Introduction
Human bocavirus (HBoV) has been detected in both
adult and children respiratory tract samples since its discovery in
2005 (1–3). The HBoV genome contains 3 open
reading frames (ORFs), encoding 2 non-structural proteins (NS1 and
NP1) and 2 viral capsid proteins (VP1 and VP2; VP2 overlaps
with VP1, but they have different start codes). HBoV strains
with larger than 10% VP1 nucleotide differences (VPD) were
distinguished as different genotypes, while VPD between 5% and 10%
was typing criteria for different subgenotypes. According to the
typing criteria, the HBoV discovered in 2005 was termed HBoV1
(4,5). Subsequently, 3 more genotypes of
HBoV (HBoV2-4) were found from human excrement (4–6).
HBoV2 can be divided into 2 subgenotypes, HBoV2A and HBoV2B. It has
been shown that inter- and intra-genotype recombinations are
present among the bocavirus (7).
In humans, the level of anti-HBoV1 antibody
increases 2 months after birth and then undergoes a continuous
decline before the age of 6 months. A continuous increase in
anti-HBoV1 antibody levels can be observed from the age of 6 months
to 6 years. By the age of 2, approximately 80% of children have
been infected by HBoV1-4 (8–10).
Serological detection has revealed that HBoV has a long-term
worldwide prevalence among individuals of all ages. Recent studies
have indicated that HBoV1 can be cultivated in human respiratory
epithelial cells in vitro (11,12). However, no animal infection model
has been established thus far; HBoV could not be confirmed as a
human pathogen according to the Koch theory (13–15). Some reports have pointed out that
HBoV1 is closely related to symptoms of wheezing in children
(8,16), while other studies have considered
HBoV1 as a passerby or co-infector virus (17,18). Evidence has shown that HBoV1-4 may
also be involved in human gastrointestinal tract infections
(1,19–21). In our previous study, HBoV1 was
found to be closely associated with acute respiratory tract
infection in children in Shanghai and most of the infected children
had symptoms of wheezing (22).
Whether HBoV2-4 is involved in acute respiratory tract infection
with symptoms of wheezing is unknown and the genetic evolutionary
relation of the epidemic HBoV1-4 strains has not been determined
yet.
During the high-occurrence season for acute
respiratory tract infections (wintertime, from December 2012 to
February 2013), we collected samples of nasopharyngeal secretion
(NPS) from hospitalized children with acute lower respiratory tract
infection and symptoms of wheezing. We detected the existence of
HBoV1-4 in the samples by nested PCR. The DNA sequences of
VP1/VP2, NP1 and NS1 in the HBoV-positive
samples were further amplified, sequenced and aligned for
phylogenetic tree construction. Our results revealed that the HBoV
strains detected from the positive samples belonged to one genotype
of HBoV1 and it was suggested that all the strains were derived
from one common ancestor.
Materials and methods
This study was approved by the Ethics Committee of
the Children’s Hospital of Fudan University, Shanghai, China. The
275 NPS samples were collected upon the agreement of the guardians
of the infected children.
Materials
A total of 275 NPS samples were collected from
children with symptoms of wheezing (within 48 h of hospitalization
during the period between December 2012 and February 2013) with
acute lower respiratory tract infection. The patients (158 males
and 117 females) were aged between 1 month to 8 years; there were
56 cases of bronchitis and 219 cases of pneumonia (including 5
cases of severe pneumonia). All patients had symptoms of coughing
and wheezing and a chest radiograph indicated the presence of
bronchitis and pneumonia. Of the 275 patients, 136 had a fever
(>38°C). Patients displaying symptoms of coughing and wheezing
after 48 h of hospitalization or with a past history of asthma were
excluded from this study.
HBoV1-4 detection
Viral RNA was extracted according to the
instructions provided with the Viral Genomic DNA/RNA Extraction kit
[Cat. no. DP 315; Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China]. The general nested PCR primer pairs were designed for
HBoV1-4 VP1/VP2 and HBoV1 NP1 (1,4,23)
(Table I). The available PCR
reaction conditions and programs were used in our study (1,4,6,23–25). The HBoV plasmid was kindly
provided by Professor Zhou Rong from Guangzhou Medical University,
Guangzhou, China.
 | Table IThe general nested PCR primer pairs
for HBoV1-4 VP1/VP2 and HBoV1 NP1 and NS1. |
Table I
The general nested PCR primer pairs
for HBoV1-4 VP1/VP2 and HBoV1 NP1 and NS1.
