Introduction
Parkinson’s disease (PD) is a progressive
neurodegenerative disorder characterized by the selective loss of
nigral dopaminergic neurons and a reduction in striatal
dopaminergic fibers, which result in tremors, rigidity,
bradykinesia and gait disturbance (1). In addition to motor dysfunction,
dementia is a widely recognized symptom of patients with PD.
Degeneration of the dopaminergic neurons during PD may affect the
hippocampal dentate gyrus (2).
Tyrosine hydroxylase (TH) is the rate-limiting
enzyme in the biosynthetic pathways of catecholamine-type
neurotransmitters, such as dopamine, epinephrine and
norepinephrine. More specifically, this enzyme converts L-tyrosine
into L-dihydroxyphenylalanine (L-DOPA), and this is the
rate-limiting step in the synthesis of dopamine (3). Since TH is an enzyme of the dopamine
biosynthetic pathway, TH activity progressively decreases following
the loss of dopaminergic neurons within the substantia nigra
(4,5).
Cell proliferation and/or neurogenesis have been
demonstrated to occur in the hippocampal dentate gyrus in the brain
of adult mammals, including humans (6,7).
The generation of new neurons in the hippocampus plays an important
role in hippocampal functions, such as learning and memory
(8,9). However, brain diseases, such as
cerebral ischemia, trauma and epileptic seizures, are accompanied
by increased neurogenesis (10–12). The induction of hippocampal
neurogenesis in pathological conditions is considered a
compensatory response to dopaminergic neuronal death (2).
Apoptosis plays a critical role in neuronal cell
death, occuring in a number of neurodegenerative diseases,
including PD, Alzheimer’s disease and epilepsy, as well as in
stroke (4,13,14). Two important groups of proteins
involved in apoptotic cell death are the members of the Bcl-2
family, Bax and Bcl-2, and a class of cysteine proteases known as
caspases (15,16). The Bcl-2 family comprises two
functionally distinct groups of proteins: the anti-apoptotic and
the pro-apoptotic proteins. Bcl-2, an anti-apoptotic protein,
protects against cell death, whereas Bax, a pro-apoptotic protein,
promotes cell death. Increasing the ratio of Bax to Bcl-2 has
commonly been used to determine the induction of apoptosis in a
number of tissues (16).
Caspase-3 is one of the key enzymes in cell apoptosis, since it can
cleave a number of proteins (15). Caspase-3 is the primary activator
of DNA fragmentation (17); the
latter is experimentally detected by the terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay.
Berberine, an isoquinoline alkaloid isolated from
Berberis vulgaris L., is known to exhibit anxiolytic,
analgesic, anti-inflammatory, antipsychotic, antidepressant and
anti-amnesic properties (18,19). Berberine has been shown to improve
spatial memory impairment in a rat model of Alzheimer’s disease and
memory impairment in a rat model of diabetes (20,21). The fact that berberine exerts
anti-inflammatory effects suggests that it may have an inhibitory
effect on the degeneration of cholinergic neurons (22).
The effects of berberine on a number of brain
diseases have been well documented (20,21,23); however, studies on the effects of
berberine on PD have reported controversial results (24,25). Bae et al (24) suggested that berberine may be
useful as a therapeutic agent in the treatment of neurological
disorders. However, Kwon et al (25) reported that treatment with
berberine reduced the number of TH-immunopositive cells in the
substantia nigra of rats and enhanced 6-hydroxydopamine
(6-OHDA)-induced cytotoxicity in PC12 cells. To our knowledge, to
date, the effects of beberine on memory function in relation to
neurogenesis and apoptosis have not been investigated in mice with
PD. Thus, in the present study, we investigated the effects of
berberine on short-term memory in relation to dopamine depletion
and hippocampal neurogenesis using a mouse model of PD, induced by
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTP/P)
treatment.
Materials and methods
Animals
Imprinting control region (ICR) male mice weighing
28±3 g (8 weeks old) were used in this study. They were kept in
controlled temperature (20±2°C) conditions under a 12-h light/12-h
dark cycle. The animals were allowed free access to food and water.
All experimental animal procedures conformed with the relevant
regulations of the National Institutes of Health (NIH) and the
guidelines of the Korean Academy of Medical Sciences. The animals
were randomly divided into 5 groups (n=10 in each group): the
control group (probenecid-injected), the MPTP/P-injected group, the
MPTP/P-injected and 20 mg/kg berberine-treated group, the
MPTP/P-injected and 50 mg/kg berberine-treated group, and the
MPTP/P-injected and 80 mg/kg berberine-treated group. All mice were
intraperitoneally administered 50 mg/kg 5-bromo-2′-deoxyuridine
(BrdU; Sigma Chemical Co., St. Louis, MO, USA) once a day 30 min
prior to berberine treatment, for 4 consecutive days. Mice in the
berberine-treated groups were orally administered berberine (Sigma
Chemical Co.) at the respective doses, once a day for 5 weeks. Mice
in the control group and the MPTP/P-injected group received the
same amount of distilled water.
