Introduction
Ultraviolet (UV) radiation found in sunlight is the
major environmental cause of skin aging and skin disorders
(1). There are three different
wavelengths of UV radiation, subdivided as UVC (290–320 nm), UVB
(290–320 nm) and UVA (320–400 nm) (2). UVC radiation contains the highest
energy, which can induce skin damage. However, the majority of UVC
radiation is blocked by the atmosphere. By contrast, UVA radiation
is, on average, 1,000-fold lower in energy than UVB radiation and
its transmission through the atmosphere is greater (3). UVB radiation reaches the dermis and
epidermis when penetrating the atmosphere. UVB radiation is
absorbed by the epidermis and dermis and causes damage to various
cells and to the extracellular matrix of the skin (4–6).
Keratinocytes are particularly influenced by UVB radiation due to
their outermost cutaneous location (7). Keratinocytes, mainly contained in
the epidermis, have been implicated in its development via
differentiation (8). In
keratinocytes, UVB radiation induces the formation of ‘sunburn
cells’ (keratinocytes undergoing apoptosis) and may result in skin
cancer (5). UVB induces
keratinocyte apoptosis by promoting DNA damage, death receptor
activation and the production of reactive oxygen species (ROS)
(9). UVB radiation can stimulate
UVB-induced signaling pathways involving p53 and MAP kinases (JNK
and p38), and can alter gene expression and subsequently induce
cell cycle arrest, apoptosis or cellular senescence (10–14). Recently, microRNAs (miRNAs) have
been implicated in UVB responses in keratinocytes (15–16). The miRNA, miR-23a, regulates DNA
damage repair and apoptosis by targeting topoisomerase-1, caspase-7
and serine/threonine kinase 4 (STK4) (17). In addition, UVB radiation induces
apoptosis by upregulating miR-141, which then leads to the
suppression of phosphatase and tensin homolog (PTEN) in
keratinocytes (18). Aberrantly
expressed miRNAs have been linked to apoptosis and cell cycle
arrest in UVB-exposed keratinocytes, where they can be used as
regulators of UVB responses (19). In the current study, we identified
a novel phytochemical that protect against UVB-induced cell death
through the regulation of miRNA expression.
Troxerutin, {vitamin P4;
3′,4′,7′-Tris[O-(2-hydroxyethyl)]rutin} is a natural flavonoid
rutin mainly found in extracts of Sophora japonica, and is a
well-known antioxidant and anti-inflammatory compound used in
experimental mouse models (20–24). In addition, troxerutin has been
shown to improve capillary function by suppressing capillary
fragility and abnormal leakage (25). It has been shown to inhibit
erythrolysis and exert anti-thrombotic, fibrinolytic,
edema-protective and rheological effects in a model of chronic
venous insufficiency (26).
In the present study, we demonstrate that troxerutin
exerts protective effects against UVB radiation by regulating miRNA
expression. The current data may enhance our understanding of the
protective mechanisms of troxerutin in UVB-exposed skin.
Materials and methods
Cell culture and reagents
HaCaT cells, an immortalized human keratinocyte cell
line, were grown as monolayers at 37°C, in a 5% CO2
atmosphere, in DMEM medium (Gibco-Invitrogen, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St.
Louis, MO, USA) and 1% penicillin/streptomycin solution
(Gibco-Invitrogen). Viability assays were carried out in 96-well
microplates seeded with 4×104 HaCaT cells. Cell cycle
analyses, DNA damage assays, and microarray analyses were performed
in 60-mm culture dishes seeded with 7×105 HaCaT cells.
Troxerutin was purchased from Sigma-Aldrich. Hydrogen peroxide
(30%) was purchased from BioShop Canada Inc. (Burlington, ON,
Canada).
Exposure of HaCaT cells to UVB
radiation
The HaCaT cells were exposed to UVB radiation using
a G8T5E lamp (Sankyo Denki, Toshima-ku, Japan). The UVB radiation
intensity was measured by a UV light meter (UV-340; Lutron, Taiwan,
Taipei). The cells were washed and resuspended in
phosphate-buffered saline (PBS) prior to exposure to UVB radiation.
Non-exposed control samples were maintained in the dark under the
same conditions. Following exposure to UVB radiation, the cells
were grown in fresh medium.
Cell viability assay
Cell viability was measured by a water-soluble
tetrazolium salt (WST-1) assay. HaCaT cells were seeded at
4×104 cells/well in 96-well microplates and incubated
for 24 h. In order to measure troxerutin toxicity, the cells were
treated with the indicated concentrations of troxerutin for 24 h.
In order to determine the protective effects of troxerutin against
UVB radiation, the cells were incubated with the indicated
concentrations of troxerutin for 4, 8 and 12 h. The
troxerutin-pre-treated HaCaT cells were then exposed to 50
mJ/cm2 of UVB radiation followed by incubation for 24 h.
WST-1 solution (EZ-Cytox Cell Viability Assay kit; ITSBio, Seoul,
Korea) was then added to the cells for 1 h. The absorbance of each
sample was measured using a microplate reader (iMark; Bio-Rad
Laboratories, Hercules, CA, USA) with filters at 450 nm and a
reference wavelength at 620 nm. The results were presented as the
relative absorbance [optical density (OD)] compared with untreated
HaCaT cells.
Cell cycle analysis
Cell cycle distribution was measured by propidium
iodide (PI; Sigma-Aldrich) staining. HaCaT cells were seeded at a
density of 7×105 cells in 60-mm culture dishes, and
incubated for 24 h. The cells were pre-treated with 5 μM troxerutin
for 8 h, and the troxerutin-pre-treated HaCaT cells were then
exposed to 50 mJ/cm2 of UVB radiation and incubated for
24 h. The cells were fixed in 70% ethanol at 4°C for 3 h, and
stained with PI staining solution containing 50 μg/ml PI
(Sigma-Aldrich), 0.5% Triton X-100 and 100 μg/ml RNase (both from
BioShop Canada Inc.) at 37°C for 1 h. The fluorescence intensity of
each cell sample was detected by the FL2-H channel of the
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Migration assay
Migration activity in the cells was measured by a
scratch wound assay. The HaCaT cells were seeded at a density of
7×105 cells in 60-mm culture dishes, and cultured to
>90% confluency. The cells were pre-treated with 5 μM troxerutin
for 8 h, and the troxerutin-pre-treated HaCaT cells were then
exposed to 50 mJ/cm2 of UVB radiation and scratched
using a 200 μl micropipette tip. At 0 and 48 h, images were
acquired of the scratch wound using a phase contrast
microscope.
DNA repair activity assay
DNA repair activity was measured by using an
impaired plasmid. HaCaT cells were seeded at a density of
7×105 cells in 60-mm culture dishes, and incubated for
24 h. The cells were transiently transfected with damaged (due to
impaired exposure response to UVB) pGL3 Luciferase reporter
vectors. pSV-β-galactosidase is a positive control vector that was
used for monitoring the transfection efficiency. The transfected
cells were pre-treated with 5 μM troxerutin for 24 h. After 24 h,
luciferase activity was measured using the Luciferase assay system
(Promega, Fitchburg, WI, USA) as described in the instruction
manual. The normalized results were presented as relative
percentages of the experimental control values.
