Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors in children and adolescents. Following the
advent of effective chemotherapy, the five-year survival rate for
patients with OS treated with intensive multidrug chemotherapy and
aggressive local control has been reported to be 55–80% (1–3).
However, numerous studies have reported that the five-year survival
rate of patients with metastatic diseases is <20% (4–6).
The development of lung metastasis is the main cause of mortatlity
in patients with OS. The identification and understanding of the
molecular mechanisms responsible for metastasis would pose a
significant impact on the management of OS.
Fatty acid synthase (FASN) is an enzyme which is
considered crucial for endogenous lipogenesis in mammals, and is
responsible for catalyzing the synthesis of long-chain fatty acids.
In the majority of normal cells, FASN expression is usually not
observed, due to the presence of abundant amounts of dietary lipids
(7). However, FASN is
overexpressed in a variety of human tumors (8–12),
and has been strongly linked to cancer cell proliferation and
apoptosis (13–16). We previously demonstrated that
FASN may contribute to the metastasis of OS cells (17,18). However, its potential molecular
mechanisms of action remain unclear.
PI3K/Akt plays a crucial role in the
cell-extracellar matrix (ECM) and cell-cell adhesion. Due to lack
of correct adhesion, the adhesion-dependent signals are
interrupted, resulting in adhesion-related apoptosis, namely
anoikis. PI3K/Akt signaling has been implicated in the regulation
of FASN expression in breast cancer and prostate cancer cells
(19,20). Wang et al reported that
there was a positive feedback regulation between Akt
phosphorylation and FASN expression in ovarian carcinoma cells
(21). However, to our knowledge,
the association between the phosphorylation of Akt and FASN protein
expression in OS has not yet been documented.
In this study, we found that the inhibition of Akt
phosphorylation by LY294002 (an inhibitor of PI3k/Akt), resulted in
the downregulation of FASN expression. In addition, the
downregulation of FASN expression inhibited Akt phosphorylation.
Based on these findings, we confirmed the existence of a positive
feedback loop between Akt phosphorylation and FASN expression; this
feedback loop may play an important role in the malignant phenotype
of OS cells.
Materials and methods
Patient specimens
A total of 24 samples of OS tissues were obtained
from patients with pulmonary metastatic disease who underwent
surgery in our hospital (The First Hospital Affiliated to Nanchang
University, Nanchang, China) from 2005 to 2012. The pulmonary
metastasis survey was performed with plain films and chest CT scans
at first diagnosis. All the patients had no history of prior
treatment with anticancer drugs or radiotherapy. The samples were
fixed with 10% formalin and embedded in paraffin and were then cut
into 4-μm-thick sections. In all cases, informed consent was
obtained from the relative departments and persons, and the study
had the approval of the Ethics Committee of Nanchang
University.
Immunohistochemistry
Immunohistochemical (S-P) staining with and
hematoxylin and eosin (H&E) was performed on the
paraffin-embedded tissue sections. Antigen retrieval was performed
by heating the sections in 10 mmol/l citrate buffer (pH 6.0) for 20
min. FASN and phosphorylated Akt (p-Akt) antibodies (rabbit
monoclonal antibody; antibody dilutions, 1:50; Epitomics, Inc.,
Burlingame, CA, USA) were used as the primary antibody at a final
dilution as corresponding product specifications. The sections were
then stained with diaminobenzidine (DAB) and counterstained using
hematoxylin. The stained sections were evaluated and scored by two
pathologists in a blinded manner without prior knowledge of the
clinical pathological characteristics of the patients. According to
the staining intensity by examining at least 500 cells in five
representative areas, the expression level of p-Akt and FASN was
measured and the intensity scores were recorded as follows: none,
0; weak, 1; moderate, 2; and intense, 3. According to the
percentage of cancer cells with a positive expression of Akt and
FASN, the percentage scores were recorded as follows: 0% (score 0);
<10% (score 1); 10–49% (score 2); 50–79% (score 3); and 80–100%
(score 4). The final score was averaged with the scores from the
two pathologists; these scores were calculated by multiplying the
intensity score by the percentage score. The sections with a final
score of <4 were considered as negative (−), those with a score
of 4–5 were considered as postivie (+), those with a score of 6–8
as double positive (++), and those with a score of 9–12 were
considered as triple positive (+++).