Mouse model of MPTP-induced PD
MPTP is a neurotoxin that selectively damages
dopaminergic cells in the substantia nigra pars compacta (SNpc),
and is widely used to induce PD in rodents and primates (26,27). MPTP hydrochloride and probenecid
(P) were purchased from Sigma Chemical Co. In order to establish
the mouse model of MPTD-induced PD, the mice were injected with
MPTP hydrochloride [20 mg/kg in saline, subcutaneously (s.c.)] with
probenecid as an adjuvant [250 mg/kg in dimethylsulfoxide,
intraperitoneally (i.p.)] 10 times for 5 weeks at 3.5-day
intervals, as previously described (4,28).
The control mice were treated with the same volume of probenecid
following the same procedure. Probenecid is known to promote the
build-up of MPTP or its derivative, 1-methyl-4-phenylpyridinium
(MPP+) in the brain and to potentiate its neurotoxic
effects by impeding the renal excretion and neuronal clearance of
MPTP and its toxic metabolites (28). Importantly, probenecid does not
affect the degeneration of dopaminergic neurons (29).
Step-down avoidance task
The latency time in the step-down avoidance task was
measured in order to evaluate short-term memory, as previously
described (9). The mice were
trained in a step-down avoidance task on the 34 days following the
start of the experiment. Two hours after training, the latency time
(sec) in each group was measured. The mice were placed on a 5×25 cm
platform of 1 cm in height facing a 42×25 cm grid of parallel
stainless steel bars, 0.1 cm in caliber, spaced 1 cm apart. In the
training sessions, the animals received a 0.5 mA scramble foot
shock for 2 sec immediately upon stepping down. The interval of
time which elapsed between the mice stepping down and placing all 4
paws on the grid was defined as the latency time. A latency time
>180 sec was registered as 180 sec.
Beam walking test
The beam walking test is a motor skill and balance
test and was conducted according to a previously described method
(30). The apparatus consisted of
an acrylic round beam (60 cm long, 1.5 cm wide) and 2 vertical
supports (60 cm high) above a round tank (150 cm in diameter). Each
mouse was given 3 successive trials and perpendicularly placed on
the center of the beam. Each trial was recorded for a maximum of 60
sec.
Tissue preparation
Following the completion of the behavioral tests,
the mice were deeply anesthetized by an injection of Zoletil
50® (1 mg/kg, i.p.; Virbac Laboratories, Carros,
France). The mice were then transcardially perfused with 0.05 M
phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in
0.5 M sodium phosphate buffer at pH 7.4. The brains were removed,
post-fixed in the same fixative solution overnight, and transferred
to a 30% sucrose solution for cryoprotection. Serial 30-μm-thick
coronal sections were made using a freezing microtome (Leica
Biosystems Nussloch GmbH, Nussloch, Germany). Brain tissues were
selected from each brain in the region spanning −2.92 to −3.64 mm
from the bregma for the SNpc and from the region spanning 0.74 to
0.26 mm from the bregma for the striatum.
Immunohistochemistry for cleaved
caspase-3
For the visualization of cleaved caspase-3,
immunohistochemistry was performed, according to a previously
described method (9). Briefly,
the sections were incubated overnight with a rabbit anti-cleaved
caspase-3 antibody (1:500; Cell Signaling Technology, Beverly, MA,
USA) and were incubated for an additional hour with the
biotinylated rabbit secondary antibody. The bound secondary
antibody was then amplified using the Elite ABC kit®
(Vector Laboratories Inc., Burlingame, CA, USA). The
antibody-biotin-avidin-peroxidase complex was visualized using
0.02% 3,3′-diaminobenzidine (DAB). The sections were mounted onto
gelatin-coated slides that were air-dried overnight at room
temperature, and the coverslips were mounted using Permount medium
(Thermo Fisher Scientific Inc., Fair Lawn, NJ, USA).
TUNEL assay
To visualize DNA fragmentation, a TUNEL assay was
performed using the In Situ Cell Death Detection
kit® (Roche Diagnostics GmbH, Mannheim, Germany),
following the manufacturer’s instructions. First, the sections were
post-fixed in an ethanol-acetic acid (2:1) mixture and rinsed.
Subsequently, the sections were incubated with 100 μg/ml proteinase
K, rinsed, incubated in 3% H2O2,
permeabilized with 0.5% Triton X-100, rinsed again, and incubated
in the TUNEL reaction mixture. The sections were rinsed, 0.02% DAB
was added and visualized using a Converter-POD. Mayer’s hematoxylin
dye (Dako, Glostrup, Denmark) was used for counterstaining, the
sections were mounted onto gelatin-coated slides that were
air-dried overnight at room temperature, and the coverslips were
mounted using Permount medium (Thermo Fisher Scientific).
