staining, PBS was
used to replace the primary antibody as the negative control.
Statistical analysis
Data were analyzed using statistical software SPSS
13.0. All data are presented as the means ± standard deviation.
Statistical analysis of the data was performed using the Student’s
t-test and one-way analysis of variance (ANOVA). A p-value <0.05
was considered to indicate a statistically significant
difference.
Results
Effect of DHJSD on the morphology of
articular cartilage
To determine the effects of DHJSD on cartilage
morphology, the sections were stained with H&E and observed
under an optical microscope. As shown in Fig. 1A, in the control group, the 4
layer structures (including surface layer, transitional layer,
radiation layer and calcification layer) of articular cartilage
were visible. The tidal line between the radiation and
calcification layer was complete. The cement line between the
calcified layer and subchondral bone was obvious, winding. The
surface was smooth and the collagen fibers formed a complete
perichondrium. The chondrocytes appeared oblate in the surface
layer and oval in the transitional and radiation layer and were
arranged regularly, with a blue nucleus and red cytoplasm. The
cartilage matrix was purple red. However, in the model group, the
articular cartilage structure was disordered. The tidal line, as
well as the cement line between the calcified layer and subchondral
bone had disappeared and bone marrow vascular invasion into the
cartilage layer was observed. The surface layer was damaged and the
complete perichondrium had disappeared, leaving a velvet-like
structure. The number of chondrocytes on the surface and deep layer
was significantly reduced, as many cells appeared necrotic. The
cartilage capsule became large and many chondrocytes appeared