Cell culture and transfection
The human OS cell line, U2-OS, was purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA), and
the cells were routinely cultured in RPMI-1640 medium (HyClone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS)
(Sigma, Lenexa, KS, USA) in a humidified 37°C incubator containing
5% CO2. The U2-OS cells were seeded in 6-well plates
till 40% confluence on the day prior to transfection. The U2-OS
cells were transfected with FASN-specific RNAi plasmid (MR-FASN)
and the negative control RNAi plasmid (MR-Neg) using Lipofectamine
2000 according to the instructions of the manufacturer (Invitrogen
Life Technologies (Carlsbad, CA, USA).
Real-time PCR
Semi-quantitive (real-time) PCR was used to detect
the FASN mRNA expression levels. Total RNA was extracted from the
cells using TRIzol reagent (Invitrogen Life Technologies). The
total RNA concentration was determined by spectrophotometry at 260
nm and the purity was determined by calculating the 260/280 ratio
with a BioPhotometer (Eppendorf, Hamburg, Germany). The two-step
kit (Promega Corp., Madison, WI, USA) was used to to obtain cDNA
according to the manufacturer’s instructions, which was then used
as the template for amplification. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as an internal standard. The gene
primer sequences are listed in Table
I. The cycling conditions were as follows: initial denaturation
at 94°C for 3 min, followed by 35 amplification cycles of 94°C for
30 sec, 55°C for 30 sec and 72°C for 12 sec. Each real-time PCR
assay contained 2 μl cDNA template, 10 μl SuperMix and 0.5 μl of
every forward and reverse primer in a 20 μl reaction mixture. All
experiments were repeated six times over multiple days.
 | Table IPrimer sequences of genes used in
real-time PCR. |
Table I
Primer sequences of genes used in
real-time PCR.
| Gene (size, bp) | Primer sequence
(5′→3′) |
|---|
| FASN (171) | F
AACTCCATGTTTGGTGTTTG
R CACATGCGGTTTAATTGTG |
| Akt (198) | F
TGCCACCATGAATGAGGTGAAT
R GCGTATGACAAAGGTGTTGGG |
| GAPDH (199) | F
CAGGGCTGCTTTTAACTCTGGT
R GATTTTGGAGGGATCTCGCT |
Western blot analysis of protein
expression
Total protein from the cells was extracted using
RIPA lysis buffer containing 60 μg/ml phenylmethylsulfonyl fluoride
(PMSF). The protein concentrations were determined using the BCA
protein assay kit (Boster Biotechnology Co., Wuhan, China). The
protein samples were denatured at 100°C for 10 min and then
preserved at −20°C for later use. The proteins were separated by
SDS-polyacrylamide gel electrophoresis and transblotted onto PVDF
membranes. The PVDF membranes were probed first with primary
antibodies PI3K, Akt and p-Akt (Ser473) antibody (1:1,000
dilution), FASN antibody (1:500 dilution) (both from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA)and β-actin antibody
(1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
overnight at 4°C. Following incubation with the appropriate
anti-rabbit or anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:5,000; Boster Biotechnology Co.) for 1.5 h at
room temperature, immunoreactive bands were visualized by
chemiluminescence dissolvent (Thermo Scientific, Rockford, IL, USA)
and exposured to X-ray film (Kodak, Rochester, NY, USA). The
determination of the grayscale value was processed using ImageJ
sofware. All experiments were repeated six times over multiple
days.
Cell proliferation assay
Cells (4×103/200 μl/well) were seeded in
96-well plates. Viable proliferating cells were detected by
3-(4,-dimethy-lthiazol-2-yl)-2,-diphenyl-tetrazoliumbromide (MTT)
assay at various time periods (24, 48 and 72 h), using five wells
per time period. Cell viability was expressed as the optical
density (OD), which was detected by an enzyme-linked
immunoabsorbent assay reader (MK3; Thermo Scientific) at a 490-nm
wavelength. All experiments were repeated six times over multiple
days.
