Introduction
Ovarian cancer is the most lethal gynecological
malignancy worldwide. The high mortality rate of patients with
ovarian cancer is attributed to the late diagnosis of this type of
tumor. Approximately 70% of patients with ovarian cancer are first
diagnosed in the advanced stage of disease (1,2).
The 5-year survival rate of patients with advanced-stage ovarian
cancer is only 30%. In women, >80% of malignant ovarian tumors
are of epithelial origin and the prognosis of epithelial ovarian
carcinoma (EOC) is poor (3).
The tumor microenvironment is composed of blood and
lymphatic vessels, tissue fluid, fibroblasts, inflammatory cells
and a large number of extracellular matrices, which are essential
for tumor cell growth (4,5). Numerous studies have found that
cancer-associated fibroblasts (CAFs) play an important role in a
wide variety of tumors, such as breast, prostate, esophageal,
pancreatic and lung cancer by promoting the initiation,
proliferation, invasion and metastasis of cancer cells; however,
the underlying mechanisms are not yet fully understood (6–9).
In our previous studies, we found that the abundance of CAFs in
ovarian cancer is associated with the advanced stage of the tumor,
lymph node metastasis and omental metastasis. We successfully
isolated primary ovarian CAFs and normal fibroblasts (NFs)
(10,11).
The enhancer of zeste homologue 2 (EZH2) is a
subunit of polycomb repressive complex 2 (PRC2), which catalyzes
the methylation of histone 3 lysine 27 (H3K27). EZH2 has been shown
to be overexpressed in many types of cancer, which contributes to
the silencing of tumor suppressor genes and is involved in the
initiation and progression of tumors (12–14). The aim of this study was to
investigate the effects of CAFs on the migration ability of ovarian
cancer cells and the involvement of EZH2 in this process, as well
as the underlying mechanisms involved.
Materials and methods
Cell culture
Three ovarian cancer cell lines (A2780, SKOV3 and
ES2) were used in the present study. A2780 cells were obtained from
the China Center for Type Culture Collection (CCTCC; Wuhan, China)
and SKOV3 and ES2 cells were purchased from the Cell bank of China
Center for Type Culture Collection, Chinese Academy of Sciences
(CTCCCAS, Shanghai, China). The A2780 cells stably transfected with
shRNA targeting EZH2 (shEZH2; A2780-shEZH2 cells) or shNC (negative
control; A2780-shNC cells) were previously established in our
laboratory (15). Primary CAFs in
EOC and ovarian NFs were isolated and identified as described in
our previous study (10). The
A2780, ES2, A2780-shEZH2 and A2780-shNC cells were cultured in
RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10%
fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA). The SKOV3 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen) supplemented with 10% FBS. Primary CAFs and NFs were
cultured in DMEM/F12 supplemented with 20% FBS. All the cell lines
were cultured at 37°C, in a humidified atmosphere of 5%
CO2.
Indirect co-culture
The A2780, SKOV3 and ES2 cells were indirectly
co-cultured with primary ovarian CAFs or NFs in 6-well Transwell
chamber plate with pores of 0.4 μm in diameter. Fibroblasts were
seeded in the lower compartment (4×104 cells/well) and
EOC cells in the upper compartment (4×104 cells/well).
These cells were indirectly co-cultured for 7 days. The EOC cells
cultured alone represented the blank group which was used as the
control. The experiment was repeated 3 times separately.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA from the cells was extracted using TRIzol
Reagent (Invitrogen) and reverse transcribed into complementary DNA
(cDNA) using the Reverse Transcription kit (Toyobo, Osaka, Japan).
The sequences of the primers used were as follows: EZH2,
5′-CTCCCGCTGAGGATGTGGATAC-3′ (forward) and
5′-GGCTCCACAAGTAAGACAGAGGTC-3′ (reverse); β-actin,
5′-GCCAACACAGTGCTGTCTGG-3′ (forward) and
5′-GCTCAGGAGGAGCAATGATCTTG-3′ (reverse). PCR was performed
according to the manufacturer's instructions using SYBR-Green PCR
Master Mix (Toyobo). The amplification protocols were as follows:
95°C for 60 sec; 40 cycles of 95°C for 15 sec, 57°C for 15 sec and
72°C for 45 sec. EZH2 mRNA was normalized to β-actin amplification.
Relative quantification was performed using the comparative
threshold cycle (Ct) method (2−ΔΔCt) as previously
described (16). All reactions
were performed in triplicate.
Western blot analysis
Total protein from the cells was extracted using
radioimmune precipitation assay (RIPA) buffer. The protein
concentration was measured by the bicinchoninic acid (BCA) assay.