denatured or necrotic on the calcification layer. The cartilage
matrix appeared red with an increase in collagen fiber content
(Fig. 1B). However, following
treatment with DHJSD, the structure of the articular cartilage
significantly improved; 2 tidal lines, as well as the cement line
between the calcified layer and subchondral bone were obvious,
winding and no bone marrow vascular invasion into the cartilage
layer was observed. The surface was much smoother and the collagen
fibers formed a complete perichondrium, compared with the model
group. The total number of chondrocytes and regularly arranged
chondrocytes were significantly increased and cell degeneration was
significantly reduced compared with the model group. The cartilage
matrix appeared purple red (Fig.
1C). Collectively, these findings suggest that DHJSD is
effective in the treatment of OA.
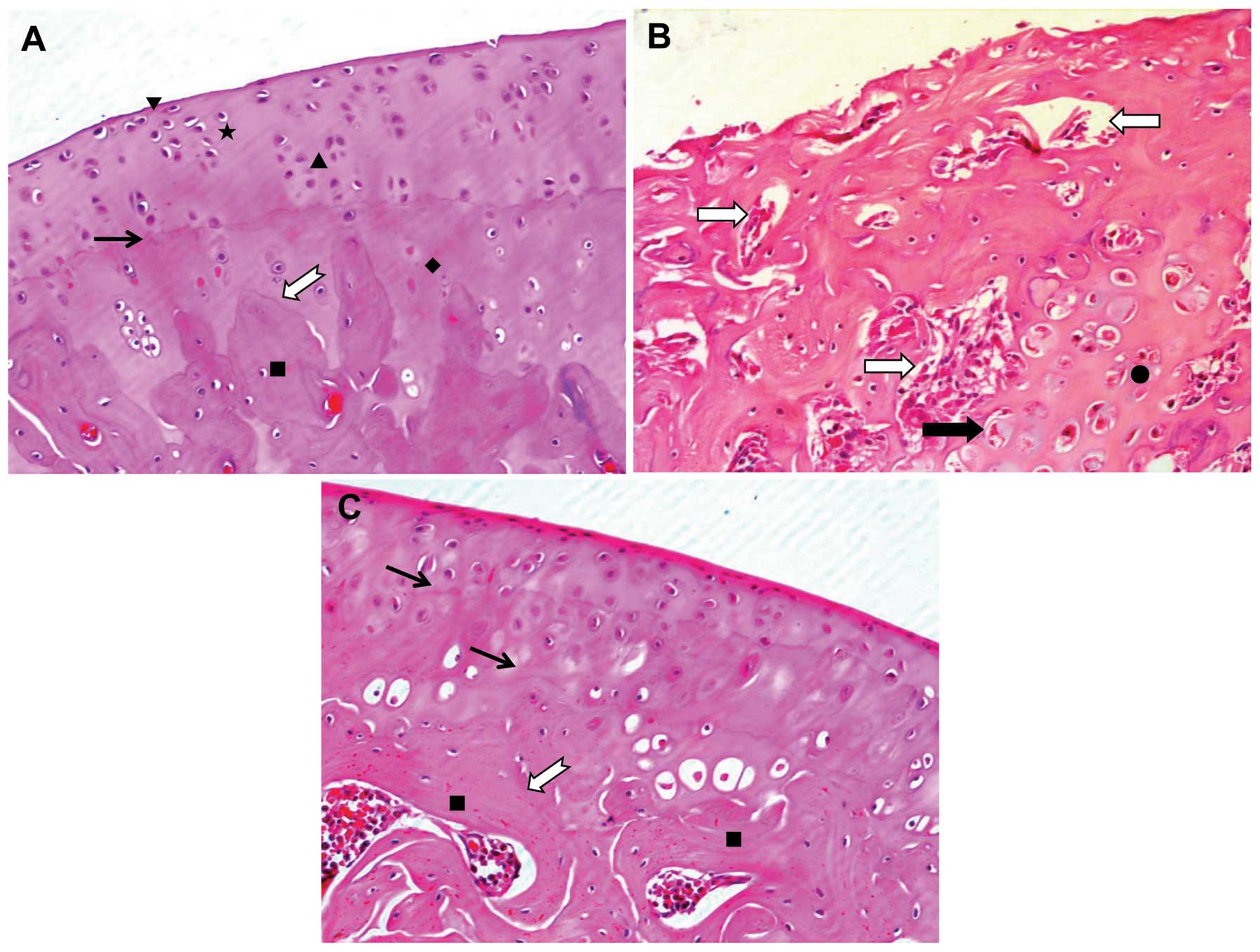 | Figure 1Effect of Duhuo Jisheng Decoction
(DHJSD) on the microstructure of cartilage tissue. Following
treatment with or without DHJSD for 8 consecutive weeks, the rats
were sacrificed and tibial plateau cartilages (inside) specimens
from each group were processed for H&E staining. (A) Control
group; (B) model group; (C) DHJSD group. The morphological changes
in the cartilage were observed under a microscope and images were
aquired at a magnification of ×200. ▼, Surface layer; ★,
transitional layer; ▲, radiation layer; ♦, calcification layer; ■,
subchondral bone; →, tidal line; ➯, cement line; ⇨, bone marrow
vascular invasion into the cartilage layer; ➡, larger cartilage
capsule on the calcification layer; ●, denatured or necrotic
chondrocytes on the calcification layer. |
Chondrocytes, the only type of cell present in
cartilage, play a central role in the equilibrium between the
