Introduction
Targeting technology is used in molecular imaging to
improve the efficacy of magnetic resonance imaging (MRI) contrast
agents and to increase their concentration in the nidus area so as
to enhance image visibility (1,2).
Numerous types of targeting technology have been used to achieve
these goals, with the peptide-based targeting vector technology
becoming increasingly popular for diagnostic and therapeutic
applications (3–5). Peptides have very good
biocompatibility, limited immunogenicity in the body and
non-specific uptake by the liver or the reticuloendothelial system
(6). The synthesis of peptides is
simple and cost-efficient, the design of the peptide chain is
flexible, while ameliorating their structure and improving their
efficacy can be easily accomplished by replacing amino acid
residues in the peptide chain. Researchers can design and
expediently synthesize the corresponding peptide to meet custom
medical purposes. The specific binding of functional peptides to
their receptors facilitates the research of targeting contrast
agents (7,8).
Integrin αvβ3 and
aminopeptidase N (CD13) are expressed in sprouting
endothelial cells and in various tumor cells, but show restricted
expression in normal cells, which renders them suitable receptors
for tumor-targeting agents (9).
Since the Arg-Gly-Asp (RGD) peptide is specific to
αvβ3 (10,11), numerous applications based on the
RGD/αvβ3 targeting delivery system have been
reported in molecular imaging (12,13). Similarly, the Asn-Gly-Arg (NGR)
peptide is a specific recognition sequence of CD13
(14). The
NGR/CD13-based targeting delivery system is frequently
reported in drug design for the treatment of cancer (15,16) and as an imaging system (17,18). However, to our knowledge, no study
to date has investigated the potential of the combination of RGD
with NGR as a targeted delivery system. Promising results from such
a study may prove meaningful for the therapy and diagnosis of
tumors.
In the present study, we designed a peptide sequence
which consists of the two motifs, RGD and NGR. The peptide sequence
was then coupled to the complex gadolinium (Gd)-1,4,
7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to
investigate its efficacy as a tumor-targeting contrast agent. The
contrast agent can target αvβ3 and
CD13 as a dual-targeting contrast agent.
αvβ3 and CD13 are
tumor biomarkers; however, these proteins are not highly expressed
in all tumor types. If each of the RGD and NGR peptides in the
dual-targeting contrast agent binds to
αvβ3-overexpressing and
CD13-overexpressing tumor cells, respectively, then the
contrast agent can detect both types of tumor, and thus the
combination of RGD and NGR may improve tumor detection rates. In
tumor cells expressing both αvβ3 and
CD13, the two motifs of RGD and NGR can simultaneously
bind to the target tissue, which enhances the incorporation of the
contrast agent; the higher concentration of the contrast agent in
the tumor area will improve imaging visibility. In this study, the
targeting behavior of the contrast agent that we designed was
examined using MDA-MB-231 human breast cancer cells (which
overexpress αvβ3) (19,20) and PC-3 human prostate cancer cells
(which overexpress CD13) (21).
Materials and methods
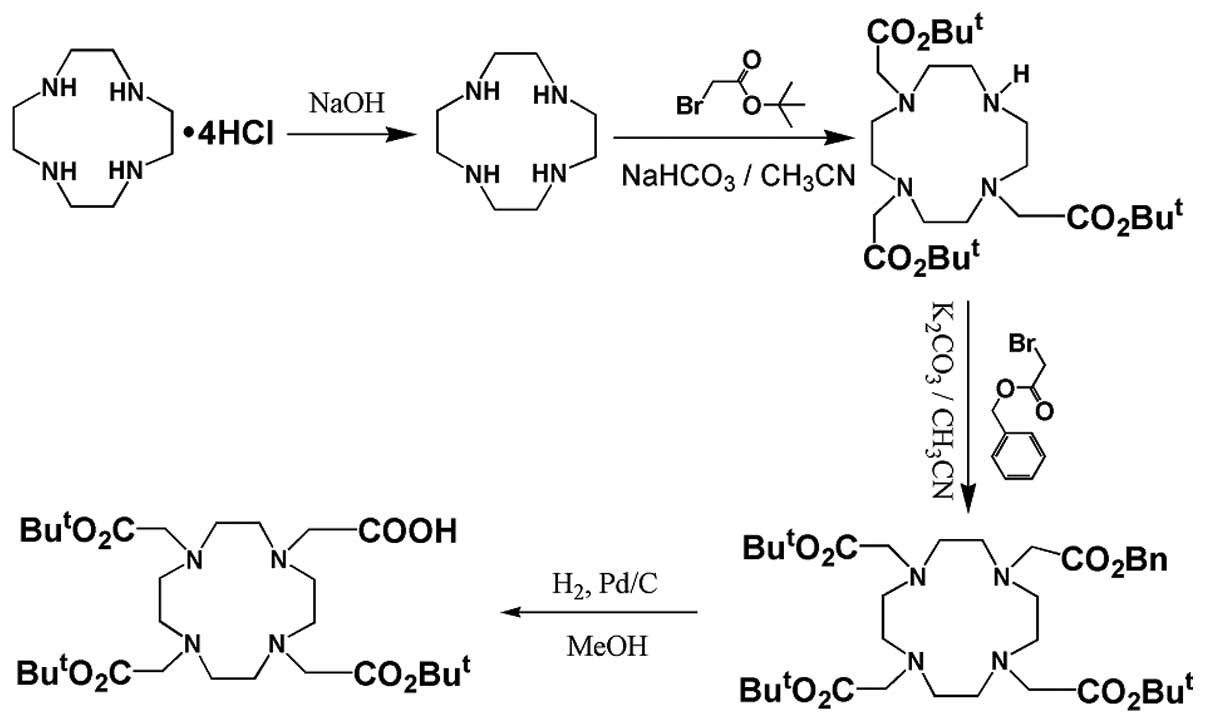
Synthesis of
4,7,10-tricarboxymethyl-tert-butyl ester 1,4,
7,10-tetraazacyclododecane-1-acetate [DOTA(tBu)3]
DOTA(tBu)3 was prepared by a multistep
synthesis procedure starting from cyclen hydrochloric salt. The
synthesis was based on a previous study (22). The procedure is outlined in
Fig. 1.
