Introduction
There has been controversy as to the safety of
ultrasound for prenatal diagnostics (1). The potential biological effects of
exposure to ultrasound on the embryo have gained increasing
attention. Certain studies however, have stated that it may not
damage the embryo, particularly during the first trimester of
pregnancy and within diagnostic dose ranges (2). A previous study proved that
diagnostic ultrasound can lead to apoptosis in chorionic villi
during early pregnancy rats; however, due to the existence of
anti-apoptotic factors, exposure to ultrasound at certain doses
causes no real damage to chorionic villi (3). This suggests that exposure to
diagnostic ultrasound deos not harm embryonic development. The
potential damage of diagnostic ultrasound to the embryo still
cannot be ignored in spite of human epidemiological studies not
having found any detrimental effects on later fetal development
(1).
In the process of embryonic development, a number of
factors can protect the embryo from the adverse effects of the
outside environment and ensure the normal development of the
embryo. Heat shock protein 70 (HSP70) is closely associated with
embryonic development and plays an important role in embryonic
development (4). Previous studies
have suggested that a number of mechanisms are involved in
HSP70-mediated embryonic protection, including acting as a
molecular chaperone involved in the folding of nascent and
mis-folded proteins under non-stressful conditions, enhancing
tolerance to heat stress, inhibiting apoptosis induced by variety
of adverse factors, resisting damage from oxygen-free radicals and
a role in immune protection (5).
To date, to our knowledge, there have been no
studies on HSP70 expression in chorionic villi exposed to
ultrasound. This study investigates the effects of exposure to
diagnostic ultrasound on chorionic villi by examining HSP70
expression and apoptosis in chorionic villi during early pregnancy
in rats exposed to ultrasound in order to provide an experimental
basis for its safe application in clinical practice.
Materials and methods
Animal housing and specimen
collection
A total of 70 adult female SPF Wistar rats (weight,
230–350 g; age, 12–16 weeks) were provided by the Animal Experiment
Center of Southern Medical University, Guangzhou, China. The rats
were then mated. They were fed a standard diet and maintained in a
temperature- and light-controlled room (21–27°C, 12/12 h light/dark
cycle, relative air humidity 40–70% and well ventilated with low
noise) in accordance with the Animal Research Committee Guidelines
of the Women and Children’s Hospital of Guangdong Province.
Pessaries were used to indicate the beginning of conception. The
pregnant rats were exposed to diagnostic ultrasound approxiamtely
6–7 days after conception. The inspection procedure was as follows:
the rats were fixed on the examination table. After local
disinfection, the body surface corresponding to the uterus was
exposed to an ultrasound instrument [Acuson 128XP/10: frequency,
2.5 MHz; spatial peak temporal average intensity (Ispta), 92
mW/cm2; spatial peak, pulse average intensity (Isppa),
104 mW/cm2; maximal intensity (Im), 266
mW/cm2] and the existence of the embryo was confirmed.
The rats were divided into four groups according to the duration of
exposure: 0 min (group A), 10 min (group B), 20 min (group C) and
30 min (group D). The pregnant rats in each group were sacrificed
24 h after exposure and the chorionic villi were collected.
Portions of the harvested tissues were flash frozen in liquid
nitrogen for 30 min and cryopreserved, while the remaining tissues
were fixed in 10% formaldehyde solution and paraffin embedded for
future immunohistochemical staining and the detection of apoptosis.
This study was approved by the Animal and Human Ethics Board of the
Women and Children’s Hospital of Guangdong Province.
Methods
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUDP nick-end labeling (TUNEL) assay
Apoptotic cells in the chorionic villi were detected
using the In Situ Cell Apoptosis Detection kit (MK1024; Wuhan
Boster Biological Engineering, Co., Ltd., Wuhan, China), with at
least five replicates for each group. Reagents were prepared
according to the manufacturer’s instructions. The fixed chorionic
villi were treated with 3% H2O2 for 10 min at
room temperature, washed three times and incubated in
permeabilization buffer for 30 min at 37°C. The chorionic villi
were then washed three times in phosphate-buffered saline (PBS) and
incubated with 50 μl TUNEL reagents for 2 h at 37°C in the dark.
The positive controls were incubated with 100 μl 1000 U/ml DNase 1
(Sigma), which cleaves all DNA, for 20 min at 37°C and washed three
times before TUNEL assay. The megative controls were incubated in
PBS in the absence of TdT. The samples then were incubated in
blocking reagent for 30 min at 37°C in the dark, followed by the
addition of 1:100 anti-DIG-biotin and 1:100 streptavidin peroxidase
for 30 min at 37°C. After TUNEL assay, the chorionic villi were
washed three times and counterstained with 50 μg/ml PI for 5 min at
room temperature to label all nuclei. Finally, the chorionic villi
were mounted on glass slides with 1,4-diazabicyclo[2.2.2]octane
(DABCO) and examined under a light microscope. The positive
reactants were noted as brown particles localized in the nuclei.
Positive criteria included separated and disorganized cells,
morphology of apoptotic nuclei (pyknotic nuclei, condensation and
margination of nuclear chromatin or nuclear fragmentation) and no
inflammatory responses around the cell. Those positive cells
without the morphology of apoptotic nuclei were not judged to be
apoptotic cells unless their staining intensity strongly contrasted
with background and the cells were singly distributed. Positive
cells were counted by a double-blinded method. Ten non-overlapping
fields were randomly observed on each slide. The percentage of
positive cells was defined as the apoptotic index (AI).
Immunohistochemistry
Protein expression was detected using the the SP
immunohistochemistry kit (Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China). Briefly, paraffin-embedded samples were
sectioned (4 μm), patched and dewaxed. Sections placed in 0.01 M
citric acid (pH 6.0) buffer were heated for 5 min for antigen
retrieval and subsequently blocked for 10 min with
H2O2. They were then incubated overnight at
4°C with primary antibodies, goat polyclonal anti-HSP70 antibody
(sc-1060) at 1:100, mouse monoclonal anti-Bcl-2 antibody (sc-7382)
at 1:100 and mouse monoclonal anti-Bax antibody (sc-7480) at 1:100
(all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
After rinsing with PBS, biotinylated goat anti-rabbit IgG secondary
antibodies were applied for 1 h, followed by streptavidin
peroxidase complexes for 30 min. Diaminobenzidine was used as the
final chromogen, and the sections were counterstained with
hematoxylin. The slices with positive expression were used as the
positive control. The negative control was established with the
primary antibody replaced by PBS. All methods were performed
according to the manufacturer’s instructions. Positive staining
presented as faint yellow, brown orange, or dark brown staining in
the cytoplasm and/or nucleus of the cells. The expression of HSP70,
Bcl-2 and Bax was semi-quantitatively analyzed by integrated
optical density (IOD) calculated using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Observation under an electron microscope
(EM)
For observation under an EM, the placental tissue
samples fixed in 3.0% glutaraldehyde were washed in cold 0.1 M
phosphate buffer (pH 7.4) for 3 h at 4°C and post-fixed in 1%
osmium tetroxide, buffered with 0.1 M phosphate, dehydrated in
graded alcohols. The tissue was embedded in epoxy resin. Sections
(1-μm-thick) were obtained using an ultramicrotome, stained with
uranyl acetate and lead citrate, and examined undr an EM (Philips
Tecnai; Philips, Eindhoven, The Netherlands).