anabolism and catabolism of the fundamental component of cartilage.
In order to further clarify the mechanisms through which DHJSD
exerts its therapeutic effects in OA, we observed the
ultrastructural changes in the chondrocytes by TEM. As shown in
Fig. 2A and B, in the control
group, the chondrocytes appeared to have an almost oval shape or a
triangular shape with many microvilli-like protrusions. The cells
contained more organelles, such as an abundant rough endoplasmic
reticulum, a mature Golgi apparatus, some mitochondria and glycogen
particles scattered in the cytoplasm. In the model group, many
chondrocytes appeared denatured and necrotic and the organelles
were destroyed or had disappeared (Fig. 2C and D). Following treatment with
DHJSD for 2 months, the chondrocytes contained more organelles and
the rough endoplasmic reticulum was abundant and the Golgi
apparatus was much more mature, suggesting that protein synthesis
and processing had increased; the mitochondria were abundant,
suggesting an enhanced cellular energy (Fig. 2E and F). More chondrocytes were
observed, with many near triangular homologous cells in the
cartilage, suggesting that DHJSD promoted chondrocyte proliferation
(Fig. 2G).
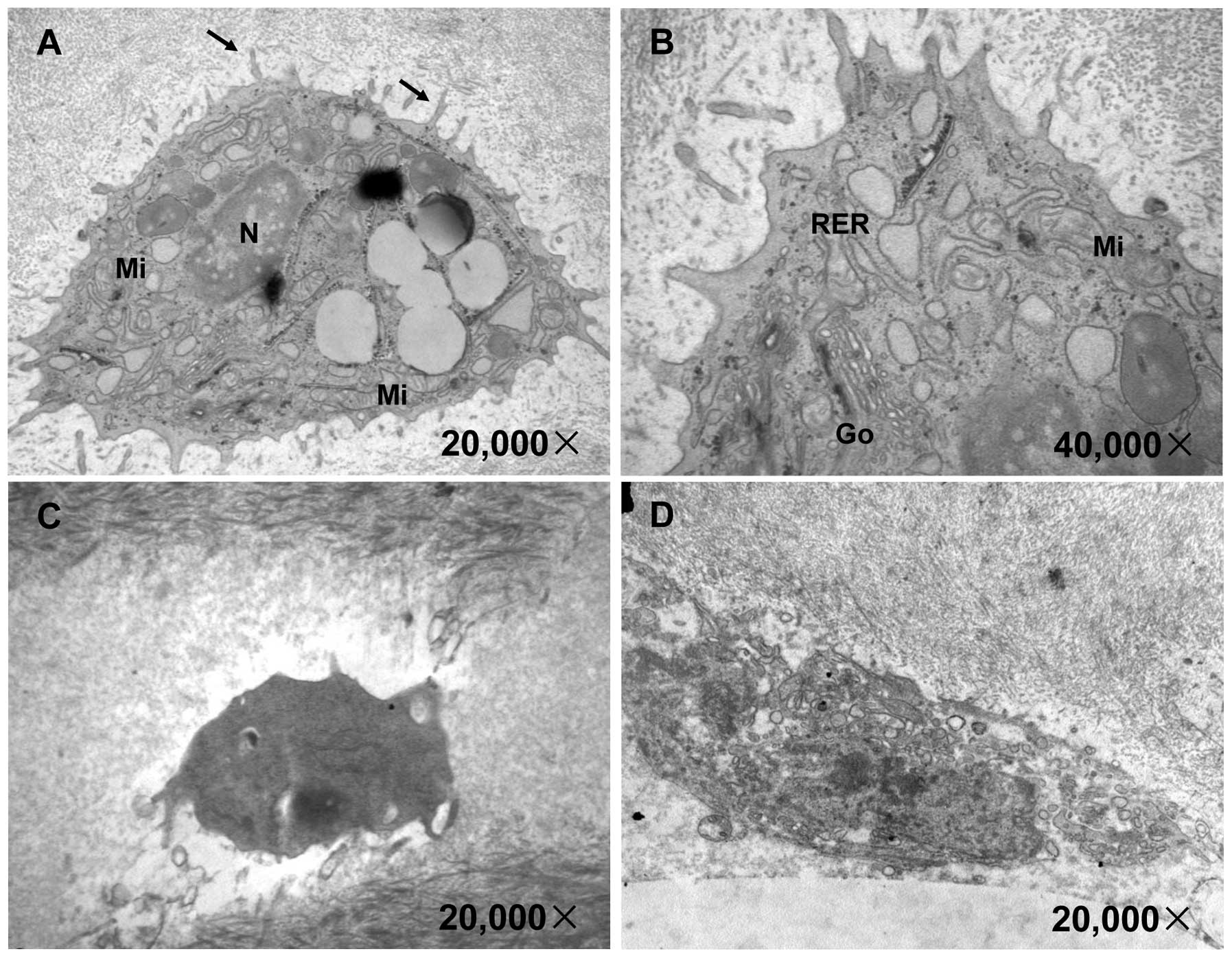 | Figure 2Effect of Duhuo Jisheng Decoction
(DHJSD) treatment on the ultrastructure of chondrocytes. Following
treatment with or without DHJSD for 8 consecutive weeks, the rats
were sacrificed and the ultrastructure of the tibial plateau
cartilage (outside) was observed under a transmission electronic
microscope. The images were acquired at a magnification of ×10,000,
×20,000 or ×40,000 magnification. (A and B) Control group; (C and
D) model group; (E, F and G) DHJSD group. N, nuclei; →,