Detection of global miRNA expression
levels
Alterations in global miRNA expression levels were
measured using a SurePrint G3 Human v16.0 miRNA microarray kit
(based on the miRBase release 19.0; Agilent Technologies, Santa
Clara, CA, USA). Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions, while
RNA stability and purity were measured using the Bioanalyzer 2100
(Agilent Technologies), and calculated as A260/A280 and
A260/A230 ratios using a MaestroNano Spectrophotometer
(Maestrogen, Las Vegas, NV, USA). The total RNA eliminated
phosphate was measured using calf intestine alkaline phosphatase
(CIP), and was then labeled with pCp-Cy3, using T4 RNA ligase (all
from Agilent Technologies). The labeled total RNA was hybridized to
the miRNA microarray. Microarrays were scanned using an Agilent
microarray scanner. The miRNA microarray data were normalized and
analyzed using GeneSpring GX version 11.5 software (Agilent
Technologies).
miRNA target gene prediction and Gene
Ontology (GO) analysis
A list of target genes for the selected miRNAs was
extracted by TargetScan (http://www.targetscan.org). The biological functions
of putative target genes were classified into different biological
processes (GO categories). Enrichment analysis of GO categories was
performed using DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/). We selected
significant GO categories using several parameters (count >600
and p-value <0.01).
Statistical analysis
Statistical significance was determined using a
Student’s t-test. Values of p<0.05 were considered to indicate
statistically significant differences.
Results
Troxerutin reduces UVB-induced
cytotoxicity in HaCaT cells
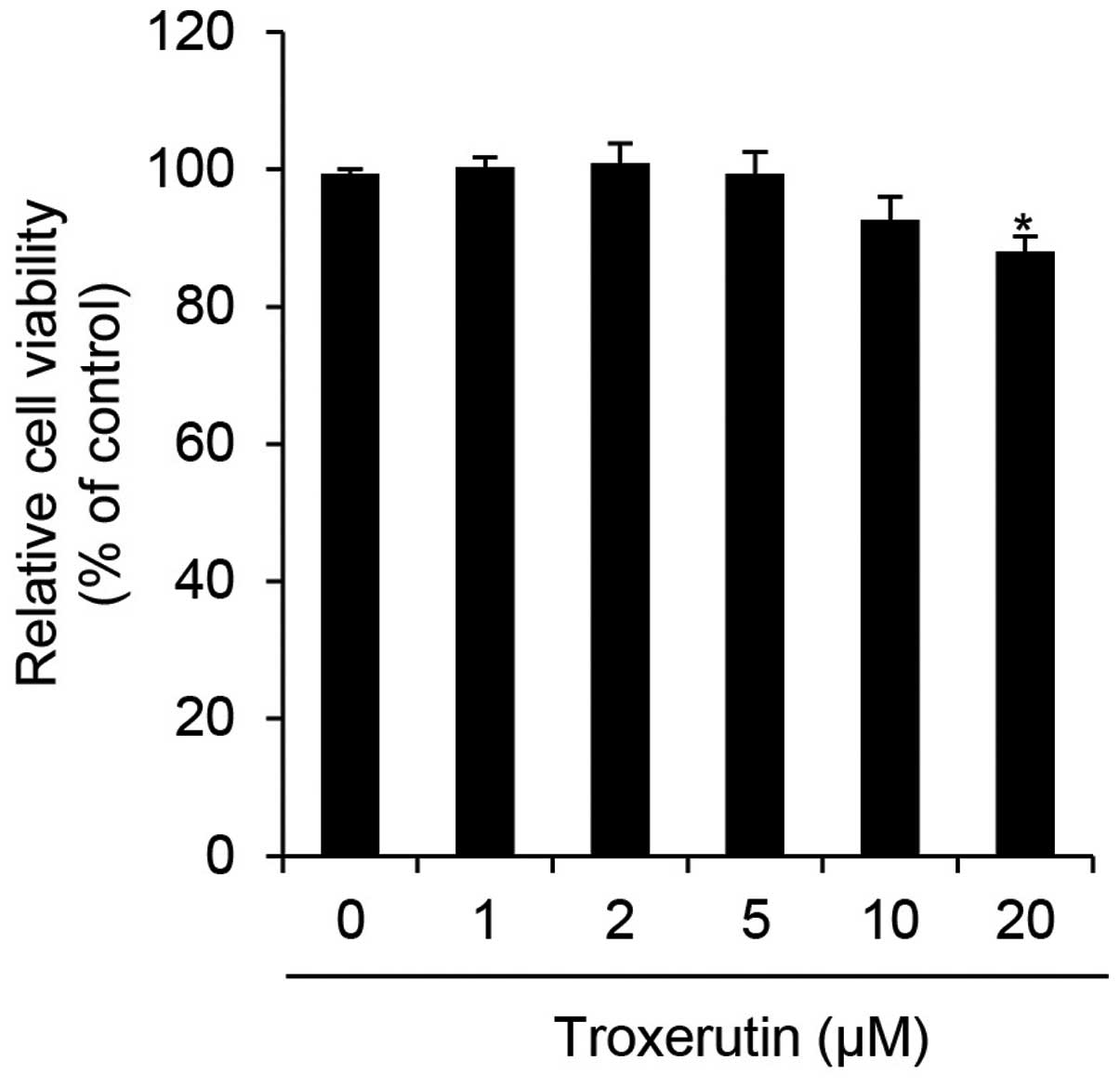
We first determined the cytotoxicity of troxerutin
in HaCaT cells by performing WST-1 assays. The HaCaT cells were
treated with the indicated concentrations of troxerutin and then
incubated for 24 h. Cytotoxicity in HaCaT cells was significantly
decreased at 20 μM troxerutin (Fig.
