Introduction
Human myopia primarily results from the abnormal
elongation of the vitreous chamber of the eye (1). A similar eye elongation associated
with myopia can readily be induced in eyes of monkeys (2), tree shrews (3), marmosets (4) and guinea pigs (5), by depriving them of form vision or
wearing negative lenses. A great deal of research has focused on
the starting point of the development of myopia, the retina, and
the end point, the sclera (6–9).
There is little research on the retinal pigment epithelium (RPE)
that may be the connection between the retina and the sclera. The
RPE separates the outer layer of the neural retina from the
capillaries of the choroid to form the outer blood-retinal barrier.
It is the first cell type to differentiate in the retina, but as
the neural retina and choroid develop around it, 40% of the RPE
transcriptome will change its expression (10). The RPE is also crucial for
maintaining the microenvironment of the sensory retina and the
choriocapillaris (11).
Retinoic acid (RA) is a biologically active
regulator that has a broad range of functions, including cell
differentiation, proliferation and apoptosis in various cell types
(12,13). It has been suggested to be a
chemical signal involved in the regulation of ocular growth
(14–18). Studies in various species have
reported that both the concentration of RA and the expression of RA
receptor-β (RAR-β) exhibit bidirectional and reversible changes in
the retina and choroid during the development of myopia.
Zonula occludens-1 (ZO-1) is a 210–225 kDa protein
found at the submembranous domain of tight junctions (TJs) in the
epithelium and endothelium. At the TJ, ZO-1 is associated with the
carboxyl terminal end of claudins through its first PDZ domain
(19), and through its second and
third PDZ domain to JAM (20),
and by its GK module to occludin (21). Numerous studies employing
cytokines, hormones and growth factors have found the abundance of
ZO-1 to be associated with the degree of tightness of the junction.
Occludin, another TJ-associated protein, was originally discovered
in avian tissues by Furuse et al (22), using anti-chick occludin antisera
prepared in rats. It was found to be localized to epithelial and
endothelial TJs, and was subsequently confirmed as the first
integral membrane TJ protein to be identified.
Recent studies have shown that RA may promote the
function of the epithelial barrier, and its bioavailability
regulates the epithelial barrier, which is accompanied by altering
the expression of TJ-associated proteins (23). The aim of this study was to
investigate the changes in the expression of TJ-associated proteins
in the RPE-choroid complex in the eyes of guinea pigs with
lens-induced myopia (LIM), and to investigate the effect of RA on
the TJs of the RPE-choroid complex of guinea pigs in
vivo.
Materials and methods
Establishment of animal model of myopia
and animal housing
Sixty clean pigmented guinea pigs 3 weeks of age
were obtained from the Animal Department of the Central South
University, Changsha, China. A concave optical resin lens of −6.00
D was provided by Hong Kong Optical Lens Co., Ltd., Hong Kong,
China. The lens diameter was 12 mm and the inner arc curvature was
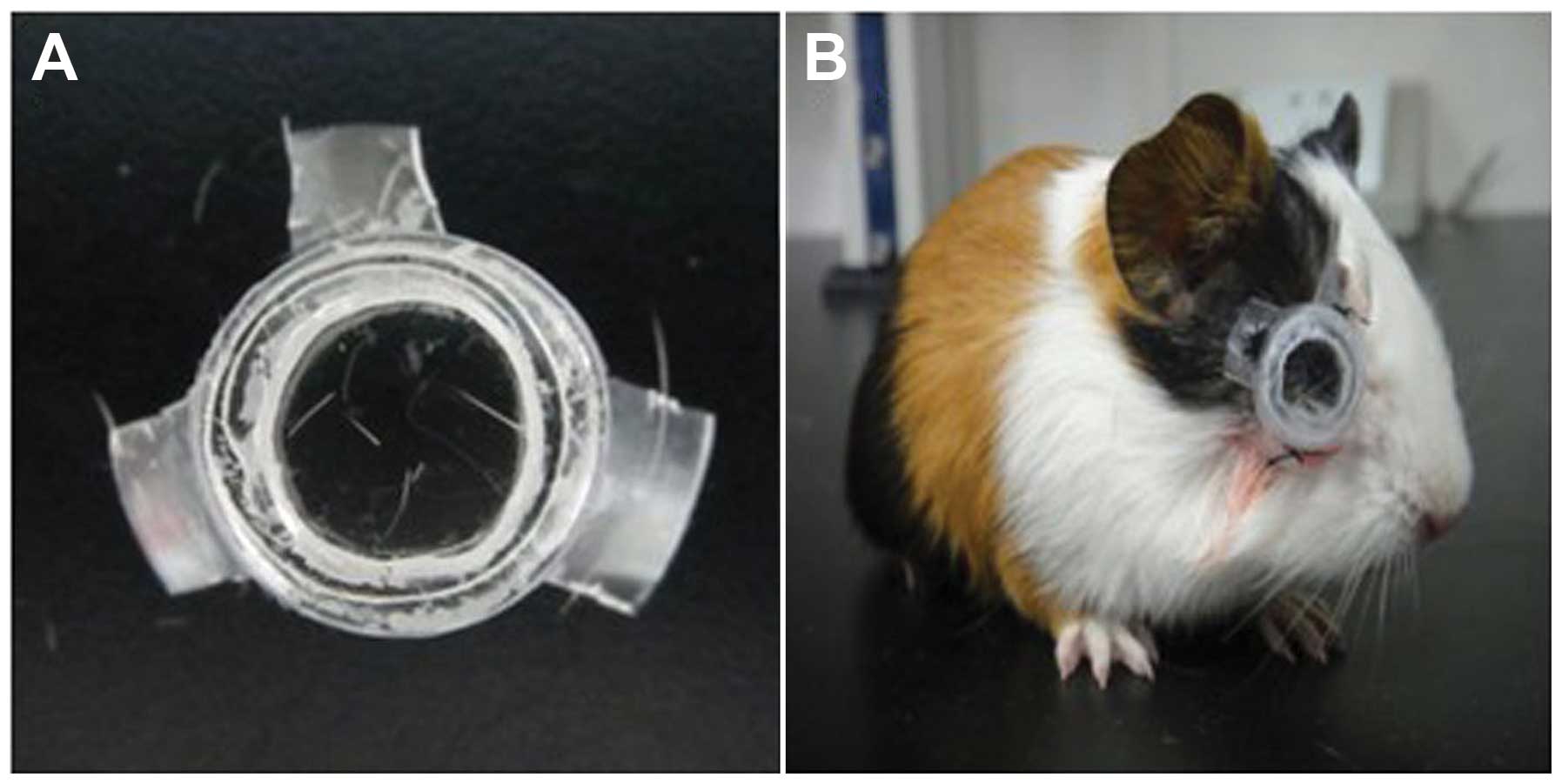