Synthesis of ligand (S7)
The peptide was assembled using manual solid phase
Fmoc peptide chemistry, as previously described (23) and outlined in Fig. 2. N-Fmoc-Lys (Dde)-OH was selected
as an anchoring site to conjugate with DOTA(tBu)3 using
its ω-amino acid. The protective group, Dde, of N-Fmoc-Lys (Dde)-OH
was removed by incubation with 2% hydrazine monohydrate in
dimethylformamide (DMF, 25 ml/g) at room temperature for 3 min. The
coupling efficiency (>99%) in every coupling step was determined
by measuring residual free amine with the quantitative ninhydrin
assay, as previously described (24).
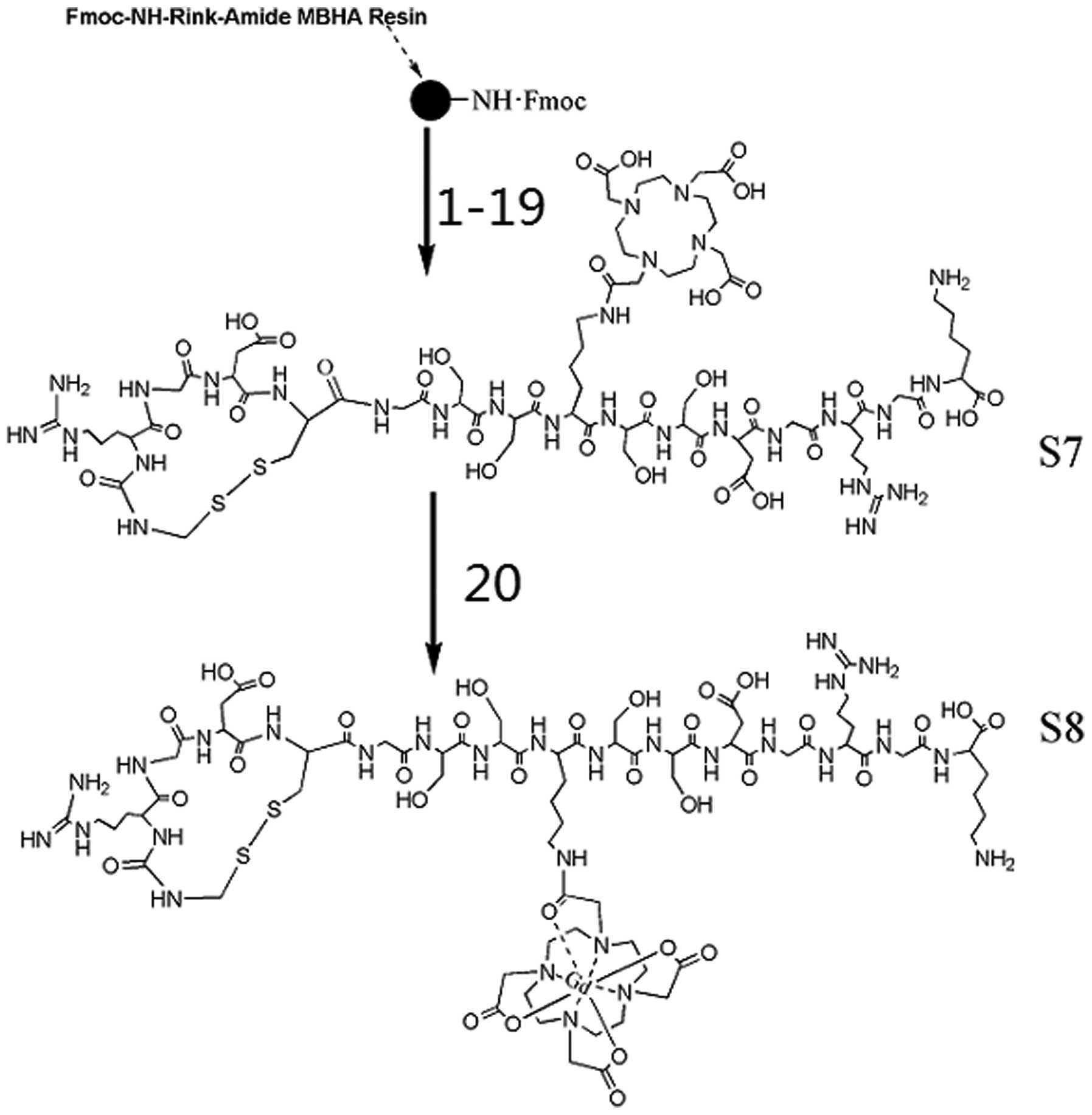 | Figure 2Synthesis of the gadolinium (Gd)
complex S8 starting from Rink Amide MBHA resin. All 16 amino acids
were coupled using the following steps. First, the
Nα-Fmoc protective group was removed by the addition of
20% piperdine in dimethylformamide (DMF) (two times) for 5 min.