RT-PCR analysis
Villous tissue was homogenized and total RNA was
then extracted using TRIzol reagent (Life Technologies Corp.,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
RNA quality was checked by running on RNase-free, 1% agarose gel
electrophoresis, and a UV300 type ultraviolet spectrophotometer
(Unicam) calculating the spectrophotometric absorbance parameters
of A260/A280 nm, where the ratio was between 1.8 and 2.0. RT-PCR
was employed to determine the expression levels of the HSP70,
Bcl-2, Bax genes using RT-PCR reagent kit (Takara Bio, Inc., Shiga,
Japan). cDNA was prepared from the total RNA (2.0 μg) using
oligo(dT) primers. The total reaction system is 29 μl including 4
μl reverse transcription product as the template. The primers were
designed on the basis of the published sequence of HSP70, Bcl-2,
Bax and β-actin. Cycling conditions were 95°C for 10 min followed
by 40 cycles at 95°C for 15 sec, 59°C for 10 sec and 72°C for 40
sec. Finally, elongation was completed at 72°C for 60 sec. For each
PCR product the melting curve was determined and the curve analysis
for each primer set showed only one peak per product. To determine
the relative quantification of gene expression for the target and
reference (β-actin) genes, the comparative threshold cycle number
(2−ΔΔCt) method was used after a validation experiment
demonstrated that the efficiencies of the target and reference
(β-actin) genes were approximately equal. Ct values define the
threshold cycle of PCR at which amplified products were detected.
ΔCt is the difference in the threshold cycles for targets and
β-actin (internal control) (8).
Each reaction was performed in triplicate and included a
non-template control. The nucleotide sequences of the primers used
in our study are presented in Table
I.
 | Table IOligonucleotide primers used for
RT-PCR in this study. |
Table I
Oligonucleotide primers used for
RT-PCR in this study.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) | Authors/(Refs.) |
|---|
| HSP70 |
TTGTCCATGTTAAGGTTTTGTGGTATA |
GTTTTTTTCATTAGTTTGTAGTGATGCAA | Le Masson and
Christians (6) |
| Bcl-2 |
CCTGTGGATGACTGAGTACC |
GAGACAGCCAGGAGAAATCA | Sharma et al
(7) |
| Bax |
GTTTCATCCAGGATCGAGCAG |
CATCTTCTTCCAGATGGT | Sharma et al
(7) |
| β-actin |
ATGGAATCCTGTGGCATCCA |
TCCACACAGAGTACTTGCGCTC | Sharma et al
(7) |
Western blot analysis
Tissue homogenates were centrifuged and proteins
were extracted. The concentration of protein in each sample was
determined using the Bradford formula. The samples were then mixed
directly with gel running buffer (Life Technologies Corp.).
Proteins were resolved by 10% SDS-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene fluoride (PVDF)
membranes (Life Technologies Corp.). The membranes were blocked for
1 h at room temperature and then incubated with primary antibodies
(at 1:1,000 for anti-HSP70, 1:200 for anti-Bcl-2 and 1:200 for
anti-Bax, respectively) at 4°C overnight, and a goat-anti human IgG
peroxide-labeled antibody was used together with the ECL
chemiluminescent system for detection using a gel imaging analysis
system (Bio-Rad). β-actin (Santa Cruz Biotechnology, Inc.) was used
as the loading control.
Statistical analysis
Data were analyzed using SPSS17.0 statistical
software. Quantitative values are presented as the means ± standard
deviation (SD). Significant differences were assessed by a one-way
ANOVA analysis. Comparisons between groups were carried out using
the LSD method except that the Dunnett T3 method was used for data
with unequal variances. A P-value refers to a comparison of a
measured parameter in the experimental group with that of the
appropriate control. P<0.05 was considered to indicate a
statistically significant difference.
Results
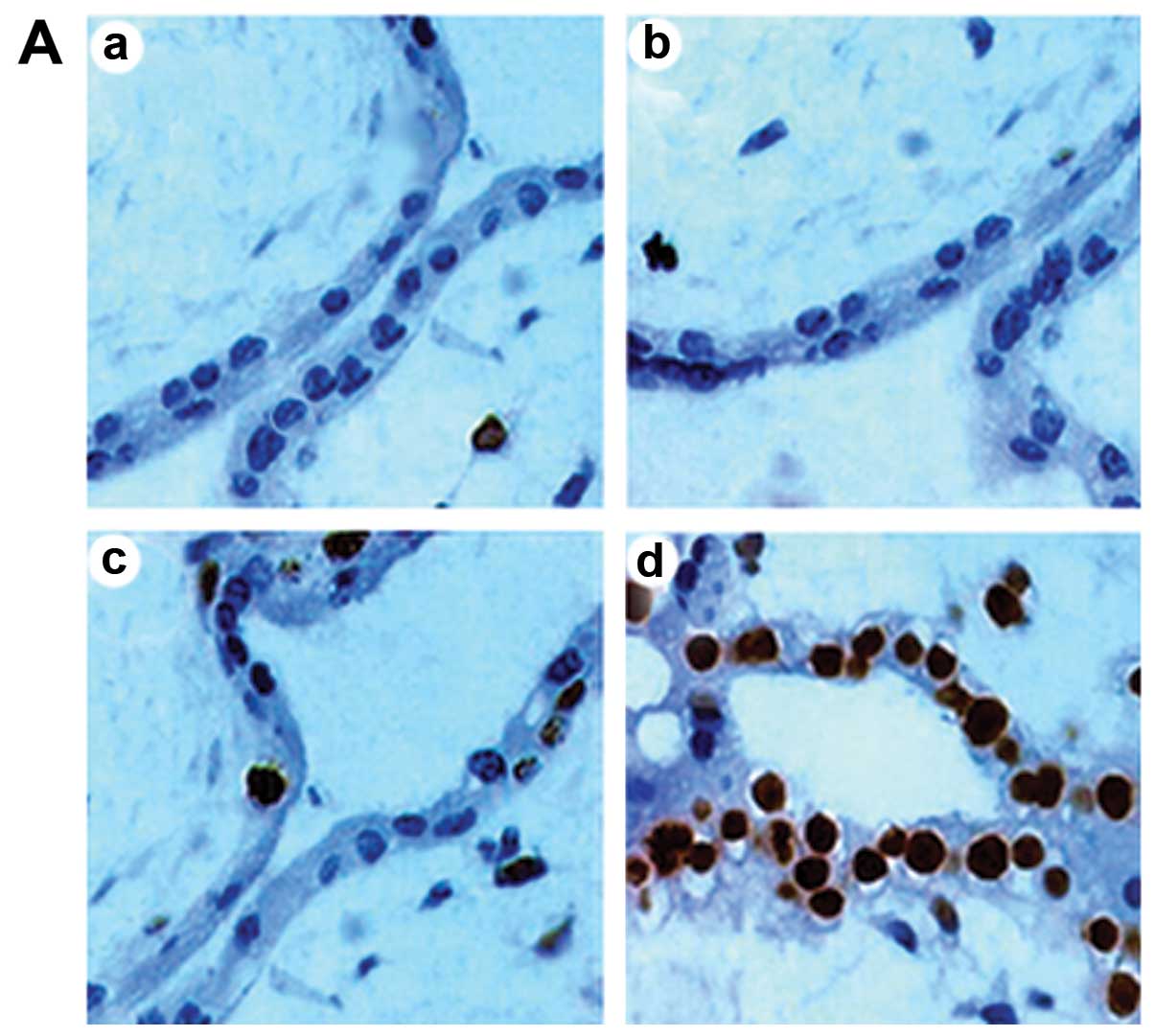
Cell apoptosis
TUNEL-positive cells were mainly observed in the
syncytiotrophoblast in chorionic villi, while they were also found
in interstitial cells and vascular endothelial cells in the villi
stroma, with a few in the cytotrophoblast. Compared with the
unexposed pregnant rats, there was no significant change in AI in
the groups exposed for 10 and 20 min (P>0.05); however, the
number of apoptotic cells in the group exposed for 30 min
significantly increased (P<0.05) (Fig. 1).
