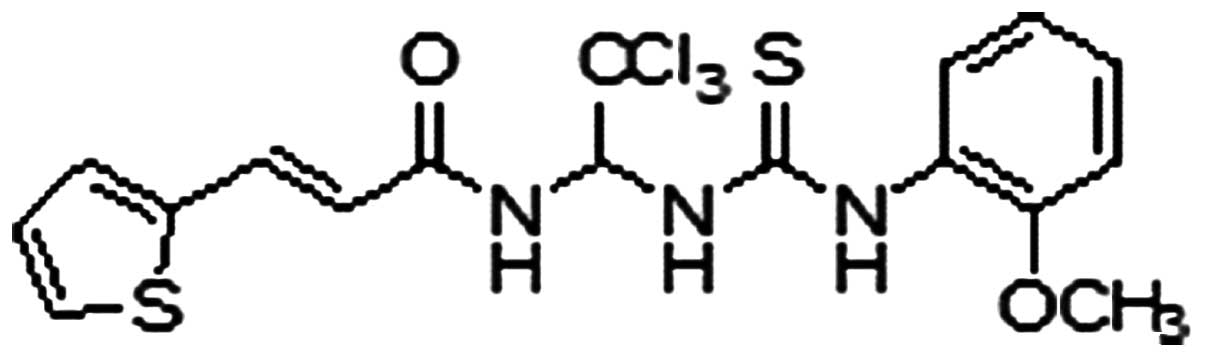
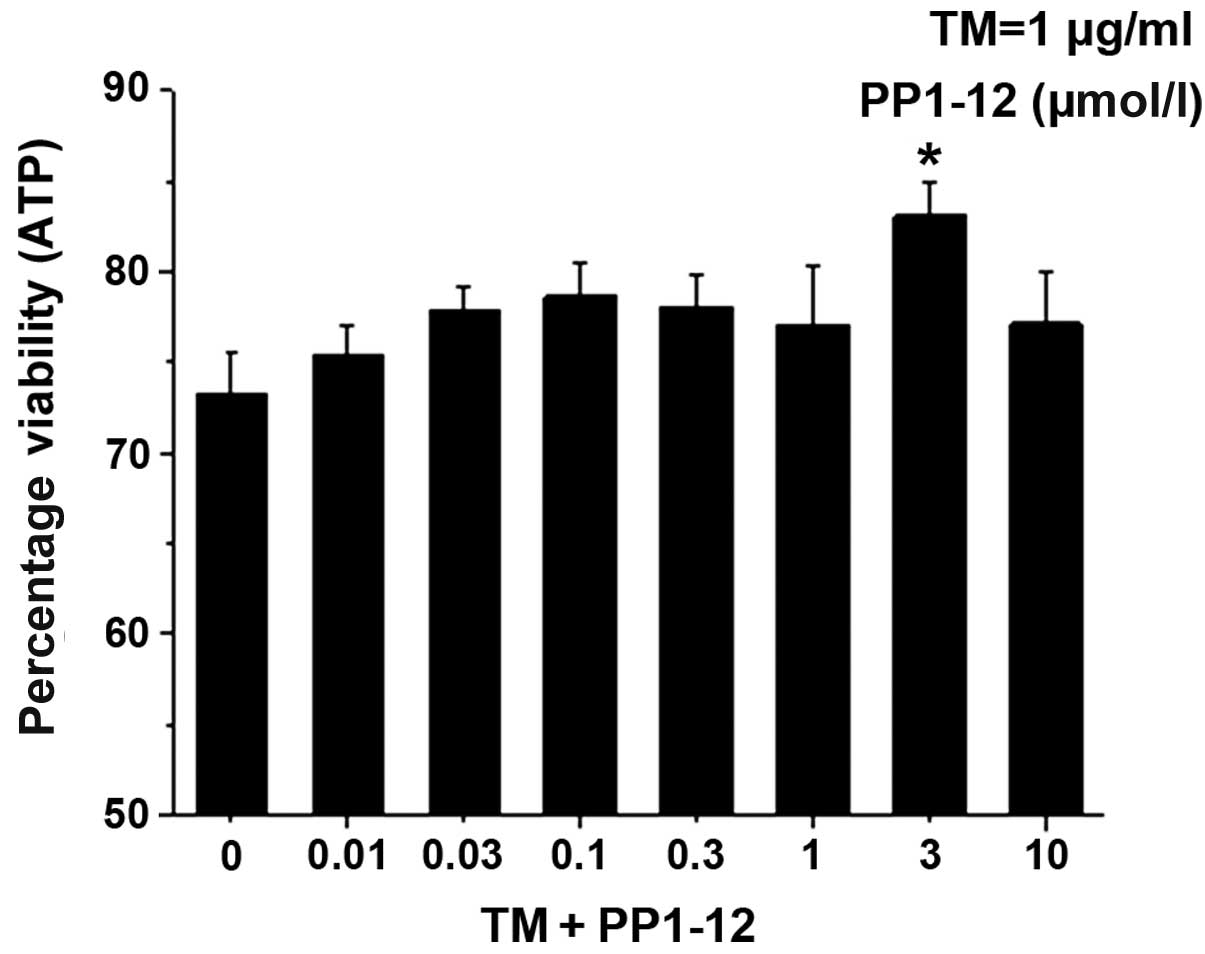