Fifty micrograms of total protein were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a nitrocellulose membrane. The membranes were
incubated at 4°C overnight with the following primary antibodies:
mouse anti-EZH2 polyclonal antibody (1:500 dilution; Cell Signaling
Technology, Beverly, MA, USA), or mouse anti-β-actin polyclonal
antibody (1:500 dilution; Santa Cruz Biotechnology, Inc., CA, USA).
The primary antibodies were detected by incubating with horseradish
peroxidase-conjugated anti-mouse secondary antibody (1:5,000
dilutions; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature and visualized using an ECL system (Beyotime, Shanghai,
China) by exposure to X-ray film. The protein bands were quantified
using Quantity One software (Bio-Rad, Hercules, CA, USA). The
experiment was repeated 3 times separately.
Cell migration assay
The 24-well Transwell chamber plate with pores of 8
μm in diameter was used to determine the migration ability of the
cells. Cells (5×104 cells/well) were resuspended in 100
μl culture medium, and then added to the Transwell inserts (Corning
Glass Works; Corning, NY, USA). The lower chamber beneath the
insert membrane was supplemented with 600 μl corresponding medium
following incubation for 16 h, and the migrated cells on the lower
surface of the membrane were fixed with 95% ethanol and stained
with crystal violet followed by cell counting. All assays were
performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
statistical software (SPSS, Chicago, IL, USA). All numerical data
are expressed as the means ± standard deviation. Statistical
significance of the differences was analyzed using a two-tailed
Student's t-test or one-way ANOVA. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
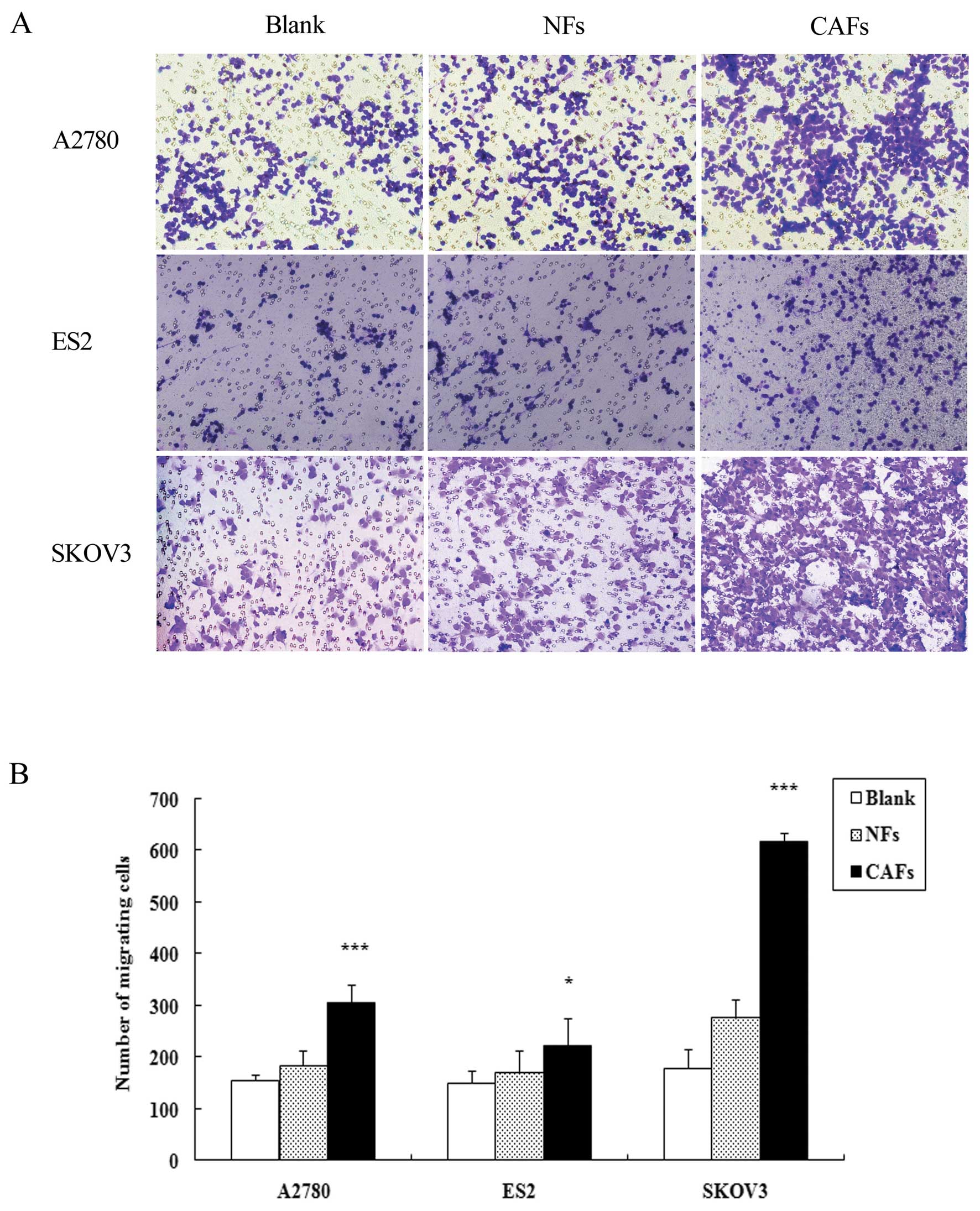
CAFs enhance the migration ability of
ovarian cancer cells
Ovarian cancer cells were cultured alone or
indirectly co-cultured with CAFs or NFs and their migration ability
was assessed. Compared with the cells cultured alone, the number of
A2780, SKOV3 and ES2 cells co-cultured with CAFs penetrating the
membrane increased by 1.97-, 3.46- and 1.49-fold, respectively;
compared with the cells cultured alone. The number of A2780, SKOV3
and ES2 cells co-cultured with NFs penetrating the membrane
increased by 1.19-, 1.54- and 1.13-fold, respectively (Fig. 1). The migration ability of the
cells co-cultured with CAFs was markedly enhanced in comparison to
the blank group and the difference was statistically significant
(PA2780<0.001, PSKOV3<0.001 and
PES2<0.05); the migration ability of the cells in the
NF group (apart from ES2 cells) was markedly enhanced in comparison
to the blank group (P<0.001).
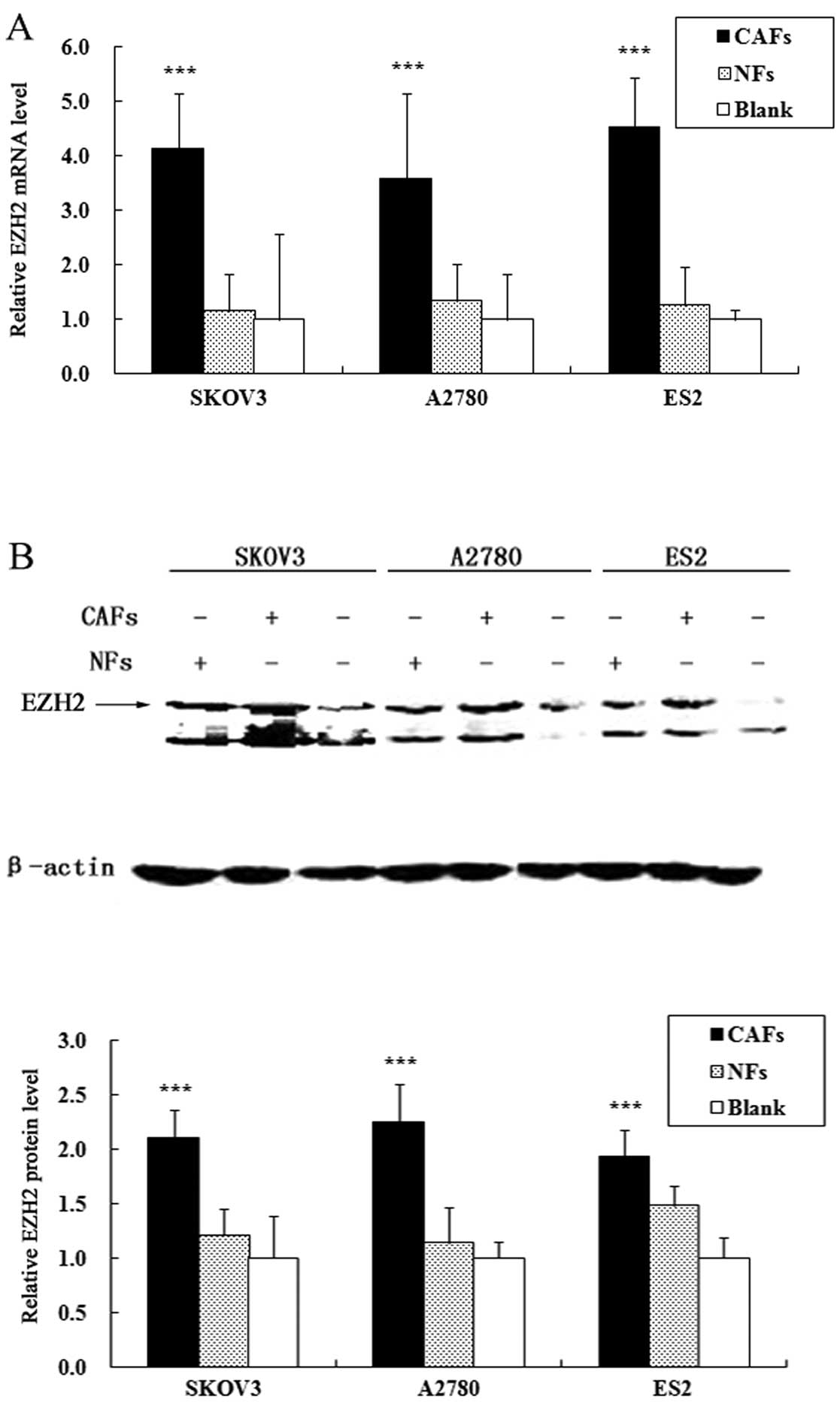