Expression of proteins in chorionic villi
by immunohistochemistry
HSP70 protein was mainly expressed in the cytoplasm.
There were small amounts of HSP70 observed in the unexposed group,
and the expression intensity of HSP70 gradually increased with the
prolonged exposure time (P<0.05). Bcl-2 protein was mainly
expressed in the cytoplasm. There was some amount of Bcl-2 in the
unexposed group, and the expression of Bcl-2 significantly
decreased following exposure to ultrasound (P<0.05), although
the levels did not differ between the groups exposed for 10 and 20
min (P>0.05). By contrast, the expression of Bcl-2 was almost
not observed in the group exposed for 30 min. Likewise, Bax protein
was also mainly expressed in the cytoplasm. There were small
amounts of Bax in the unexposed group, and the expression intensity
of Bax gradually increased with the prolonged exposure time
(P<0.05) (Fig. 2).
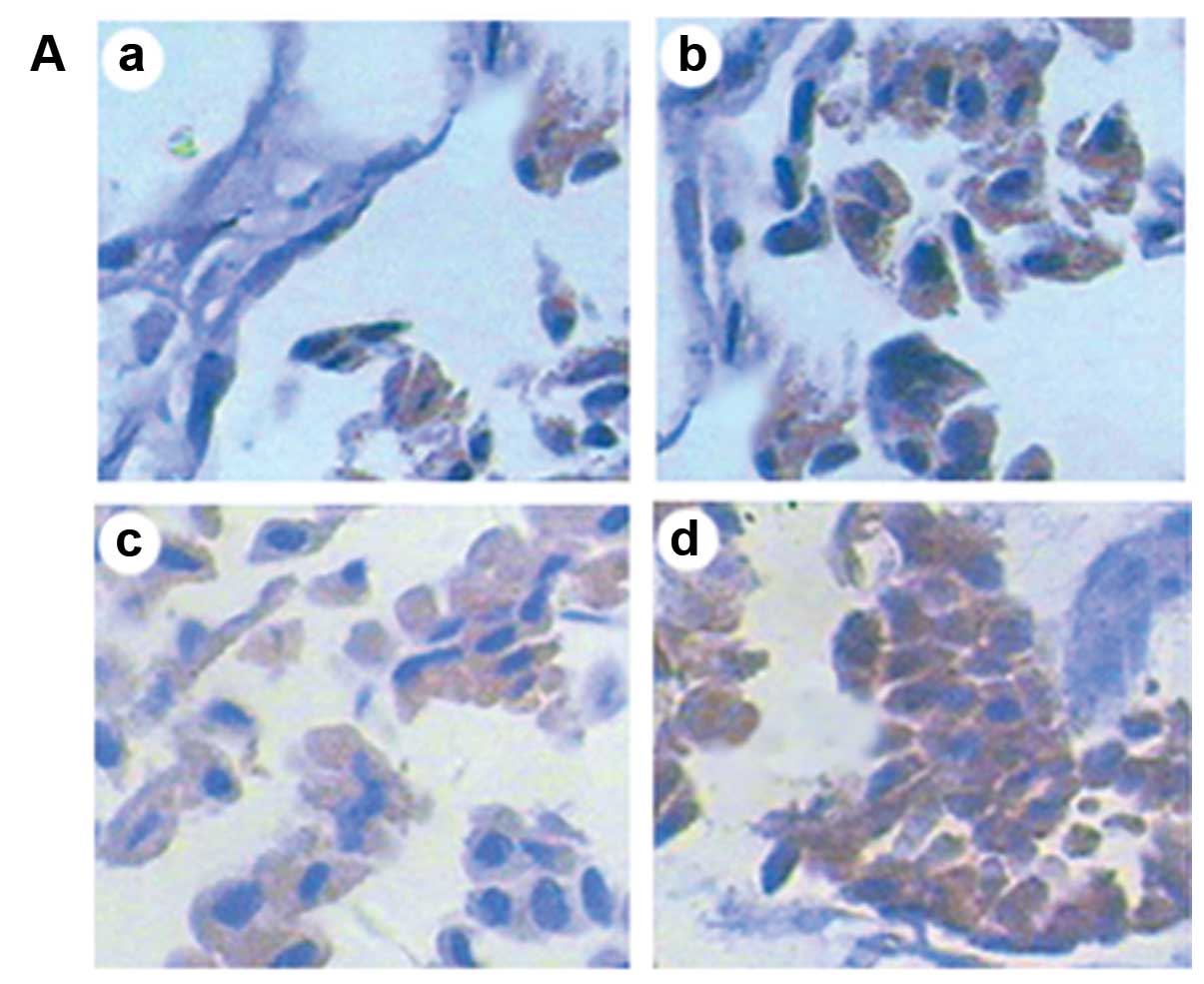 | Figure 2Protein expression analyzed by SP
immunohistochemistry (magnification, ×400). (A) Protein expression
of HSP70 in chorionic villi in the different groups following
exposure to ultrasound. There were small amounts of HSP70 in the
unexposed group, and the expression intensity of HSP70 gradually
increased with the prolonged exposure time. (B) Protein expression
of Bcl-2 in the different groups Bcl-2 protein was mainly expressed
in the cytoplasm. There was some amount of Bcl-2 in the unexposed
group, and the expression of Bcl-2 significantly decreased
following exposure to ultrasound, although the levels did not
differ between the groups exposed for 10 and 20 min. The expression
of Bcl-2 was almost not found in the group exposed for 30 min. (C)
Protein expression of Bax in the different groups. Bax protein was
also mainly expressed in the cytoplasm. There were small amounts of
Bax in the unexposed group, and the expression intensity of Bax
gradually increased with the prolonged exposure time. a, group A
(unexposed group); b, group B (exposed for 10 min); c, group C
(exposed for 20 min); d, group D (exposed for 30 min). (D)
Integrated optical density (IOD) of each protein in the different
groups (compared to other groups, *P<0.05; * vs.