After alternate washes with DMF and dichloromethane (DCM), a
mixture solution of amino acid (three equivalents), HBTU (three
equivalents), HOBt (three equivalents) and DIPEA (six equivalents)
in 10 ml DMF was added into the reaction. The reaction was
completed by frothing agitation with nitrogen for 2 h to generate a
peptide bond. The Nω-Dde protective group of middle Lys
was removed using a solution of 2% hydrazine monohydrate in DMF (25
g/ml) at rt for 3 min and then the DOTA(tBu)3 was
coupled with the middle Lys using the same method as described
above for the amino acids. Coupling sequences: 1, Fmoc-lys(Boc)-OH;
2, Fmoc-Gly-OH; 3, Fmoc-Arg(Pbf)-OH; 4, Fmoc-Gly-OH; 5,
Fmoc-Asp(otBu)-OH; 6, Fmoc-Ser(tBu)-OH; 7, Fmoc-Ser(tBu)-OH; 8,
Fmoc-lys(Dde)-OH; 9, DOTA(tBu)3; 10, Fmoc-Ser(tBu)-OH;
11, Fmoc-Ser(tBu))-OH; 12, Fmoc-Gly-OH; 13, Fmoc-Cys(Acm)-OH; 14,
Fmoc-Asn(Trt)-OH; 15, Fmoc-Gly-OH; 16, Fmoc-Arg(Pbf)-OH; 17,
Fmoc-Cys(Acm)-OH; 18, 5 M I2 in
DMF/Na2S2O3·5H2O, aq;
19, TFA:p-cresol:thioanisole:ethanedithiol (90:2:5:3); 20,
GdCl3·6H2O/NH3·H2O pH
6.5–7.0, room temperature, 3 h. |
The intramolecular disulfide bond was formed between
the two cysteine (Cys) residues. A 5 M I2 solution in
DMF was added to the reaction system with continuous stirring for 3
h, to remove the side-protection group, Acm, from Cys (Acm)-OH and
to simultaneously oxidize the two sulfydryls, so as to form a
disulfide bridge. Excessive iodine was removed with sodium
thiosulphate
(Na2S2O3·5H2O, aq).
The crude product was cleaved from the
4-(2′,4′-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamido-norleucyl
(Rink Amide) MBHA resin by treatment with a mixture of 90%
trifluoroacetic acid (TFA), 2% p-cresol, 5% thioanisole and 3%
ethanedithiol for 4 h at 0°C. Residual protective groups were
removed. Subsequently, the free peptide was precipitated in cold
Et2O and centrifuged. The sediment was collected and
dissolved in water. Semi-preparative reversed phase (RP)
high-performance liquid chromatography (HPLC) purification and
subsequent lyophilization yielded the white powder product, S7
(230.1 mg, yield 21.5% based on resin loading). Analytical RP HPLC
estimated the purity of S7 at 98%, and the product was eluted in a
linear gradient of 0–30% CH3CN-H2O in 0.1%
TFA for 30 min.
Gd complex S8
The ligand S7 (20.2 mg from a 10.2 μmol
comcentration) was dissolved in 10 ml of demineralized water. The
pH of the aqueous solution was adjusted to 6.5–7.0 by the addition
of 0.25% NH4OH(aq). Subsequently, a solution of 3.75 mg
Gd chloride hexahydrate in 1.0 ml water (10.0 μmol, 0.99
equivalents) was added. The mixture was vigorously stirred for 4 h
at room temperature with the pH maintained at 6.5–7.0 by the
addition of 0.25% NH4OH. The crude product was purified
using a dialysis bag, and was condensed and lyophilized. The
resulting Gd complex, S8, was a white hygroscopic powder (20.97 mg,
yield 97.2%).
Cell culture
MDA-MB-231 cells were cultured in Dulbecco’s
modified Eagle’s (DMEM) medium supplemented with 10 U/ml
penicillin, 10 μg/ml streptomycin and 10% fetal bovine serum (FBS).
All media, serum and antibiotics were provided by Invitrogen Life
Technologies (Carlsbad, CA, USA). Cells were cultured in a 5%
CO2 atmosphere at 37°C under 95% humidity, within a
ThermoFisher Scientific (Rockford, IL, USA) incubator 3111. PC-3
cells were cultured as described above, in F12 medium.
Relaxivity measurements
To determine the longitudinal relaxivity
(r1) value, seven different concentrations (0, 0.05,
0.1, 0.2, 0.4, 0.8 and 1.6 mM) of the contrast agents, S8 and
Magnevist (serving as the control), were prepared. The relaxation
time (T1) of these solutions was measured on a Bruker
AVANCE III 500WB Spectrometer (Bruker NMR, Germany) with an 89-mm
vertical-bore magnet of 11.7 T with the following parameters: 20°C,
Multi-Slice Multi-Echo repeat time (TR), 5,000 msec; echo time
(TE), 7.6 msec; and average signal, 2. Linear fitting of the
reciprocal of the T1 relaxation time vs. the Gd
concentration (mM) was used to estimate 1/T1
(r1, mM−1sec−1).
Cellular uptake of contrast agents
MDA-MB-231 or PC-3 cells were seeded in 24-well
culture plates (5×105 cells/well) and were cultured
overnight to allow the cells to adhere to the plate wall. The cells
were then divided into two groups: group 1 was treated in
triplicate with eight different concentrations (0.05–1.2 mM) of S8
for 8 h, and group 2 was treated with Magnevist with the same
method. Following incubation, the nutrient solution was discarded
and the cells were washed twice with phosphate-buffered saline
(PBS) to remove unbound Gd complexes. The Gd content of the cells
was determined by inductively coupled plasma atomic emission
spectroscopy (ICP-AES) in a PerkinElmer (Waltham, MA, USA) Optima
8000 spectrometer. The results are expressed as the means ± SD.
Cell imaging
MDA-MB-231 or PC-3 cells were seeded in 24-well
culture plates (5×106 cells/well). The cells were then
grouped and incubated as described above. All samples were fixed in
1% agarose and visualized using a 11.7 T NMR spectrometer.