CAFs increase the expression of EZH2 in
ovarian cancer cells
The alterations in the expression levels of EZH2 in
the co-cultured ovarian cancer cell lines were assessed by qRT-PCR
and western blot analysis. The results of qRT-PCR revealed that the
CAFs significantly increased the mRNA expression of EZH2 in the
ovarian cancer cells. Compared with the cells cultured alone, the
mRNA expression of EZH2 in the A2780, SKOV3 and ES2 cells
co-cultured with CAFs increased by 3.41-, 4.12-, 4.85-fold,
respectively (PA2780<0.001,
PSKOV3<0.001 and PES2<0.001); the
ovarian NFs did not significantly increase the mRNA expression of
EZH2 in the ovarian cancer cells (Fig. 2A). Similarly, western blot
analysis revealed that the CAFs significantly increased the protein
expression of EZH2 in the ovarian cancer cells. Compared with cells
cultured alone, the protein expression of EZH2 in the A2780, SKOV3
and ES2 cells co-cultured with CAFs increased by 2.25-, 2.1- and
1.94-fold, respectively (PA2780<0.001,
PSKOV3<0.001 and PES2<0.001); the
ovarian NFs did not significantly increase the protein expression
of EZH2 in the ovarian cancer cells (Fig. 2B).
CAFs enhance the migration ability of
ovarian cancer cells by increasing EZH2 expression
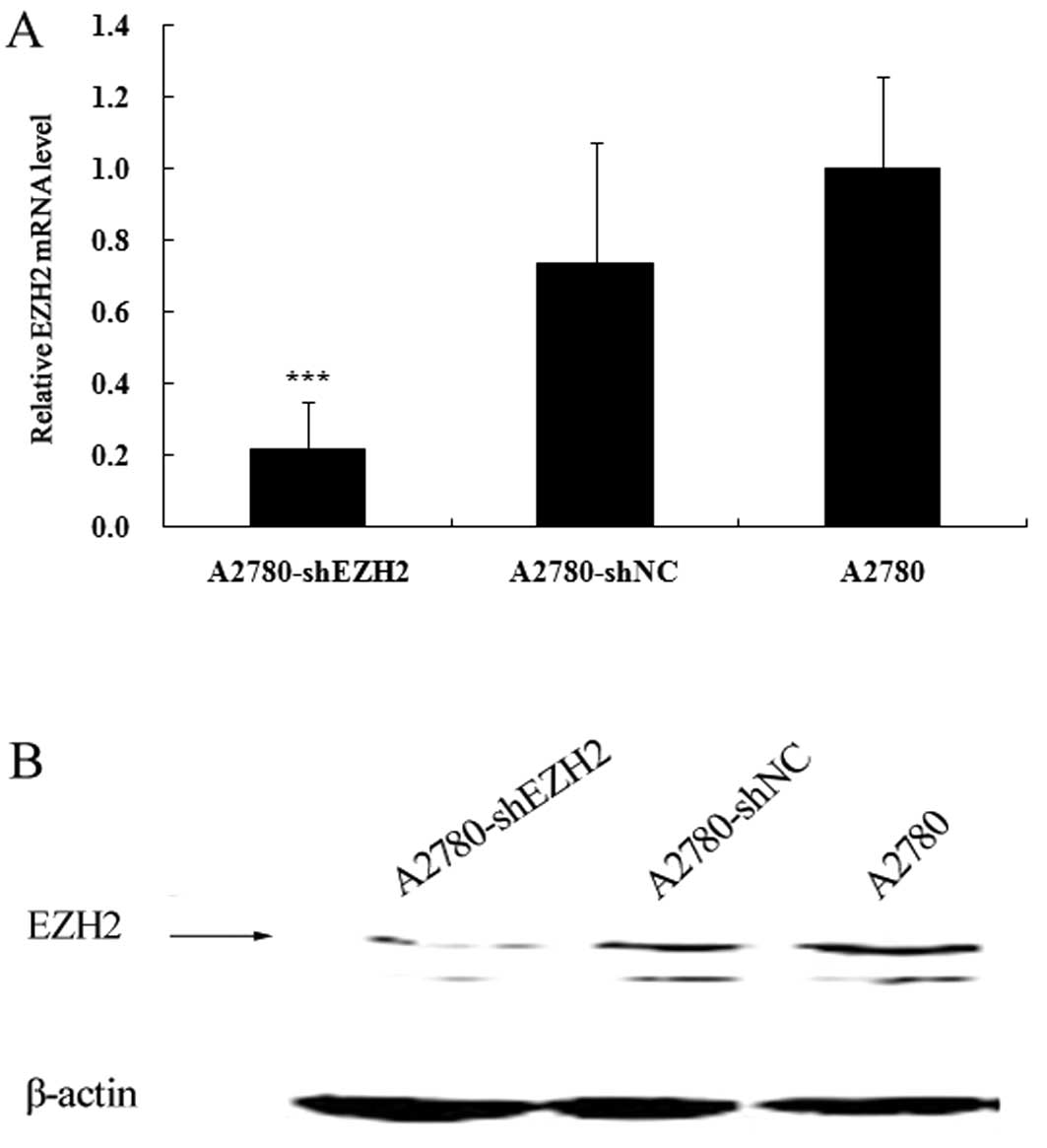
The expression levels of EZH2 in the A2780-shEZH2,