1). In a previous study, troxerutin was shown to exert limited,
if any, cytotoxic effects at a dose of 20 μg/ml (approximately
26.93 μM) in V79 Chinese hamster lung fibroblasts (27). Therefore, a similar concentration
range of troxerutin (0–10 μM) was used in the current study. To
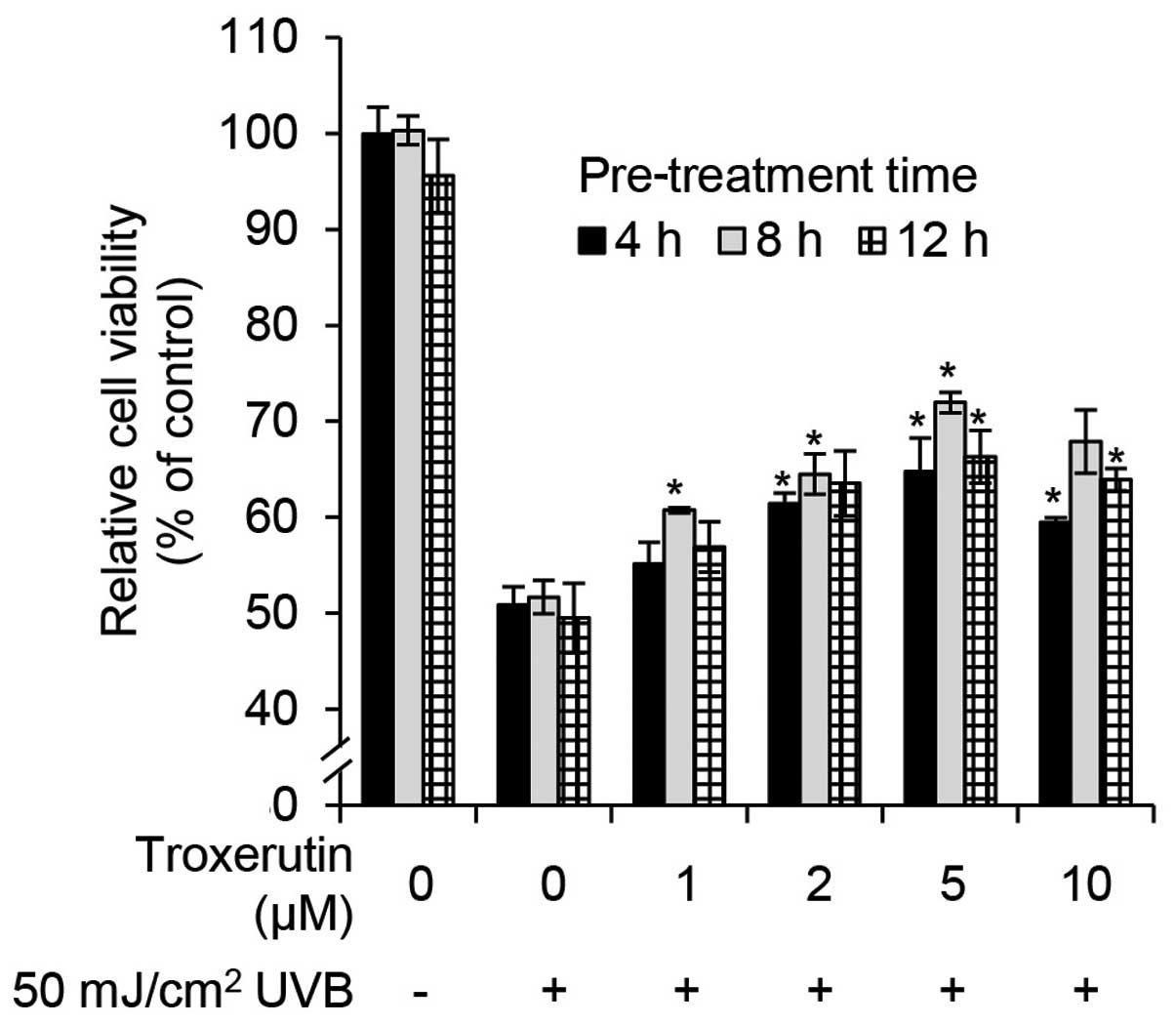
determine whether troxerutin protects against UVB-induced growth
arrest in HaCaT cells, we performed cell viability assays. The
HaCaT cells were pre-treated with the indicated concentrations of
troxerutin for 4, 8 and 12 h, and then exposed to 50
mJ/cm2 of UVB radiation and incubated for 24 h.
Troxerutin prevented UVB-mediated growth arrest in a dose-dependent
manner (Fig. 2). Troxerutin (5
μM) effectively protected the cells against UVB-mediated growth
arrest. Notably, pre-treatment with 5 μM troxerutin for 8 h
increased cell viability by 20.27% compared with untreated
UVB-exposed HaCaT cells (Fig. 2).
Overall, pre-treatment with troxerutin protected the cells from
UVB-mediated growth arrest.
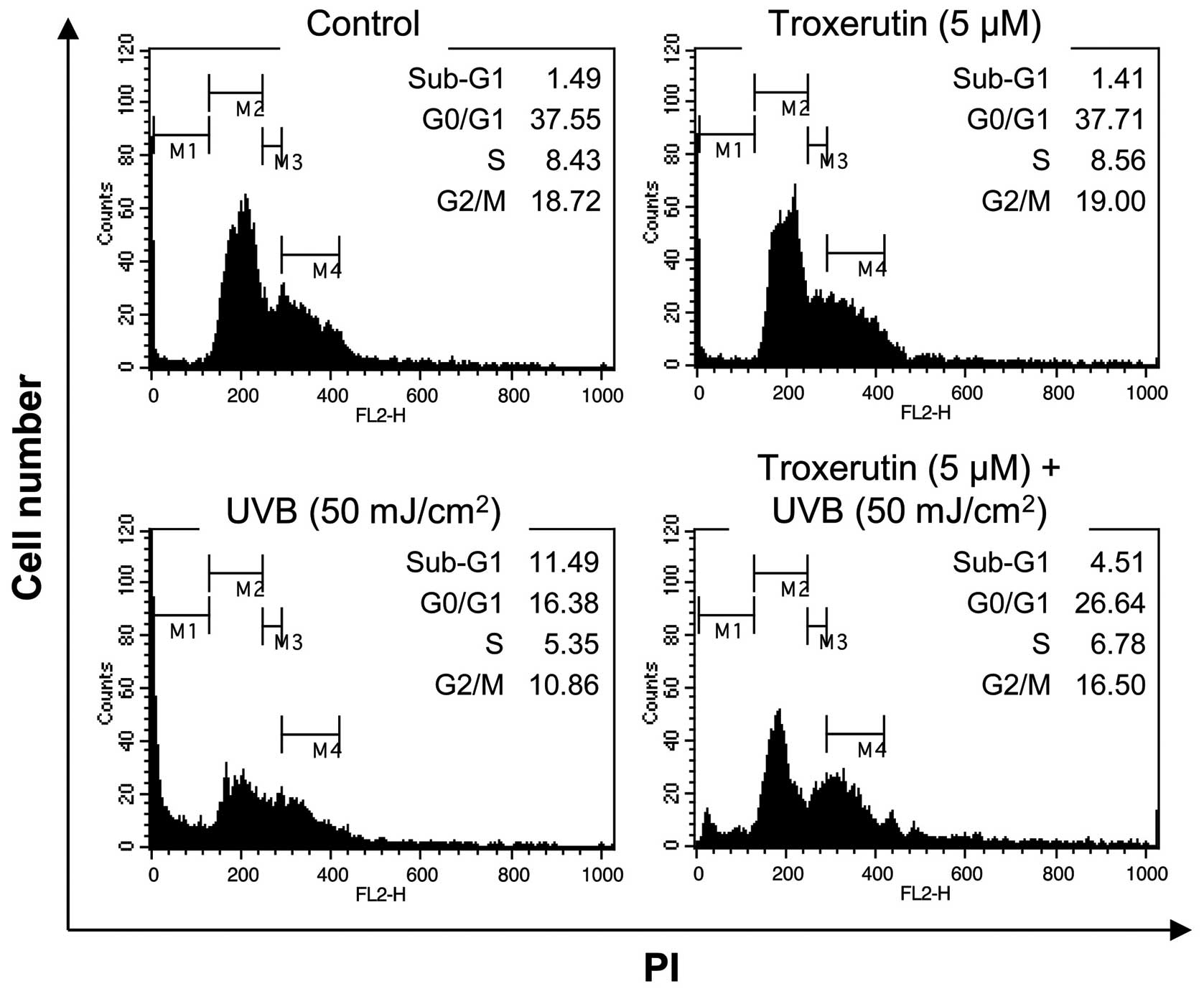
Troxerutin reduces UVB-induced death in
HaCaT cells
As growth arrest and cell death often cause a
decrease in cell viability, in the current study, we determined
whether the troxerutin-mediated protective effects against UVB
exposure were due to the suppression of growth arrest and cell
death. In normal HaCaT cells, 50 mJ/cm2 of UVB radiation
caused an increase in the number of cells in the sub-G1 phase
(1.49–11.49%). However, in the HaCaT cells pre-treated with
troxerutin, the UVB-mediated increase in the number of sub-G1 phase
cells decreased to 4.51% (Fig.
3). As the sub-G1 phase indicates a population of dead cells,
the troxerutin-mediated recovery of cell viability may involve the
blockade of cell death-associated pathways.