Analysis of cell apoptosis
Cells (5×105) were harvested, washed with
PBS and resuspended in binding buffer, followed by mixing with
Annexin V-FITC and propidium iodide (both from KeyGen Biotech. Co.,
Ltd., Nanjing, China). The cells were analyzed by a BD FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). All experiments
were repeated six times over multiple days.
Transwell invasion assays
Cell invasion was measured in 24-well plates by
Transwell assay using a chamber containing the polyethylene
terephthalate filter membrane with 8-μm pores (BD Biosciences). The
cells (6×104/200 μl/chamber) were seeded in the upper
chamber with RPMI-1640 medium containing 10 g/l BSA, and the lower
well was filled with 500 μl RPMI-1640 medium supplemented with 10%
FBS as a chemoattractant. Following incubation for 24 h, the
chambers were stained with crystal violet. The invaded cells were
counted from ten randomly selected fields under an inverted
microscope. All experiments were repeated six times over multiple
days.
Wound healing assays
Cell migration was assessed by determining the
ability of the cells to move into a cellular space in a
two-dimensional in vitro ‘wound healing assay’. In brief,
the cells were grown to confluence in 6-well tissue culture plastic
dishes to a density of 5×106 cells/well. The cells were
denuded by dragging a rubber policeman (Fisher Scientific, Hampton,
NH, USA) through the center of the plate. The cultures were rinsed
with PBS and replaced with fresh DMEM alone or containing 10% FBS,
following which the cells were incubated at 37°C for 24 h. Images
were captured at 0 and 24 h and the migrated distance was measured
using ImageJ software (NIH, Bethesda, MD, USA). All experiments
were repeated six times over multiple days.
Statistical analysis
Statistical comparisons were performed using SPSS
software version 13.0 (SPSS Inc., Chicago, IL, USA). The
correlation of FASN with p-Akt protein in the OS tissues was
evaluated using the Wilcoxon rank sum test. All measurement data
are presented as the means ± SD, and the one-way ANOVA with a
post-hoc test (Student-Newman-Keuls test) was performed for
statistical analysis. A P-value <0.05 was considered to indicate
a statistically significant difference.
Results
Correlation between FASN and p-Akt
protein expression in OS
To explore the possible association between FASN
expression and the phosphorylation of Akt in OS, FASN and p-Akt
(Ser473) protein expression in the OS tissues from patients with
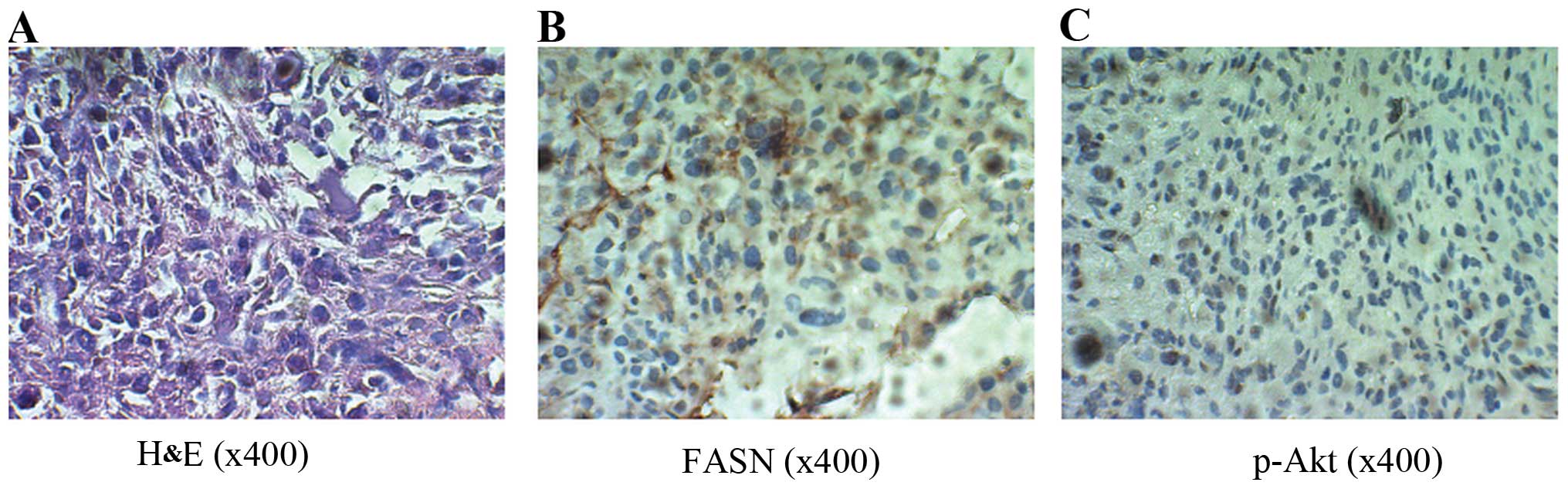
pulmonary metastatic disease was detected. The results revealed
that FASN protein was expressed in the cytoplasm of the OS tissues,
and the p-Akt protein was expressed in the nucleus and cytoplasm
(Fig. 1A–C). There was a
significant positive relationship between FASN and p-Akt expression
(R=0.469, P=0.04). These data suggest that a possible connection
between FASN expression and the phosphorylation of Akt exists in
OS.