9.61 mm. A single use Murphy dropper was trimmed to make a frame
for the lens (Fig. 1A). Two small
apertures were left at the bottom of the frame for cleaning and air
circulation. Pentobarbital sodium (0.3%) at a dosage of 30 mg/kg
was injected into the abdominal cavity of the guinea pigs for
anesthesia, and the hairs around the fissura orbitalis were
sheared. The self-made frame was sutured and fixed to the soft
tissue around the fissura orbitalis of the right eye (Fig. 1B). Guinea pigs were randomly
divided into 4 groups: group A, normal control group (n=15, no lens
on each eye); group B, LIM group (n=15, −6.00 D optical lens on the
right eye); group C, LIM plus phosphate-buffered saline
(PBS)-injected group (n=15, PBS was injected into the vitreous
chamber of the eye with LIM); and group D, LIM plus LE540-injected
group (n=15, LE540 was injected into the vitreous chamber of the
eye with LIM). The animals were housed in plastic boxes (60×40×20
cm) with wire mesh lids. The boxes contained a small hiding shelf
at one end (32×16×14 cm) and were lined with wood shavings. Water
(supplemented with vitamin C) and food (guinea pig pellets, hay and
occasionally, fresh vegetables) were freely available. Lighting was
provided by ceiling fluorescent tubes (36 W) on a 12-h light/dark
cycle (lights on at 10 am). The room temperature was kept at 22°C.
The treatment and care of the animals were conducted according to
the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research, and were approved by the Committee for Animal Welfare of
the Xiangya Hospital of Central South University.
Biometric measurements
Tropicamide (0.25%) was administered 3 times at
5-min intervals to dilate the pupil. Refractive error (RE)
measurements were obtained using streak retinoscopy 45 min after
the first mydriasis. Axial length was measured with a CineScan A/B
ultrasonographic machine (Optikon 2000 S.p.A., Rome, Italy;
sensitivity, 0.01 mm). A topical anesthesia (0.5% proparacaine
hydrochloride; Alcon, Puurs, Belgium) was administered prior to the
ultrasound measurement. The ultrasound probe was placed in direct
contact with the cornea during the axial measurement. RE were
presented as the mean spherical equivalent refractive error (MSE).
Due to the powerful accommodation ability of guinea pig eyes, a
retinoscopy was conducted in the central zone shortly after the
ciliary muscle was completely paralyzed. The measurements above
were performed on days 0, 3, 7 and 14 after lense placement. All
refraction data were presented as the means derived from 5 repeated
measurements.
Collection of RPE-choroid complex
samples
The guinea pigs were sacrificed on days 0, 3, 7 and
14 when necessary with an overdose of pentobarbital sodium. The
eyes were removed and dissected along the ora serrata, and were
subsequently washed immediately in Hank’s balanced salt solution
(HBSS; Gibco, Grand Island, NY, USA) with penicillin and
streptomycin (200 μg/ml penicillin/streptomycin), and gentamicin
sulfate (400 μg/ml) (all from Invitrogen, Carlsbad, CA, USA). After
dissection of the anterior part of the eye, and the vitreous and
neural retina, the RPE-choroid complex was carefully removed from
the sclera. The procedures were performed on ice and under subdued
lighting.