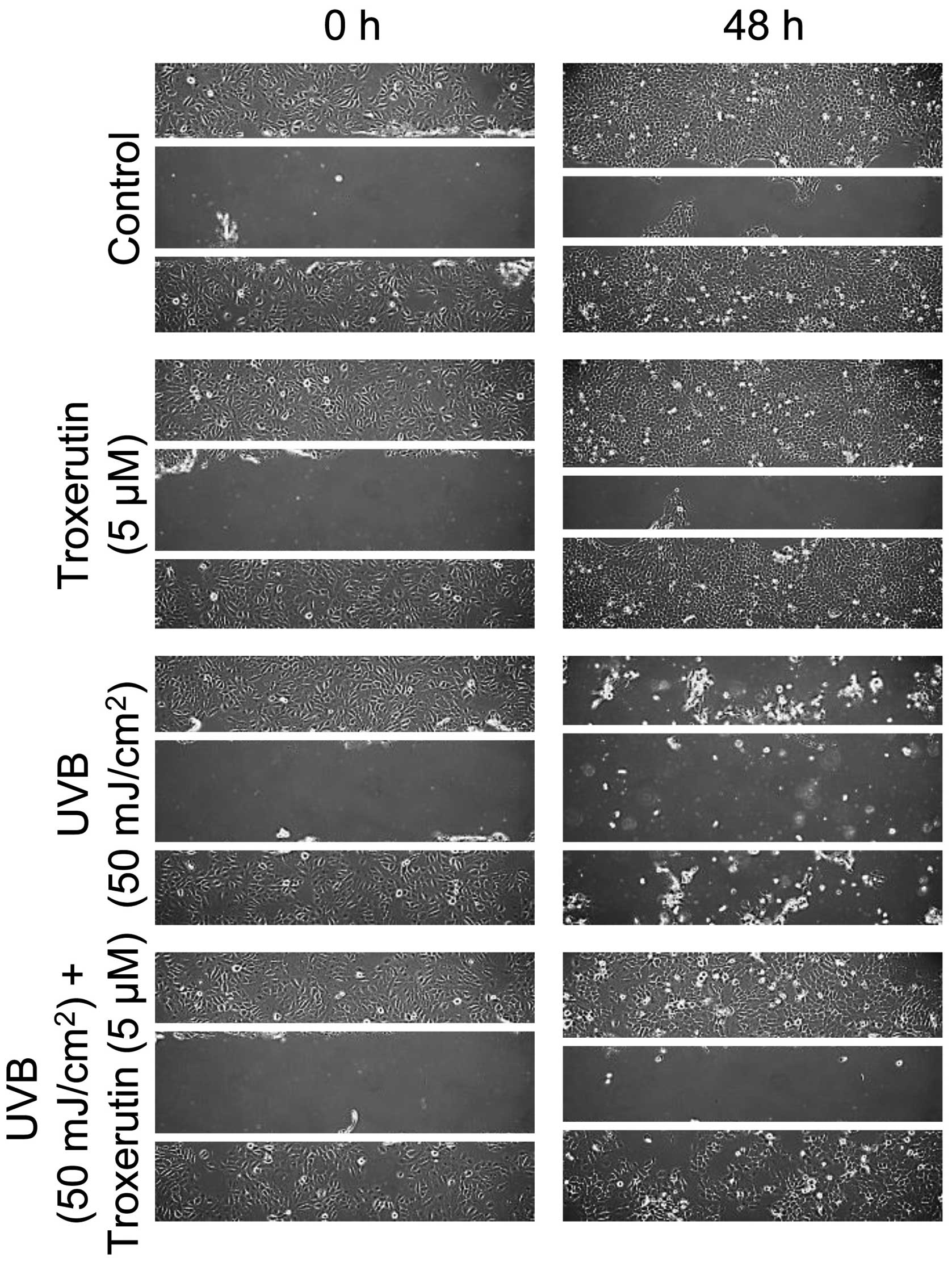
Troxerutin enhances migration activity in
HaCaT cells
We then determined whether troxerutin affects
migration activity in HaCaT cells. To assess migration activity,
the scratched wound healing assay was used as described in
Materials and methods. The migration activity was increased by
treatment with troxerutin in HaCaT cells as compared to the
untreated cells. Exposure to UVB radiation attenuated migration in
HaCaT cells, whereas troxerutin restored the migration activity to
the control levels (Fig. 4).
Troxerutin induces DNA repair activity in
HaCaT cells
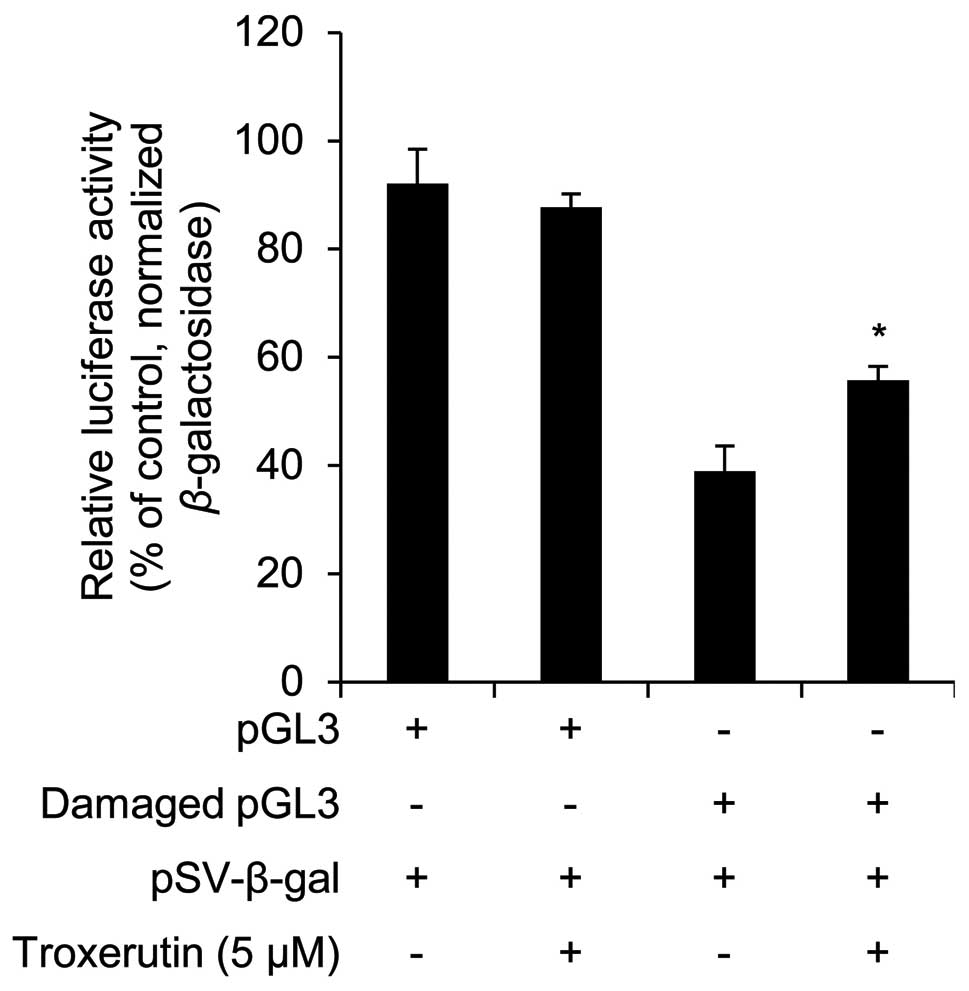
To determine whether troxerutin increases DNA repair
activity, we examined its effects using the damaged luciferase
reporter vector. Troxerutin had no effects on luciferase activity
in non-damaged pGL3 vector-transfected HaCaT cells (Fig. 5). However, in the damaged-pGL3
vector-transfected HaCaT cells, troxerutin increased luciferase
activity (Fig. 5). The increased
luciferase activity was used to measure DNA repair activity;
troxerutin increased DNA repair activity in HaCaT cells. Similarly,
in a previous study, Maurya et al (28) demonstrated that troxerutin exerted
protective effects against gamma radiation-induced damage in mouse
cellular DNA.