Inhibiting the phosphorylation of Akt
suppresses the malignant phenotype of U2-OS cells
To determine the effects of the inhibition of Akt
phosphorylation on the malignant phenotype of OS cells, the U2-OS
cells were treated with LY294002 (an inhibitor of PI3k/Akt) at
various concentrations (0, 5, 10, 20, 40, 80 and 160 μM) for 24, 48
and 72 h. Cell proliferation and apoptosis were assessed by MTT and
FACS assays, respectively. The migration and invasion abitility of
the cells was investigated using wound healing and Transwell
assays, respectively. In the MTT assays, the results revealed that
LY294002 induced U2-OS cell apoptosis and inhibited cell growth in
a dose- and time-dependent manner (Fig. 2A) and the IC50 was
47.96 μM for 24 h. The concentration of 40 μM was selected for
further experiments. Furthermore, in FACS assays, the results
revealed that LY294002 induced U2-OS cell apoptosis in a dose- and
time-dependent manner (Fig. 2B and
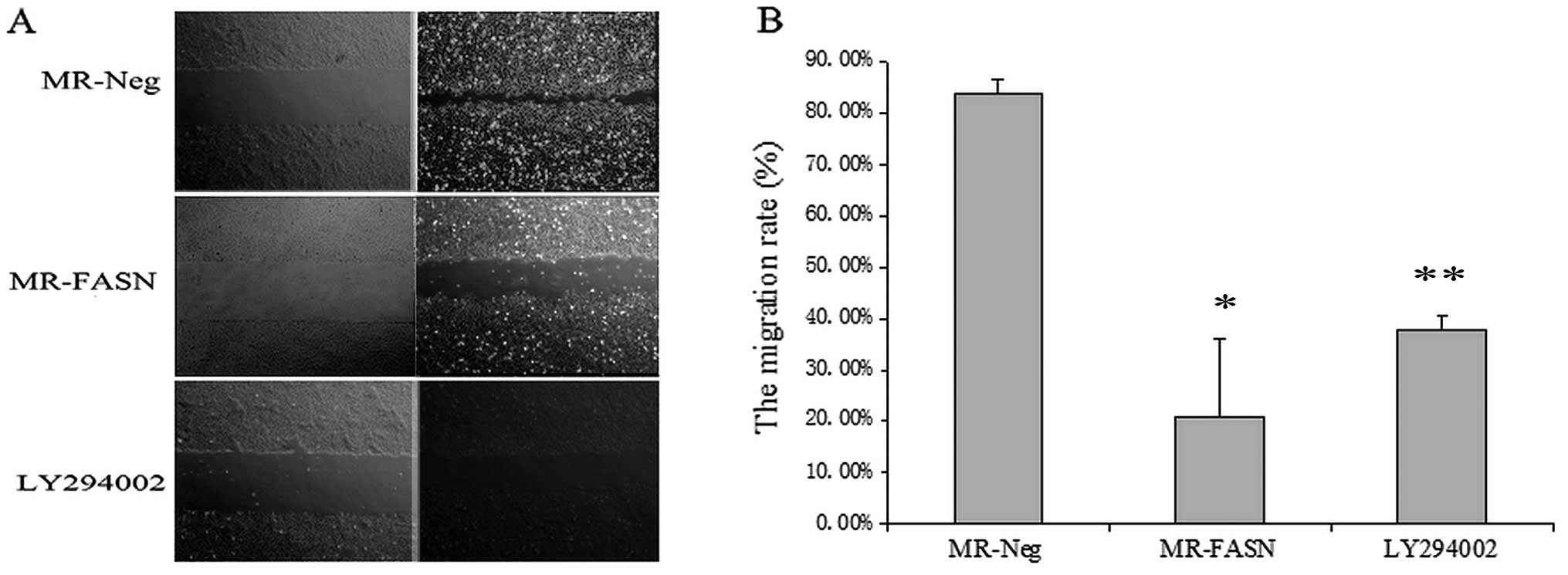
C). In the the wound healing assay, the migration rate of the
cells treated with 40 μM LY294002 was significantly lower than that
of the control (untreated) cells (P<0.05) (Fig. 3). The percentage of invading cells
in the group treated with the p-Akt inhibitor (LY294002) was
36.1±5.0%, compared with 89.1±6.4% in the control group (P<0.05)
(Fig. 4).