T1-weighted images were obtained by multi-slice
multi-echo imaging, using inversion times 50, 100, 200, 400, 700,
1,400, 2,000 and 2,800 msec, a TE of 6 msec and an echo train
length of 8 at a TR of 300 msec. All images were obtained from a
single axial slice of 3 mm in a 20×15 cm2 field of view
(FOV), on a 125×125×125 matrix and at one excitation.
WST
The WST assay was performed to examine cell
proliferation and cytotoxicity. The MDA-MB-231 or PC-3 cells were
seeded in quintuplicate in 96-well plates (1×104 cells
in 100 μl culture medium) and were maintained for 24 h. The cells
were then incubated for 48 h with S8 at different Gd
concentrations, the nutrient solution was discarded and the cells
were washed with PBS buffer three times. Subsequently, fresh
culture medium and WST were added. Cell viability was measured
following the manufacturer’s instructions (Beyotime Institute of
Biotechnology (Haimen, China). The data are expressed as the means
± SD. The same concentration of Magnevist was used in the control
experiments.
Results
Target product
The Gd complex S8 is a white hygroscopic powder.
Chemical characterization of S7 and S8 was performed by high
resolution electron spray ionization mass spectrometry (HRESIMS)
using the 6220 Accurate-Mass TOF LC/MS spectrometer (Agilent
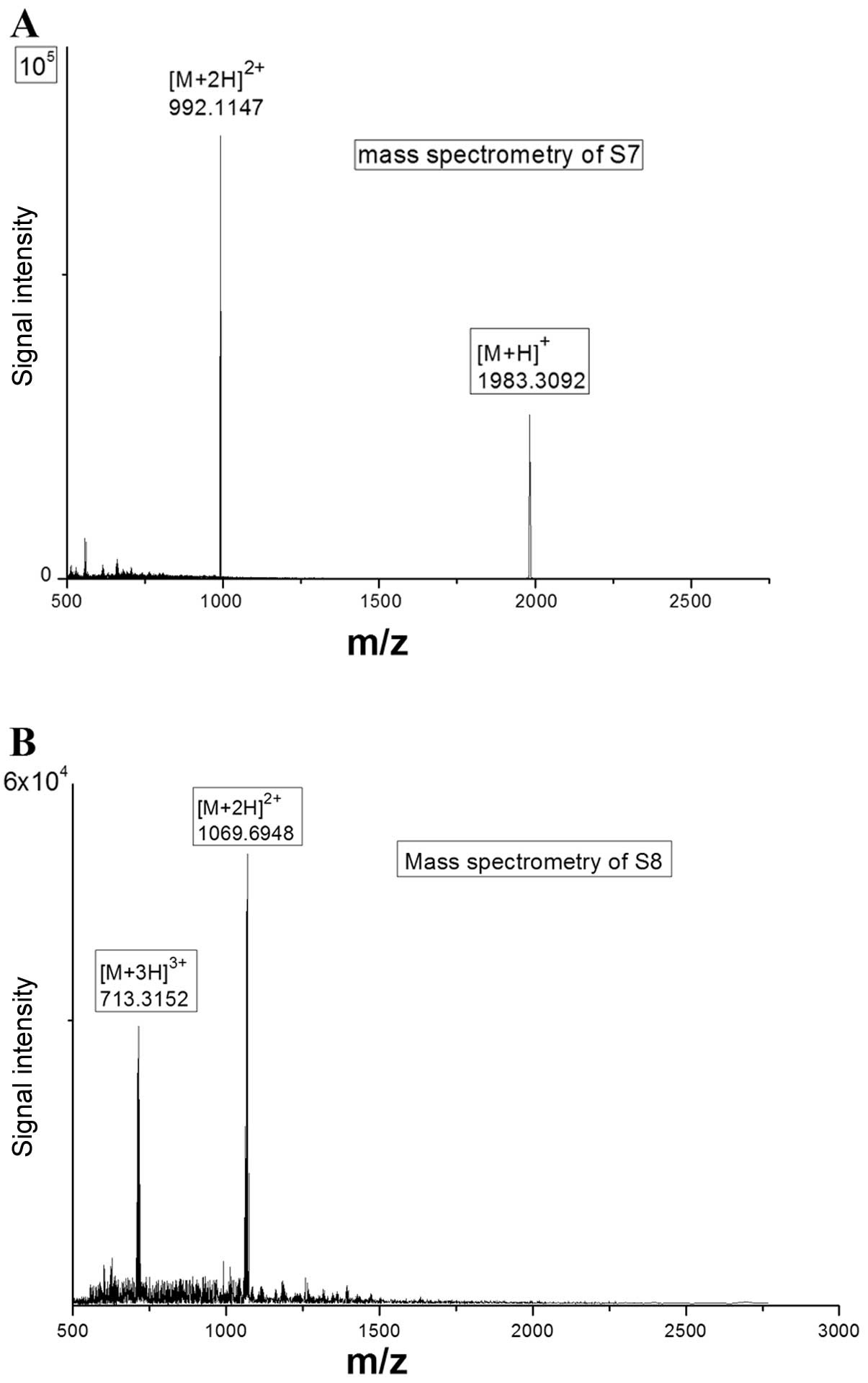
Technologies, Santa Clara, CA, USA). HRESIMS revealed that the S7
[M+H]+ mass was 1983.3092 and the S7 [M+2H]2+
mass was 992.1147 (Fig. 3A), in
agreement with their theoretical mass values (1983.8530 for
[M+H]+ and 992.4265 for [M+2H]2+,
respectively). The S8 [M+2H]2+ mass was similarly shown
to be 1069.6948 and the S8 [M+3H]3+ mass was 713.3152
(Fig. 3B), in agreement with
their theoretical mass values (1069.8770 for [M+2H]2+
and 713.5847 for [M+3H]3+, respectively). The Gd content
of S8 was determined by ICP-AES. The observed value of the Gd
content was 98.3% of the theoretical value.