microvilli-like protrusions; RER, rough endoplasmic reticulum; Go,
Golgi apparatus; Mi, mitochondria; ▲, homologous chondrocytes. |
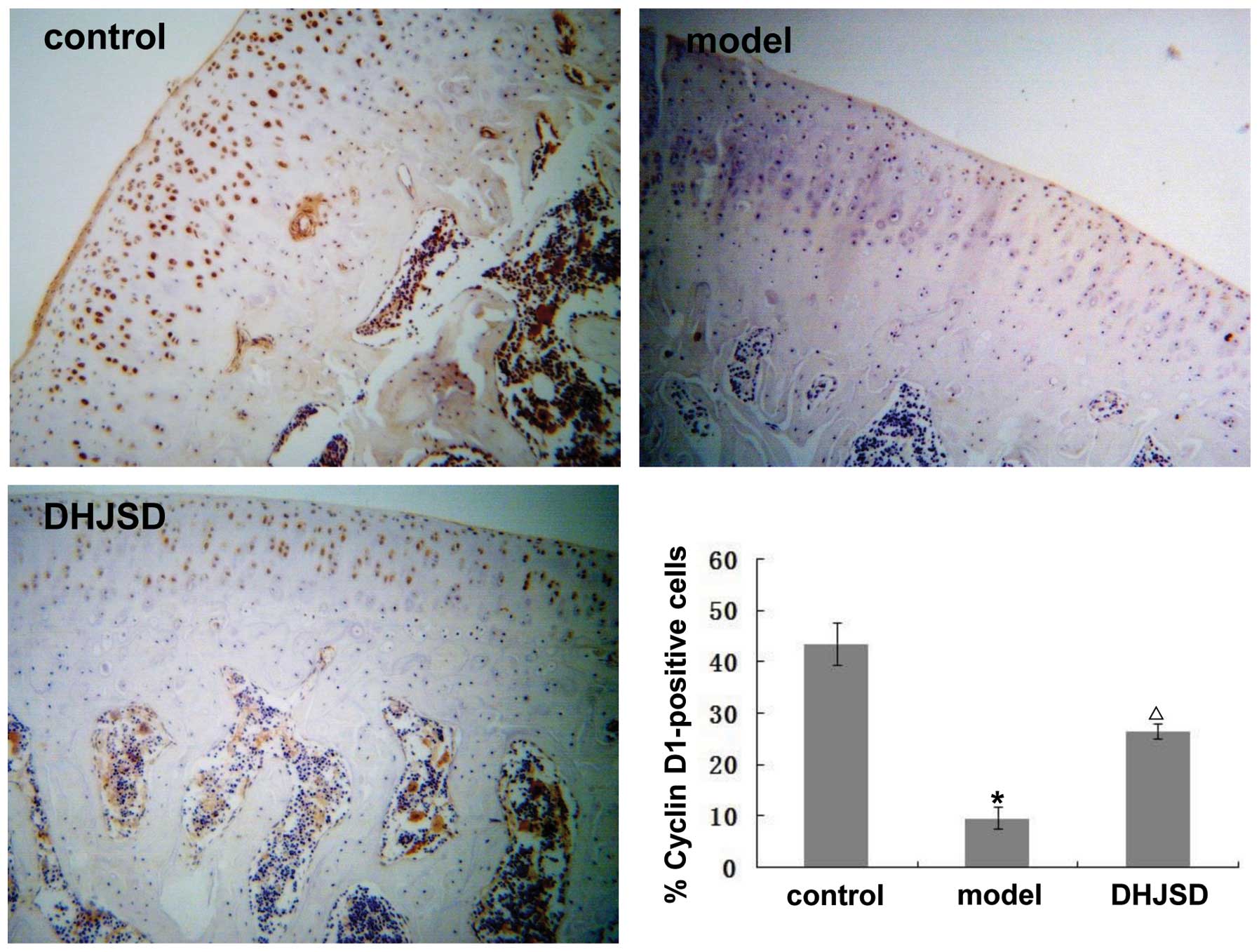
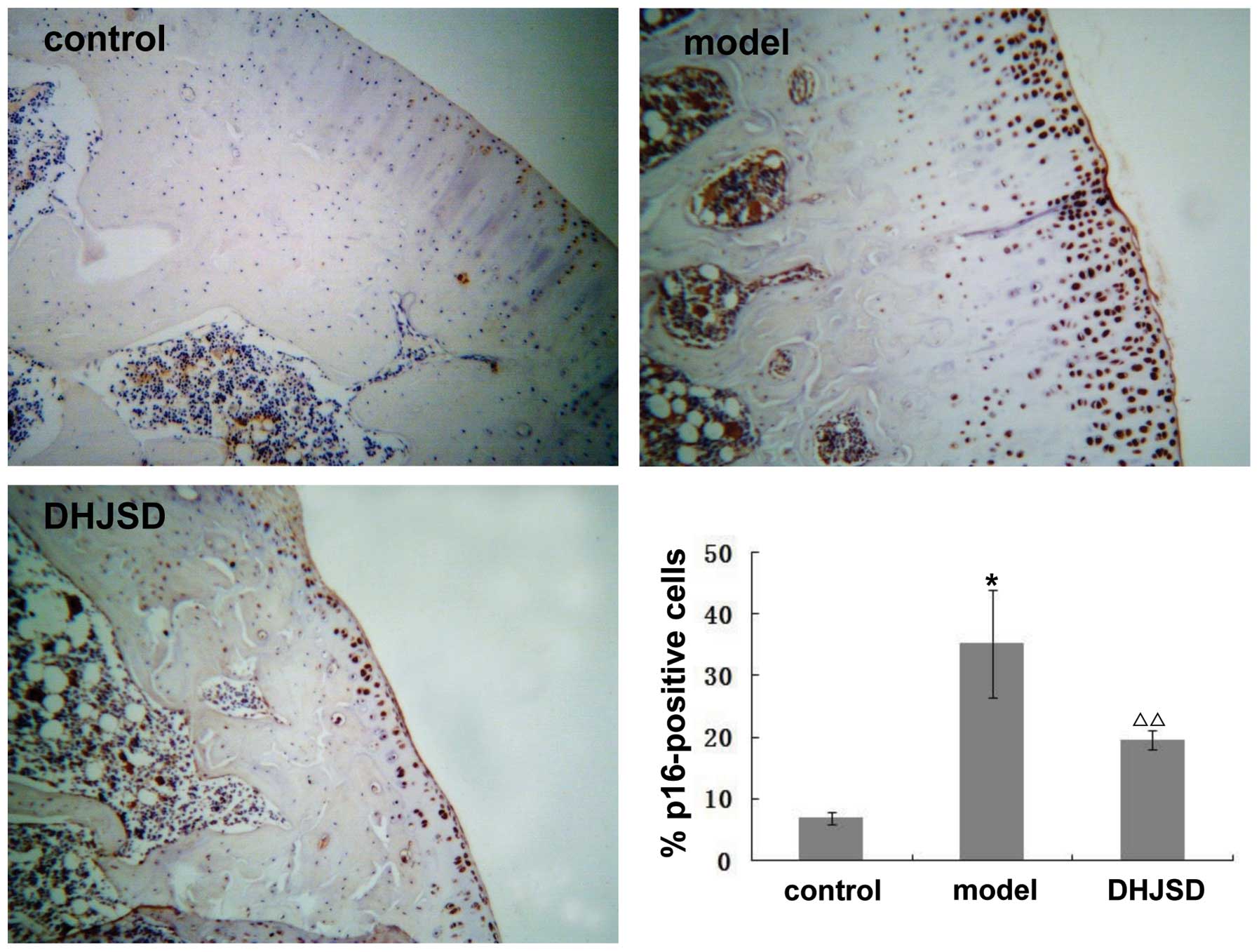
Effect of DHJSD on the expression of
cyclin D1, CDK4, CDK6, Rb and p16
It is well known that the G1/S transition is
dependent on the activity of cyclin D1-CDK4/6. Once activated,
these CDK complexes phosphorylate Rb, resulting in its dissociation
from the transcription factors, predominantly members of the
E2F family, which then activate the many genes required
for the progression of the cell cycle to the S phase. This
progression is controlled by p16, which causes transient or
permanent cell cycle arrest in cells with DNA damage. To further
explore the mechanism behind the promotion of chondrocyte
proliferation by DHJSD, we analyzed the mRNA and protein expression
levels of cyclin D1, CDK4, CDK6, Rb and p16 following treatment
with DHJSD using RT-PCR and immunohistochemistry, respectively. As
shown in Fig. 3A, the amplified
products of cyclin D1, CDK4, CDK6, Rb and p16 were clearly visible
on the agarose gel. Quantification of the PCR products indicated
that the levels of cyclin D1, CDK4, CDK6, Rb were significantly
lower, but those of p16 were higher in the model group compared
with the control group (p<0.01 or 0.05). However, following
treatment with DHJSD, the levels of cyclin D1, CDK4, CDK6, Rb
increased and those of p16 decreased, significantly versus the
model group (p<0.01 or 0.05); the protein expression pattern of
cyclin D1, CDK4, CDK6, Rb and p16 was similar to their respective
mRNA levels (Figs. 4–8). These results suggest that DHJSD
promotes chondrocyte proliferation by upregulating the expression
of cyclin D1, CDK4, CDK6 and Rb and downregulating the expression