Endogenous levels of RA after visual
manipulations
The level of endogenous RA in the RPE-choroid
complex was determined using high-performance liquid chromatography
(HPLC). The RPE-choroid complex from 4 eyes was pooled and
homogenized with a Polytron homogenizer (Brinkmann, Westbury, NY,
USA) in 2 ml of water. The all-trans RA (atRA) standard was
purchased from the Sigma-Aldrich
(C20H28O2; molecular mass,
300.44). A Waters uBondapak C18 reversion phase chromatography
column (150×3.9 mm) was used and the chromatographic conditions
were as follows: mobile phase, V (acetonitrile):V (0.1% glacial
acetic acid solution), 86:14; flow rate, 1.0 ml/min; detected wave
length, 350 nm; column temperature, 25°C; sample size, 20 μl. The
RA contents (ng) in the RPE-choroid complex/100 mg were
calculated.
Western blot analysis
Western blot analysis was perforemd to detect the
protein expression of ZO-1 and occludin in the RPE-choroid complex.
The RPE-choroid complex of the guinea pig eyes was collected and
lysed in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 M
MEDTA, 1% NP-40) containing protease inhibitors (Boehringer,
Mannheim, Germany). Total protein was resolved by SDS
polyacrylamide gel electrophoresis, and then was transferred onto a
nitrocellulose membrane. The membrane was incubated at 4°C
overnight with rabbit anti-ZO-1 polyclonal antibody (1:250
dilution; no. 61–7300; Invitrogen) and mouse anti-occludin
monoclonal antibody (1:500 dilution; no. 33–1500; Invitrogen).
Peroxidase-conjugated secondary antibodies were used as secondary
detection reagents with an enhanced chemiluminescence kit (GE
Healthcare, New York, NY, USA). Chemiluminescent signals were
visualized by exposure to X-ray film. Band intensities were
quantified with BandScan software (version 5.0). Levels of GAPDH
were used for standardization. The relative expression of the
target protein was calculated. Independent experiments were
performed, and repeated 3 times.
Indirect immunofluorescence
Immunofluorescence was carried out to detect the
distribution of ZO-1 and occludin proteins in the RPE-choroid
complex in all groups. Briefly, fixed tissues were washed 3 times
with PBS, covered with 10% normal donkey serum diluted in PBS, and
incubated for 20 min at 37°C. Rabbit anti-ZO-1 polyclonal antibody
was used at a 1:100 dilution (no. 40–2200; Invitrogen) and mouse
anti-occludin monoclonal antibody at a 1:250 dilution (no. 33–1500;
Invitrogen). PBS was used as a control for the primary antibody.
Following overnight incubation with the primary antibody at 4°C
temperature, the slides were rinsed 3 times with PBS and AlexaFluor
488 was added at a dilution of 1:500 for 1 h at 37°C.
Statistical analysis
All data are presented as the means ± SEM.
Statistical analyses used repeated measures (RM) or one-way ANOVA
(SPSS 11.0) as specified. Post hoc analyses used Tukey’s least
significant difference (LSD) test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Effects of negative lens on the ocular
refractive state
RE and axial lengths of all left eyes in each group
developed at a normal rate. The right eyes developed differently in
each group. In group A, refraction developed with respect to the
direction of emmetropization. The REs and axial lengths of the
right eyes did not differ significantly as compared to the left
eyes at each time point (P>0.05). In group B and C, the diopter
developed in the direction of myopia as time progressed in the eyes
of guinea pigs with LIM. The most relative myopia was approximately
4.7 D on the 14th day, and differed significantly compared to the
opposite eyes and normal control eyes (P<0.05) (Table I). Similarly, the axial length of
the eyes of guinea pigs with LIM extended more rapidly than that in
the contralateral eyes. However, the development of myopia and the
speed of axial length growth in the eyes of guinea pigs with LIM
were inhibited following the injection of LE540 into the vitreous,
and there was statistically significant difference as compared with
group B and C on the 7th and 14th day (P<0.05) (Table I).
 | Table IEffect of negative lens on the ocular
refractive state (n=15). |
Table I
Effect of negative lens on the ocular
refractive state (n=15).