Inhibition FASN suppresses U2-OS cell
migration and invasion
In a previous study, we demonstrated that the
suppression of FASN expression induces U2-OS cell apoptosis and
inhibits cell growth in vivo and in vitro (17). Therefore, in this study, we
evaluated the effects of the downregulation FASN on U2-OS cell
migration and invasion. The migration and invasion ability of the
cells was significantly lower in the cells transfected with the
FASN-specific RNAi plasmid than those transfected with the negative
RNAi plasmid (P<0.05) (Figs. 3
and 4).
Downregulation of FASN expression
inhibits the phosphorylation of Akt
To investigate the effects of silencing FASN on the
phosphorylation of Akt in OS, the U2-OS OS cells were transfected
with the FASN-specific RNAi plasmid to inhibit FASN expression. The
mRNA expression of FASN and Akt was detected by real-time PCR, and
western blot analysis was used to measure the protein expression of
FASN, PI3K, Akt and p-Akt. The downregulation of FASN inhibited the
activation of the PI3K/Akt signaling pathway. However, the mRNA
expression of Akt was not affected by the downregulation of FASN in
the U2-OS cells (Fig. 5A and
B).
Inhibition of Akt phosphorylation
downregulates FASN in U2-OS cells
In order to determine the effects of the inhibition
of the phosphorylation of Akt on the mRNA and protein expression of
FASN in OS, the U2-OS cells were treated with LY294002 (an
inhibitor of PI3K/Akt). The mRNA and protein expression of FASN was
measured by RT-PR and western blot analysis, respectively. The
results revealed that both the mRNA and protein expression of FASN
was decreased by the inhibition of Akt phosphorylation (Fig. 5).
Discussion
Previous studies have demonstrated that cancers with
a high expression of FASN always undergo a significant endogenous
fatty acid biosynthesis and display a biologically aggressive
subset (22,23). Moreover, the overexpression of
FASN is an early event in tumor development and is more pronounced
in tumors with a poor prognosis (24). Importantly, we previously
demonstrated that the inhibition of FASN with pharmacological
inhibitors is selectively cytotoxic to human OS cells and leads to
a significant antitumor effect (17). Although very little is known about
the mechanisms underlying the upregulation of the FAS protein in
cancer cells, studies have revealed that FASN is also upregulated
at the mRNA level (25,26), and increasing evidence indicates
that Akt activity modulates FASN expression in tumor cells
(20,27). Previous studies have revealed that
activated PI3K stimulates the binding of sterol regulatory
element-binding protein (SREBP)-1c, a SREBP family transcription
factor, which controls genes involved in lipogenesis to a
SREBP-binding site in the FASN promoter, thus inducing FASN
transcription (28–30). Of note, the inhibition of FASN
activity by either cerulenin or C75 has been shown to inhibit the
prodution of p-Akt (19,31). These findings suggest the
existence of a positive bidirectional association between p-Akt and
FASN expression in cancer cells.