of p16.
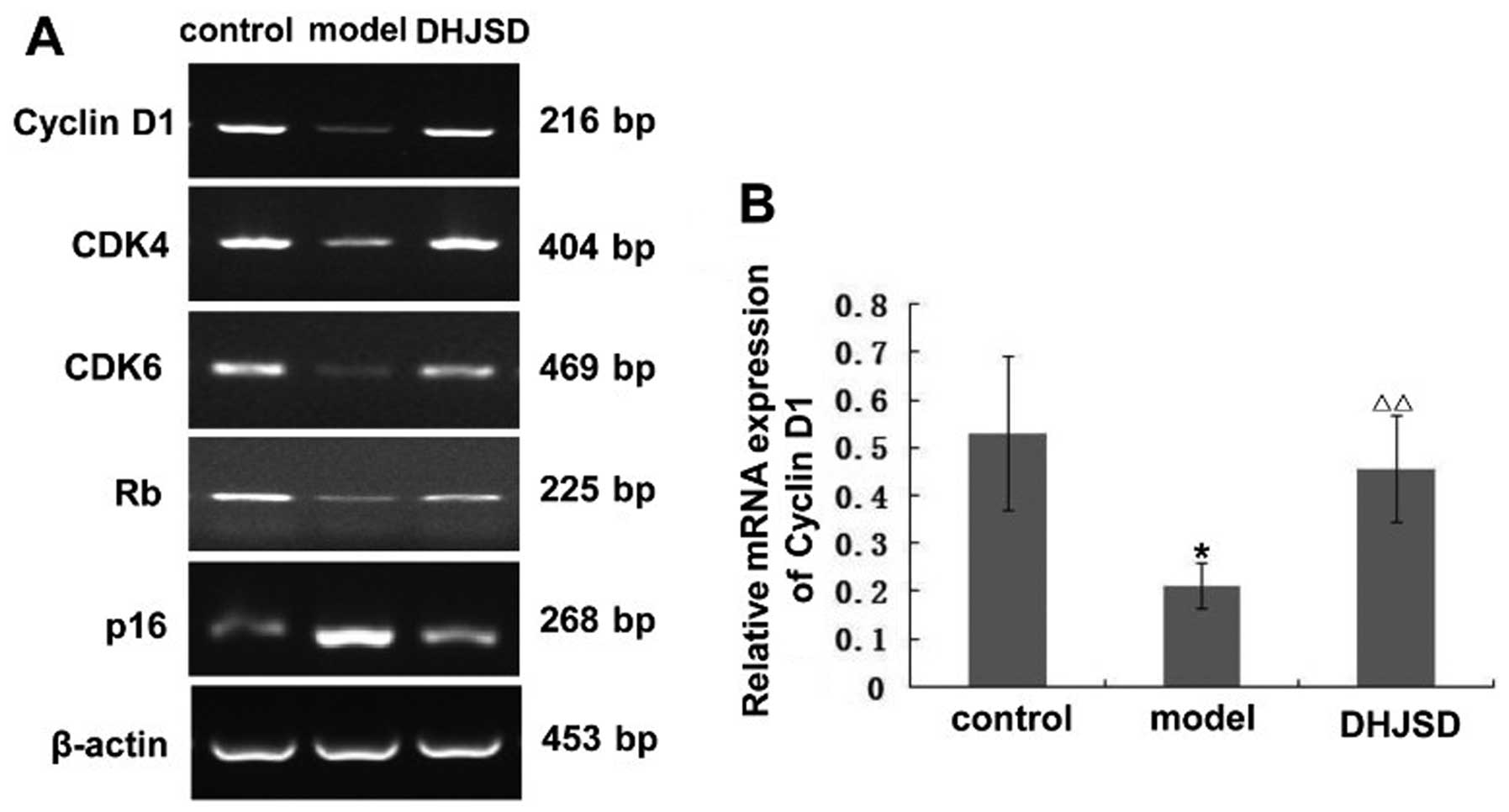 | Figure 3Effect of Duhuo Jisheng Decoction
(DHJSD) on the mRNA expression of cyclin D1, CDK4, CDK6, Rb and
p16. Following treatment with or without DHJSD for 8 consecutive
weeks, the rats were sacrificed and tibial plateau cartilage
specimens from each group were obtained. The mRNA levels of cyclin
D1, CDK4, CDK6, Rb and p16 in the chondrocytes were determined by
RT-PCR. β-actin was used as an internal control. For each group,
RT-PCR analyses were performed in triplicate, as the cartilage from
3 rats was randomly mixed together once. Data shown are the means ±
SD (error bars). (A) The representative amplified products of
cyclin D1, CDK4, CDK6, Rb and p16. The relative mRNA expression of
(B) cyclin D1, (C) CDK4, (D) CDK6, (E) Rb and (F) p16.
*p<0.01, **p<0.05, statistically
significant versus control group. Δp<0.01,
ΔΔp<0.05, statistically significant versus model
group. |
Discussion
OA is caused by multiple molecular abnormalities,
rather than being the result of a single effect (23). In this regard, the application of
combinational drugs or multi-target drugs, in which more than 2
drugs interact with multiple targets simultaneously, may be a
rational and effective treatment strategy for OA (20). Notably, TCM is a holistic approach
to health that attempts to bring the body, mind and spirit into
harmony; thus, it advocates drug-combined administrations (24). Furthermore, previous studies have
demonstrated that Chinese medicinal herbs have the potential to
ameliorate the progression of OA and such herbs have received
increasing attention (25,26).
DHJSD is a classical formula, which is commonly used for the
treatment OA. Our study demonstrates that treatment with DHJSD
promotes the cell cycle G1/S transition in chondrocytes by