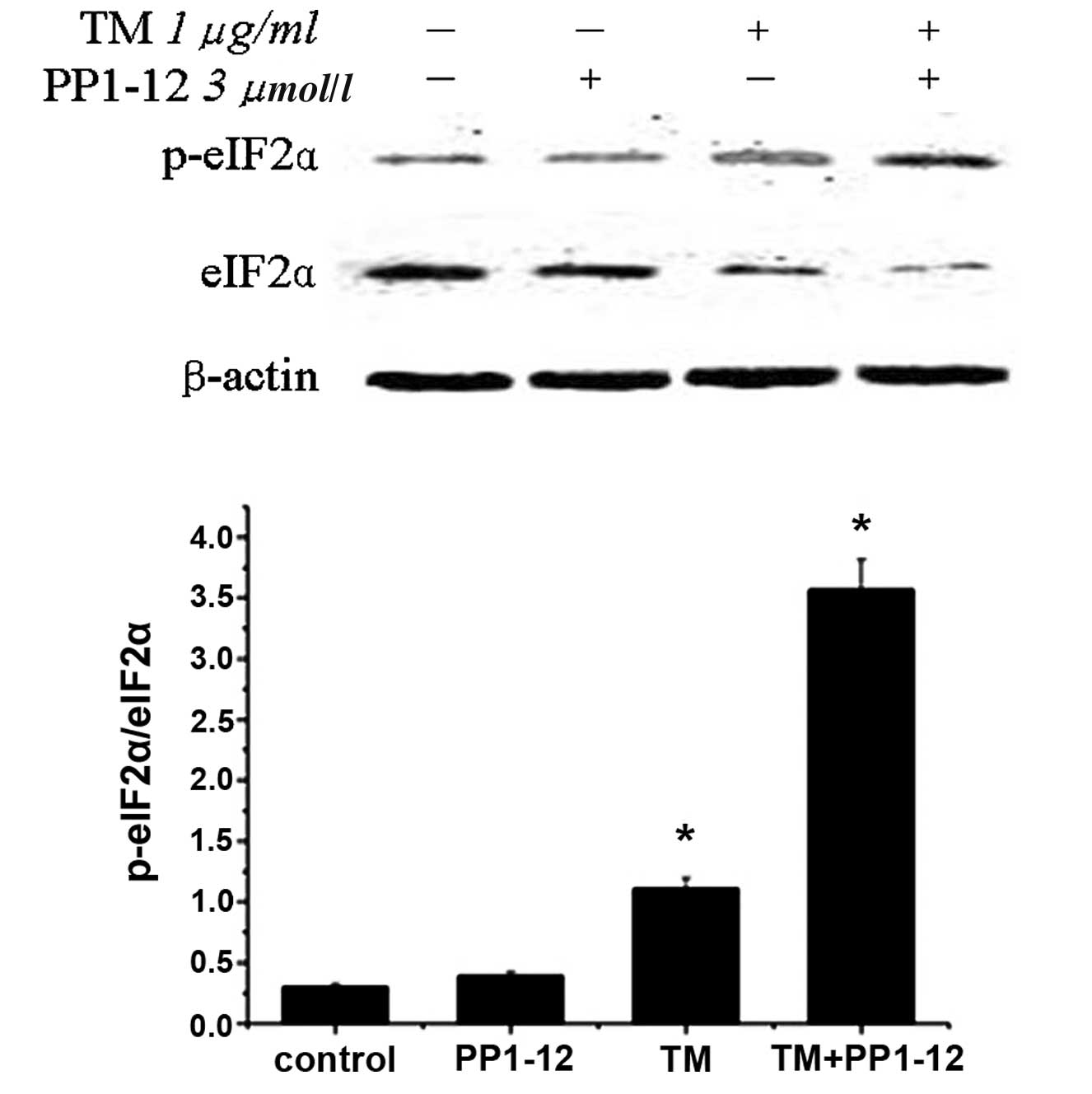
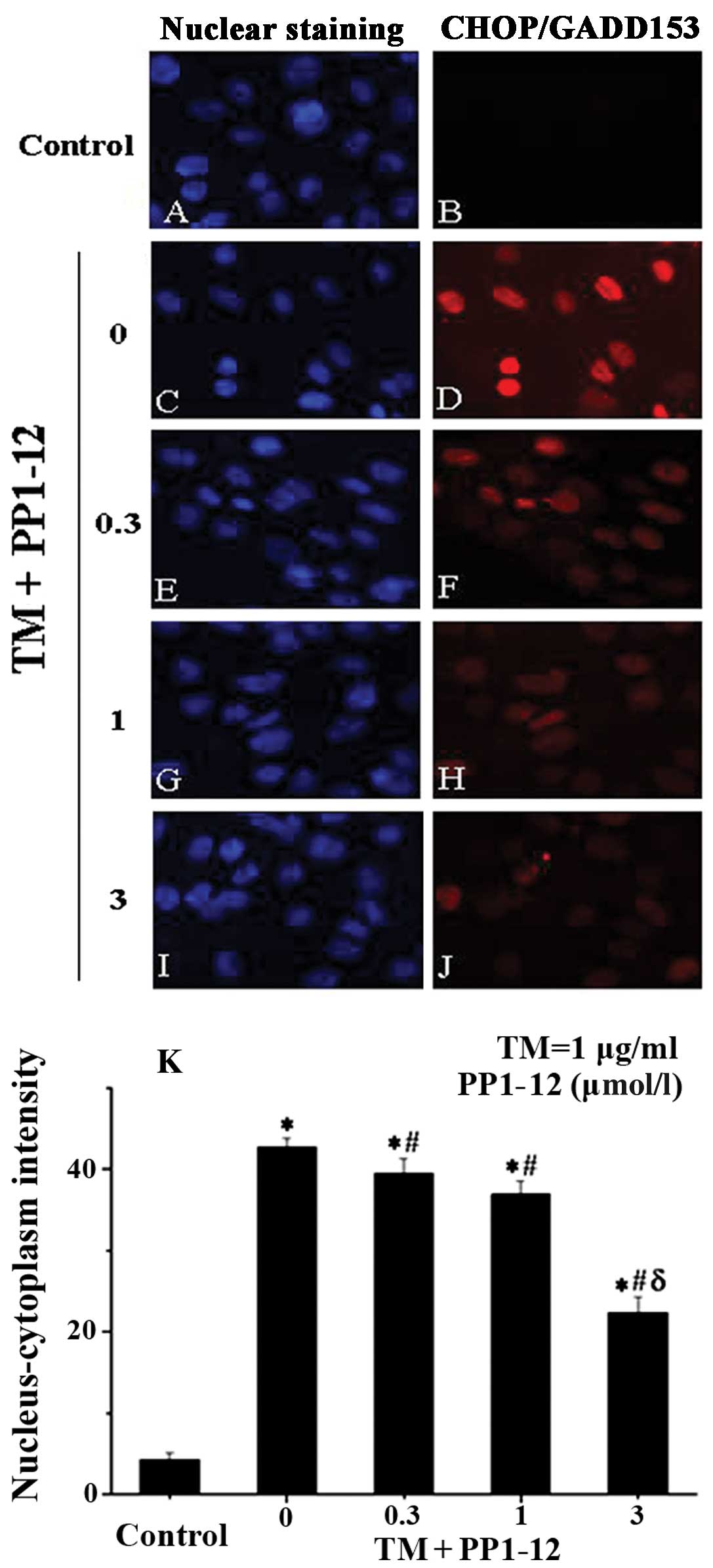
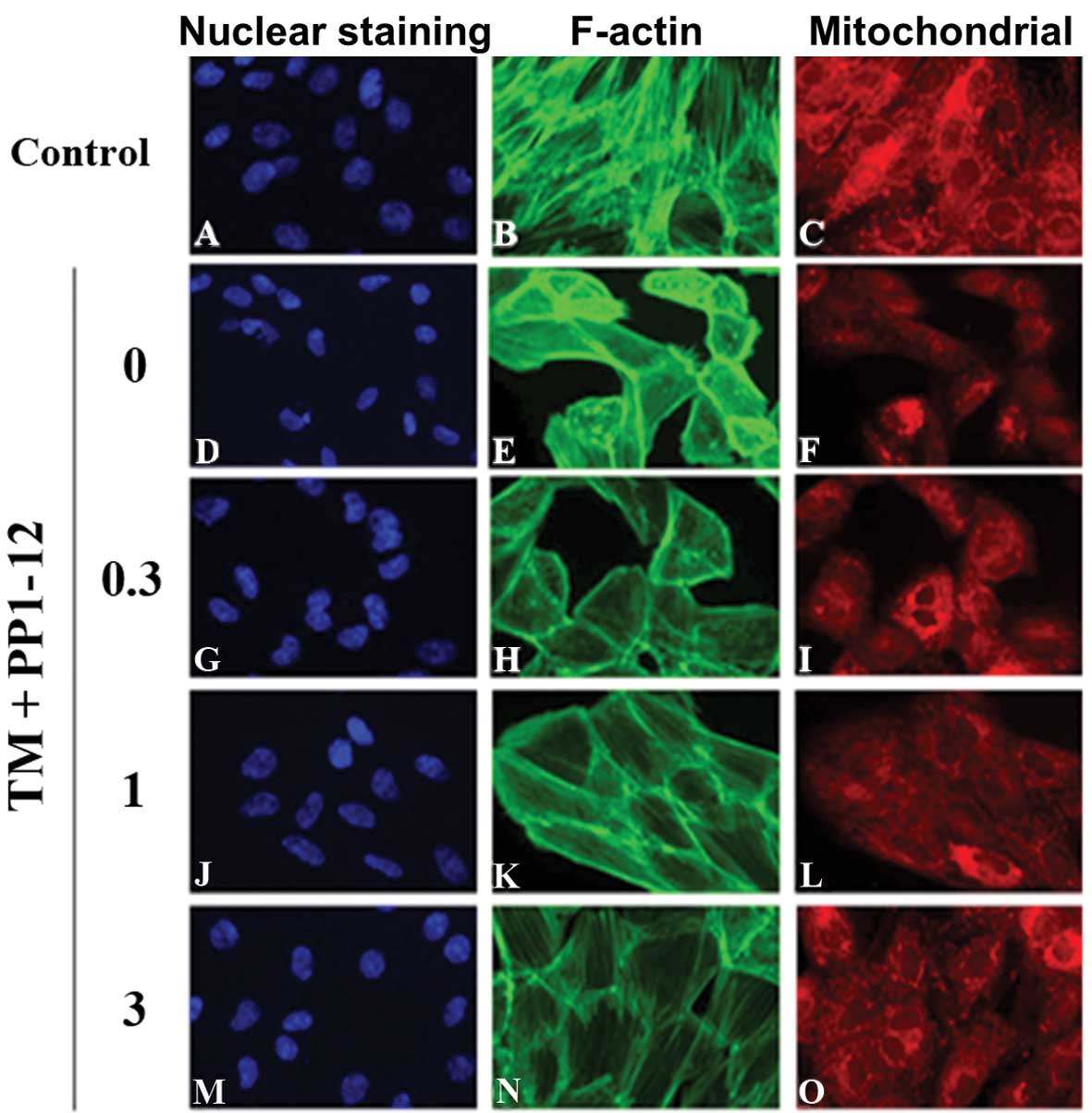