| Gene name | Primer name | Primer sequence |
|---|
| HBoV1-4
VP1 | AK-VP-F1 |
5′-CGCCGTGGCTCCTGCTCT-3′ |
| AK-VP-R1 |
5′-TGTTCGCCATCACAAAAGATGTG-3′ |
| HBoV1-4
VP2 | AK-VP-F2 |
5′-GGCTCCTGCTCTAGGAAATAAAGAG-3′ |
| AK-VP-R2 |
5′-CCTGCTGTTAGGTCGTTGTTGTATGT-3′ |
| HBoV1 NP1 | HBoV_2204F |
5′-GAGACATCGCAAGTGGACTAT-3′ |
| HBoV_3101R |
5′-TTGAGCAGCGCGATCAGCGTTA-3′ |
| HBoV_2321F |
5′-GCACAGCCACGTGACGAAGATGA-3′ |
| HBoV_3056R |
5′-GGATTAAATGGCCCAAGATA-3′ |
| HBoV1 NS1 | Adel-OF |
5′-AGGTAAAACAAATATTGCAAAGGCCATAGTC-3′ |
| Adel-OR |
5′-TGGGAGTTCTCTCCGTCCGTATC-3′ |
| Adel-IF |
5′-AGGGTTTGTCTTTAACGATTGCAGACAAC-3′ |
| Adel-IR |
5′-TATACACAGAGTCGTCAGCACTATGAG-3′ |
Alignment analysis of HBoV1 VP1/VP2, NP1
and NS1 DNA sequences
The DNA sequences obtained were sequenced and
aligned using online BLAST software. The DNA sequences were
analyzed and phylogenetic trees were generated using MEGA5.1.
Detection of other common respiratory
tract viruses and pathogens
The direct immune fluorescence technique
(Respiratory Panel IFA Kit; Chemion) was used for antigen detection