&, P<0.05; & vs. &, P>0.05). |
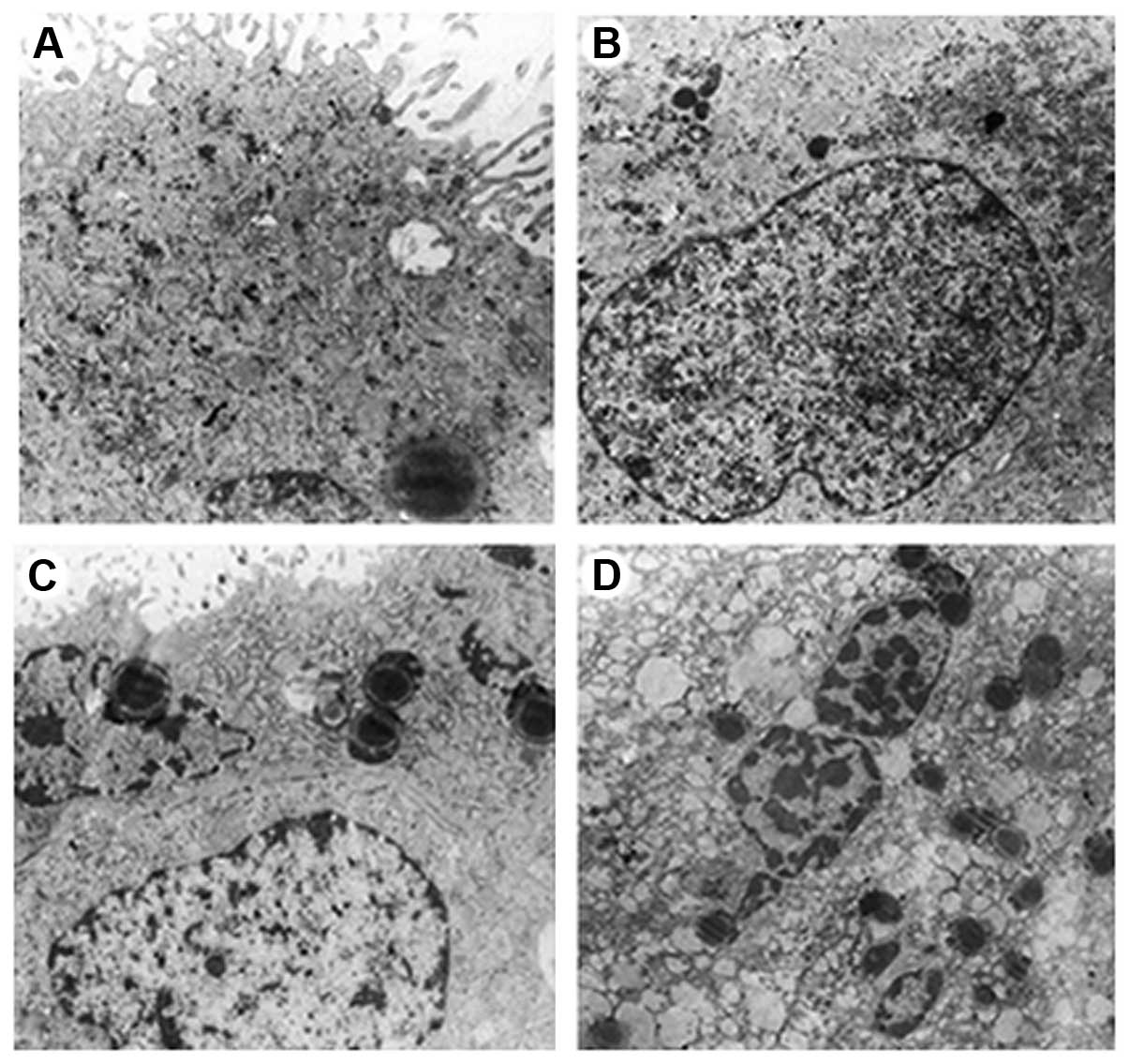
Ultrastructural findings
In the unexposed group, both the syncytiotrophoblast
and cytotrophoblast cells were characterized by round nuclei,
nuclear membrane integrity and an obvious nucleolus, in which
chromatin was uniformly distributed. Additionally, in the
cytoplasm, there are abundant Golgi complexes, scattered glycogen
particles, round or oval mitochondria and a rough endoplasmic
reticulum with the ribosomes adhering on their surface. The
intercellular junctions were tight (Fig. 3A).
In the group exposed for 10 min, both the
syncytiotrophoblast and cytotrophoblast cells were still
characterized by nuclei with a regular shape, a mildly swollen
nuclear membrane and a still obvious nucleolus, in which chromatin
was still uniformly distributed and nuclear pyknosis was
occasionally observed. Additionally, in the cytoplasm, there were
slightly decreased Golgi complexes and glycogen particles, round or
oval but mildly swollen mitochondria and a mildly expanded rough
endoplasmic reticulum with the ribosomes on the surface falling
off. There were also occasional lipid droplets and vacuoles. The
intercellular junctions were still tight. Generally, compared with
the unexposed group, there were no significant differences in the
structure of chorionic villi in the group exposed for 10 min
(Fig. 3B).
In the group exposed for 20 min, both the
syncytiotrophoblast and cytotrophoblast cells were still
characterized by nuclei with a regular shape, a mildly swollen
nuclear membrane and a still complete nucleolus, in which chromatin
was not very uniformly distributed, including a part of lump-like
euchromatin and margination of heterochromatin and nuclear pyknosis
was occasionally observed. Additionally in the cytoplasm, there
were slightly decreased Golgi complexes and glycogen particles,
round or oval but mildly swollen mitochondria and a mildly
expansive rough endoplasmic reticulum with the ribosomes on the
surface falling off. There were also slightly increased lipid
droplets and vacuoles. The gap between the intercellular junctions
was slightly widened. Generally, compared with the unexposed group,
there were no significant differences in the whole structure of
chorionic villi in the group exposed for 20 min (Fig. 3C).
In the group exposed for 30 min, both the
syncytiotrophoblast and cytotrophoblast cells were characterized by
nuclei with an irregular shape, an obviously swollen nuclear
membrane and a nucleolus which had disappeared, in which chromatin
was very unevenly distributed, including large amount of lump-like
euchromatin and margination of heterochromatin and nuclear pyknosis
was widely observed. Additionally in the cytoplasm, there were
obviously decreased Golgi complexes and clustered glycogen
particles, obviously swollen mitochondria with decreased and
irregularly arranged cristae and a clearly expanded rough
endoplasmic reticulum with the ribosomes on the surface falling
off. There were also significantly increased lipid droplets and
vacuoles. The gap between the intercellular junctions widened and
looked blurry. Typical apoptotic bodies were observed, which
contained some organelles, such as mitochondria. Generally,
compared with the unexposed group, there were significant changes
of in the structure of chorionic villi in the group exposed for 30
min that indicated damage (Fig.
3D).