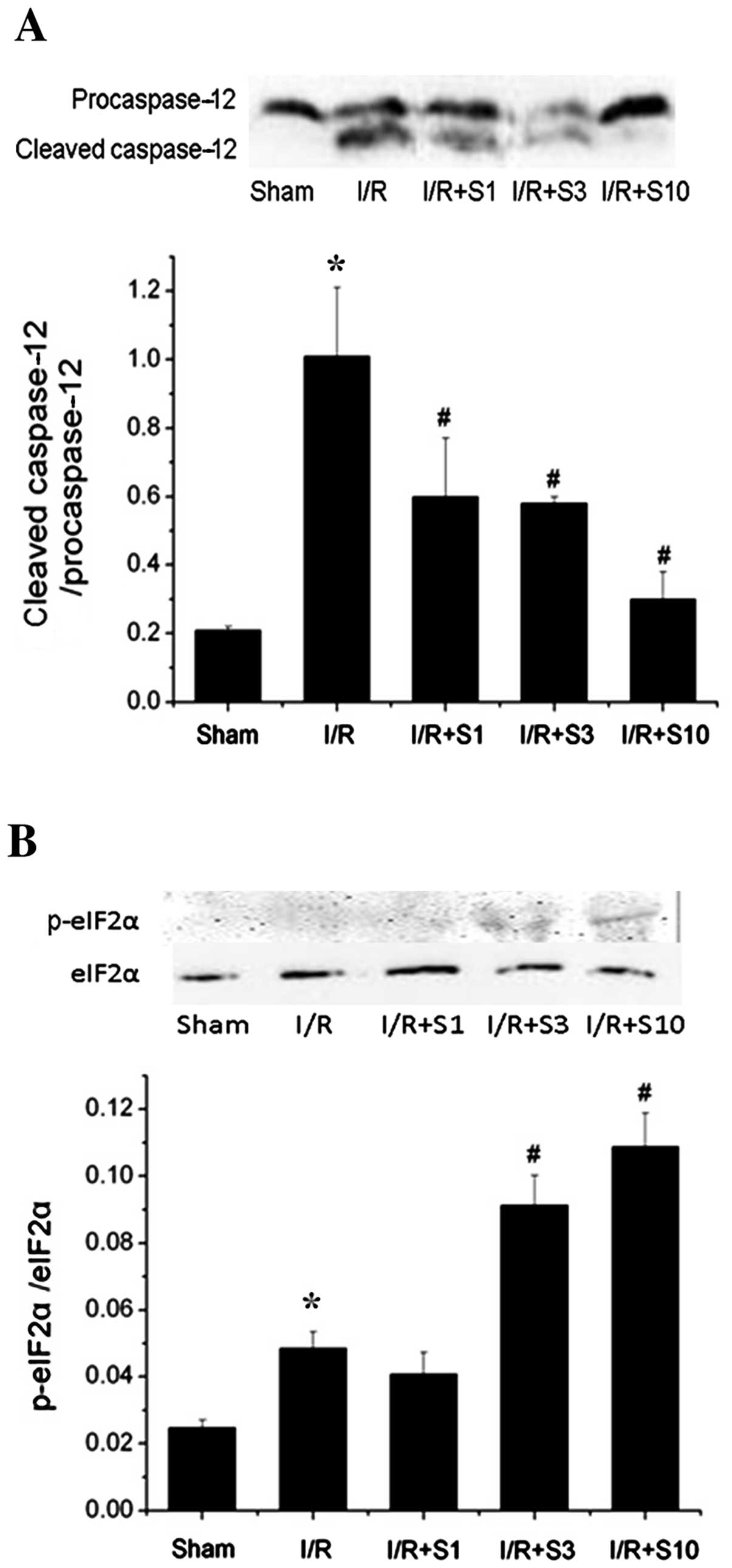
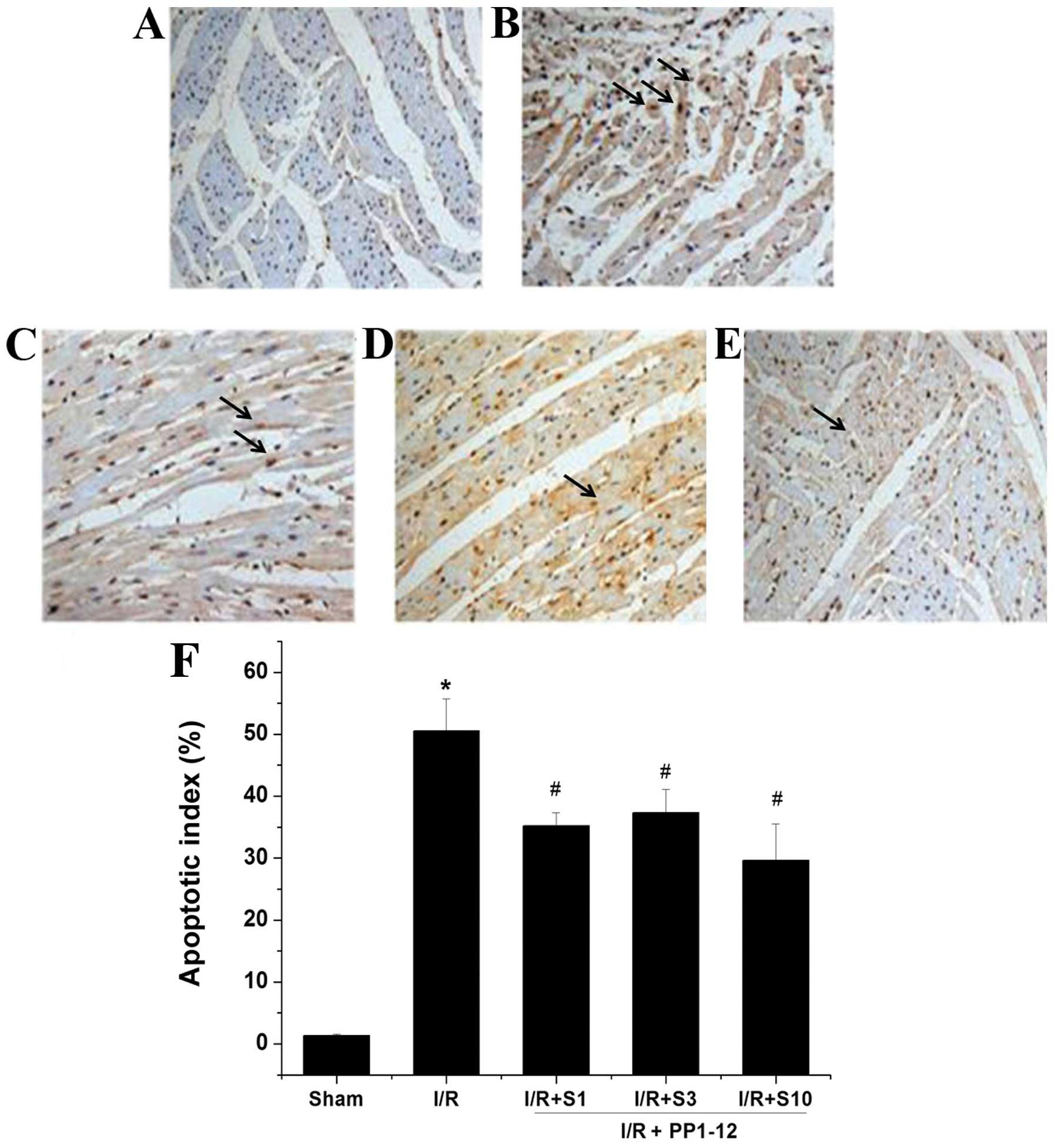