for respiratory syncytial virus (RSV), adenovirus (ADV), influenza
virus (IFV)-A, IFV-B and parainfluenza virus (PIV)1–3. The
fluorescent PCR and bacteria cultivation results of Mycoplasma
pneumoniae and Chlamydia trachomatis for the 275 NPS
samples were also obtained.
Results
Detection of HBoV1-4
The nested PCR results revealed that 15 of the 275
NPS samples were HBoV1-positive; the detection rate was 5.45%. With
the use of common primers for HBoV1-4 VP1/VP2, HBoV1
NP1 and HBoV1 NS1, 15, 15, and 8 positive samples
were detected, respectively, by nested PCR. Seven samples with
positive signals of VP1/VP2 and NP1 failed to yield
nested RT-PCR products using NS1 primers. The clinical
information of the 15 HBoV1-positive samples is summarized in
Table II.
 | Table IIClinical information of children
infected by HBoV1. |
Table II
Clinical information of children
infected by HBoV1.
| No. | Age | Gender | Clinical
diagnosis | Diarrhea | Co-infected
pathogens |
|---|
| 1 | 3 Months | Female | Pneumonia | Yes | RSV |
| 2 | 11 Months | Male | Pneumonia | Yes | RSV, hMPV |
| 3 | 3 Years | Male | Bronchitis | No | Hi |
| 4 | 2 Years | Female | Pneumonia | Yes | |
| 5 | 3 Years | Female | Pneumonia | No | ADV, MC |
| 6 | 8 Months | Female | Bronchitis | Yes | RSV |
| 7 | 6 Years | Male | Pneumonia | No | MP |
| 8 | 2 Years | Male | Bronchitis | No | RSV |
| 9 | 8 Years | Male | Pneumonia | No | MP, Strep |
| 10 | 11 Months | Male | Pneumonia | No | RSV, Hi |
| 11 | 2 Years | Female | Pneumonia | Yes | IAV |
| 12 | 8 Months | Male | Bronchitis | Yes | |
| 13 | 3 Years | Female | Pneumonia | No | RSV |
| 14 | 4 Years | Female | Pneumonia | No | ADV |
| 15 | 2 Years | Male | Pneumonia | Yes | |
All the PCR products obtained were sequenced. All
the sequences were pooled together with the HBoV1 whole genome
(available in GeneBank) for homology analysis using ClustalW
(MEGA5.1). The detailed homology information for VP1/VP2,
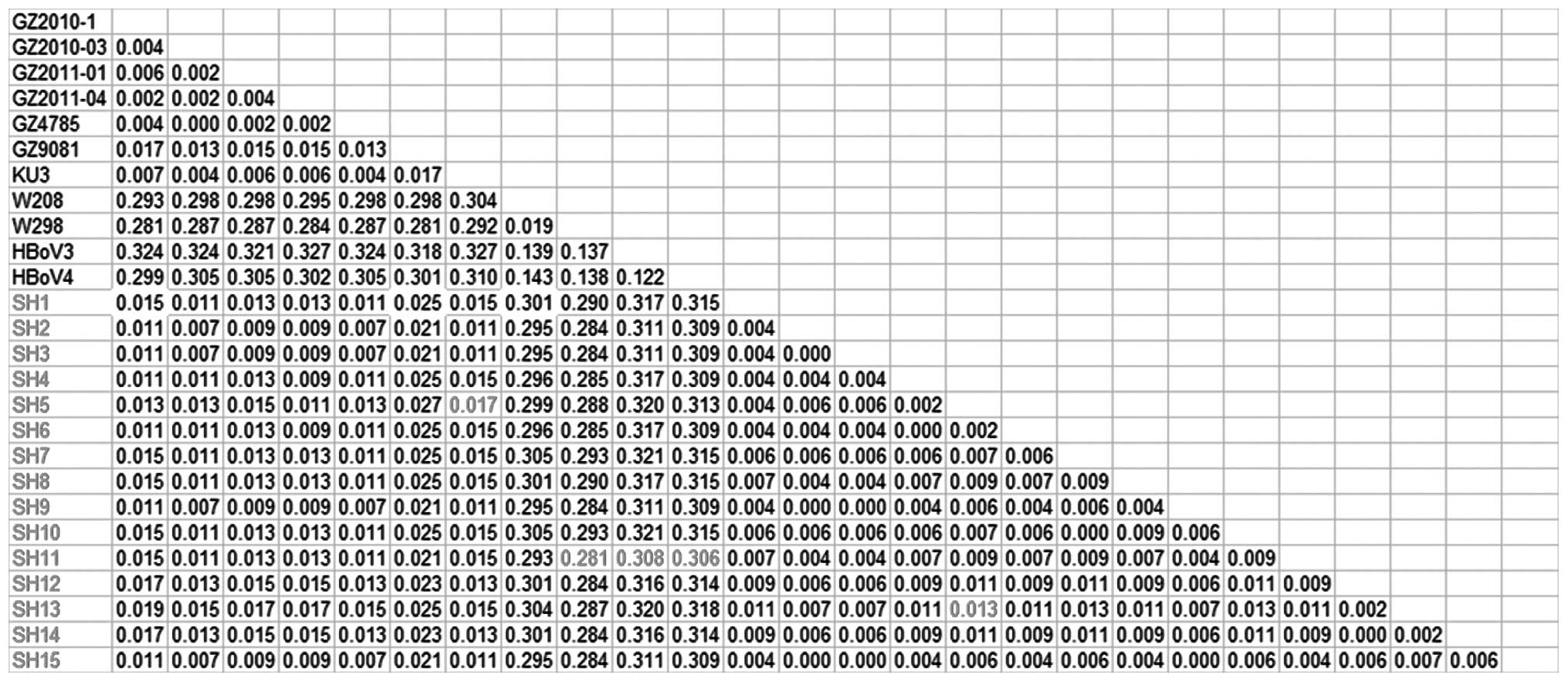
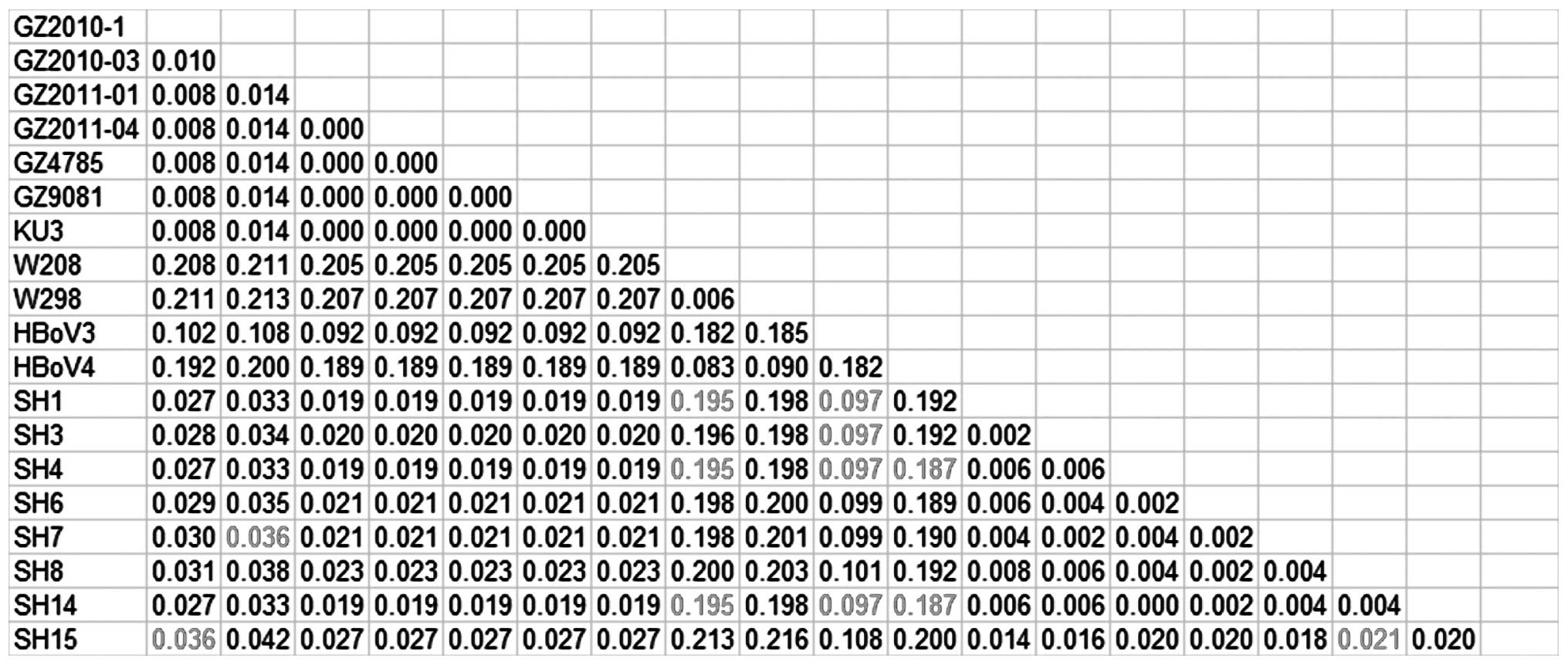
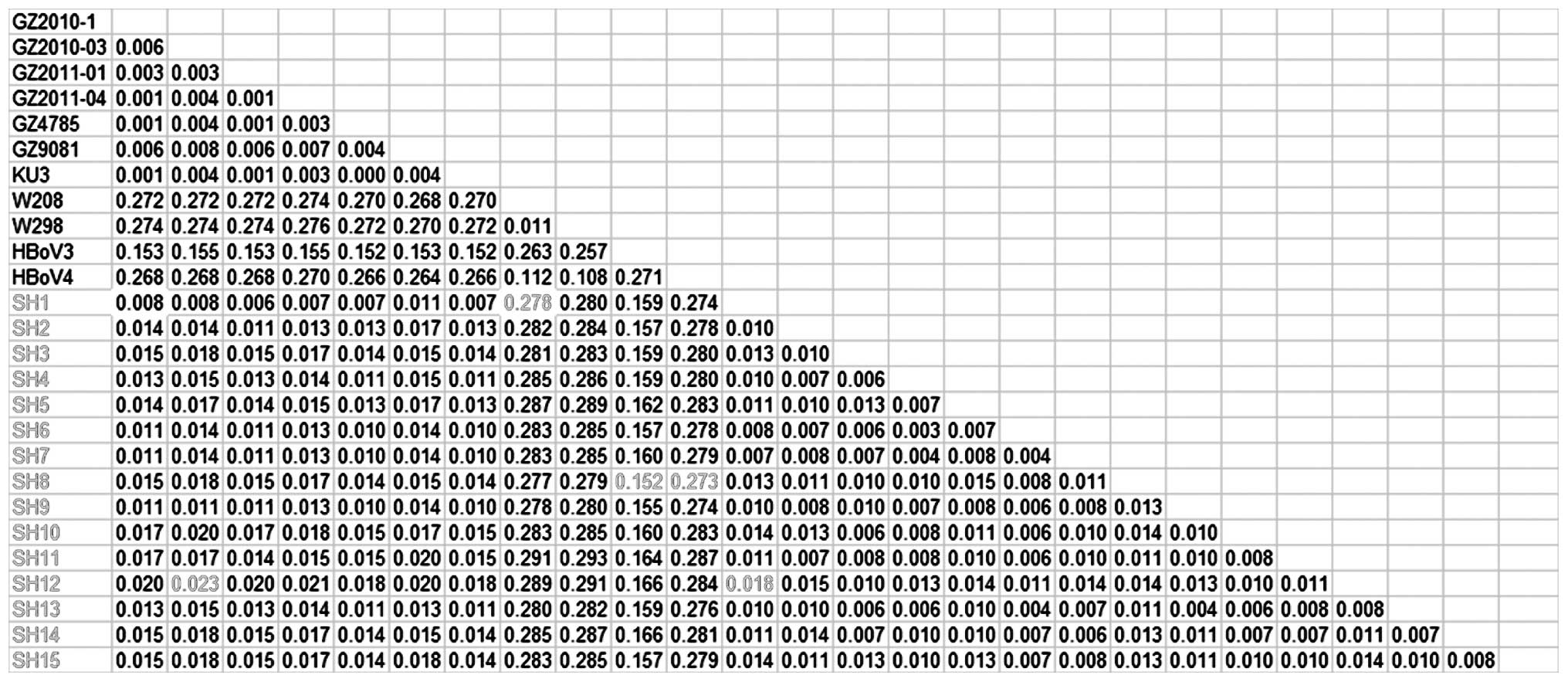
NP1 and NS1 is shown in Figs. 1–3, respectively.
