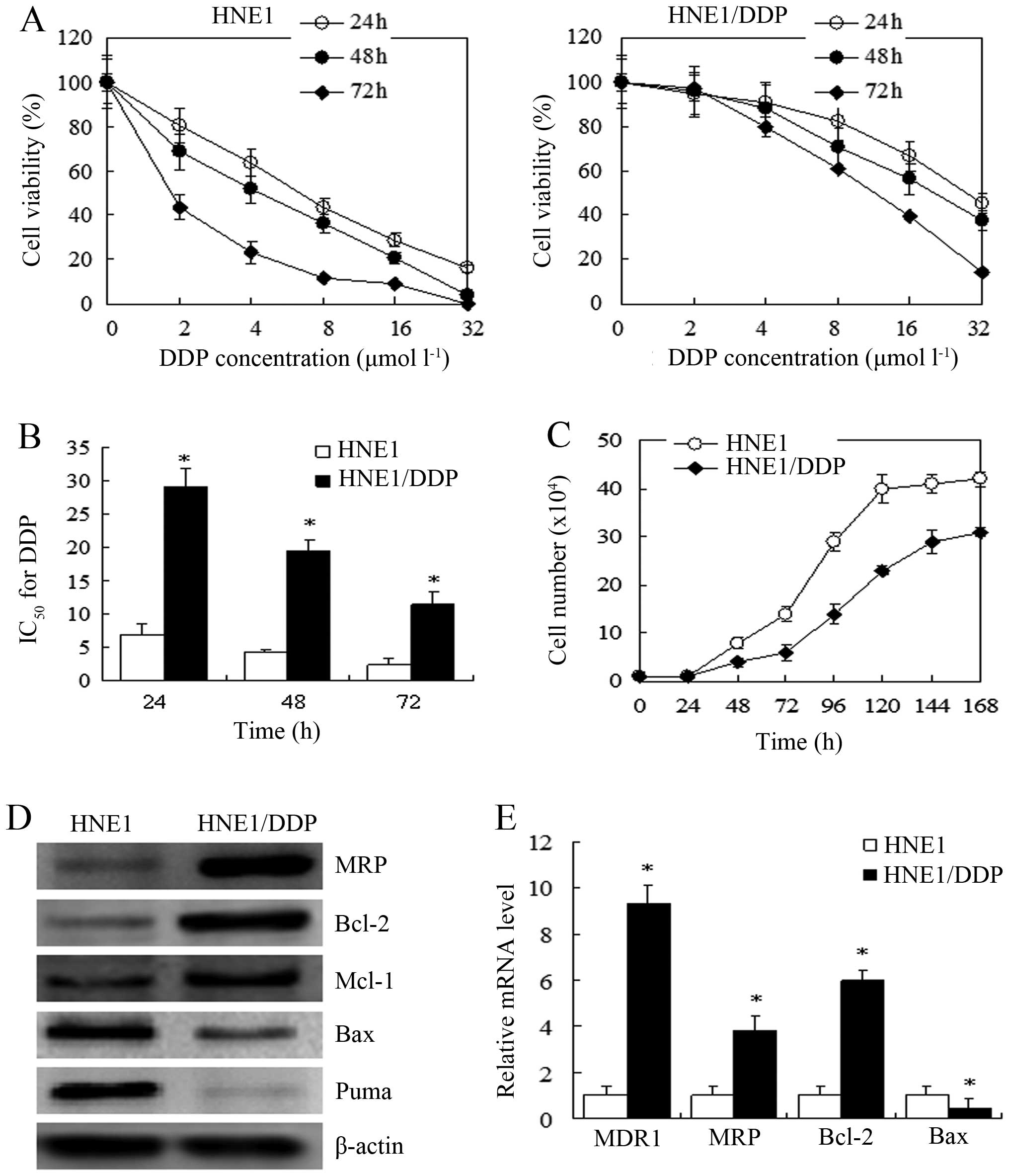
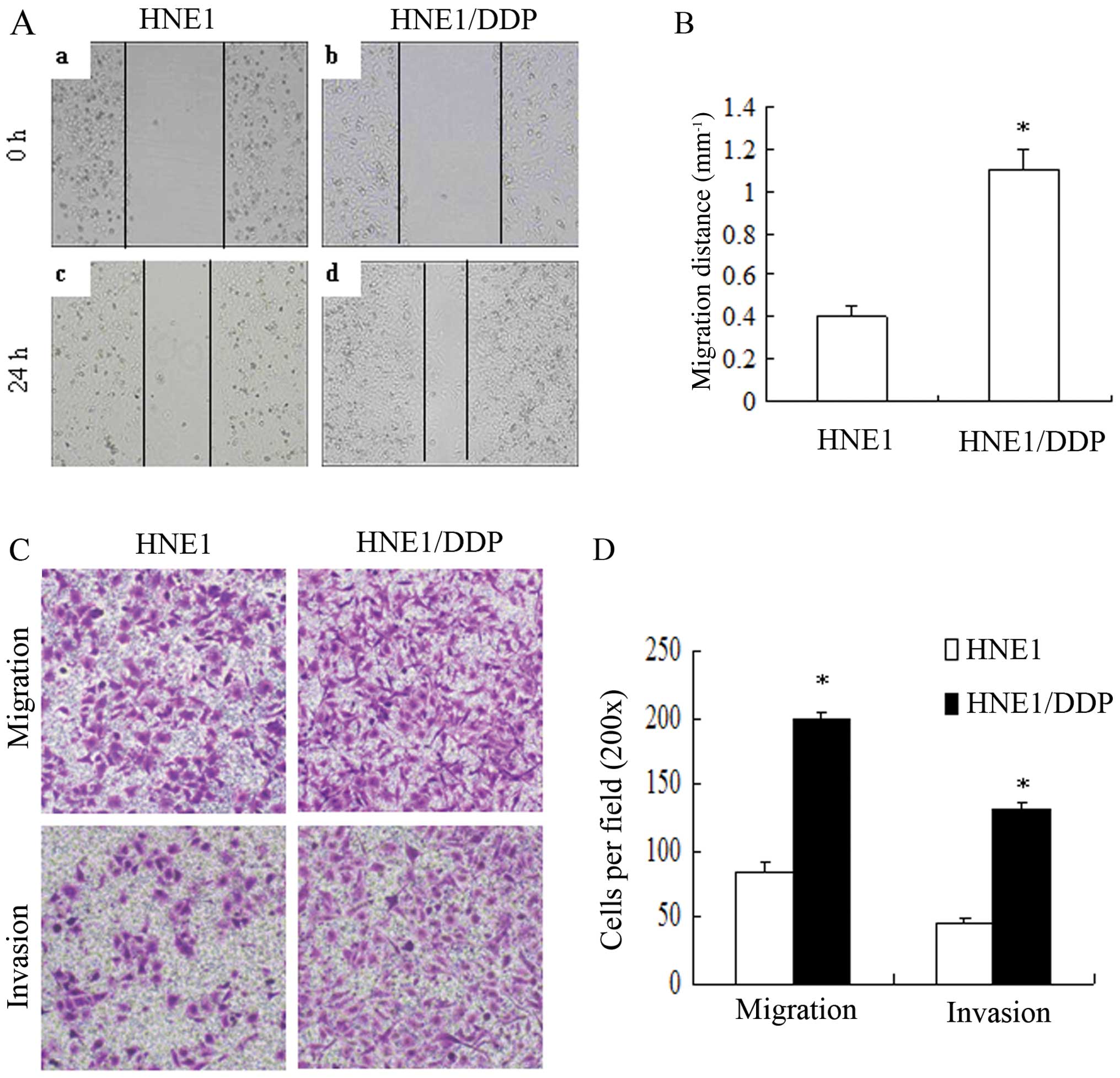
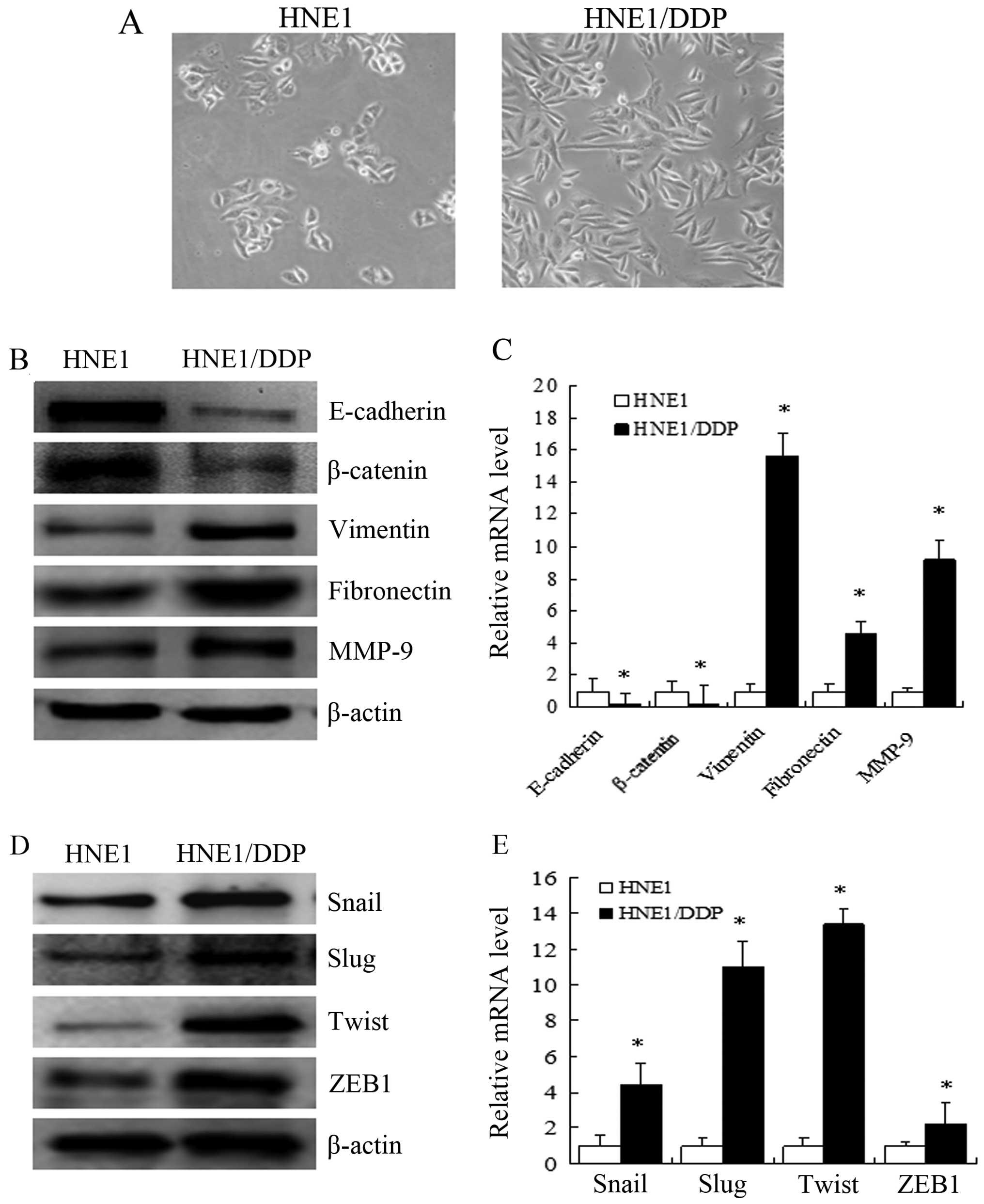
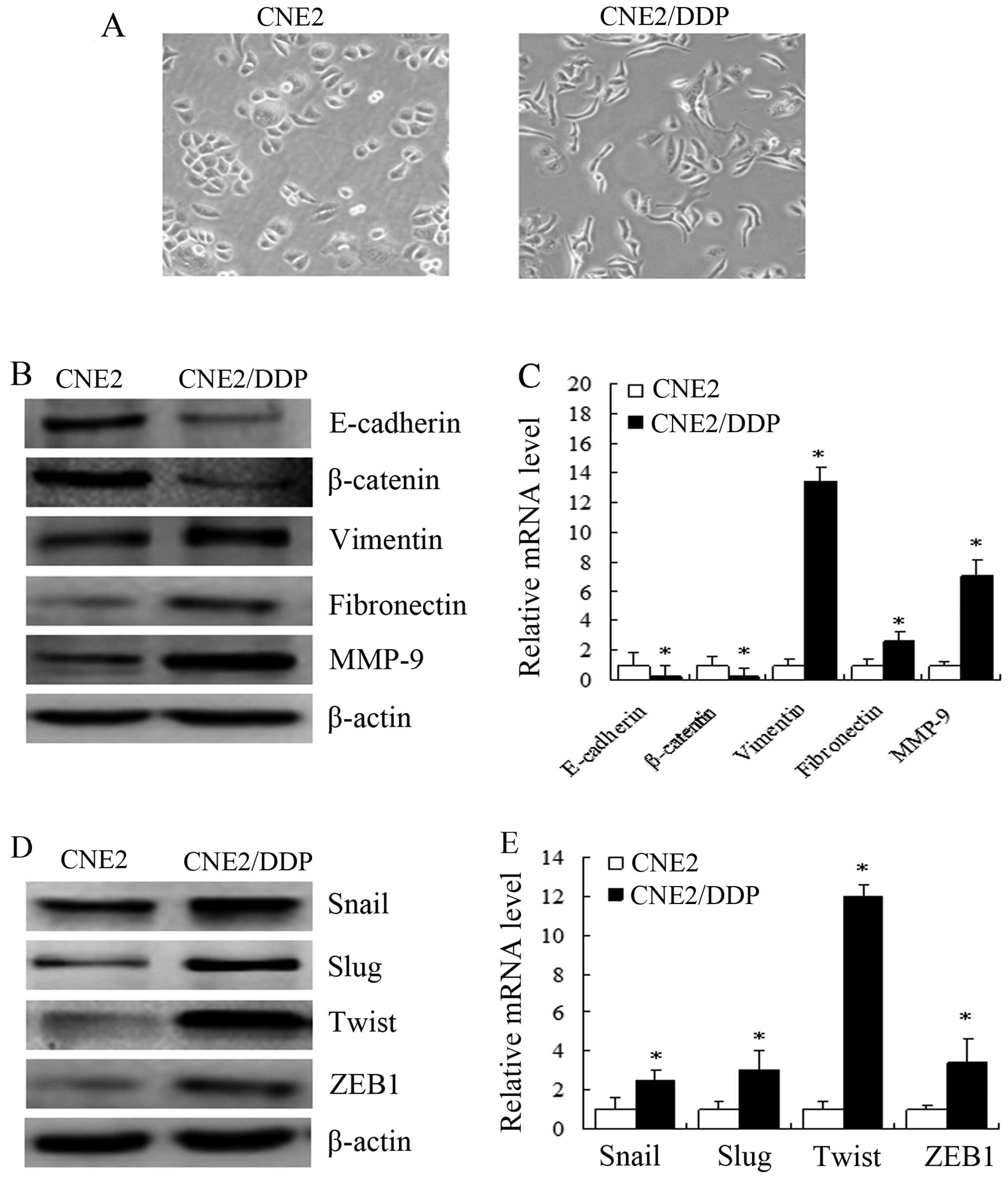
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Chen QY, Wen YF, Guo L, et al: Concurrent
chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal
carcinoma: phase III randomized trial. J Natl Cancer Inst.
103:1761–1770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabec V and Kasparkova J: Modifications
of DNA by platinum complexes. Relation to resistance of tumors to
platinum antitumor drugs. Drug Resist Updat. 8:131–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Q, Kawamura K, Yamanaka M, et al:
Upregulated p53 expression activates apoptotic pathways in
wild-type p53-bearing mesothelioma and enhances cytotoxicity of
cisplatin and pemetrexed. Cancer Gene Ther. 19:218–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Xiang S, Williams KA, et al:
Depletion of HDAC6 enhances cisplatin-induced DNA damage and
apoptosis in non-small cell lung cancer cells. PLoS One.