Relaxivity
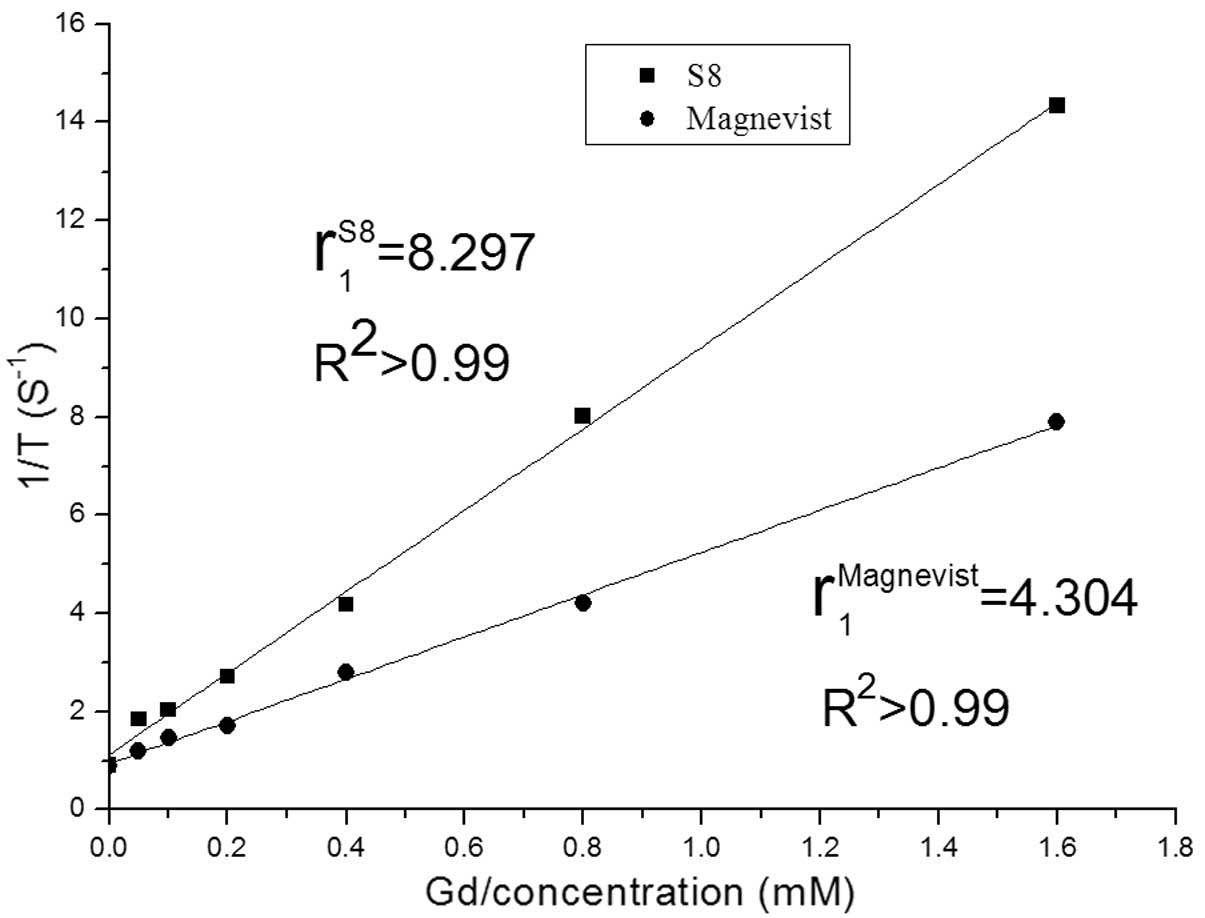
The r1 value of S8 was estimated to be
8.297 mM−1sec−1 (Fig. 4), that is, higher than the
r1 value of Magnevist (4.30
mM−1sec−1), which is a ripe contrast agent
widely used in clinical applications.
Targeting behavior of S8
We investigated the targeting behavior of S8 in two
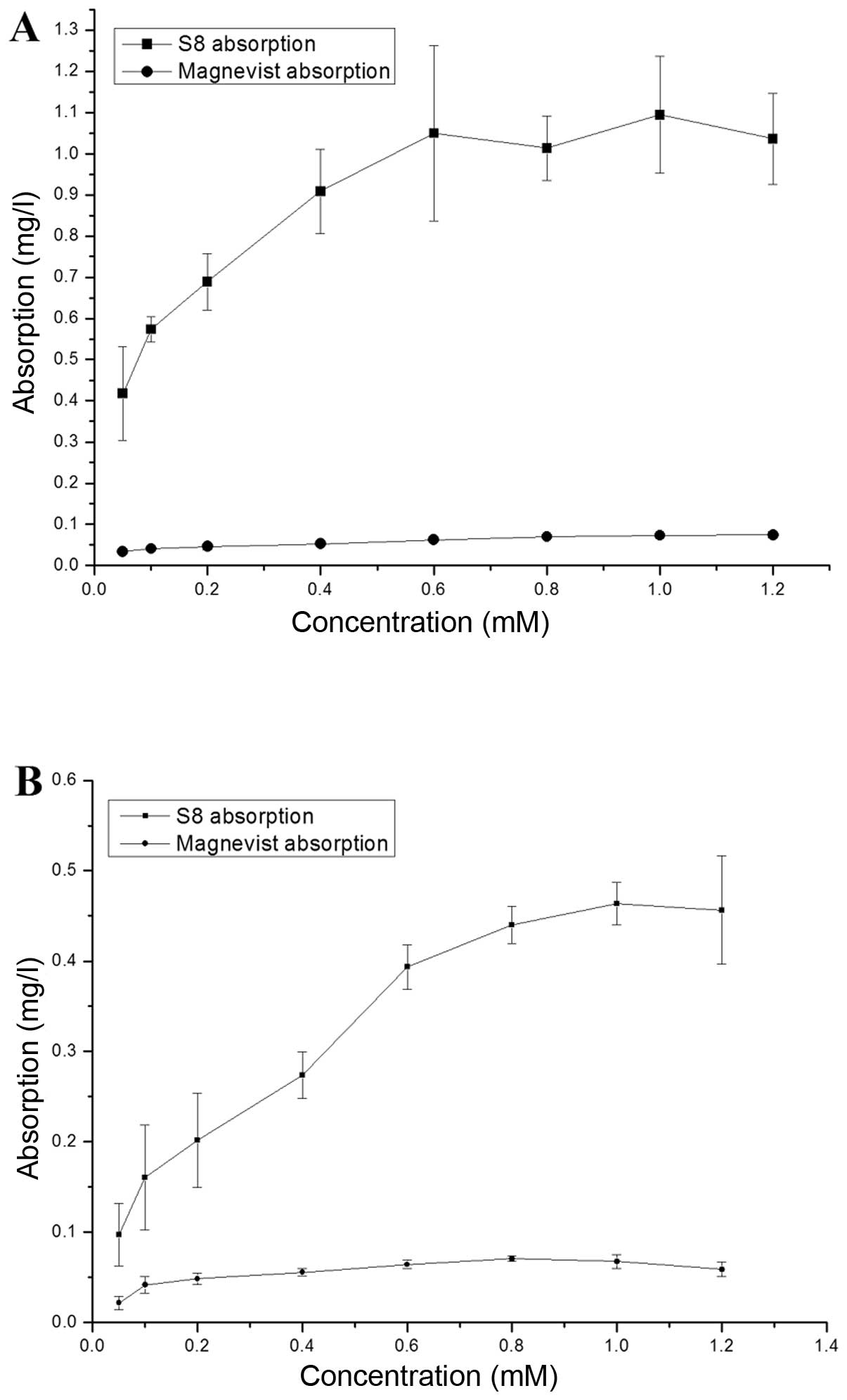
cell lines (MDA-MB-231 and PC-3). Fig. 5 shows the contrast agent uptake
quantity of MDA-MB-231 and PC-3 cells at different concentrations
of S8 or Magnevist.
We found that, within a certain concentration range,
the uptake quantity increased linearly with the increase in the
concentration of S8 added into the cells. In the MDA-MB-231 cells,
the uptake quantity reached saturation at 0.6 mM of added S8, while
in the PC-3 cells, saturation was reached at 1.0 mM of S8. At the
same concentration of S8, the quantity absorbed by the MDA-MB-231
cells was higher than that absorbed by the PC-3 cells. A possible
explanation for this may be that αvβ3
expression in the MDA-MB-231 cells is higher than CD13
expression in the PC-3 cells. The two cell lines displayed
comparable uptake quantity for Magnevist. The uptake quantity was
in the low range and were altered only slightly in response to
changes in the concentrations of S8 added to the cells.
Cell imaging
For both cell lines examined, much clearer and more
visible images of cells incubated with S8 (above a certain
concentration value) were obtained compared with the cells
incubated with Magnevist, (Fig.
6). In the MDA-MB-231 cells, this value was 0.2 mM and in the
PC-3 cells, it was 0.6 mM.
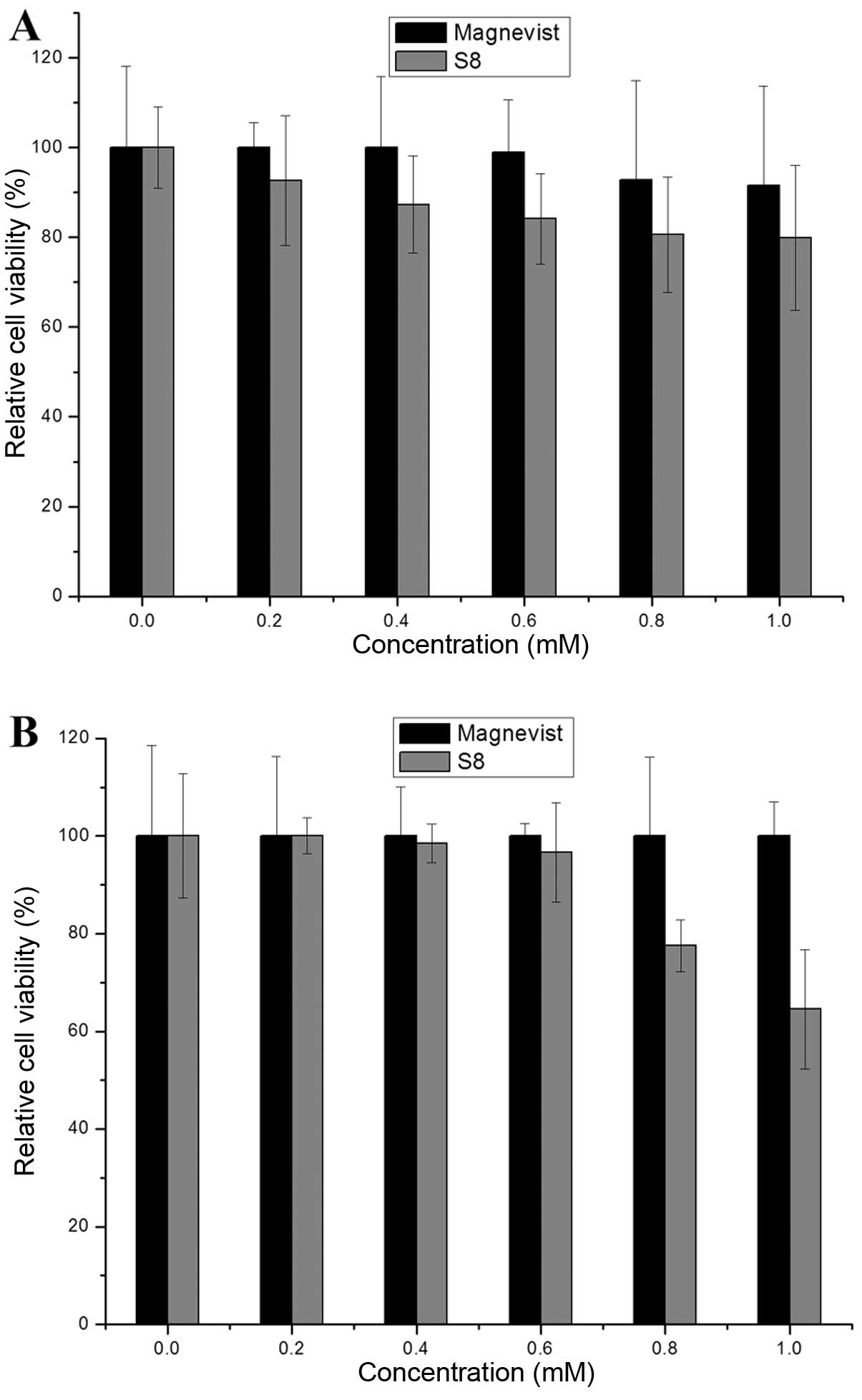
Cytotoxicity of S8
Through testing the activity of dehydrogenase in the
mitochondria, one can indirectly investigate the viability and
proliferation of cells. It was found that S8 inhibited the
proliferation of tumor cells, and this ability was enhanced with
the increasing concentrations. At a concentration of 1.0 mM, the
relative MDA-MB-231 cell viability was 80% (Fig. 7A) and that of PC-3 cells was 64.5%