| Groups | Time point (day) | Refraction (D) | Axial length
(mm) |
|---|
|
|
|---|
| R | L | R | L |
|---|
| A: Normal control
group (n=15) | 0 | 3.14±0.71 | 3.21±0.55 | 7.58±0.07 | 7.56±0.06 |
| 3 | 2.60±0.80 | 2.68±0.49 | 7.62±0.10 | 7.60±0.08 |
| 7 | 2.01±0.65 | 2.07±0.43 | 7.65±0.04 | 7.64±0.05 |
| 14 | 1.39±0.74 | 1.42±0.61 | 7.68±0.10 | 7.69±0.09 |
| B: LIM group
(n=15) | 0 | 3.15±0.82 | 3.18±0.49 | 7.59±0.05 | 7.56±0.04 |
| 3 | 1.93±0.80 | 2.68±0.49 | 7.64±0.11 | 7.61±0.04 |
| 7 | −0.21±0.65a,b | 2.07±0.43 | 7.73±0.03a,b | 7.64±0.07 |
| 14 | −3.29±0.74 | 1.42±0.61 | 7.88±0.09a,b | 7.68±0.06 |
| C: LIM plus
PBS-injected group (n=15) | 0 | 3.13±0.67 | 3.15±0.43 | 7.60±0.05 | 7.58±0.08 |
| 3 | 1.90±0.80 | 2.65±0.39 | 7.64±0.05 | 7.62±0.06 |
| 7 | −0.23±0.56a,b | 2.05±0.53 | 7.74±0.04a,b | 7.64±0.05 |
| 14 | −3.27±0.44a,b | 1.44±0.71 | 7.89±0.10a,b | 7.67±0.06 |
| D: LIM plus
LE540-injected group (n=15) | 0 | 3.18±0.45 | 3.21±0.57 | 7.58±0.11 | 7.57±0.05 |
| 3 | 2.54±0.39 | 2.64±0.40 | 7.63±0.08 | 7.61±0.08 |
| 7 | 1.82±0.37a,c | 2.07±0.35 | 7.66±0.06c | 7.63±0.07 |
| 14 | 0.85±0.56a,c | 1.46±0.49 | 7.71±0.08c | 7.68±0.09 |
Effect of vision manipulation on the
level of RA in the RPE-choroid complex
The level of RA exhibited no significant change in
group A, and there was no difference observed between the right and
left eyes (P>0.05). The level of RA increased with time in group
B and C. In group B and C, the RA content in the RPE-choroid
complex of the eyes of guinea pigs with LIM was 12.40±0.31 ng/100
mg and 12.33±0.23 ng/100 mg, respectively before LIM, and
significantly increased to 137.85±1.02 ng/100 mg and 132.09±0.44
ng/100 mg on the 14th day. Moreover, the difference in the RA level
was also significant between the right and left eyes on days 3, 7
and 14 (all P<0.05) (Table
II). In group D, the RA level in the eyes of guinea pigs with
LIM decreased from 12.18±01.23 ng/100 mg to 2.55±0.18 ng/100 mg on
the 14th day after the injection of LE540, which was significantly
lower than the level observed in the normal control eyes or
contralateral eyes at the same time point. There was a
statistically significant difference as compared to group B and C
on days 3, 7 and 14 (all P<0.05) (Table II).
 | Table IILevels of retinoic acid (RA) in the
retinal pigment epithelium (RPE)-choroid complex after different
vision manipulations (n=4). |
Table II