upregulating the expression of cyclin D1, CDK4, CDK6 and Rb and
downregulating the expression of p16; this may be one of the
mechanisms through which DHJSD exerts its therapeutic effects.
Chondrocytes are the only cell type present in
mature cartilage; they are responsible for extracellular signals
and regulate the maintenance of cartilage homeostasis. Therefore,
the functional changes of chondrocytes play an important role by
contributing to the degradation of articular cartilage and thus to
the pathogenesis of OA (27,28). Several studies have reported that
osteoarthritic chondrocytes have a very low proliferative activity;
thus, promoting chondrocyte proliferation may be an efficient
treatment strategy to cure or delay the progression of OA (29,30). Therefore, in the present study, we
focused on chondrocyte proliferation and the anti-degenerative
effects of DHJSD on cartilage. Our microstructural observation of
cartilage tissue demonstrated that DHJSD is effective in the
treatment of OA. Using TEM, we further clarified that the
mechanisms behind the therapeutic effects of DHJSD in OA involve
the promotion of chondrocyte proliferation.
The cell cycle plays an important role in the
division and duplication of chondrocytes and can be divided into 4
phases: G1 phase, preparation for DNA synthesis; S phase, DNA
synthesis; G2 phase, preparation for mitosis; and M phase, mitosis
(31), where cell division occurs
(32). The G1/S transition is one
of the 2 main checkpoints used by a cell to regulate the
progression of the cell cycle and following that, chondrocytes can
pass through to the S phase and complete cell division (33). Progression through the G1 phase of
the cell cycle is tightly regulated by the successive activation of
D-type cyclins and CDKs (34).