A2780-shNC and A2780 cells were assessed by qRT-PCR and western
blot analysis. The mRNA expression of EZH2 decreased by
approximately 78.2% in the A2780-shEZH2 cells (P<0.001)
(Fig. 3A), whereas there was no
significant difference between the A2780-shNC and A2780 cells
(Fig. 3A). The knockdown of EZH2
in the A2780-shEZH2 cells was further confirmed by western blot
analysis (P<0.001) (Fig. 3B).
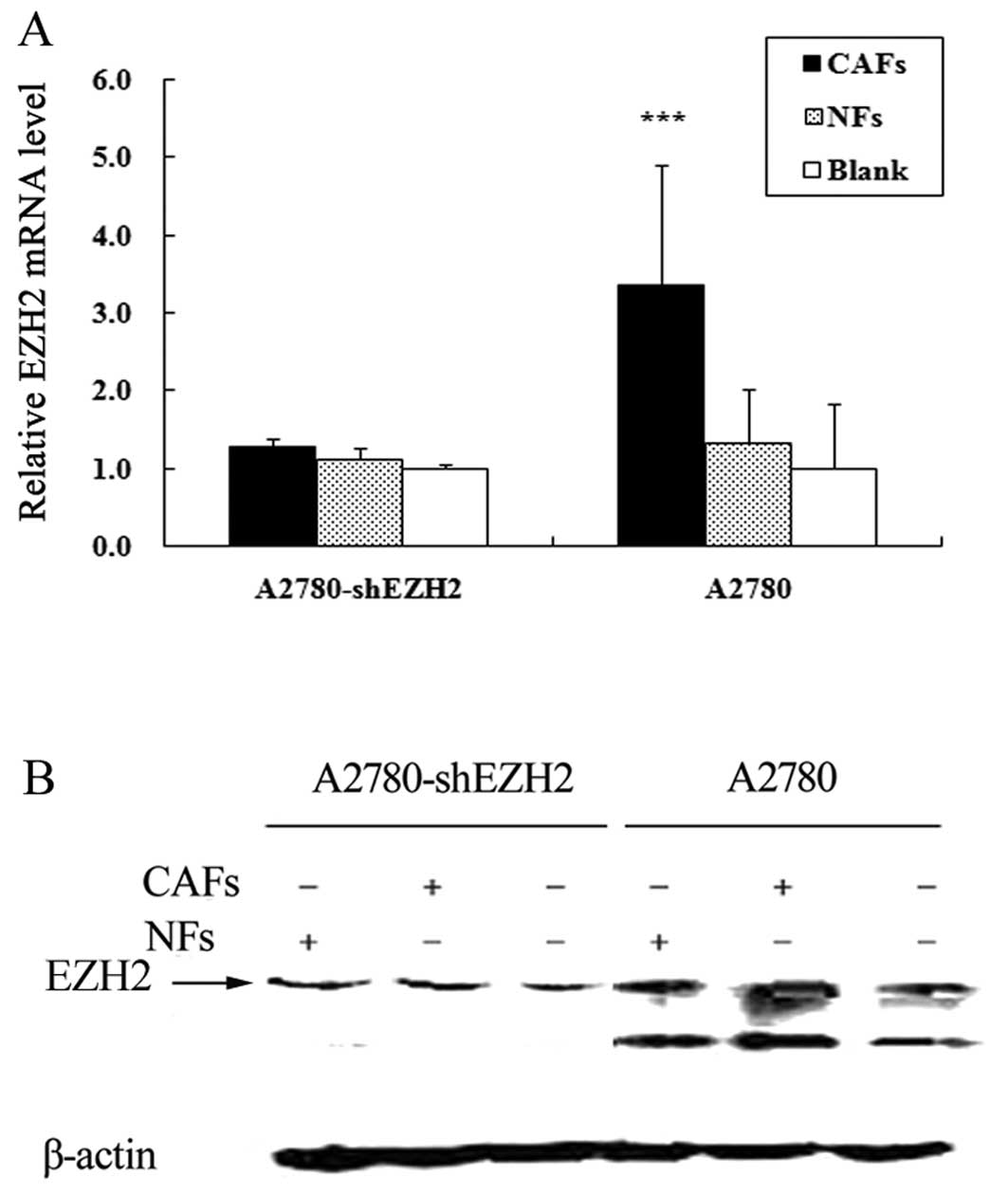
Compared with the cells cultured alone, the expression of EZH2 in
the A2780-shEZH2 cells co-cultured with CAFs was not significantly
increased (P>0.05) (Fig. 4).
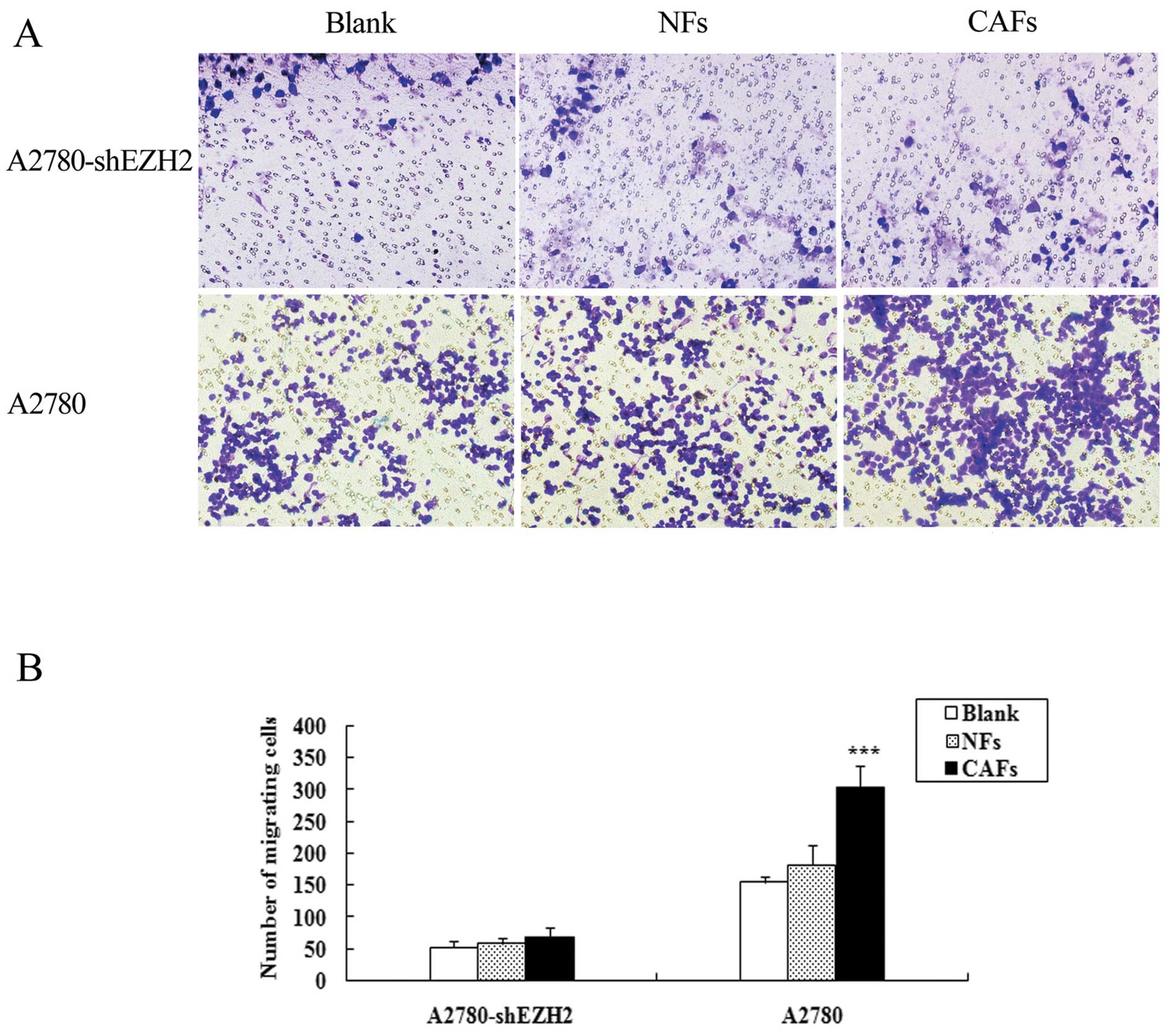
The number of A2780-shEZH2 cells co-cultured with CAFs penetrating
the membrane was 1.26-fold in comparison to the cells cultured
alone, and the number of A2780-shEZH2 cells co-cultured with NFs
penetrating the membrane was 1.11-fold in comparison to the cells
cultured alone. Transwell assay demonstrated that the CAFs did not
enhance the migration ability of the A2780-shEZH2 cells compared
with the blank group (P>0.05) (Fig. 5); the CAFs and NFs enhanced the
migration ability of the A2780 cells, and the expression of EZH2 in
the A2780 cells was much higher than that in the A2780-shEZH2
cells.
Discussion
The tumor microenvironment is thought to affect
malignant transformation and tumor progression. The immune cells in