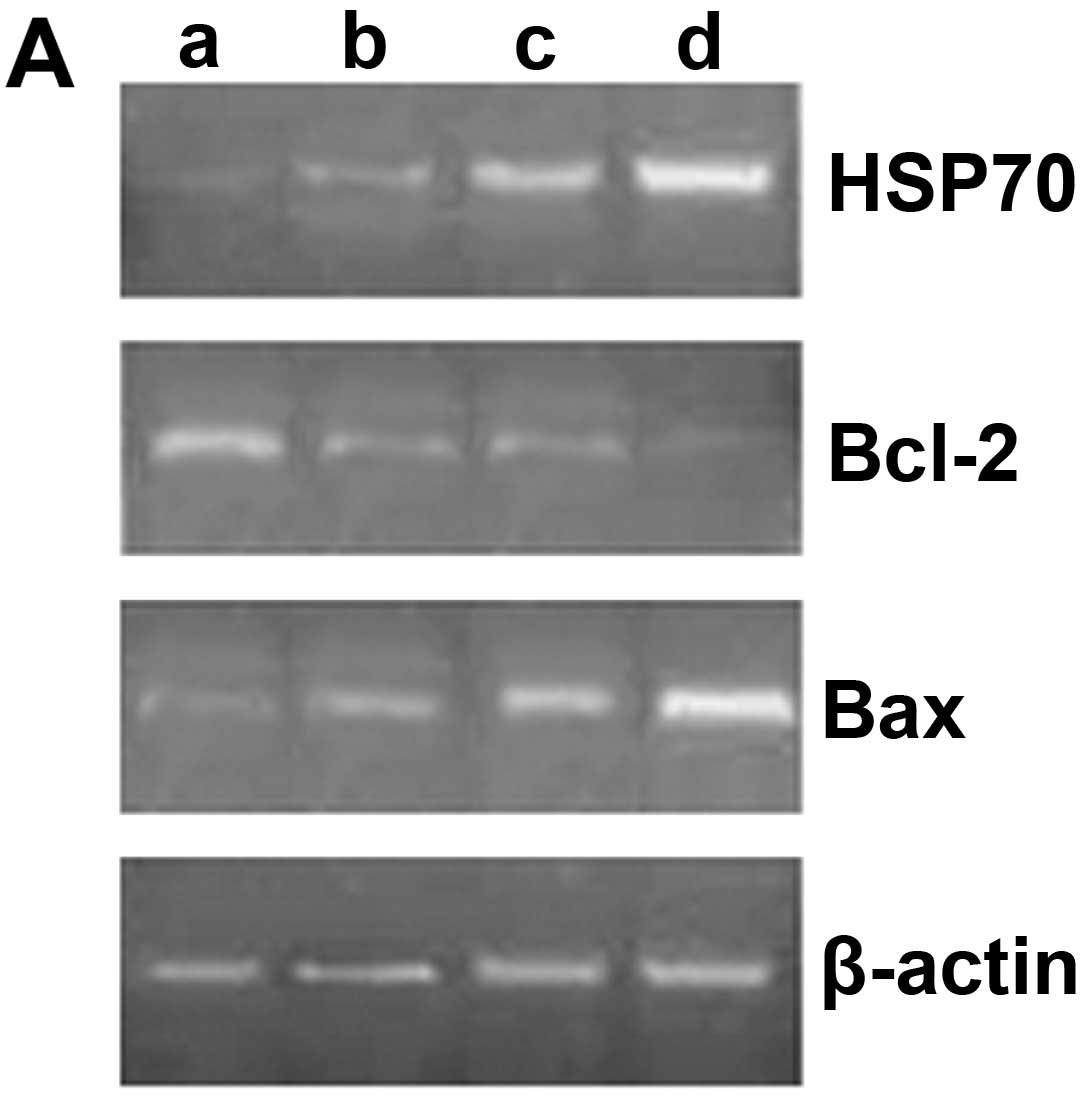
Expression of HSP70, Bcl-2 and Bax
mRNA
Compared with the unexposed group, the mRNA
expression of HSP70 gradually increased with the prolonged exposure
time (P<0.05). The mRNA expression of Bcl-2 significantly
decreased following exposure to ultrasound (P<0.05), although
there were no significant differences between the groups exposed
for 10 and 20 min (P>0.05). The mRNA expression of Bcl-2 was
lower in the group exposed for 30 min than in the groups exposed
for 10 and 20 min (P<0.05). Likewise, the mRNA expression of Bax
gradually increased with the prolonged exposure time (P<0.05)
(Fig. 4).
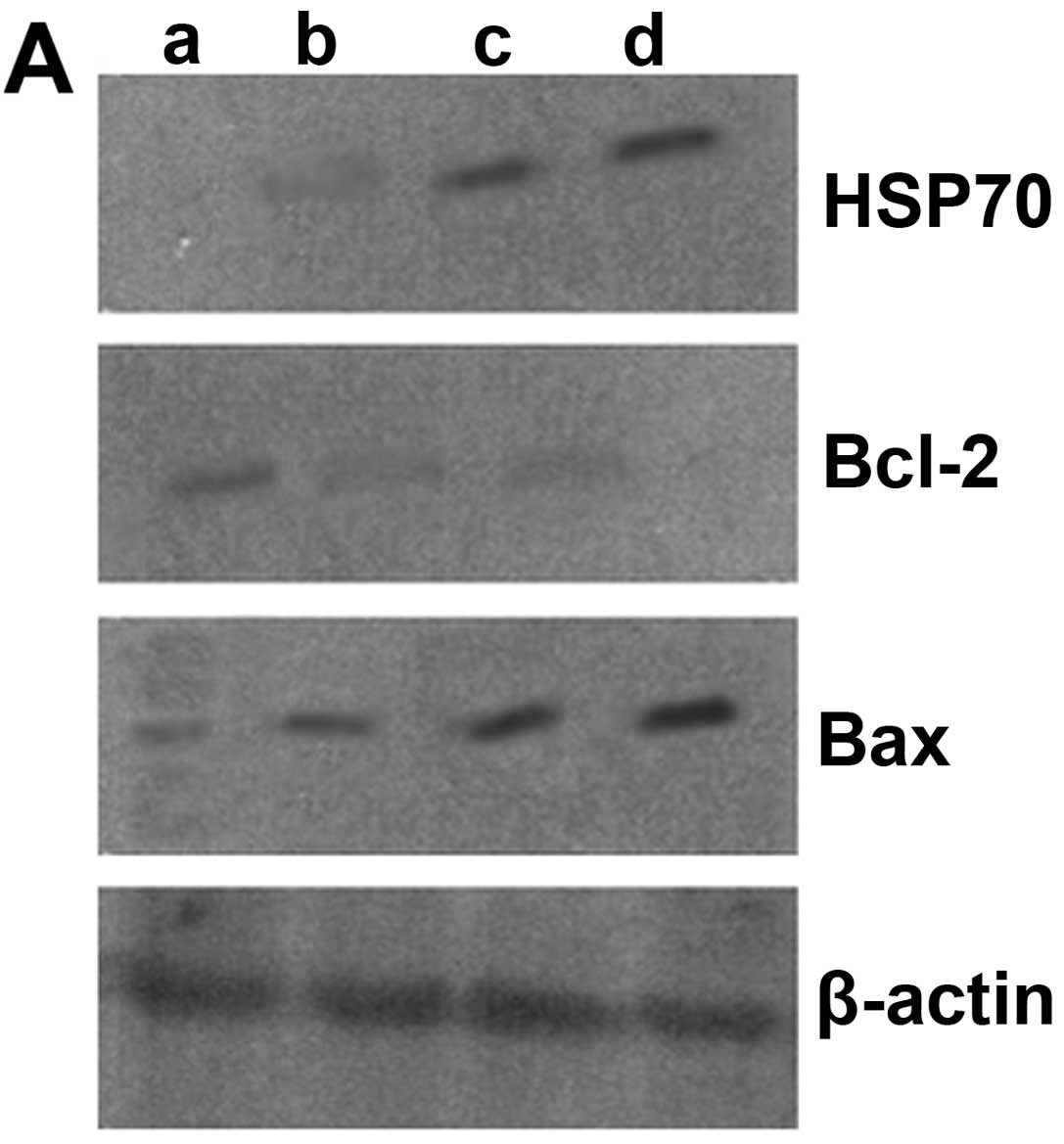
Protein expression in chorionic villi as
measured by western blot analysis
Compared with the unexposed group, the protein
expression of HSP70 gradually increased with the prolonged exposure
time (P<0.05). The protein expression of Bcl-2 significantly
decreased following exposure to ultrasound (P<0.05), although
there were no significant differences between the groups exposed
for 10 and 20 min (P>0.05). The protein expression of Bcl-2 was
lower in the group exposed for 30 min than in the groups exposed
for 10 and 20 min (P<0.05). Likewise, the protein expression of
Bax gradually increased with the prolonged exposure time
(P<0.05) (Fig. 5).