Sequence homology analysis of the HBoV1
VP1/VP2 gene
The 15 HBoV1 strains in our study showed a maximum
homology difference of 1.7% with previously discovered strains
[strains reported in 2010 and 2011 in Guangzhou:
GZ2010-1(JN128956), GZ2010-03(JN128953), GZ2011-01(JN128954) and
GZ2011-04(JN128955); strains reported in 2012 in Guangzhou:
GZ4785(JN794565) and GZ9081(JN794566); strains reported in 2012 in
the US: KU3(JQ411251)]. A minimum difference of 28.1% was shown
between the 2009 Australian HBoV2 strains [W298(FJ948860) and
W208(EU082214)] and the newly discovered strains. A minimum
difference of 30.8% was shown between the 2009 Australian
HBoV3(NC_012564) and the new HBoV1 strains. A minimum difference of
30.6% was shown between the 2010 American HBoV4(NC_012729) and the
new HBoV1 strains. The maximum difference among the 15 new HBoV1
strains was 1.3%, which indicated that they belonged to the same
genotype (Fig. 1).
Sequence homology analysis of the HBoV1
NP1 gene
The homology differences among the newly discovered
HBoV strains and previously discovered strains were also calculated
based on the NP1 sequences. A maximum difference in homology
of 2.3% was observed between the 15 HBoV1 strains and the
previously discovered strains (GZ2010-1, GZ2010-03, GZ2011-01,
GZ2011-04, GZ4785, GZ9081 and KU3). The minimum difference between
W298 and W208 and the newly discovered HBoV1 strains was 27.8%; the
minimum difference between the new strains and HBoV3 was 15.2%; the
minimum difference between the new strains and HBoV4 was 27.3%; the
maximum difference between the 15 new HBoV1 strains was 1. 8%,
which indicated that they belonged to the same genotype (Fig. 2).
Sequence homology analysis of the HBoV1
NS1 gene
The homology differences among the newly discovered
HBoV strains and the previously discovered strains were also
calculated based on the 8 NS1 sequences. A maximum
difference in homology of 3.6% was observed between the 8 HBoV1
strains and the previously discovered strains (GZ2010-1, GZ2010-03,
GZ2011-01, GZ2011-04, GZ4785, GZ9081 and KU3). A minimum difference
of 19.5% was shown among the new strains and W298 and W208; the
minimum difference between the new strains and HBoV3 was 9.7%; the
minimum difference between the new strains and HBoV4 was 18.7%. The
maximum difference between the 8 new HBoV1 strains was 2.1%, which
indicated that they belonged to the same genotype (Fig. 3). The 8 HBoV1 strains showed a
minimum difference of 10% with HBoV3, which supported the
assumption that HBoV3 was derived from the recombination of HBoV1
and HBoV2 (4,25,26).