Troxerutin alters miRNA expression
profiles in UVB-exposed HaCaT cells
As described in the Introduction, miRNAs play a
crucial role in UVB responses through specific binding to target
genes (19). Therefore, in
UVB-exposed HaCaT cells, we examined the effects of troxerutin on
global miRNA expression levels. To analyze the global miRNA
expression levels and identify putative alterations, we used miRNA
gene microarrays, which contained 1,368 probes representing 1,205
human miRNAs. Total RNA, labeled with Cy3, a green fluorescent dye,
was extracted from the HaCaT cells treated with or without
troxerutin and then exposed to UVB radiation. Each Cy3-labeled
sample was hybridized onto an miRNA microarray. Using analytical
software (GeneSpring GX version 11.5; Agilent), we first normalized
the data from each sample using global normalization. To obtain
refined data, we did not take into consideration the absent and
undetectable data in all samples. Lastly, significantly altered
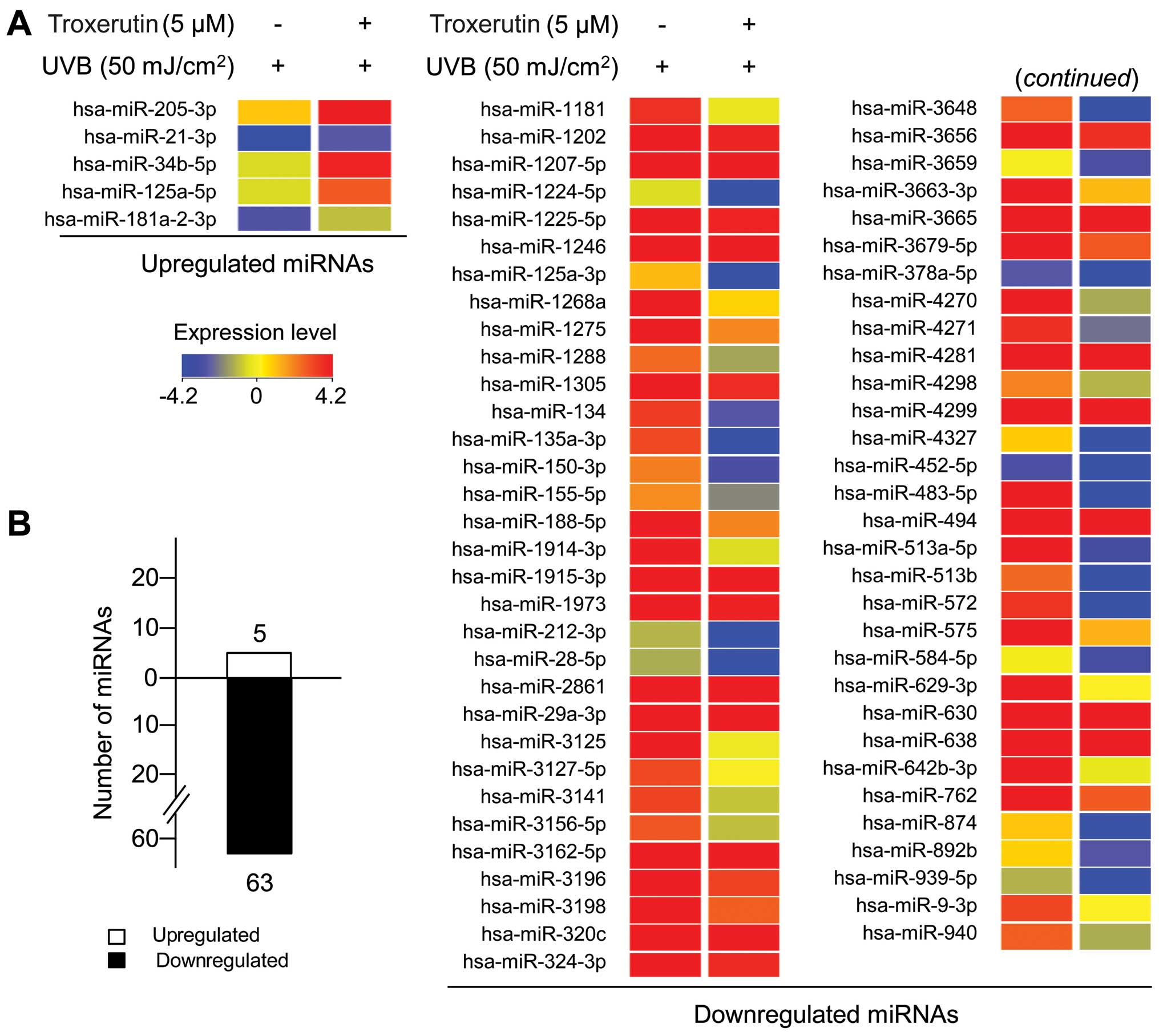
miRNAs were designated as miRNAs with a ≥2-fold change in
expression. A total of 68 miRNAs, 5 upregulated miRNAs and 63
downregulated miRNAs, were selected for analyses (Fig. 6 and Table I). miR-205-3p expression was
increased by 4.29-fold, whereas miR-513b, miR483-5p and miR-3648
expression was significantly decreased by 23.10-, 16.61- and
11.55-fold, respectively. Overall, our results demonstrate that
troxerutin protects HaCaT cells from UVB-induced damage through the
specific regulation of miRNA expression.
 | Table IUpregulated and downregulated miRNAs
in UVB-exposed HaCaT cells pre-treated with troxerutin. |
Table I
Upregulated and downregulated miRNAs
in UVB-exposed HaCaT cells pre-treated with troxerutin.