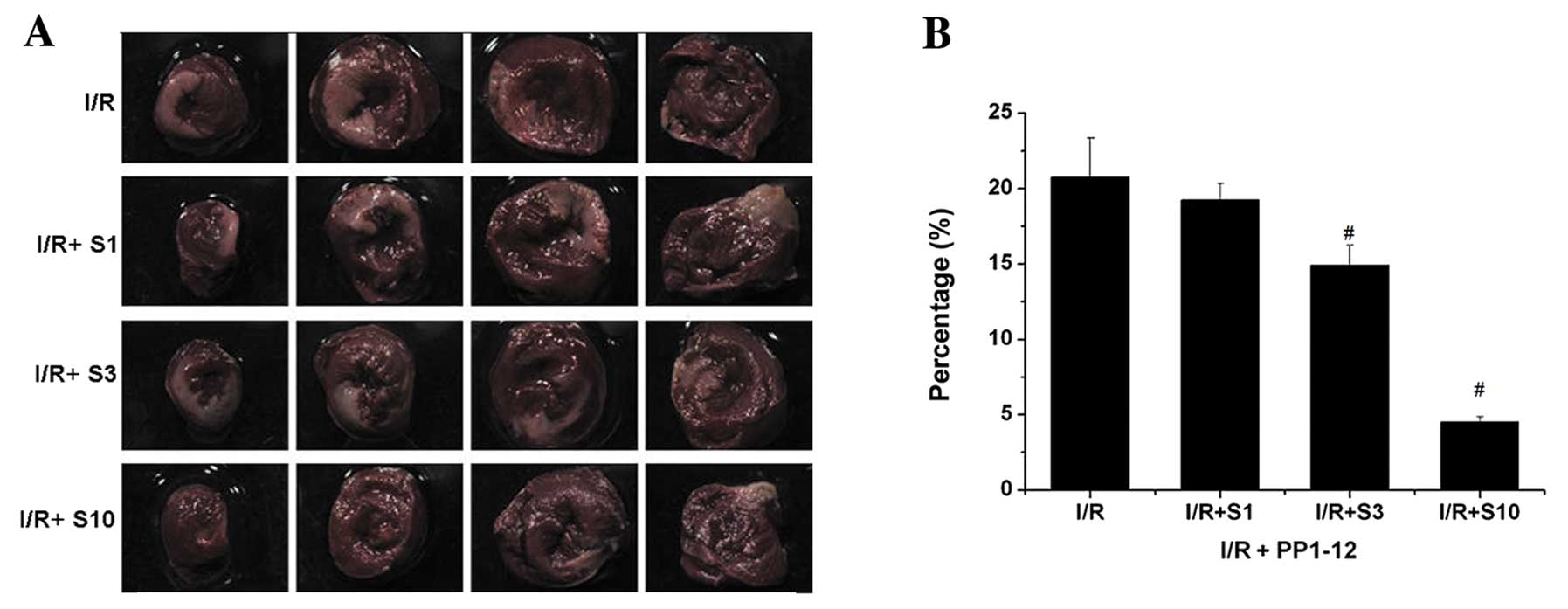
|
1
|
Moscardó A, Vallés J, Piñón M, et al:
Regulation of cytosolic PlA2 activity by PP1/PP2A serine/threonine
phosphatases in human platelets. Platelets. 17:405–415.
2006.PubMed/NCBI
|
|
2
|
Novoa I, Zeng H, Harding HP and Ron D:
Feedback inhibition of the unfolded protein response by
GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol.