Cyclin D1 forms complexes with CDK4 or CDK6, which play an
important role in the G1/S transition by phosphorylating Rb
(35). As a consequence of Rb
phosphorylation, E2F is released from the
Rb/E2F complexes and then triggers cell progression from
the G1 to the S phase (36).
During the progression of the cell cycle, CDK activity can be
blocked by the binding of CDKIs, such as p16, a potent inhibitor
protein of CDK/cyclin complexes, which plays an important role in S
phase arrest (37). In
conclusion, cell cycle regulatory factors, including cyclin D1,
CDK4, CDK6, Rb and p16 may regulate the G1/S transition in
chondrocytes. In this study, we demonstrate that treatment with
DHJSD enhances cyclin D1, CDK4, CDK6 and Rb mRNA expression and
decreases p16 mRNA expression in chondrocytes, indicating that
treatment with DHJSD promotes the progression of chondrocytes from
the G1 to the S phase by affecting cyclin D1, CDK4, CDK6, Rb and
p16 at the transcriptional level. In order to further confirm our
results, we determined the effects of DHJSD on the protein
expression of cyclin D1, CDK4, CDK6, Rb and p16 by
immunohistochemistry. The results revealed that the protein
expression of cyclin D1, CDK4, CDK6 and Rb increased and that the
expression of p16 decreased following treatment with DHJSD, which
is in accordance with the pattern of their mRNA expression.
In conclusion, to our knowledge, the data presented
in this study demonstrate for the first time that treatment with
DHJSD promotes the progression of chondrocytes from the G1 to the S
phase through the upregulation of the expression of cyclin D1,
CDK4, CDK6, Rb and the downregulation of p16, suggesting that DHJSD
may be a potential novel therapeutic agent for the treatment of
OA.
Acknowledgements
This study was supported by the Special Research
Fund for Doctor Discipline in College (20123519110001).
References
|
1
|
Heinegard D, Bayliss M and Lorenzo P:
Biochemistry and metabolism of normal and osteoarthritic cartilage.
Osteoarthritis. Brandt KD, Doherty M and Lohmander LS: Oxford
University Press; New York, NY: pp. 74–84. 1998
|
|
2
|
Pritzker K: Pathology of osteoarthritis.
Osteoarthritis. Brandt KD, Doherty M and Lohmander LS: Oxford
University Press; New York, NY: pp. 50–61. 1998
|
|
3
|
Kim HA and Blanco FJ: Cell death and
apoptosis in osteoarthritic cartilage. Curr Drug Targets.
8:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Chen L, Xu X, Li C, Huang C and
Deng CX: TGF-β/Smad3 signals repress chondrocyte hypertrophic
differentiation and are required for maintaining articular
cartilage. J Cell Biol. 153:35–46. 2001.
|
|
5
|
Iannone F, De Bari C, Scioscia C, Patella
V and Lapadula G: Increased Bcl-2/p53 ratio in human osteoarthritic
cartilage: a possible role in regulation of chondrocyte metabolism.
Ann Rheum Dis. 64:217–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou HW, Lou SQ and Zhang K: Recovery of
function in osteoarthritic chondrocytes induced by
p16INK4a-siRNA in vitro. Rheumatology. 43:555–568. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tetlow LC and Woolley DE: Histamine
stimulates the proliferation of human articular chondrocytes in
vitro and is expressed by chondrocytes in osteoarthritic cartilage.
Ann Rheum Dis. 62:991–994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hulleman E, Bijvelt JJ, Verkleij AJ,
Verrips CT and Boonstra J: Nuclear translocation of
mitogen-activated protein kinase p42MAPK during the ongoing cell
cycle. J Cell Physioll. 180:325–333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ekholm SV, Zickert P, Reed SI and
Zetterberg A: Accumulation of cyclin E is not a prerequisite for
passage through the restriction point. Mol Cell Biol. 21:3256–3265.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004.
|
|
11
|
Tashiro E, Tsuchiya A and Imoto M:
Functions of cyclin D2 as an oncogene and regulation of cyclin D1
expression. Cancer Sci. 98:629–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Poi M and Tsai MD: Regulatory
mechanisms of tumor suppressor P16(INK4A) and their relevance to
cancer. Biochemistry. 50:5566–5582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Witkiewicz AK, Knudsen KE, Dicker AP and
Knudsen ES: The meaning of p16(ink4a) expression in tumors:
functional significance, clinical associations and future
developments. Cell Cycle. 10:2497–2503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bensky D, Clavey S, Stöger E and Gamble A:
Chinese Herbal Medicine: Materia Medica. 3rd edition. Eastland
Press; Seattle, WA: 2004
|
|
16
|
Ho LJ and Lai JH: Chinese herbs as
immunomodulators and potential disease-modifying antirheumatic
drugs in autoimmune disorders. Curr Drug Metab. 5:181–192. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng HR: Grand Dictionary of Chinese
Medicinal Formula (Zhong Yi Fang Ji Da Ci Dian). 1. 1st edition.