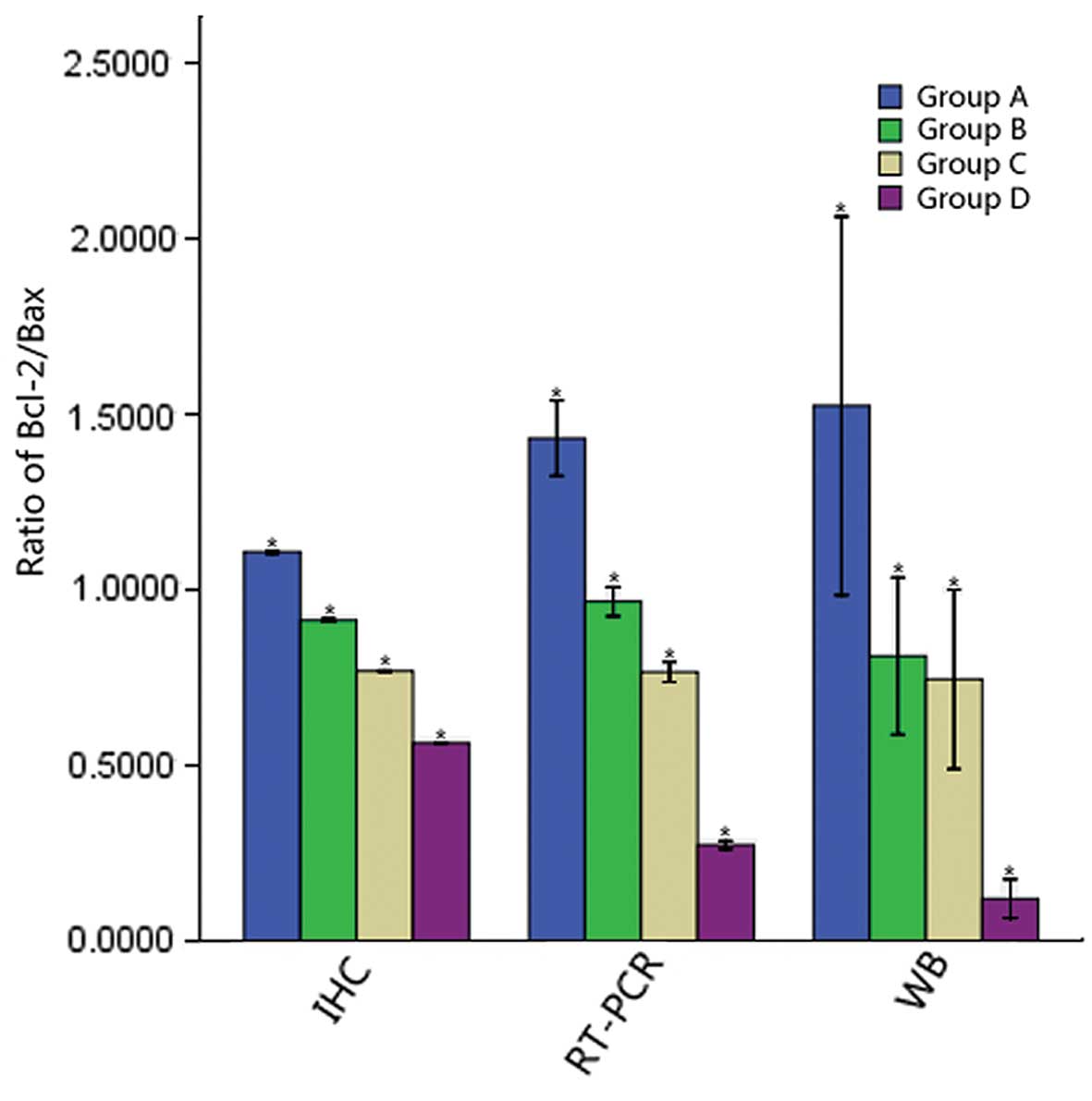
Ratio of Bcl-2/Bax in the different
groups
With the prolonged exposure time, the ratio of
Bcl-2/Bax in the different groups gradually decreased (P<0.05)
(Fig. 6).
Discussion
The mechanisms of interaction of ultrasound with
tissue that may lead to biological effects are often broadly
divided into two categories, thermal and non-thermal (9). The absorption of energy within
tissue leads primarily to a rise in local temperature when a
certain tissue is exposed to ultrasound. The magnitude of the
thermal effect of ultrasound is related to organizational
characteristics (including density, absorption coefficient, thermal
conductivity and local blood perfusion), ultrasonic intensity and
the time of ultrasonic exposure per unit volume, as well as
ultrasonic pulse repetition frequency. For example, the thermal
effects of ultrasound on bone are the most significant, and these
effects are related to its dense structure and good conductivity,
followed by the tendons and adipose tissue.
The non-thermal effects of ultrasound refer to the
‘mechanical’ effects of ultrasonic pressure waves through tissue,
mainly the cavitation effect. The cavitation effect refers to
various dynamic behaviors of gas bubbles in a certain sound field.
The negative pressure in an ultrasonic pulse can draw gas out of a
solution in tissue. These gas bubbles grow from existing nuclei in
tissue. Once a bubble has been formed, it may expand and contract,
in what are often termed breathing oscillations, in response to the
pressure wave (non-inertial or stable cavitation), or if at a size
that is resonant for the drive frequency of the ultrasound beam,
the bubble will greatly expand, followed by rapid contraction
(inertial cavitation) and break up thereby damaging local tissue
(1). To date, the cavitation
effects of diagnostic ultrasound are generally thought not to have
detrimental impacts on tissue while its damage to local tissue is
mainly caused by the thermal effects (2,10).