7:e442652012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shellard SA, Fichtinger-Schepman AM, Lazo
JS and Hill BT: Evidence of differential cisplatin-DNA adduct
formation, removal and tolerance of DNA damage in three human lung
carcinoma cell lines. Anticancer Drugs. 4:491–500. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torigoe T, Izumi H, Ishiguchi H, et al:
Cisplatin resistance and transcription factors. Curr Med Chem
Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung HW, Jin DY, Ling MT, et al: Mitotic
arrest deficient 2 expression induces chemosensitization to a
DNA-damaging agent, cisplatin, in nasopharyngeal carcinoma cells.
Cancer Res. 65:1450–1458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial - mesenchymal and mesenchymal - epithelial transitions
in carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MR, Choi HK, Cho KB, Kim HS and Kang
KW: Involvement of Pin1 induction in epithelial-mesenchymal
transition of tamoxifen-resistant breast cancer cells. Cancer Sci.
100:1834–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajiyama H, Shibata K, Terauchi M, et al:
Chemoresistance to paclitaxel induces epithelial-mesenchymal
transition and enhances metastatic potential for epithelial ovarian
carcinoma cells. Int J Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
13
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu MF, Liu LZ, He LJ, et al: Sequential
chemoradiotherapy with gemcitabine and cisplatin for locoregionally
advanced nasopharyngeal carcinoma. Int J Cancer. 132:215–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie SM, Fang WY, Liu TF, Yao KT and Zhong
XY: Association of ABCC2 and CDDP-resistance in two sublines
resistant to CDDP derived from a human nasopharyngeal carcinoma
cell line. J Oncol. 2010:9150462010.PubMed/NCBI
|
|
16
|
Wong RS and Cheong SK: Leukaemic stem
cells: drug resistance, metastasis and therapeutic implications.
Malays J Pathol. 34:77–88. 2012.PubMed/NCBI
|
|
17
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danila DC, Heller G, Gignac GA, et al:
Circulating tumor cell number and prognosis in progressive
castration-resistant prostate cancer. Clin Cancer Res.
13:7053–7058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Y, Meleady P, Cleary I, McDonnell S,
Connolly L and Clynes M: Selection with melphalan or paclitaxel
(Taxol) yields variants with different patterns of multidrug
resistance, integrin expression and in vitro invasiveness.
Eur J Cancer. 37:1041–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haga A, Nagase H, Kito H and Sato T:
Invasive properties of cadmium-resistant human fibrosarcoma HT-1080
cells. Cancer Biochem Biophys. 15:275–284. 1997.PubMed/NCBI
|
|
21
|
Wang Z, Li Y, Ahmad A, et al: Targeting
miRNAs involved in cancer stem cell and EMT regulation: An emerging
concept in overcoming drug resistance. Drug Resist Updat.
13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haslehurst AM, Koti M, Dharsee M, et al:
EMT transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pai MH, Kuo YH, Chiang EP and Tang FY:
S-Allylcysteine inhibits tumour progression and the
epithelial-mesenchymal transition in a mouse xenograft model of
oral cancer. Br J Nutr. 108:28–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing Y, Han Z, Liu Y, et al: Mesenchymal
stem cells in inflammation microenvironment accelerates
hepatocellular carcinoma metastasis by inducing
epithelial-mesenchymal transition. PLoS One. 7:e432722012.
View Article : Google Scholar
|
|
26
|
Comijn J, Berx G, Vermassen P, et al: The
two-handed E box binding zinc finger protein SIP1 downregulates
E-cadherin and induces invasion. Mol Cell. 7:1267–1278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Ling MT, Guan XY, et al:
Identification of a novel function of TWIST, a bHLH protein, in the
development of acquired taxol resistance in human cancer cells.
Oncogene. 23:474–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudin CM, Yang Z, Schumaker LM, et al:
Inhibition of glutathione synthesis reverses Bcl-2-mediated
cisplatin resistance. Cancer Res. 63:312–318. 2003.PubMed/NCBI
|
|
29
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: a cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barr MP, Gray SG, Hoffmann AC, et al:
Generation and characterisation of cisplatin-resistant non-small
cell lung cancer cell lines displaying a stem-like signature. PLoS
One. 8:e541932013. View Article : Google Scholar : PubMed/NCBI
|