153:1011–1022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan T, Luo BL, Wei TH, et al: Salubrinal
protects against cigarette smoke extract-induced HBEpC apoptosis
likely via regulating the activity of PERK-eIF2alpha signaling
pathway. Arch Med Res. 43:522–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyce M, Bryant K, Jousse CJ, et al: A
selective inhibitor of eIF2alpha dephosphorylation protects cells
from ER stress. Science. 307:935–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CL, Li X, Hu GL, et al: Salubrinal
protects against tunicamycin and hypoxia induced cardiomyocyte
apoptosis via the PERK-eIF2alpha signaling pathway. J Geriatr
Cardiol. 9:258–268. 2012.PubMed/NCBI
|
|
6
|
Liu J, He KL, Li X, et al: SAR, cardiac
myocytes protection activity and 3D-QSAR studies of salubrinal and
its potent derivatives. Curr Med Chem. 19:6072–6079. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gasparetto M, Gentry T, Sebti S, et al:
Identification of compounds that enhance the anti-lymphoma activity
of rituximab using flow cytometric high-content screening. J
Immunol Methods. 292:59–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faitova J, Krekac D, Hrstka R and Vojtesek
B: Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett.
11:488–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chatterjee J and Kohn M: Targeting the
untargetable: recent advances in the selective chemical modulation
of protein phosphatase-1 activity. Curr Opin Chem Biol. 17:361–368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pathak A, Monte F, Zhao W, et al:
Enhancement of cardiac function and suppression of heart failure
progression by inhibition of protein phosphatase 1. Circ Res.
96:756–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicolaou P, Hajjar RJ and Kranias EG: Role
of protein phosphatase-1 inhibitor-1 in cardiac physiology and
pathophysiology. J Mol Cell Cardiol. 47:365–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada M, Ikeda Y, Yano M, et al:
Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery
ameliorates heart failure progression in genetic cardiomyopathy.
FASEB J. 20:1197–1199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaya H, Utsumi T, Shimoke K, et al:
Nicotine suppresses tunicamycin-induced, but not
thapsigargin-induced, expression of GRP78 during ER stress-mediated
apoptosis in PC12 cells. J Biochem. 144:251–257. 2008. View Article : Google Scholar
|
|
15
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W, Brush MH, Choy MS and Shenolikar
S: Association with endoplasmic reticulum promotes proteasomal
degradation of GADD34 protein. J Biol Chem. 286:21687–21696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Gong M, Yan M and Zhang X:
Sevoflurane induces endoplasmic reticulum stress mediated apoptosis
in hippocampal neurons of aging rats. PLoS One. 8:e578702013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhang C, Hong Z, et al: C/EBP
homologous protein (CHOP) mediates neuronal apoptosis in rats with
spinal cord injury. Exp Ther Med. 5:107–111. 2013.PubMed/NCBI
|
|
19
|
Oh JG, Kim J, Jang SP, et al: Decoy
peptides targeted to protein phosphatase 1 inhibit
dephosphorylation of phospholamban in cardiomyocytes. J Mol Cell
Cardiol. 56:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanana H, Talarmin H, Pennec JP, Droguet
M, Morel J and Dorange G: Effect of okadaic acid on cultured clam
heart cells: involvement of MAP kinase pathways. Biol Open.
1:1192–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Fan P, Xu A, et al: Improved
functional recovery to I/R injury in hearts from lipocalin-2
deficiency mice: restoration of mitochondrial function and
phospholipids remodeling. Am J Transl Res. 4:60–71. 2012.PubMed/NCBI
|
|
22
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic reticulum-specific apoptosis and
cytotoxicity by amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vidavalur R, Swarnakar S, Thirunavukkarasu
M, et al: Ex vivo and in vivo approaches to study mechanisms of
cardioprotection targeting ischemia/reperfusion (i/r) injury:
useful techniques for cardiovascular drug discovery. Curr Drug
Discov Technol. 5:269–278. 2008. View Article : Google Scholar : PubMed/NCBI
|