In the current study, we found that there was a
positive correlation between p-Akt and FASN protein expression in
OS tissues. This indicates that a possible connection between the
phosphorylation of Akt and FASN expression may exist. In order to
investigate whether the inhibition of the phosphorylation of Akt
suppresses FASN expression in OS, LY294002, an inhibitor of
PI3k/Akt, was used to downregulate the phosphorylation of Akt in
U2-OS cells. The results revealed that the mRNA and protein
expression of FASN was markedly inhibited by LY294002, which
suggests that p-Akt regulates FASN by affecting the transcription
and translation in U2-OS cells.
To determine the effects of the inhibition of FASN
on the phosphorylation of Akt in OS cells, the U2-OS cells were
transfected with the FASN-specific RNAi plasmid to inhibit FASN
expression. We found that the protein expression of both total Akt
and p-Akt was decreased in the FASN-silenced U2-OS cells. However,
the mRNA expression of Akt did not differ between the cells treated
with the FASN-specific RNAi plasmid and those treated with the
negative RNAi plasmid. These data suggest that FASN regulates
PI3K/Akt at the translational rather than the transcriptional
level. However, the mechanism(s) responsible for the inhibition of
Akt activity by the downregulation of FASN remain unclear.
Currently, several mechanisms are likely to contribute to
FASN-mediated p-Akt regulation: i) FASN-mediated lipogenesis
produces phospholipids that are incorporated into cell membranes
and partition into lipid rafts, which accommodate ErbBs and form
signaling platforms. FASN blockade destabilizes these lipid rafts,
which triggers the degradation of ErbBs and impedes the membrane
recruitment of downstream mediators of Akt, thereby causing the
downregulation of p-Akt (32,33); ii) the FASN promoter contains
several sterol regulatory element-binding protein-1 (Sp1) sites
enabling FASN to activate Akt (34,35).
Recently, a number of studies have demonstrated that
FASN and PI3K/Akt play an important role in the proliferation,
invasion and migration of cancer cells (36–39). In this study, we observed that the
suppression of FASN expression by RNAi or the inhibition of the
activity of PI3K/Akt blocked cell proliferation and migration and
increased the apoptotic rate in the U2-OS cells. This suggests that
FASN and PI3K/Akt play a key role in the maintenance of the
malignant phenotype of OS cells.
In conclusion, the data presented in this study,
confirm the existence of a positive feedback loop between Akt
phosphorylation and FASN expression. Moreover, this feedback loop
plays an important role in the malignant phenotype of OS cells.
However, the detailed mechanisms of the bidirectional association
between FASN expression and the phosphorylation of Akt in OS cells
are currently unknown. Thus, further studies are required to
provide further clarification.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81260400) and the
Natural Science Fundation of Jiangxi Province (no.
20114BAB205093).
References
|
1
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, et al: Osteosarcoma: a
randomized, prospective trial of the addition of ifosfamide and/or
muramyl tripeptide to cisplatin, doxorubicin, and high-dose
methotrexate. J Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Forni C, Longhi A, Ferrari S,
Mercuri M, Bertoni F, et al: Local recurrence and local control of
non-metastatic osteosarcoma of the extremities: a 27-year
experience in a single institution. J Surg Oncol. 96:118–123.
2007.PubMed/NCBI
|
|
3
|
Jawad MU, Cheung MC, Clarke J, Koniaris LG
and Scully SP: Osteosarcoma: improvement in survival limited to
high-grade patients only. J Cancer Res Clin Oncol. 137:597–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, et al: Metastatic osteosarcoma at
diagnosis: prognostic factors and long-term outcome--the French
pediatric experience. Cancer. 04:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss
J, Szendroi M, et al: Good prognosis of localized osteosarcoma in
young patients treated with limb-salvage surgery and chemotherapy.
Pediatr Blood Cancer. 57:415–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stokkel MP, Linthorst MF, Borm JJ,
Taminiau AH and Pauwels EK: A reassessment of bone scintigraphy and
commonly tested pretreatment biochemical parameters in newly
diagnosed osteosarcoma. J Cancer Res Clin Oncol. 128:393–399. 2002.