| miRNA | Fold change | miRNA | Fold change | miRNA | Fold change |
|---|
| Upregulated
miRNA |
| hsa-miR-205-3p | 4.29 | hsa-miR-34b-5p | 2.65 |
hsa-miR-181a-2-3p | 2.00 |
| hsa-miR-21-3p | 2.97 |
hsa-miR-125a-5p | 2.62 | | |
| Downregulated
miRNA |
| hsa-miR-513b | −23.10 | hsa-miR-630 | −4.88 | hsa-miR-3141 | −3.15 |
| hsa-miR-483-5p | −16.61 | hsa-miR-188-5p | −4.83 |
hsa-miR-1915-3p | −3.08 |
| hsa-miR-3648 | −11.55 | hsa-miR-1202 | −4.78 | hsa-miR-1181 | −3.02 |
|
hsa-miR-513a-5p | −9.66 |
hsa-miR-1224-5p | −4.66 | hsa-miR-940 | −2.98 |
|
hsa-miR-135a-3p | −9.49 | hsa-miR-4299 | −4.58 | hsa-miR-320c | −2.97 |
| hsa-miR-572 | −9.26 |
hsa-miR-1207-5p | −4.45 | hsa-miR-324-3p | −2.96 |
| hsa-miR-575 | −8.01 |
hsa-miR-3663-3p | −4.44 |
hsa-miR-3156-5p | −2.89 |
| hsa-miR-4327 | −7.32 |
hsa-miR-3162-5p | −4.35 | hsa-miR-1288 | −2.87 |
| hsa-miR-874 | −7.15 | hsa-miR-212-3p | −4.14 | hsa-miR-1305 | −2.86 |
| hsa-miR-638 | −6.68 | hsa-miR-3198 | −4.08 | hsa-miR-1973 | −2.78 |
| hsa-miR-4270 | −6.44 |
hsa-miR-642b-3p | −3.96 | hsa-miR-155-5p | −2.76 |
| hsa-miR-1246 | −6.27 | hsa-miR-150-3p | −3.90 | hsa-miR-28-5p | −2.59 |
|
hsa-miR-3679-5p | −5.78 |
hsa-miR-1914-3p | −3.77 | hsa-miR-452-5p | −2.54 |
|
hsa-miR-1225-5p | −5.64 | hsa-miR-762 | −3.66 |
hsa-miR-378a-5p | −2.48 |
| hsa-miR-3656 | −5.60 | hsa-miR-3196 | −3.62 | hsa-miR-892b | −2.47 |
| hsa-miR-1275 | −5.59 | hsa-miR-3125 | −3.43 | hsa-miR-4298 | −2.40 |
| hsa-miR-494 | −5.53 | hsa-miR-1268a | −3.30 |
hsa-miR-3127-5p | −2.38 |
| hsa-miR-2861 | −5.44 | hsa-miR-9-3p | −3.28 | hsa-miR-939-5p | −2.33 |
| hsa-miR-134 | −5.18 | hsa-miR-629-3p | −3.21 | hsa-miR-29a-3p | −2.26 |
| hsa-miR-4271 | −5.03 | hsa-miR-4281 | −3.21 | hsa-miR-584-5p | −2.16 |
|
hsa-miR-125a-3p | −4.91 | hsa-miR-3665 | −3.15 | hsa-miR-3659 | −2.08 |
Analysis of putative target genes and GO
analysis of troxerutin-specific miRNAs using bioinformatics
From the detection of global miRNA expression
levels, our results identified 68 troxerutin-specific miRNAs.
miRNAs regulate biological functions through the interference of
their target genes (29). To
identify biological functions of the miRNAs, we used TargetScan to
predict putative target genes of troxerutin-specific miRNAs in
UVB-exposed HaCaT cells. Using a seed sequence-based target
prediction system (TargetScan), we analyzed the 5 miRNAs
upregulated by troxerutin and identified 1,755 putative target
genes. For the 63 downregulated miRNAs, 13,768 putative target
genes were identified. We then analyzed the categorized functions,
using GO analysis within the putative target pool. Following
classification into GO categories, we selected significant GO
categories using parameters, such as a count of >600, a
percentage (%) of >4% and a p-value <0.01. Our analyses
revealed the following distribution of biological processes:
regulation of transcription (2,050; number of GO term-related genes
in the putative target pool), intracellular signaling cascades
(1,021), phosphate metabolic processes (792), protein localization
(712), regulation of programmed cell death (653), regulation of
cell proliferation (642), cell cycle (611) and ion transport (603)
(Table II).
 | Table IIGO analysis of potential target genes
of troxerutin-regulated miRNAs. |
Table II
GO analysis of potential target genes
of troxerutin-regulated miRNAs.
| Accession no. | GO term | Count | Percentage (%) | p-value |
|---|
| GO:0045449 | Regulation of
transcription | 2,050 | 15.01 | 4.83E-18 |
| GO:0007242 | Intracellular
signaling cascade | 1,021 | 7.48 | 6.80E-15 |
| GO:0006793 | Phosphate metabolic
processes | 792 | 5.80 | 6.75E-12 |
| GO:0008104 | Protein
localization | 712 | 5.21 | 1.74E-09 |
| GO:0043067 | Regulation of
programmed cell death | 653 | 4.78 | 2.87E-08 |
| GO:0042127 | Regulation of cell
proliferation | 642 | 4.70 | 4.15E-10 |
| GO:0007049 | Cell cycle
regulation | 611 | 4.47 | 1.69E-05 |
| GO:0006811 | Ion transport | 603 | 4.42 | 3.54E-05 |
The aforementioned effects of troxerutin were
further grouped into four cell functional groups: cell death and
apoptosis, cell proliferation and the cell cycle, migration and DNA
repair. These groups included the following: cell death and
apoptosis, 16 GO terms; cell proliferation and cell cycle, 19 GO
terms; migration, 8 GO terms; and DNA repair, 2 GO terms (Table III). Overall, these results
demonstrated that troxerutin exerted protective effects against
UVB-induced damage, which was related to an alteration in cellular
miRNA expression profiles.
 | Table IIIGroupring of GO terms into four cell
functional groups reflecting the effects of troxerutin. |
Table III
Groupring of GO terms into four cell
functional groups reflecting the effects of troxerutin.
| Accession no. | GO term | Count | Percentage (%) | p-value |
|---|
| Cell death and
apoptosis (16 GO terms) |
| GO:0043067 | Regulation of
programmed cell death | 653 | 4.78 | 2.87E-08 |
| GO:0010941 | Regulation of cell
death | 653 | 4.78 | 8.51E-08 |
| GO:0042981 | Regulation of
apoptosis | 647 | 4.74 | 2.75E-08 |
| GO:0016265 | Death | 569 | 4.17 | 4.95E-05 |
| GO:0008219 | Cell death | 564 | 4.13 | 7.79E-05 |
| GO:0012501 | Programmed cell
death | 474 | 3.47 | 1.65E-03 |
| GO:0006915 | Apoptosis | 466 | 3.41 | 2.44E-03 |
| GO:0043068 | Positive regulation
of programmed cell death | 344 | 2.52 | 4.31E-04 |
| GO:0010942 | Positive regulation
of cell death | 344 | 2.52 | 7.94E-04 |
| GO:0043065 | Positive regulation
of apoptosis | 341 | 2.50 | 5.82E-04 |
| GO:0043069 | Negative regulation
of programmed cell death | 289 | 2.12 | 2.55E-04 |
| GO:0060548 | Negative regulation
of cell death | 289 | 2.12 | 3.66E-04 |
| GO:0012502 | Induction of
programmed cell death | 251 | 1.84 | 1.16E-02 |
| GO:0006917 | Induction of
apoptosis | 250 | 1.83 | 1.27E-02 |
| GO:0006916 | Anti-apoptosis | 162 | 1.19 | 3.08E-02 |
| GO:0043524 | Negative regulation
of neuron apoptosis | 49 | 0.36 | 8.44E-05 |
| Cell proliferation
and cell cycle (19 GO terms) |
| GO:0042127 | Regulation of cell
proliferation | 642 | 4.70 | 4.15E-10 |
| GO:0007049 | Cell cycle | 611 | 4.47 | 1.69E-05 |
| GO:0022402 | Cell cycle
process | 442 | 3.24 | 7.41E-04 |
| GO:0008283 | Cell
proliferation | 345 | 2.53 | 7.21E-04 |
| GO:0008284 | Positive regulation
of cell proliferation | 336 | 2.46 | 2.11E-05 |
| GO:0022403 | Cell cycle
phase | 331 | 2.42 | 2.36E-04 |
| GO:0008285 | Negative regulation
of cell proliferation | 292 | 2.14 | 1.24E-04 |