Levels of retinoic acid (RA) in the
retinal pigment epithelium (RPE)-choroid complex after different
vision manipulations (n=4).
| | RA content (ng/100
mg) |
|---|
| |
|
|---|
| Groups | Time point
(day) | R | L |
|---|
| A: Normal control
group (n=15) | 0 | 12.17±0.22 | 12.17±0.24 |
| 3 | 12.20±0.20 | 12.19±0.19 |
| 7 | 12.18±0.28 | 12.16±0.16 |
| 14 | 12.21±0.18 | 12.20±0.27 |
| B: LIM group
(n=15) | 0 | 12.40±0.31 | 12.37±0.29 |
| 3 | 18.15±0.34a | 12.34±0.35 |
| 7 | 46.71±0.40a | 13.01±0.35 |
| 14 | 137.85±1.02a | 12.67±0.40 |
| C: LIM plus
PBS-injected group (n=15) | 0 | 12.33±0.23 | 12.39±0.30 |
| 3 | 19.06±0.36a | 12.41±0.39 |
| 7 | 48.68±0.56a | 12.47±0.34 |
| 14 | 132.09±0.44a | 12.50±0.29 |
| D: LIM plus
LE540-injected group (n=15) | 0 | 12.18±0.23 | 12.21±0.27 |
| 3 | 5.24±0.24a,b | 12.19±0.30 |
| 7 | 4.05±0.27a,b | 12.17±0.25 |
| 14 | 2.55±0.18a,b | 12.23±0.29 |
Effect of vision manipulation on
TJ-associated proteins in the RPE-choroid complex as determined by
western blot analysis
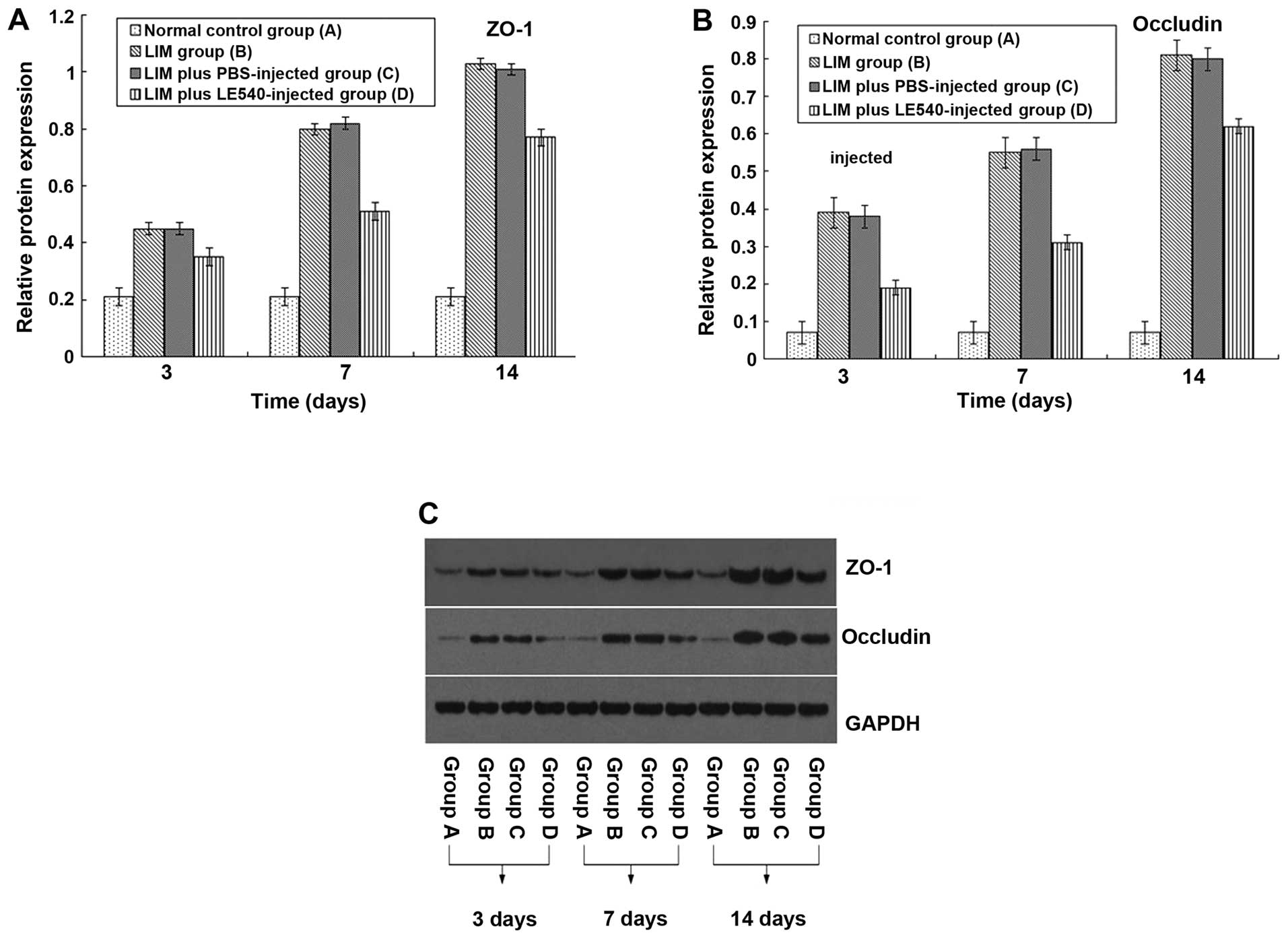
The expression of ZO-1 and occludin in the normal
control group showed no obvious change (Fig. 2). After LIM, ZO-1 and occludin
protein expression was upregulated in the RPE-choroid complex
within 14 days and the differences on days 3, 7 and 14 were
statistically significant between groups B and C and group A
(P<0.05). By contrast, the increase in ZO-1 and occludin
expression was partly inhibited by the injection of LE540, and
there was a statistically significant difference when compared with
the eyes of guinea pigs with LIM with or without the PBS injection
(P<0.05).
Effect of vision manipulation on
TJ-associated proteins in the RPE-choroid complex as determined by
immunofluorescence
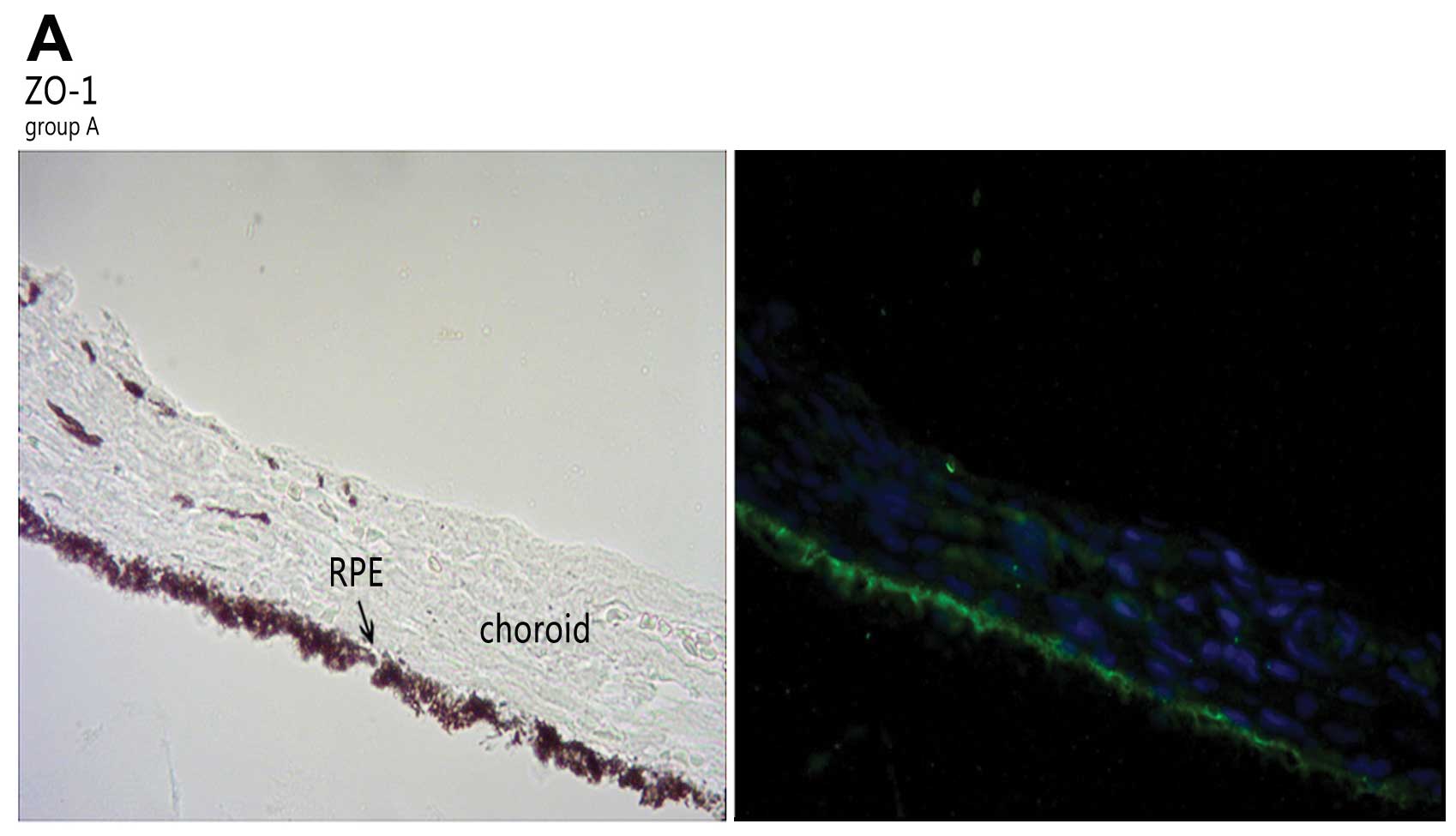
In accordance with the results from western blot
analysis, ZO-1 and occludin were expressed in the RPE (Fig. 3). The 2 proteins were upregulated
in group B and C. The expression levels of TJ-associated proteins
varied slightly at the different time points in group A. In the
eyes of the guinea pigs with LIM, the immunostaining of ZO-1 and
occludin was most intense in the RPE and choroid; this was not
observed in the normal control group. After the injection of LE540,
the expression of ZO-1 and occludin decreased.
Discussion
A large amount of research has focused on the
pathogenesis of myopia and RA has been determined to be a factor in