(Fig. 7B). The relative cell
viability in response to Magnevist was >90% at all
concentrations (0–1.0 mM).
Discussion
The product synthesized in the present study
contains two target-specific sequences, RGD and NGR. The two
peptide moieties were connected by four serine (Ser) and one
glycine (Gly). Ser is a hydrophilic amino acid, which can enhance
peptide flexibility and water solubility. The four Ser and one Gly
separate RGD from NGR to minimize potential steric interactions
which may hamper binding.
Cyclic peptides are considerably more stable than
their linear counterparts. Dathe et al (25) reported that cyclic peptides
display improved binding affinity to their cellular targets owing
to minimal entropy costs for binding. Colomb et al (26) demonstrated that the use of
disulfide-bridged cyclic NGR peptide led to a 10-fold increase in
the efficacy of targeting to tumor sites compared to its linear
analog. Therefore, in this study, we linked the two Cys by
disulfide bridge in order to improve the stability and the binding
affinity. The synthesis of the ligand was completed on the solid
resin substrate. Solid phase synthesis offers many advantages, such
as a high coupling efficiency and convenient purification
procedures. The various impurities and the excess reaction
substances are easily removed from the reaction system by washing
with the appropriate solvent. The Gd complex S8 was prepared by
adding 0.99 equivalents of Gd chloride to a solution of S7 in
water. A slight excess of S7 was used to ensure the absence of free
Gd, which is highly toxic.
From Fig. 5, one
can see that the quantity of S8 absorbed by the two cell lines was
markedly higher compared with the one absorbed by Magnevist. The
difference may be due to the overexpression of
αvβ3 in the MDA-MB-231 cells and the
overexpression of CD13 in the PC-3 cells, which leads to
the binding of RGD and NGR peptides of S8 to
αvβ3 and CD13, respectively. This
result indicates that S8 can specifically target tumor cells
overexpressing αvβ3 and CD13. In