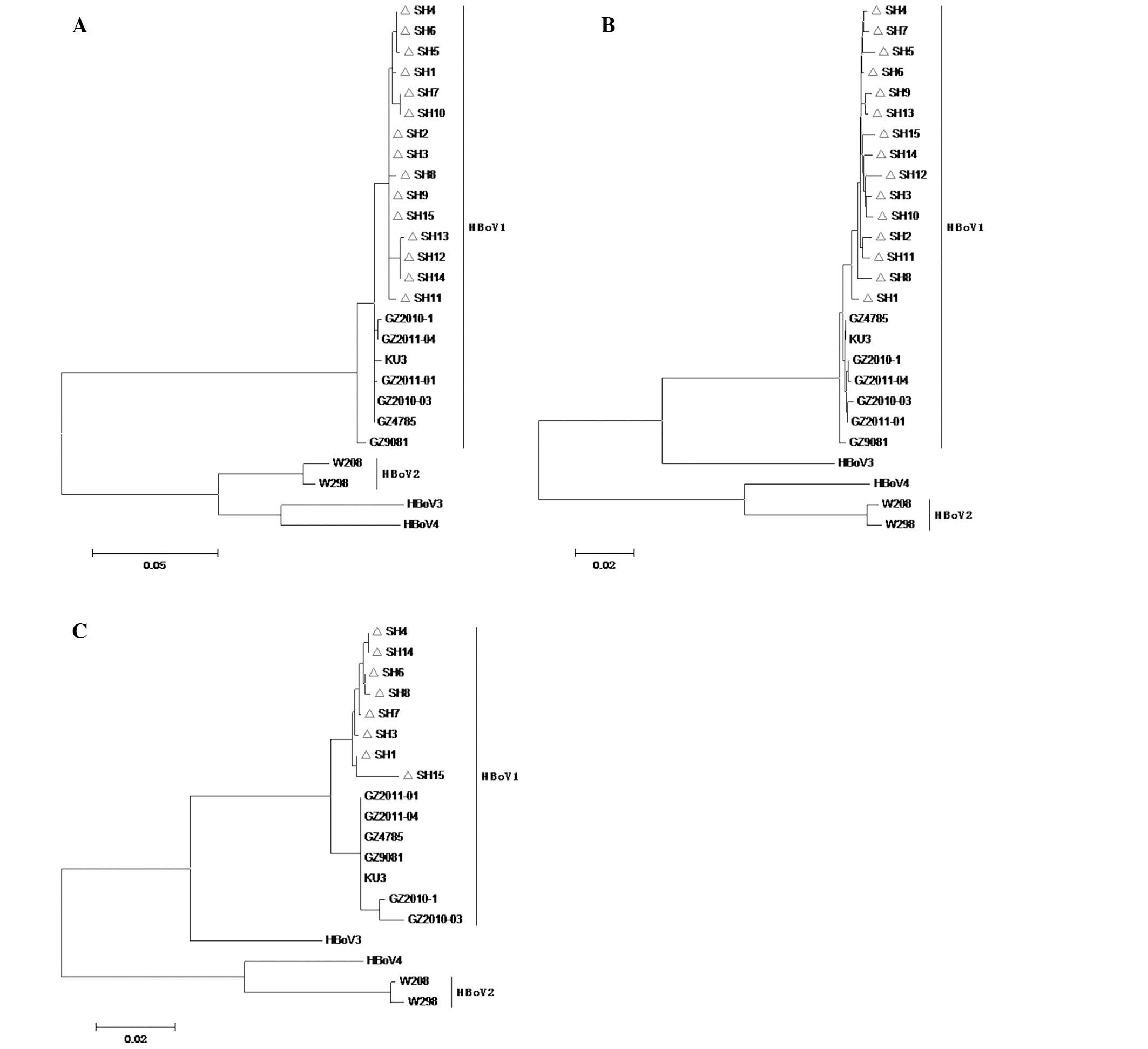
Phylogenetic tree generation for the
HBoV1 gene
The positive PCR products of 15 VP1/VP2
genes, 15 NP1 genes and 8 NS1 genes were pooled for
phylogenetic tree construction (Fig.
4). All 3 phylogenetic trees showed that the 15 detected viral
strains were scattered on the same branch, which indicated that
they belonged to the same HBoV1 genotype.
Detection of other common pathogens
The detection rates for 8 common viruses [RSV, ADV,
IFA/B, PIV1-3 and human metapneumovirus (hMPV)] were 44.73, 2.18,
1.45, 1.45 and 3.64%, respectively. The total detection rate was
53.4% (147/275); RSV was the most common pathogen (44.73%) and the
detection rate of HBoV1 (5.45%) was second to RSV. The 15
HBoV1-positive samples were co-detected with 6 cases of RSV and 2
cases of hMPV. Thirteen cases of Mycoplasma pneumoniae and
Chlamydia trachomatis were identified by qRT-PCR (4.73%);
the positive rate of the bacterial culture was 24.36% (67/275); the
co-infection rate was 16.0% (44/275); the total detection rate of
all the pathogens was 72.0% (198/275).