View Article : Google Scholar
|
|
7
|
Kuhajda FP: Fatty acid synthase and
cancer: new application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alo PL, Amini M, Piro F, Pizzuti L,
Sebastiani V, Botti C, et al: Immunohistochemical expression and
prognostic significance of fatty acid synthase in pancreatic
carcinoma. Anticancer Res. 27:2523–2527. 2007.PubMed/NCBI
|
|
9
|
Walter K, Hong SM, Nyhan S, Canto M,
Fedarko N, Klein A, et al: Serum fatty acid synthase as a marker of
pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev.
18:2380–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okawa Y, Hideshima T, Ikeda H, Raje N,
Vallet S, Kiziltepe T, et al: Fatty acid synthase is a novel
therapeutic target in multiple myeloma. Br J Haemato. 141:659–671.
2008. View Article : Google Scholar
|
|
11
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, et al: Fatty acid synthase: a metabolic enzyme
and candidate oncogene in prostate cancer. J Natl Cancer Inst.
101:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silva SD, Cunha IW, Younes RN, Soares FA,
Kowalski LP and Graner E: ErbB receptors and fatty acid synthase
expression in aggressive head and neck squamous cell carcinomas.
Oral Dis. 16:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saati GE and Archer MC: Inhibition of
fatty acid synthase and Sp1 expression by 3,3′-diindolylmethane in
human breast cancer cells. Nutr Cancer. 63:790–794. 2011.
|
|
14
|
Notarnicola M, Pisanti S, Tutino V, Bocale
D, Rotelli MT, Gentile A, et al: Effects of olive oil polyphenols
on fatty acid synthase gene expression and activity in human
colorectal cancer cells. Genes Nutr. 6:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Notarnicola M, Messa C, Refolo MG, Tutino
V, Miccolis A and Caruso MG: Polyunsaturated fatty acids reduce
fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene
expression and promote apoptosis in HepG2 cell line. Lipids Health
Dis. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zecchin KG, Rossato FA, Raposo HF, Melo
DR, Alberici LC, Oliveira HC, et al: Inhibition of fatty acid
synthase in melanoma cells activates the intrinsic pathway of
apoptosis. Lab Invest. 91:232–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long XH, Mao JH, Peng AF, Zhou Y, Huang SH
and Liu ZL: Tumor suppressive microRNA-424 inhibits osteosarcoma
cell migration and invasion via targeting fatty acid synthase. Exp
Ther Med. 5:1048–1052. 2013.PubMed/NCBI
|
|
18
|
Liu ZL, Wang G, Peng AF, Luo QF, Zhou Y
and Huang SH: Fatty acid synthase expression in osteosarcoma and
its correlation with pulmonary metastasis. Oncol Lett. 4:878–882.
2012.PubMed/NCBI
|
|
19
|
Liu X, Shi Y, Giranda VL and Luo Y:
Inhibition of the phosphatidylinositol 3-kinase/Akt pathway
sensitizes MDA-MB468 human breast cancer cells to cerulenin-induced
apoptosis. Mol Cancer Ther. 5:494–501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van de Sande T, De Schrijver E, Heyns W,
Verhoeven G and Swinnen JV: Role of the phosphatidylinositol
3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty
acid synthase in LNCaP prostate cancer cells. Cancer Res.
62:642–646. 2002.
|
|
21
|
Wang HQ, Altomare DA, Skele KL, Poulikakos
PI, Kuhajda FP, Di Cristofano A, et al: Positive feedback
regulation between AKT activation and fatty acid synthase
expression in ovarian carcinoma cells. Oncogene. 24:3574–3582.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menendez JA and Lupu R: Fatty acid
synthase-catalyzed de novo fatty acid biosynthesis: from
anabolic-energy-storage pathway in normal tissues to
jack-of-all-trades in cancer cells. Arch Immunol Ther Exp (Warsz).
52:414–426. 2004.
|
|
23
|
Menendez JA, Ropero S, Mehmi I, Atlas E,
Colomer R and Lupu R: Overexpression and hyperactivity of breast
cancer-associated fatty acid synthase (oncogenic antigen-519) is
insensitive to normal arachidonic fatty acid-induced suppression in
lipogenic tissues but it is selectively inhibited by tumoricidal
α-linolenic and gamma-linolenic fatty acids: A novel mechanism by
which dietary fat can alter mammary tumorigenesis. Int J Oncol.