addition, it has been demonstrated that the absorption of Magnevist
by cells is non-specific. These results are in agreement with our
original hypothesis that the combination of the RGD and NGR
peptides can create a more efficient targeting contrast agent and
can improve tumor detection rates.
Cell imaging experiments have demonstrated that in
order to achieve effective cell visualization, relatively high
concentrations of the contrast agent must be used. The two cell
lines used in this study showed the same uptake rule. However, the
effective concentration of S8 was 0.2 mM in MDA-MB-231 and 0.6 mM
in PC-3 cells. The imaging of the two cell lines showed that
αvβ3 is expressed at higher levels in
MDA-MB-231 than CD13 is in PC-3 cells, which is
consistent with the absorption experiment results. Overall, the
dual-targeting contrast agent can bind to tumor cells
overexpressing αvβ3 and to tumor cells
overexpressing CD13.
The cytotoxicity of Magnevist is lower compared to
S8. A possible explanation for this is that Magnevist can rapidly
enter and be released from cells due to its low molecular weight.
Another explanation may be that the Magnevist quantity absorbed by
the cells is limited due to the non-specific nature of Magnevist
cell uptake, and therefore, this compound cannot exert cytotoxic
effects. Nevertheless, as shown by our results, S8 inhibits the
proliferation of tumor cells at high concentrations, which
indicates that S8 can bind to tumor cells.
In conclusion, we designed a dual-targeting contrast
agent containing RGD and NGR, which has a high r1 value
(8.297 mM−1sec−1) and can target tumor cells
overexpressing αvβ3 and CD13.
Thereby, a single contrast agent can improve tumor detection
rates.
Acknowledgements
This study was financially supported by a Strategic
Priority Research Program of the Chinese Academy of Sciences
(XDA01030203) and a Basic Research Project from the Ministry of
Science and Technology of China (2011CB965004). The MRI facility
used in this study is funded by the Chinese Academy of Sciences.