| GO:0040008 | Regulation of
growth | 265 | 1.94 | 1.61E-02 |
| GO:0000279 | M phase | 260 | 1.90 | 3.88E-03 |
| GO:0051726 | Regulation of cell
cycle | 260 | 1.90 | 6.85E-03 |
| GO:0000087 | M phase of mitotic
cell cycle | 173 | 1.27 | 6.99E-02 |
| GO:0001558 | Regulation of cell
growth | 151 | 1.11 | 6.26E-02 |
| GO:0010564 | Regulation of cell
cycle process | 96 | 0.70 | 3.86E-03 |
| GO:0007050 | Cell cycle
arrest | 87 | 0.64 | 5.25E-03 |
| GO:0051329 | Interphase of
mitotic cell cycle | 85 | 0.62 | 1.91E-02 |
| GO:0051327 | M phase of meiotic
cell cycle | 78 | 0.57 | 9.53E-02 |
| GO:0045927 | Positive regulation
of growth | 66 | 0.48 | 4.23E-02 |
| GO:0000082 | G1/S transition of
mitotic cell cycle | 49 | 0.36 | 1.34E-02 |
| GO:0045787 | Positive regulation
of cell cycle | 47 | 0.34 | 9.65E-02 |
| Migration (8 GO
terms) |
| GO:0048870 | Cell motility | 244 | 1.79 | 3.03E-03 |
| GO:0016477 | Cell migration | 227 | 1.66 | 1.11E-04 |
| GO:0042060 | Wound healing | 151 | 1.11 | 2.78E-02 |
| GO:0030334 | Regulation of cell
migration | 143 | 1.05 | 2.08E-04 |
| GO:0030335 | Positive regulation
of cell migration | 79 | 0.58 | 3.86E-04 |
| GO:0030336 | Negative regulation
of cell migration | 48 | 0.35 | 5.24E-02 |
| GO:0010595 | Positive regulation
of endothelial cell migration | 13 | 0.10 | 8.71E-02 |
| GO:0002685 | Regulation of
leukocyte migration | 19 | 0.14 | 5.47E-02 |
| DNA repair (2 GO
terms) |
| GO:0051052 | Regulation of DNA
metabolic process | 90 | 0.66 | 9.55E-02 |
| GO:0051054 | Positive regulation
of DNA metabolic process | 47 | 0.35 | 5.15E-02 |
Discussion
In the skin, UVB radiation is a crucial inducer of
apoptosis and growth arrest (5).
In the epidermis, the accumulation of UVB-induced damaged
keratinocytes results in sunburn, psoriasis and skin cancer
(30). Therefore, protection from
UVB-induced keratinocyte damage, including cell death, growth
arrest, DNA damage and decreased migration, is essential in order
to maintain epidermal homeostasis.
In the present study, we demonstrated that
troxerutin protected cells from UVB-induced decrease in cell growth
through the suppression of apoptosis. Pre-treatment with troxerutin
protected the HaCaT cells from UVB-induced growth arrest (Fig. 2) by preventing apoptosis (Fig. 3). Also, migration assay results
deonstrated that troxerutin increased cell migration activity
(Fig. 4). In previous studies,
troxerutin-induced DNA repair enhanced its protective effects
against gamma radiation (28,30). Similarly, in the present study,
DNA repair activity was increased by troxerutin in HaCaT cells
(Fig. 5). In addition, using
miRNA microarray analysis and bioinformatics tools, we identified
specific troxerutin-induced miRNAs and found a link between
troxerutin-induced miRNAs, as well as anti-apoptotic, enhanced
migration and DNA repair effects. In UVB-exposed HaCaT cells,
troxerutin altered the expression of 68 miRNAs, with a ≥2-fold up-
or downregulation (Fig. 3).
In our study, in UVB-exposed HaCaT cells,
miR-181a-5p was upregulated 2-fold by troxerutin. The miR-181a, a
mature form of miR-181a-1, and miR-181a-2, have been implicated in
proliferation, migration and invasion, and target BIM, a
member of the apoptotic BCL-2 family (32,33). In the present study, in the
downregulated miRNA group, miR-513a-5p was markedly decreased by
9.66-fold in troxerutin-treated HaCaT cells. This miRNA induces
apoptosis by targeting the X-linked inhibitor of apoptosis (XIAP)
in endothelial cells (34).
miR-874 was also decreased by 7.15-fold in troxerutin-treated HaCaT
cells and has been shown to contribute to cell proliferation
through its effects on histone deacetylase 1 (35). miR-874 has been implicated in cell
proliferation and migration by targeting aquaporin-3 (36). In this study, the expression
levels of miR-1246 and miR-494 were significantly decreased in
troxerutin-pre-treated HaCaT cells exposed to UVB. The expression
of miR-1246 has been reported to be regulated by p53, a crucial
transcription factor in the DNA damage response, and can repress
the translation of dual-specificity tyrosine-(Y)-phosphorylation
regulated kinase 1A (DYRK1A), an inducer of proliferation (37). miR-494 has been implicated in
TRAIL-induced apoptosis through the repression of BIM
(38). In addition, miR-494
regulates three pro-apoptotic proteins [PTEN, Rho-associated,
coiled-coil containing protein kinase 1 (ROCK1) and
Ca2+/calmodulin-dependent protein kinase δ CaMKIIδ] and
two anti-apoptotic proteins [fibroblast growth factor receptor 2
(FGFR2) and leukemia inhibitory factor (LIF)] (39). Therefore, our results suggest that
troxerutin protects cells from UVB-induced DNA damage and apoptosis
by regulating miRNA expression.
Furthermore, we predicted target genes of
troxerutin-regulated miRNAs and analyzed the GO terms of potential
target genes using DAVID bioinformatics resources. DAVID functional
annotation suggested that the target genes of troxerutin-regulated
miRNAs have a regulatory role in transcription, intracellular
signaling cascades, phosphate metabolic processes, protein
localization, programmed cell death, cell proliferation, the cell
cycle and ion transport (Table
II). Indeed, we grouped the GO terms into four functional
groups induced by troxerutin in UVB-damaged cells: cell death and
apoptosis, cell proliferation and the cell cycle, cell migration
and DNA repair (Table III).
Overall, the current study provides evidence of the
protective effects of troxerutin against UVB-induced damage in
HaCaT cells. The present study demonstrates a significant
correlation between the four cell function groups with alterations
in miRNA expression, troxerutin-regulated miRNA function, and GO
analysis of the miRNA target genes.
Acknowledgements
We would like to thank all other members of Coreana
Cosmetics Co., Ltd. for their support. This study was supported by
the KU Research Professor Program of Konkuk University and a grant
from the Ministry of Science, ICT and Future Planning (no.
20110028646) of the Republic of Korea.
References
|
1
|
Oresajo C, Pillai S, Manco M, Yatskayer M
and McDaniel D: Antioxidants and the skin: understanding
formulation and efficacy. Dermatol Ther. 25:252–259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campbell C, Quinn AG, Angus B, Farr PM and
Rees JL: Wavelength specific patterns of p53 induction in human
skin following exposure to UV radiation. Cancer Res. 53:2697–2699.