People’s Health Press; Beijing: pp. 11131993
|
|
18
|
Scheid V, Bensky D, Ellis A and Barolet R:
Chinese Herbal Medicine: Formulas and Strategies. 2nd edition.
Eastland Press; Seattle, WA: 2009
|
|
19
|
Sun SM: Bei Ji Qian Jin Yao Fang. 8. 1st
edition. People’s Medical Publishing House; Beijing: pp. 166–167.
1982
|
|
20
|
Zheng CS, Xu XJ, Ye HZ, Wu GW, Li XH,
Huang SP and Liu XX: Computational approaches for exploring the
potential synergy and polypharmacology of Duhuo Jisheng Decoction
in the therapy of osteoarthritis. Mol Med Rep. 7:1812–1818.
2013.PubMed/NCBI
|
|
21
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance Suggestions for the Care
and Use of Laboratory Animals. 2006.
|
|
22
|
Murat N, Karadam B, Ozkal S, Karatosun V
and Gidener S: Quantification of papain-induced rat osteoarthritis
in relation to time with the Mankin score. Acta Orthop Traumatol
Turc. 41:233–237. 2007.PubMed/NCBI
|
|
23
|
Garstang SV and Stitik TP: Osteoarthritis:
epidemiology, risk factors, and pathophysiology. Am J Phys Med
Rehabil. 85(Suppl 11): S2–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan E, Tan M, Xin J, Sudarsanam S and
Johnson DE: Interactions between traditional Chinese medicines and
Western therapeutics. Curr Opin Drug Discov Devel. 13:50–65.
2010.PubMed/NCBI
|
|
25
|
Khanna D, Sethi G, Ahn KS, Pandey MK,
Kunnumakkara AB, Sung B, Aggarwal A and Aggarwal BB: Natural
products as a gold mine for arthritis treatment. Curr Opin
Pharmacol. 7:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huh JE, Lee WI, Seo BK, Baek YH, Lee JD,
Choi DY and Park DS: Gastroprotective and safety effects of
WIN-34B, a novel treatment for osteoarthritis, compared to NSAIDs.
J Ethnopharmacol. 137:1011–1017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shortkroff S and Yates KE: Alteration of
matrix glycosaminoglycans diminishes articular chondrocytes’
response to a canonical Wnt signal. Osteoarthritis Cartilage.
15:147–154. 2007.PubMed/NCBI
|
|
28
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang JG, Xia C, Zheng XP, et al:
17β-Estradiol promotes cell proliferation in rat osteoarthritis
model chondrocytes via PI3K/Akt pathway. Cell Mol Biol Lett.
16:564–575. 2011.
|
|
30
|
Kashiwagi A, Schipani E, Fein MJ, Greer PA
and Shimada M: Targeted deletion of Capn4 in cells of the
chondrocyte lineage impairs chondrocyte proliferation and
differentiation. Mol Cell Biol. 30:2799–2810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang M, Xie R, Hou W, et al: PTHrP
prevents chondrocyte premature hypertrophy by inducing
cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation
and proteasomal degradation. J Cell Sci. 122:1382–1389. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HP, Chen YL, Shen HC, Lo WH and Hu YC:
Baculovirus transduction of rat articular chondrocytes: roles of
cell cycle. J Gene Med. 9:33–43. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Löwenheim H, Reichl J, Winter H, et al: In
vitro expansion of human nasoseptal chondrocytes reveals distinct
expression profiles of G1 cell cycle inhibitors for replicative,
quiescent, and senescent culture stages. Tissue Eng. 11:64–75.
2005.
|
|
34
|
Aszodi A, Hunziker EB, Brakebusch C and
Fässler R: Beta1 integrins regulate chondrocyte rotation, G1
progression, and cytokinesis. Genes Dev. 17:2465–2479. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh S, Johnson J and Chellappan S: Small
molecule regulators of Rb-E2F pathway as modulators of
transcription. Biochim Biophys Acta. 1799:788–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhaduri S and Pryciak PM: Cyclin-specific
docking motifs promote phosphorylation of yeast signaling proteins
by G1/S Cdk complexes. Curr Biol. 21:1615–1623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu D, Liu J, Lin B, et al: Lewis y
regulate cell cycle related factors in ovarian carcinoma cell RMG-I
in vitro via ERK and Akt signaling pathways. Int J Mol Sci.
13:828–839. 2012. View Article : Google Scholar : PubMed/NCBI
|