Previous studies on ultrasonic damage to the embryo
have been frequently reported. An older study found that the body
weight of newborns of cynomolgus macaques exposed to ultrasound
with a 7.5 MHz scanhead significantly decreased during the first
three months after birth with short-term abnormal behavior
(11). In the study by Ang et
al (12), the authors found
that the distribution of neurons of the cerebral neocortex in
newborn mice differed significantly from control mice on the 10th
day after birth when exposed to ultrasound wave for a total of 30
min or longer during the period of neuronal migration. Md Dom et
al (13) suggested that
parathyroid hormone (PTH) levels in rabbit fetal bodies exposed to
ultrasound at different gestational stages were significantly
decreased. A study on human chorionic villi indicated that the
cleavage products of active caspase-3 and cytochrome c
release were significantly increased in a time-dependent manner
when exposed to transvaginal ultrasound in the first trimester of
pregnancy (14). Moreover, the
degree of damage diagnostic ultrasound has on the embryo differ at
various developmental stages and tissues, in which embryo
pre-implantation and the nervous system are the most sensitive
stage and tissue, respectively (2,15,16).
For the mammalian embryo to successfully complete
development, it must not only incur proper timing of internal
machinery, but also protect itself from potentially harmful
external stimuli. To counterbalance these potential detriments,
embryonic cells have some complex protective machinery (17). Early pregnancy is a key stage of
embryonic development and the period during which the embryo is the
most sensitive to risk factors. Thus, it is crucial to investigate
the roles of this protective machinery during early pregnancy.
Heat shock proteins (HSPs) are highly conserved
cellular stress proteins present in every organism from bacteria to
man and are meant to protect cells from damage. HSPs can be broadly
placed into five major families according to their molecular
weight, amino acid sequence homologies and functions: HSP100
family, HSP90 family, HSP70 family, HSP60 family and the small HSP
family (18). Previous studies
have indicted that HSPs may play important protective roles in
animal embryonic development. Zhang et al (19) found that more than ten HSP genes
had differential expression between degenerate cattle embryos and
blastocysts, whereas others were not expressed at all. This
suggests the important roles of HSPs in fertilization and early
development of the embryo. King et al (20) found that the small heat shock
protein, p26, aids the development of encysted Artemia
embryos, prevents spontaneous diapause termination and protects
against stress. A study on embryonic stem cells (ESCs) suggested
that HSP90 is essential for mouse ESC pluripotency by regulating
multiple pluripotency factors, including Oct4, Nanog and signal
transducer and activator of transcription 3 (STAT3) (21).
HSPs almost exist in all cells in an organism. The
HSP70 family represents one of the most highly conserved classes of
the HSPs, of which they are also the most abundant and appear the
most when stress occurs (22).
Under normal circumstances, there is a small amount of HSP70
expressed in the cytoplasm, whereas its synthesis rapidly increases
with stress, the amount of which may account for 15% of total
proteins in the stressed cells (23). The factors inducing HSP70
expression include changes in temperature, the presence of free
oxygen radicals, viral and bacterial infections, heavy metals,
ethanol and ischemia (24). HSP70
is crucial for the maintenance of cellular homeostasis during
normal cell growth and for survival during and after various
cellular stresses (25). In 1998,
Neuer et al (26)
indicated that mouse embryonic growth was markedly inhibited when
monoclonal anti-HSP70 antibodies were supplemented to mouse embryos
at the 2-cell stage, thus proving that HSP70 plays important roles
in protecting normal embryonic development.
In the present study, we investigated the
correlation between HSP70 and apoptosis in chorionic villi when the
rat uterus was exposed to diagnostic ultrasound for different
periods of time. The expression of HSP70 gradually increased with
the prolonged exposure time. After 20 min of exposure, apoptosis
and the ultrastructure of the exposed chorionic villi did not
differ significantly from the unexposed pregnant rats, whereas the
ultrastructure of the chorionic villi was remarkably damaged with
obvious apoptosis following exposure to ultrasound for 30 min.
Functionally, Bcl-2 and Bax are opposing genes, of
which the former may maintain mitochondrial homeostasis, blocking
the release of cytochrome c, inhibiting apoptosis, whereas
the latter may increase mitochondrial permeability, enhance the
release of cytochrome c and promote apoptosis. The ratio of
Bcl-2/Bax is usually used to judge the presence of apoptosis
(27). Our results demonstrated
that the expression of Bcl-2 in chorionic villi significantly
decreased when exposed to ultrasound, although the levels did not
significantly differ between the groups exposed for 10 and 20 min.
By contrast, the expression of HSP70 gradually increased with the
prolonged exposure time. Additionally, the ratio of Bcl-2/Bax
gradually decreased with the prolonged exposure time, indicating a
gradual increase in pro-apoptotic factors over time.
Based on the above results, it can by hypothesized
that HSP70 may play important protective roles in rat chorionic
villi exposed to ultrasound, protecting them from apoptosis. The
thermal effects of ultrasound may increase the expression of HSP70,
thereby protecting chorionic villi from apoptosis induced by heat
stress. The regulation of Bcl-2 and Bax by HSP70 may be one of the
important mechanisms by which HSP70 inhibits apoptosis. A previous
study found that the gene transfer of HSP72 can protect the cornu
ammonis 1 region of the hippocampus from global cerebral ischemia,
partly by increasing Bcl-2 expression (28). Nagashima et al (29) suggested that HSP70 inhibits the
activation of Bax through the suppression of the JNK/BIM pathway,
thereby playing an anti-apoptotic role. The findings regarding the
expression of Bcl-2 and Bax in the present study also indicate
their regulation by HSP70. However, over a prolonged exposure to
ultrasound, the pro-apoptotic factors become gradually predominant,
which may prevail over the protective effects of HSP70. This
results in the characteristic apoptotic and ultrastructural changes
in the chorionic villi following exposure to ultrasound for 30
min.
However, the overexpression of HSP70 also has
detrimental effects on the embryo. Previous studies have suggested
that HSP70 expression in the rat embryo in a stressed environment
is frequently accompanied by the abundant co-expression of c-fos
and the c-myc gene, which may interfere with the normal procedure
of embryonic organogenesis. This may, however, also reflect damage,
restoration and the regeneration of cells and tissues in the embryo
(30,31). In this study, as described above,
the chorionic villi in the group exposed to ultrasound for 30 min
presented apoptotic and ultrastructural changes characteristic of
damage. Whether these changes were caused by the overexpression of
HSP70 or other significantly increased pro-apoptotic factors need
to be further investigated.
Additionally, apart from HSP70, a number of other
factors can protect embryonic development. For example, the
epidermal growth factor (EGF) plays an important role in protecting
the cell or embryo from damage by promoting the synthesis of
glutathione (GSH) (32). Stem
cell factor (SCF) may improve the culture of mouse embryos exposed
to unfavorable milieu by inhibiting apoptosis (33). Insulin-like growth factor 1 (IGF1)
can protect the normal development of the pre-implantation bovine
embryo by inhibiting apoptosis during heat stress (34). Thus, it may prove valuable to
investigate the changes in these factors and their correlation with
HSP70 in chorionic villi exposed to ultrasound.
Taken together, the data from this study suggest
that HSP70 protects chorionic villi exposed to ultrasound by
inhibiting apoptosisy. However, after prolonged exposure, apoptosis
begins to occur as a result of an increase in pro-apoptotic factors
or possibly HSP70-induced damage. Therefore, the prudent use of
ultrasound during the early stages of pregnancy should be
advocated.