Discussion
HBoV is a newly discovered Parvoviridae virus. HBoV
particles can be detected in respiratory secretions and digestive
excrements. A number of scientists have considered that HBoV is
closely connected with human respiratory tract infections. However,
there is still controversy as to the involvement of HBoV1-4 in
children with symptoms of wheezing. The detection of HBoV DNA or
particles in the secretions of patients with symptoms of wheezing
and the positive transition of serological HBoV antibody were used
in this study as alternative methods of examining the infections,
although no animal model was available for an HBoV infection
study.
We discovered 15 HBoV1-positive samples from the 275
NPS collected from hospitalized children with acute lower
respiratory tract infection with symptoms of wheezing; the
detection rate was 5.45%, which was second to RSV. This indicates
that HBoV1 may be one of the common pathogens responsible for the
hospitalization of children with acute lower respiratory tract
infection with symptoms of wheezing in Shanghai. As we did not
include a control group with no symptoms, no serological and
viremia detection were perrormed in our study; the 15 HBoV1 strains
may be co-pathogens only or just viruses being carried by the
patients. No HBoV2-4 signals were detected, which indicated that
HBoV2-4 could not be the pathogens responsible for the
hospitalization of children with acute lower respiratory tract
infection with symptoms of wheezing in the winter of 2012; this is
consistent with the low detection rate of HBoV2-4 in respiratory
tract secretions (27–29). Although 7 HBoV1-positive cases
displayed symptoms of diarrhea, it could not be confirmed that the
digestive tract removed the viral particles, as no excrement
samples of the HBoV1-positive cases were collected and detected.
Further studies are required to fully investigate this issue.
The homology differences among the newly discovered
HBoV strains and the previously discovered strains were calculated
based on the VP1/VP2, NP1 and NS1 sequences.
All the results revealed that the 15 new HBoV1 strains belonged to
the same genotype (Figs.
1–3). All the 15 strains were
scattered on the same branch in the 3 phylogenetic trees (Fig. 4). This indicated that the 15 new
HBoV1 strains were very likely derived from one common HBoV1
ancestor with pathogenicity, which underwent evolution through
inter-genotype recombination.
The discovery of HBoV1-4 indicated that HBoV has a
feature of genetic diversities. Being consistent with a recent
report (29), the analysis of
whole genome sequences in GenBank revealed that the gene
recombinations occurred not only among HBoV1-4, but also among the
members in the same genotypes. The analysis of different ORFs led
to the same conclusion that all the newly discovered HBoV1 strains
belonged to the same genotype. Further persistent monitoring is
required to determine whether the HBoV1 genotype is closely related
with digestive tract infections in children with symptoms of
wheezing in Shanghai.
In our study, only HBoV1 was detected by nested PCR
from the 275 NPS samples collected from the hospitalized children
with symptoms of wheezing in the winter of 2012. The detection rate
of HBoV1 was 5.45% (15/275), which was second only to RSV. HBoV1
had a high co-infection rate with other potential pathogens in most
of the samples. The 15 newly discovered HBoV1 strains belonged to
the same subgroup and thus may be derived from one common ancestor.
Our results indicated that HBoV1 may be one of the common pathogens
responsible for the hospitalization of children with acute lower
respiratory tract infection with symptoms of wheezing in
Shanghai.
Acknowledgements
This study was supported by a grant from the Key
Discipline Construction of Public Health in Shanghai
(08GWZX0102).
References
|
1
|
Khamrin P, Malasao R, Chaimongkol N, et
al: Circulating of human bocavirus 1, 2, 3, and 4 in pediatric
patients with acute gastroenteritis in Thailand. Infect Genet and
Evol. 12:565–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu WK, Chen DH, Liu Q, et al: Detection
of human bocavirus from children and adults with acute respiratory
tract illness in Guangzhou, southern China. BMC Infect Dis.
11:3452011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo L, Wang Y, Zhou H, et al: Differential
seroprevalence of human bocavirus species 1–4 in Beijing, China.
PLoS One. 7:e396442012.PubMed/NCBI
|
|
4
|
Kapoor A, Simmonds P, Slikas E, et al:
Human bocaviruses are highly diverse, dispersed, recombination
prone, and prevalent in enteric infections. J Infect Dis.
201:1633–1643. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapoor A, Slikas E, Simmonds P, et al: A
newly identified bocavirus species in human stool. J Infect Dis.