24:1369–1383. 2004.PubMed/NCBI
|
|
24
|
Porter D, Lahti-Domenici J, Keshaviah A,
Bae YK, Argani P, Marks J, et al: Molecular markers in ductal
carcinoma in situ of the breast. Mol Cancer Res. 362–375.
2003.PubMed/NCBI
|
|
25
|
Swinnen JV, Vanderhoydonc F, Elgamal AA,
Eelen M, Vercaeren I, Joniau S, et al: Selective activation of the
fatty acid synthesis pathway in human prostate cancer. Int J
Cancer. 88:176–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.PubMed/NCBI
|
|
27
|
Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY
and Lin JK: Suppression of fatty acid synthase in MCF-7 breast
cancer cells by tea and tea polyphenols: a possible mechanism for
their hypolipidemic effects. Pharmacogenomics J. 3:267–276.
2003.PubMed/NCBI
|
|
28
|
Bandyopadhyay S, Pai SK, Watabe M, Gross
SC, Hirota S, Hosobe S, et al: FAS expression inversely correlates
with PTEN level in prostate cancer and a PI 3-kinase inhibitor
synergizes with FAS siRNA to induce apoptosis. Oncogene.
24:5389–5395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swinnen JV, Heemers H, Deboel L, Foufelle
F, Heyns W and Verhoeven G: Stimulation of tumor-associated fatty
acid synthase expression by growth factor activation of the sterol
regulatory element-binding protein pathway. Oncogene. 19:5173–81.
2000. View Article : Google Scholar
|
|
30
|
Yang Y, Morin PJ, Han WF, Chen T, Bornman
DM, Gabrielson EW and Pizer ES: Regulation of fatty acid synthase
expression in breast cancer by sterol regulatory element binding
protein-1c. Exp Cell Res. 282:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alli PM, Pinn ML, Jaffee EM, McFadden JM
and Kuhajda FP: Fatty acid synthase inhibitors are chemopreventive
for mammary cancer in neu-N transgenic mice. Oncogene. 24:39–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scheid MP, Marignani PA and Woodgett JR:
Multiple phosphoinositide 3-kinase-dependent steps in activation of
protein kinase B. Mol Cell Biol. 22:6247–6260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li N, Lu H, Chen C, Bu X and Huang P: Loss
of fatty acid synthase inhibits the ‘HER2-PI3K/Akt axis’ activity
and malignant phenotype of Caco-2 cells. Lipids Health Dis.
12:832013.
|
|
34
|
Jin HO, An S, Lee HC, Woo SH, Seo SK, Choe
TB, et al: Hypoxic condition- and high cell density-induced
expression of Redd1 is regulated by activation of hypoxia-inducible
factor-1alpha and Sp1 through the phosphatidylinositol 3-kinase/Akt
signaling pathway. Cell Signal. 19:1393–1403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roder K, Wolf SS, Larkin KJ and Schweizer
M: Interaction between the two ubiquitously expressed transcription
factors NF-Y and Sp1. Gene. 234:61–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deepa PR, Vandhana S and Krishnakumar S:
Fatty acid synthase inhibition induces differential expression of
genes involved in apoptosis and cell proliferation in ocular cancer
cells. Nutr Cancer. 65:311–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahman MT, Nakayama K, Ishikawa M, Rahman
M, Katagiri H, Katagiri A, et al: Fatty acid synthase is a
potential therapeutic target in estrogen receptor-/progesterone
receptor-positive endometrioid endometrial cancer. Oncology.
84:166–173. 2013. View Article : Google Scholar
|
|
38
|
Zhou JD, Shen F, Ji JS, Zheng K, Huang M
and Wu JC: FAM9C plays an anti-apoptotic role through activation of
the PI3K/Akt pathway in human hepatocellular carcinoma. Oncol Rep.
2013. View Article : Google Scholar
|
|
39
|
Yothaisong S, Dokduang H, Techasen A,
Namwat N, Yongvanit P, Bhudhisawasdi V, et al: Increased activation
of PI3K/AKT signaling pathway is associated with cholangiocarcinoma
metastasis and PI3K/mTOR inhibition presents a possible therapeutic
strategy. Tumour Biol. 34:3637–3648. 2013. View Article : Google Scholar : PubMed/NCBI
|