the development of myopia. Seko et al (24) reported that RA levels were
increased in the retina of chicks with form-deprived myopia. Merts
and Wallman (16) reported that
the synthesis of choroidal RA is modulated by those visual
manipulations that influence ocular elongation and that this RA may
reach the sclera in concentrations adequate to modulate scleral
proteoglycan formation. However, the results of the association
between RA and myopia have varied according to the species
examined. Previously, McFadden et al (25) found that feeding RA to chickens
can accelerate the speed of eye elongation and they concluded that
RA may act at the level of a non-visual mechanism which regulates
ocular growth. In this study, the level of RA in the RPE-choroid
complex of the eyes of guinea pig was upregulated by wearing a
negative lens. These results were consistent with those from the
study by McFadden et al (25), namely that the level of RA was
upregulated in the choroid during the development of myopia. On the
contrary, the increase in the RA level was partly inhibited and the
development of myopia was much slower when LE540, an antagonist of
RARs (26), was injected into the
vitreous chamber of the eyes of guinea pigs with LIM.
TJs that are synthesized and assembled during
epithelial differentiation are the most apical structures of the
junctional complex. They serve as a barrier to regulate the flow of
solutes and fluid from the choroidal vasculature into the outer
retina, and to control the pathway of ions and small molecules
through paracellular channels. Occludin and claudins are linked to
the cytoskeleton by the intracellular membrane-associated guanylate
kinase homologs, ZO-1, ZO-2, ZO-3 and claudin-1 (27). The combination of claudin-1 and
occludin is required for the establishment of an effective
paracellular barrier (28).
Numerous studies that have employed cytokines, hormones and growth
factors have shown that the ZO-1 level is associated with the
degree of tightness of the junction. The results from this study
demonstrated that ZO-1 and occludin were upregulated in the
RPE-choroid complex in the eyes of guinea pigs with LIM. Thus, we
hypothesized that the TJs were reinforced by the 14th day in the
eyes of guinea pigs with LIM. The reason for this finding is
uncertain, but RA may be a regulator of TJ-associated proteins.