199:196–200. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arthur JL, Higgins GD, Davidson GP, Givney
RC and Ratcliff RM: A novel bocavirus associated with acute
gastroenteritis in Australian children. PLoS Pathog.
5:e10003912009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu X, Wang X, Ni B, et al: Recombination
analysis based on the complete genome of bocavirus. Virology J.
8:1822011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kantola K, Hedman L, Arthur J, et al:
Seroepidemiology of human bocaviruses 1–4. J Infect Dis.
204:1403–1412. 2011.
|
|
9
|
Endo R, Ishiguro N, Kikuta H, et al:
Seroepidemiology of human bocavirus in Hokkaido prefecture, Japan.
J Clin Microbiol. 45:3218–3223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hustedt JW, Christie C, Hustedt MM,
Esposito D and Vazquez M: Seroepidemiology of human bocavirus
infection in Jamaica. PLoS One. 7:e382062012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng X, Yan Z, Luo Y, et al: In vitro
modeling of human bocavirus 1 infection of polarized primary human
airway epithelia. J Virol. 87:4097–4102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Q, Deng X, Yan Z, et al:
Establishment of a reverse genetics system for studying human
bocavirus in human airway epithelia. PLoS Pathog. 8:e10028992012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rivers TM: Viruses and Koch’s Postulates.
J Bacteriol. 33:1–12. 1937.
|
|
14
|
Weissbrich B, Neske F, Schubert J, et al:
Frequent detection of bocavirus DNA in German children with
respiratory tract infections. BMC Infect Dis. 6:1092006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lusebrink J, Schildgen V, Tillmann RL, et
al: Detection of head-to-tail DNA sequences of human bocavirus in
clinical samples. PLoS One. 6:e194572011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Y, Gu X, Zhao X, et al: High viral
load of human bocavirus correlates with duration of wheezing in
children with severe lower respiratory tract infection. PLoS One.
7:e343532012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longtin J, Gubbay JB, Patel S and Low DE:
High prevalence of asymptomatic bocavirus in daycare: is otitis
media a confounder? J Infect Dis. 202:16172010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Debiaggi M, Canducci F, Ceresola ER and
Clementi M: The role of infections and coinfections with newly
identified and emerging respiratory viruses in children. Virol J.
9:2472012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Proenca-Modena J, Martinez M, Amarilla A,
et al: Viral load of human bocavirus-1 in stools from children with
viral diarrhoea in Paraguay. Epidemiol Infect. Feb 21–2013.(Epub
ahead of print).
|
|
20
|
Risku M, Kätkä M, Lappalainen S, Räsänen S
and Vesikari T: Human bocavirus types 1, 2 and 3 in acute
gastroenteritis of childhood. Acta Paediatr. 101:e405–e410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khamrin P, Thongprachum A, Shimizu H, et
al: Detection of human bocavirus 1 and 2 from children with acute
gastroenteritis in Japan. J Med Virol. 84:901–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng M, Zhu QR, Wang XH, Yu H and Shen J:
Human bocavirus in children with respiratory tract infection in
Shanghai: a retrospective study. World J Pediatr. 6:65–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitui MT, Tabib SM, Matsumoto T, et al:
Detection of human bocavirus in the cerebrospinal fluid of children
with encephalitis. Clin Infect Dis. 54:964–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghietto LM, Cámara A, Zhou Y, et al: High
prevalence of human bocavirus 1 in infants with lower acute
respiratory tract disease in Argentina, 2007–2009. Braz J Infect
Dis. 16:38–44. 2012.PubMed/NCBI
|
|
25
|
Cheng W, Chen J, Xu Z, et al: Phylogenetic
and recombination analysis of human bocavirus 2. BMC Infect Dis.
11:502011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chieochansin T, Simmonds P and Poovorawan
Y: Determination and analysis of complete coding sequence regions
of new discovered human bocavirus types 2 and 3. Arch Virol.
155:2023–2028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koseki N, Teramoto S, Kaiho M, et al:
Detection of human bocaviruses 1 to 4 from nasopharyngeal swab
samples collected from patients with respiratory tract infections.
J Clin Microbiol. 50:2118–2121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu L, He X, Zhang DM, et al: Surveillance
and genome analysis of human bocavirus in patients with respiratory
infection in Guangzhou, China. PLoS One. 7:e448762012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdel-Moneim AS, Kamel MM, Al-Ghamdi AS
and Al-Malky MI: Detection of bocavirus in children suffering from
acute respiratory tract infections in Saudi Arabia. PLoS One.
8:e555002013. View Article : Google Scholar : PubMed/NCBI
|