Acknowledgements
This study was supported by a grant from the Science
and Technology Plan Program for Social Development of Guangdong
Province (2011B061300093).
References
|
1
|
ter Haar Gail: Ultrasonic imaging: safety
considerations. Interface Focus. 1:686–697. 2011.PubMed/NCBI
|
|
2
|
Advisory Group on Non-ionising Radiation.
Health effects of exposure to ultrasound and infrasound. Health
Protection Agency; UK: 2010
|
|
3
|
Li T, Shi HT, Yang WX and Xiong RP: Effect
of diagnostic dose of color doppler ultrasound on apoptosis and
Bcl-xl mRNA, Caspase3 mRNA express in rats embryos cells. Zhongguo
Yi Xue Wu Li Xue Za Zhi. 3:1242–1245. 2009.(In Chinese).
|
|
4
|
Luft JC and Dix DJ: Hsp70 expression and
function during embryogenesis. Cell Stress Chaperones. 3:162–170.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hristova I: Role of heat shock proteins
(Hsp) in human and mammalian fertilization and pregnancy. Part I
Akush Ginekol (Sofiia). 5:45–49. 2012.
|
|
6
|
Le Masson F and Christians E: HSFs and
regulation of Hsp70.1 (Hspa1b) in oocytes and preimplantation
embryos: new insights brought by transgenic and knockout mouse
models. Cell Stress Chaperones. 3:275–285. 2011.PubMed/NCBI
|
|
7
|
Sharma L, Kaur J and Shukla G: Role of
oxidative stress and apoptosis in the placental pathology of
Plasmodium berghei infected mice. PLoS One. 3:e326942012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
|
|
9
|
Fowlkes JB; Bioeffects Committee of the
American Institute of Ultrasound in Medicine. American Institute of
Ultrasound in Medicine consensus report on potential bioeffects of
diagnostic ultrasound: executive summary. J Ultrasound Med.
27:503–515. 2008.PubMed/NCBI
|
|
10
|
Sheiner E, Shoham-Vardi I, Hussey MJ,
Pombar X, Strassner HT, Freeman J and Abramowicz JS:
First-trimester sonography: is the fetus exposed to high levels of
acoustic energy? J Clin Ultrasound. 35:245–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarantal AF and Hendrickx AG: Evaluation
of the bioeffects of prenatal ultrasound exposure in the cynomolgus
macaque (Macaca fascicularis). II. Growth and behavior
during the first year. Teratology. 39:149–162. 1989.
|
|
12
|
Ang ES Jr, Gluncic V, Duque A, Schafer ME
and Rakic P: Prenatal exposure to ultrasound waves impacts neuronal
migration in mice. Proc Natl Acad Sci. 103:12903–12910. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Md Dom S, Abdul Razak HR, Ahmad Zaiki FW,
Saat NH, Abd Manan K, Che Isa IN and Hashim UF: Ultrasound exposure
during pregnancy affects rabbit foetal parathyroid hormone (PTH)
level. Quant Imaging Med Surg. 3:49–53. 2013.PubMed/NCBI
|
|
14
|
Zhang J, Zhou F, Song Y, Ying W and Zhang
Y: Long dwell-time exposure of human chorionic villi to
transvaginal ultrasound in the first trimester of pregnancy induces
activation of caspase-3 and cytochrome C release. Biol Reprod.
67:580–583. 2002. View Article : Google Scholar
|
|
15
|
Abramowicz JS, Barnett SB, Duck FA,
Edmonds PD, Hynynen KH and Ziskin MC: Fetal thermal effects of
diagnostic ultrasound. J Ultrasound Med. 27:541–563.
2008.PubMed/NCBI
|
|
16
|
Ziskin MC and Barnett SB: Ultrasound and
the developing nervous system. Ultrasound Med Biol. 27:875–876.
2001. View Article : Google Scholar
|
|
17
|
Driver AM and Khatib H: Physiology and
Endocrinology Symposium: heat shock proteins: potentially powerful
markers for preimplantation embryonic development and fertility in
livestock species. J Anim Sci. 91:1154–1161. 2013. View Article : Google Scholar
|
|
18
|
Gupta SC, Sharma A, Mishra M, Mishra RK
and Chowdhuri DK: Heat shock proteins in toxicology: how close and
how far? Life Sci. 86:377–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Peñagaricano F, Driver A, Chen H
and Khatib H: Differential expression of heat shock protein genes
and their splice variants in bovine preimplantation embryos. J
Dairy Sci. 94:4174–4182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
King AM and MacRae TH: The small heat
shock protein p26 aids development of encysting Artemia
embryos, prevents spontaneous diapause termination and protects
against stress. PLoS One. 7:e437232012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bradley E, Bieberich E, Mivechi NF,
Tangpisuthipongsa D and Wang G: Regulation of embryonic stem cell
pluripotency by heat shock protein 90. Stem Cells. 8:1624–1633.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayer MP and Bukau B: Hsp70 chaperones,
cellular functions and molecular mechanism. Cell Mol Life Sci.
62:670–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welch WJ: How cells respond to stress. Sci
Am. 268:56–64. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindquist S and Craig EA: The heat shock
proteins. Annu Rev Genet. 22:631–677. 1988. View Article : Google Scholar
|
|
25
|
Rokutan K, Hirakawa T, Teshima S, et al:
Implications of heat shock/stress proteins for medicine and
disease. J Med Invest. 44:137–147. 1998.PubMed/NCBI
|
|
26
|
Neuer A, Mele C, Liu HC, et al: Monoclonal
antibodies to mammalian heat shock proteins impair mouse embryo
development in vitro. Hum Reprod. 4:987–990. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelly S, Zhang ZJ, Zhao H, Xu L, et al:
Gene transfer of HSP72 protects cornu ammonis 1 region of the
hippocampus neurons from global ischemia: influence of Bcl-2. Ann
Neurol. 52:160–167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagashima M, Fujikawa C, Mawatari K, Mori
Y and Kato S: HSP70, the earliest-induced gene in the zebrafish
retina during optic nerve regeneration: its role in cell survival.
Neurochem Int. 58:888–895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharp FR, Massa SM and Swanson RA:
Heat-shock protein protection. Trends Neurosci. 22:97–99. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka K and Mizushima T: Protective role
of HSF1 and HSP70 against gastrointestinal diseases. Int J
Hyperthermia. 25:668–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim MK, Fibrianto YH, Oh HJ, et al: Effect
of beta-mercaptoethanol or epidermal growth factor supplementation
on in vitro maturation of canine oocytes collected from dogs with
different stages of the estrus cycle. J Vet Sci. 5:253–258.
2004.PubMed/NCBI
|
|
33
|
Glabowski W, Wiszniewska B and Kurzawa R:
Protective potential of SCF for mice preimplantation embryos
cultured in vitro in suboptimal conditions. J Assist Reprod Genet.
25:395–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonilla AQ, Oliveira LJ, Ozawa M, Newsom
EM, Lucy MC and Hansen PJ: Developmental changes in
thermoprotective actions of insulin-like growth factor-1 on the
preimplantation bovine embryo. Mol Cell Endocrinol. 332:170–179.
2011. View Article : Google Scholar : PubMed/NCBI
|