The authors would like to thank the Center of Analysis and Test at
the Huazhong University of Science and Technology (Wuhan, China)
for assistance with ESI-MS and RP-HPLC analyses.
References
|
1
|
Riccabona G and Decristoforo C: Peptide
targeted imaging of cancer. Cancer Biother Radiopharm. 18:675–687.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudin M and Weissleder R: Molecular
imaging in drug discovery and development. Nat Rev Drug Discov.
2:123–131. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li ZJ and Cho CH: Peptides as targeting
probes against tumor vasculature for diagnosis and drug delivery. J
Transl Med. 10(Suppl 1): S12012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caravan P: Protein-targeted
gadolinium-based magnetic resonance imaging (MRI) contrast agents:
design and mechanism of action. Acc Chem Res. 42:851–862. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra A, Nan A, Papadimitriou JC, et al:
Polymer-peptide conjugates for angiogenesis targeted tumor
radiotherapy. Nucl Med Biol. 33:43–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pawar PV, Gohil SV, Jain JP, et al:
Functionalized polymersomes for biomedical applications. Polym
Chem. 4:3160–3176. 2013. View Article : Google Scholar
|
|
7
|
Seward GK, Wei Q and Dmochowski IJ:
Peptide-mediated cellular uptake of cryptophane. Bioconjug Chem.
19:2129–2135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye F, Wu X, Jeong EK, Jia Z, et al: A
peptide targeted contrast agent specific to fibrin-fibronectin
complexes for cancer molecular imaging with MRI. Bioconjug Chem.
19:2300–2303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen XM, Wang X, Huang Y, et al: A
comparison study of the targeting properties of NGR liposomes and
RGD-liposomes towards human umbilical vein endothelial cells. J
Chinese Pharm Sci. 18:162–169. 2009.
|
|
10
|
Beer AJ and Schwaiger M: Imaging of
integrin alphavbeta3 expression. Cancer Metastasis Rev. 27:631–644.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S: Radiolabeled cyclic RGD peptides as
integrin alpha(v)beta(3)-targeted radiotracers: maximizing binding
affinity via bivalency. Bioconjug Chem. 20:2200–2213.
2009.PubMed/NCBI
|
|
12
|
Dijkgraaf I, Kruijtzer JA, Liu S, et al:
Improved targeting of the alpha(v)beta(3) integrin by
multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging.
34:267–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haukkala J, Laitinen I, Luoto P, et al:
68Ga-DOTA-RGD peptide: biodistribution and binding into
atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging.
36:2058–2067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie J, Chen K, Lee HY, et al: Ultrasmall
c(RGDyK)-coated Fe3O4 nanoparticles and their
specific targeting to integrin alpha(v)beta3-rich tumor cells. J Am
Chem Soc. 130:7542–7543. 2008.PubMed/NCBI
|
|
15
|
Wang RE, Niu Y, Wu H, et al: Development
of NGR peptide-based agents for tumor imaging. Am J Nucl Med Mol
Imaging. 1:36–46. 2011.PubMed/NCBI
|
|
16
|
Albrecht S, Al-Lakkis-Wehbe M, Orsini A,
et al: Amino-benzosuberone: a novel warhead for selective
inhibition of human aminopeptidase-N/CD13. Bioorg Med
Chem. 19:1434–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soudy R, Ahmed S and Kaur K: NGR Peptide
ligands for targeting CD13/APN identified through
peptide array screening resemble fibronectin sequences. ACS Comb
Sci. Oct 11–2012.(Epub ahead of print).
|
|
18
|
Negussie AH, Miller JL, Reddy G, et al:
Synthesis and in vitro evaluation of cyclic NGR peptide targeted
thermally sensitive liposome. J Control Celease. 143:265–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sloan EK, Pouliot N, Stanley KL, et al:
Tumor-specific expression of alphavbeta3 integrin promotes
spontaneous metastasis of breast cancer to bone. Breast Cancer Res.
8:R202006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goswami LN, Ma L, Cai Q, et al: cRGD
peptide-conjugated icosahedral closo-B12(2-) core
carrying multiple Gd3+-DOTA chelates for
α(v)β3 integrin-targeted tumor imaging (MRI). Inorg
Chem. 52:1701–1709. 2013.PubMed/NCBI
|
|
21
|
Ndinguri MW, Solipuram R, Gambrell RP, et
al: Peptide targeting of platinum anti-cancer drugs. Bioconjug
Chem. 20:1869–1878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mishra AK and Chatal JF: Synthesis of
macrocyclic bifunctional chelating agents:
1,4,7-tris(carboxymethyl)-10-(2-aminoethyl)-1,4,7,10-tetraazacyclododecane
and 1,4,8-tris
(carboxymethyl)-11-(2-aminoethyl)-1,4,8,11-tetraazacyclotetradecane.
New J Chem. 25:336–339. 2001. View
Article : Google Scholar
|
|
23
|
Kates SA, Sole NA, Johnson CR, et al: A
novel, convenient, 3-dimensional orthogonal strategy for
solid-phase synthesis of cyclic-peptides. Tetrahedron Lett.
34:1549–1552. 1993. View Article : Google Scholar
|
|
24
|
Sarin VK, Kent SBH, Tam JP and Merrifield
RB: Quantitative monitoring of solid-phase peptide synthesis by the
ninhydrin reaction. Anal Biochem. 117:147–157. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dathe M, Nikolenko H, Klose J and Bienert
M: Cyclization increases the antimicrobial activity and selectivity
of arginine- and tryptophan-containing hexapeptides. Biochemistry.
43:9140–9150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colombo G, Curnis F, De Mori GM, et al:
Structure-activity relationships of linear and cyclic peptides
containing the NGR tumor-homing motif. J Biol Chem.
277:47891–47897. 2002. View Article : Google Scholar : PubMed/NCBI
|