the tumor microenvironment can be quantitatively assessed through
epigenetic markers, providing a novel method for the early
diagnosis of cancer (17). What
is more, cancer epigenetics may be regulated by the tumor
microenvironment (such as hypoxia) (18). It has been reported that CAFs
promote the growth and invasion of cancer cells; however, the
specific mechanisms involved remain unclear (19–21). EZH2 is overexpressed in many types
of cancer and plays a crucial role in the tumorigenesis of several
types of human cancer (12–14). In ovarian cancer, the
overexpression of EZH2 promotes cell proliferation and invasion
(22). The aim of this study was
to investigate the effects of CAFs on the migration ability of
ovarian cancer cells and to determine the involvement of EZH2 in
this process, as well as the underlying mechanisms.
In the present study, we found that the migration
ability of ovarian cancer cells indirectly co-cultured with CAFs or
NFs was significantly enhanced, particularly that of cells
indirectly co-cultured with CAFs. Additionally, CAFs significantly
increased the mRNA and protein expression of EZH2 in ovarian cancer
cells. However, the NFs did not significantly increase the
expression of EZH2 in ovarian cancer cells. We therefore considered
that CAFs may induce epigenetic alterations in cancer cells.
Moreover, CAFs did not enhance the migration ability of the
A2780-shEZH2 cells compared with the cells cultured alone.
The exact mechanisms responsible for CAFs enhancing
the migration ability of ovarian cancer cells may include two
aspects: CAFs induced the upregulation of EZH2 in ovarian cancer
cells through paracrine or autocrine mechanisms, subsequently
enhancing the migration ability of the ovarian cancer cells; CAFs
and EZH2 interacted with each other, which enhanced the migration
ability of the ovarian cancer cells.
Our study revealed that ovarian CAFs enhanced the
migration ability of the ovarian cancer cells by increasing the
expression of EZH2. CAFs enhanced the expression of EZH2 in ovarian
cancer cells, which may be due to the function of certain factors
secreted by CAFs. Several studies have found that certain genes are
upreglated in CAFs in contrast to NFs, such as growth factors
associated with metastasis [platelet-derived growth factor
(PDGF)-A, fibroblast growth factor 1 (FGF1), transforming growth
factor (TGF)-β and interleukin (IL)-6] and factors promoting
angiogenesis [vascular endothelial growth factor (VEGF),
cyclooxygenase (COX)-2, vimentin and α-smooth muscle actin (α-SMA)]
(23,24). CAFs may provide the most suitable
microenvironment for the growth of ovarian cancer cells. Lu et
al confirmed that VEGF induced the upregulation of EZH2 in
ovarian cancer cells (25).