Based on detection in F9 cells, in a colitis model, and in some
cancer tissues (29–31), RA is believed to be an obligatory
component in the differentiation of epithelial cells that leads to
the establishment of epithelial integrity. In their study, Rong and
Liu (23) observed that the
expression of ZO-1 and occludin increased in ARPE-19 cultures
treated with atRA, suggesting that atRA has a barrier function in a
process involving a specific increase in these TJ-associated
proteins. Of note, in this study, the increase in the expression of
ZO-1 and occludin in the eyes of guinea pigs with LIM was partly
inhibited following the injection of LE540 into the vitreous
chamber. These results led us to hypothesize that although RA may
play an important role in forming functional TJs, many other
factors also regulate the expression of TJ-associated proteins
during the development of myopia.
Myopia induced by negative lenses may be related to
the myopia clinically observed in young humans who spend many hours
reading, suggesting that insufficient accommodation (the ‘lag of
accommodation’) also imposes hyperopic defocus. The majority of
researchers have concluded that local modulation is the key factor
in the development of myopia. This suggests that the neural retina
itself has to be the source of growth-regulating signals, and that
the sclera is the target of these signals. Thus, the RPE-choroid
complex may play a critical role in signal transduction as a whole
system. In this study, we found that both RA and TJ-associated
proteins in the RPE-choroid complex were affected by optical
manipulation in guinea pigs. However, it is not clear as to why the
TJs were upregulated in the eyes of the guinea pigs with LIM and
whether there is an association between RA and TJ-associated
proteins. RA had been reported as a possible mediator of the
changes in eye growth (15). It
has been reported that retinal atRA levels are increased in myopic
eyes with accelerated elongation, and decreased in eyes with
inhibited elongation (32). As
previously demonstrated, functional atRA can regulate the
permeability of various cell types in vitro, and impaired
atRA signaling leads to the disruption of functional TJs (29–31). It has been reported that changes
in the blood-retinal barrier (BRB) are observed at 45 days after
form deprivation, which suggests that abnormal BRB function is
secondary to the development of myopia rather than the cause of
myopia. The results of this study suggest that RA may play a
protective role in the integrity of the TJs in the RPE-choroid
complex, but whether its association with this complex is direct or
indirect remains to be elucidated.
In the present study, when myopia developed, the
vitreous chamber of the eyes of the guinea pigs became longer.
Generally speaking, the barrier of the TJs may be disrupted due to
the elongated axial length. However, we found that the TJs were
enhanced rather than disrupted in this study, and the TJ-associated
proteins in the RPE-choroid complex were upregulated. We speculate
there are two possible reasons for this finding: i) TJs may have a
compensatory mechanism within 14 days of the induction of myopia.
The RPE cell number would not change, but the expansion of
individual RPE cells and the increment of TJs between epithelial
cells were most likely the responses to the ‘pulling force’ applied
to RPE cells. ii) RA may play a protective role with respect to the
TJs in the RPE-choroid complex. The upregulation of RA may be a
negative feedback mechanism for the regulation of ocular growth,
which led to the enhancement of TJs. However, if the vitreous
chamber was excessively elongated, the TJs cannot compensate and RA
cannot protect the integrity of the TJs.
The results of the present study indicate that RA is
beneficial to TJs in the RPE-choroid complex. We speculate that the
disruption of TJ function during the development of myopia is
likely not the cause of myopia, but only the pathological phenomena
of high myopia. These findings may be helpful for further research
regarding the pathogenesis of myopia.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81070752) and the National
Natural Science Youth Foundation of China (grant no. 81100691).
References
|
1
|
Curtin BJ: The Myopias: Basic Science and
Clinical Management. Philadelphia: Harper and Row; pp. 4951985
|
|
2
|
Wiesel TN and Raviola E: Myopia and eye
enlargement after neonatal lid fusion in monkeys. Nature.
266:66–68. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherman SM, Norton TT and Casagrande VA:
Myopia in the lid-sutured tree shrew (Tupaia glis). Brain
Res. 124:154–157. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Troilo D and Judge SJ: Ocular development
and visual deprivation myopia in the common marmoset (Callithrix
jacchus). Vision Res. 33:1311–1324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howlett MH and McFadden SA:
Form-deprivation myopia in the guinea pig (Cavia porcellus).
Vision Res. 46:267–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McBrien NA and Gentle A: Role of the
sclera in the development and pathological complications of myopia.
Prog Retin Eye Res. 22:307–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang F, Pan M, Yan T, Tao Y, Wu H, Liu X,
Qu J and Zhou X: The role of cGMP in ocular growth and the
development of form-deprivation myopia in guinea pigs. Invest
Ophthalmol Vis Sci. 54:7887–7902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujikado T, Kawasaki Y, Suzuki A, Ohmi G
and Tano Y: Retinal function with lens-induced myopia compared with
form-deprivation myopia in chicks. Graefes Arch Clin Exp
Ophthalmol. 235:320–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xi X, Chu R, Zhou X, Lu Y and Liu X:
Retinal dopamine transporter in experimental myopia. Chin Med J
(Engl). 115:1027–1030. 2002.PubMed/NCBI
|
|
10
|
Rizzolo LJ, Chen X, Weitzman M, Sun R and
Zhang H: Analysis of the RPE transcriptome reveals dynamic changes
during the development of the outer blood-retinal barrier. Mol Vis.