1993.PubMed/NCBI
|
|
3
|
de Gruijl FR and Van der Leun JC: Estimate
of the wavelength dependency of ultraviolet carcinogenesis in
humans and its relevance to the risk assessment of a stratospheric
ozone depletion. Health Phys. 67:319–325. 1994.PubMed/NCBI
|
|
4
|
Chainiaux F, Magalhaes JP, Eliaers F,
Remacle J and Toussaint O: UVB-induced premature senescence of
human diploid skin fibroblasts. Int J Biochem Cell Biol.
34:1331–1339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumura Y and Ananthaswamy HN: Toxic
effects of ultraviolet radiation on the skin. Toxicol Appl
Pharmacol. 195:298–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka M, Koyama Y and Nomura Y: Effects
of collagen peptide ingestion on UV-B-induced skin damage. Biosci
Biotechnol Biochem. 73:930–932. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Everett MA, Yeargers E, Sayre RM and Olson
RL: Penetration of epidermis by ultraviolet rays. Photochem
Photobiol. 5:533–542. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suter MM, Schulze K, Bergman W, Welle M,
Roosje P and Müller EJ: The keratinocyte in epidermal renewal and
defense. Vet Dermatol. 20:515–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kulms D, Zeise E, Pöppelmann B and Schwarz
T: DNA damage, death receptor activation and reactive oxygen
species contribute to ultraviolet radiation-induced apoptosis in an
essential and independent way. Oncogene. 21:5844–5851. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bode AM and Dong Z: Mitogen-activated
protein kinase activation in UV-induced signal transduction. Sci
STKE. 2003.RE22003.PubMed/NCBI
|
|
11
|
Assefa Z, Van Laethem A, Garmyn M and
Agostinis P: Ultraviolet radiation-induced apoptosis in
keratinocytes: on the role of cytosolic factors. Biochim Biophys
Acta. 1755:90–106. 2005.PubMed/NCBI
|
|
12
|
Rezvani HR, Mazurier F, Cario-André M, et
al: Protective effects of catalase overexpression on UVB-induced
apoptosis in normal human keratinocytes. J Biol Chem.
281:17999–18007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henseleit U, Zhang J, Wanner R, Haase I,
Kolde G and Rosenbach T: Role of p53 in UVB-induced apoptosis in
human HaCaT keratinocytes. J Invest Dermatol. 109:722–727. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Enk CD, Jacob-Hirsch J, Gal H, et al: The
UVB-induced gene expression profile of human epidermis in vivo is
different from that of cultured keratinocytes. Oncogene.
25:2601–2614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou BR, Xu Y, Permatasari F, et al:
Characterization of the miRNA profile in UVB-irradiated normal
human keratinocytes. Exp Dermatol. 21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou BR, Xu Y and Luo D: Effect of UVB
irradiation on microRNA expression in mouse epidermis. Oncol Lett.
3:560–564. 2012.PubMed/NCBI
|
|
17
|
Guo Z, Zhou B, Liu W, et al: MiR-23a
regulates DNA damage repair and apoptosis in UVB-irradiated HaCaT
cells. J Dermatol Sci. 69:68–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Di W, Hua L, Zhou B, Guo Z and Luo
D: UVB suppresses PTEN expression by upregulating miR-141 in HaCaT
cells. J Biomed Res. 25:135–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pothof J, Verkaik NS, van IJcken W, et al:
MicroRNA-mediated gene silencing modulates the UV-induced
DNA-damage response. EMBO J. 28:2090–2099. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan SH, Zhang ZF, Zheng YL, et al:
Troxerutin protects the mouse kidney from D-galactose-caused injury
through anti-inflammation and anti-oxidation. Int Immunopharmacol.
9:91–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang ZF, Fan SH, Zheng YL, et al:
Troxerutin protects the mouse liver against oxidative
stress-mediated injury induced by D-galactose. J Agric Food Chem.
57:7731–7736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Wu DM, Hu B, et al: Chronic
administration of troxerutin protects mouse brain against
D-galactose-induced impairment of cholinergic system. Neurobiol
Learn Mem. 93:157–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF and
Wang YJ: NGF-dependent activation of TrkA pathway: a mechanism for
the neuroprotective effect of troxerutin in D-galactose-treated
mice. Brain Pathol. 20:952–965. 2010.PubMed/NCBI
|
|
24
|
Lu J, Wu DM and Zhang ZF, Zheng YL, Hu B
and Zhang ZF: troxerutin protects against high cholesterol-induced
cognitive deficits in mice. Brain. 134:783–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budzianowski J, Korzeniowska K, Chmara E
and Mrozikiewicz A: Microvascular protective activity of flavonoid
glucuronides fraction from Tulipa gesneriana. Phytother Res.
13:166–168. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boisseau MR, Taccoen A, Garreau C, Vergnes
C, Roudaut MF and Garreau-Gomez B: Fibrinolysis and hemorheology in
chronic venous insufficiency: A double blind study of troxerutin
efficiency. J Cardiovasc Surg (Torino). 36:369–374. 1995.PubMed/NCBI
|
|
27
|
Ping X, Junqing J, Junfeng J and Enjin J:
Radioprotective effects of troxerutin against gamma irradiation in
V79 cells and mice. Asian Pac J Cancer Prev. 12:2593–2596.
2011.PubMed/NCBI
|
|
28
|
Maurya DK, Balakrishnan S, Salvi VP and
Nair CK: Protection of cellular DNA from gamma-radiation-induced
damages and enhancement in DNA repair by troxerutin. Mol Cell
Biochem. 280:57–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambros V: microRNAs: tiny regulators with
great potential. Cell. 107:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raj D, Brash DE and Grossman D:
Keratinocyte apoptosis in epidermal development and disease. J
Invest Dermatol. 126:243–257. 2006. View Article : Google Scholar
|
|
31
|
Maurya DK, Salvi VP and Krishnan Nair CK:
Radioprotection of normal tissues in tumor-bearing mice by
troxerutin. J Radiat Res. 45:221–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013.
|
|
33
|
Li S, Niu X, Cui A, He Y and Wu W: The
effects of miR-181a on proliferation, migration and invasion
abilities of esophageal carcinoma cell line TE11. Tumor.
31:613–618. 2011.
|
|
34
|
Shin S, Moon KC, Park KU and Ha E:
MicroRNA-513a-5p mediates TNF-α and LPS induced apoptosis via
downregulation of X-linked inhibitor of apoptotic protein in
endothelial cells. Biochimie. 94:1431–1436. 2012.PubMed/NCBI
|
|
35
|
Nohata N, Hanazawa T, Kinoshita T, et al:
Tumour-suppressive microRNA-874 contributes to cell proliferation
through targeting of histone deacetylase 1 in head and neck
squamous cell carcinoma. Br J Cancer. 108:1648–1658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang B, Li Z, Zhang W, et al: miR-874
Inhibits cell proliferation, migration and invasion through
targeting aquaporin-3 in gastric cancer. J Gastroenterol. June
26–2013.(Epub ahead of print).
|
|
37
|
Zhang Y, Liao JM, Zeng SX and Lu H: p53
downregulates Down syndrome-associated DYRK1A through miR-1246.
EMBO Rep. 12:811–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Romano G, Acunzo M, Garofalo M, et al:
MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced
apoptosis in non-small-cell lung cancer through BIM
down-regulation. Proc Natl Acad Sci USA. 109:16570–16575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Zhang X, Ren XP, et al:
MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins
protects against ischemia/reperfusion-induced cardiac injury.
Circulation. 122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|