In conclusion, our results suggest that CAFs enhance
the migration ability of ovarian cancer cells partly by increasing
EZH2 expression. We established the link between epigenetic
mechanisms and the microenvironment. Further studies are required
to identify the mechanisms responsible for CAFs increasing the
expression of EZH2 in ovarian cancer cells.
Acknowledgements
We would like to thank the Department of Obstetrics
and Gynecology, Union Hospital, Wuhan, China. This study was
supported by the National Natural Science Foundation of China
(81072134 and 81272860).
References
|
1
|
Sarojini S, Tamir A, Lim H, et al: Early
detection biomarkers for ovarian cancer. J Oncol. 2012:7090492012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bijron JG, Bol GM, Verheijen RH and van
Diest PJ: Epigenetic biomarkers in the diagnosis of ovarian cancer.
Expert Opin Med Diagn. 6:421–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naora H and Montell DJ: Ovarian cancer
metastasis: integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chou J and Werb Z: MicroRNAs play a big
role in regulating ovarian cancer-associated fibroblasts and the
tumor microenvironment. Cancer Discov. 2:1078–1080. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra AK, Zillhardt M, Hua Y, et al:
MicroRNAs reprogram normal fibroblasts into cancer-associated
fibroblasts in ovarian cancer. Cancer Discov. 2:1100–1108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Finak G, Bertos N, Pepin F, et al: Stromal
gene expression predicts clinical outcome in breast cancer. Nat
Med. 14:518–527. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wintzell M, Hjerpe E, Avall Lundqvist E
and Shoshan M: Protein markers of cancer-associated fibroblasts and
tumor-initiating cells reveal subpopulations in freshly isolated
ovarian cancer ascites. BMC Cancer. 12:3592012. View Article : Google Scholar
|
|
8
|
Ko SY, Barengo N, Ladanyi A, et al: HOXA9
promotes ovarian cancer growth by stimulating cancer-associated
fibroblasts. J Clin Invest. 122:3603–3617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schauer IG, Sood AK, Mok S and Liu J:
Cancer-associated fibroblasts and their putative role in
potentiating the initiation and development of epithelial ovarian
cancer. Neoplasia. 13:393–405. 2011.PubMed/NCBI
|
|
10
|
Zhang Y, Tang H, Cai J, et al: Ovarian
cancer-associated fibroblasts contribute to epithelial ovarian
carcinoma metastasis by promoting angiogenesis, lymphangiogenesis
and tumor cell invasion. Cancer Lett. 303:47–55. 2011. View Article : Google Scholar
|
|
11
|
Cai J, Tang H, Xu L, et al: Fibroblasts in
omentum activated by tumor cells promote ovarian cancer growth,
adhesion and invasiveness. Carcinogenesis. 33:20–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao R, Wang L, Wang H, et al: Role of
histone H3 lysine 27 methylation in Polycomb-group silencing.
Science. 298:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirmizis A, Bartley SM, Kuzmichev A, et
al: Silencing of human polycomb target genes is associated with
methylation of histone H3 Lys 27. Genes Dev. 18:1592–1605. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu S, Yu L, Li Z, et al: Overexpression of
EZH2 contributes to acquired cisplatin resistance in ovarian cancer
cells in vitro and in vivo. Cancer Biol Ther. 10:788–795. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sehouli J, Loddenkemper C, Cornu T, et al:
Epigenetic quantification of tumor-infiltrating T-lymphocytes.
Epigenetics. 6:236–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shahrzad S, Bertrand K, Minhas K and
Coomber BL: Induction of DNA hypomethylation by tumor hypoxia.
Epigenetics. 2:119–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
20
|
Shimoda M, Mellody KT and Orimo A:
Carcinoma-associated fibroblasts are a rate-limiting determinant
for tumour progression. Semin Cell Dev Biol. 21:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci (Landmark Ed). 15:166–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Cai Q, Godwin AK and Zhang R:
Enhancer of zeste homolog 2 promotes the proliferation and invasion
of epithelial ovarian cancer cells. Mol Cancer Res. 8:1610–1618.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor and the Met system as a mediator of tumor-stromal
interactions. Int J Cancer. 119:477–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
25
|
Lu C, Han HD, Mangala LS, et al:
Regulation of tumor angiogenesis by EZH2. Cancer Cell. 18:185–197.
2010. View Article : Google Scholar : PubMed/NCBI
|