13:1259–1273. 2007.PubMed/NCBI
|
|
11
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Durston AJ, Timmermans JP, Hage WJ,
Hendriks HF, de Vries NJ, Heideveld M and Nieuwkoop PD: Retinoic
acid causes an anteroposterior transformation in the developing
central nervous system. Nature. 340:140–144. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osanai M and Petkovich M: Expression of
the retinoic acid-metabolizing enzyme CYP26A1 limits programmed
cell death. Mol Pharmacol. 67:1808–1817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bitzer M, Feldkatmper M and Schaeffel F:
Visually induced changes in components of the retinoic acid system
in fundal layers of the chick. Exp Eye Res. 70:97–106. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McFadden SA, Howlett MH and Mertz JR:
Retinoic acid signals the direction of ocular elongation in the
guinea pig eye. Vision Res. 44:643–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mertz JR and Wallman J: Choroidal retinoic
acid synthesis: a possible mediator between refractive error and
compensatory eye growth. Exp Eye Res. 70:519–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Troilo D, Nickla DL, Mertz JR and Summers
Rada JA: Change in the synthesis rates of ocular retinoic acid and
scleral glycosaminoglycan during experimentally altered eye growth
in marmosets. Invest Ophthalmol Vis Sci. 47:1768–1777. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan DS, Zhou XT, Chen XY, Lü F, Wang J, Hu
DN and Qu J: Expression of retinoid acid receptors in human scleral
fibroblasts, regulation of growth of fibroblasts by retinoic acid.
Zhonghua Yan Ke Za Zhi. 43:750–753. 2007.(In Chinese).
|
|
19
|
Itoh M, Furuse M, Morita K, Kubota K,
Saitou M and Tsukita S: Direct binding of three tight
junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH
termini of claudins. J Cell Biol. 147:1351–1363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebnet K, Schulz CU, Meyer Zu, Brickwedde
MK, Pendl GG and Vestweber D: Junctional adhesion molecule
interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J
Biol Chem. 275:27979–27988. 2000.PubMed/NCBI
|
|
21
|
Schmidt A, Utepbergenov DI, Krause G and
Blasig IE: Use of surface plasmon resonance for real-time analysis
of the interaction of ZO-1 and occludin. Biochem Biophys Res
Commun. 288:1194–1199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: a novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar
|
|
23
|
Rong J and Liu S: Effect of all-trans
retinoic acid on the barrier function in human retinal pigment
epithelial cells. Biochem Biophys Res Commun. 407:605–609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seko Y, Shimizu M and Tokoro T: Retinoic
acid increases in the retina of the chick with form deprivation
myopia. Opthalmic Res. 306:361–367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McFadden SA, Howlett MH, Mertz JR and
Wallman J: Acute effects of dietary retinoic acid on ocular
components in the growing chick. Exp Eye Res. 83:949–961. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satoh T, Higuchi Y, Kawakami S, Hashida M,
Kagechika H, Shudo K and Yokoyama M: Encapsulation of the synthetic
retinoids Am80 and LE540 into polymeric micelles and the retinoids’
release control. J Control Release. 136:187–195. 2009.PubMed/NCBI
|
|
27
|
Miyoshi J and Takai Y: Molecular
perspective on tight-junction assembly and epithelial polarity. Adv
Drug Deliv Rev. 57:815–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furuse M, Hata M, Furuse K, Yoshida Y,
Haratake A, Sugitani Y, Noda T, Kubo A and Tsukita S: Claudin-based
tight junctions are crucial for the mammalian epidermal barrier: a
lesson from claudin-1-deficient mice. J Cell Biol. 156:1099–1111.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osanai M, Nishikiori N, Murata M, Chiba H,
Kojima T and Sawada N: Cellular retinoic acid bioavailability
determines epithelial integrity: role of retinoic acid receptor
alpha agonists in colitis. Mol Pharmacol. 71:250–258. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubota H, Chiba H, Takakuwa Y, Osanai M,
Tobioka H, Kohama G, Mori M and Sawada N: Retinoid X receptor alpha
and retinoic acid receptor gamma mediate expression of genes
encoding tight-junction proteins and barrier function in F9 cells
during visceral endodermal differentiation. Exp Cell Res.
263:163–172. 2001. View Article : Google Scholar
|
|
31
|
de Thé H: Altered retinoic acid receptors.
FASEB J. 10:955–960. 1996.
|
|
32
|
Seko Y, Shimokawa H and Tokoro T: In vivo
and in vitro association of retinoic acid with form-deprivation
myopia in the chick. Exp Eye Res. 63:443–52. 1996. View Article : Google Scholar : PubMed/NCBI
|