BrdU/NeuN immunofluorescence
Immunofluorescence staining was used to detect the
number of BrdU-positive and neuronal nuclear antigen
(NeuN)-positive cells in the hippocampal dentate gyrus following a
previously described method (9).
Briefly, the brain sections were permeabilized by incubation with
0.5% Triton X-100 in PBS for 20 min, then incubated with 50%
formamide-2X standard saline citrate (SSC) at 65°C for 2 h,
denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100
mM sodium borate (pH 8.5). The sections were incubated overnight
with rat anti-BrdU antibody (1:500; Abcam, Cambridge, UK) and mouse
anti-NeuN antibody (1:500; EMD Millipore Corp., Billerica, MA,
USA). The sections were then incubated for 2 h with Cy3-conjugated
anti-rat secondary antibody (1:200) for the BrdU assay and with
fluorescein isothiocyanate (FITC)-conjugated anti-mouse (1:200)
antibody (both from Jackson ImmunoResearch Laboratories Inc., West
Grove, PA, USA) for the NeuN assay. The sections were then mounted
on gelatin-coated glass slides, and the coverslips were mounted
using fluorescent mounting medium (Dakocytomation, Carpinteria, CA,
USA). Images were captured under a confocal laser scanning
microscope (LSM-700; Carl Zeiss Microscopy GmbH, Oberkochen,
Germany). On average, 6 sections were selected from each brain,
from the dentate gyrus area spanning −1.34 to −3.28 mm from the
bregma. The number of BrdU/NeuN-labeled cells was counted under a
confocal laser scanning microscope. The results were expressed as
the number of BrdU/NeuN-labeled cells/mm2.
TH immunohistochemistry
The expression level of TH in the SNpc and striatum
was assessed by immunohistochemistry, following a previously
described method (4). The
sections were rinsed in PBS and incubated with 3%
H2O2 for 20 min to block the endogenous
peroxidase activity. After washing with PBS, the sections were
incubated with blocking serum (10% horse serum and 0.1% Triton
X-100 in PBS) for 30 min, followed by incubation with an anti-TH
mouse monoclonal antibody solution (1:1,000; BD Biosciences,
Franklin Lakes, NJ, USA) for 24 h at room temperature. The sections
were then incubated for 1 h with biotinylated anti-mouse IgG
secondary antibody (1:300; Vector Laboratories Inc.). The sections
were subsequently incubated with the avidin-biotin-peroxidase
complex (Vector Laboratories Inc.) for 1 h at room temperature.
Immunoreactivity was visualized by incubating the sections for 3
min with a solution consisting of 0.05% DAB and 0.01%
H2O2 in 50 mM Tris buffer (pH 7.6). The
sections were mounted on gelatine-coated slides and coverslipped
using Permount mounting medium (Thermo Fisher Scientific). Cell
counting and optical density measurements were performed using the
computer-assisted Image-Pro® Plus (Media Cybernetics,
Inc., Silver Spring, MD, USA) image analysis system, attached to a
light microscope (Olympus, Tokyo, Japan).
Western blot analysis
In order to measure Bax and Bcl-2 protein
expression, western blot analysis was performed, following a
previously described protocol (9). Hippocampal samples were dissected
from the mice, and stored at −70°C until further analysis. The
samples were lysed by incubation, for 30 min at 4°C, with ice-cold
lysate buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10%
glycerol, 1% Triton X-100, 1.5 mM magnesium chloride hexahydrate, 1
mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N′-tetraacetic acid
(EGTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/ml
leupeptin, 1 μg/ml pepstatin, 1 mM sodium orthovanadate and 100 mM
sodium fluoride. The tissue debris was then removed by
microcentrifugation, while the supernatant was immediately frozen.
Protein concentrations were measured using a colorimetric protein
assay kit (Bio-Rad, Hercules, CA, USA). Proteins (30 μg) were
separated on SDS-polyacrylamide gels and transferred onto a
nitrocellulose membrane (Whatman Inc., Clifton, NJ, USA). Mouse
anti-Bax (1:1,000) and anti-Bcl-2 (1:1,000) (both from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were used as the primary
antibodies. Horseradish peroxidase-conjugated anti-mouse (1:3,000;
Vector Laboratories Inc.) were used as the secondary antibodies.
Band detection was performed using the enhanced chemiluminescence
(ECL) detection system (Stanta Cruz Biotechnology, Inc.). The bands
were then quantified using the computer-assisted Image-Pro Plus
analysis system.
Statistical analysis
All data were analyzed using SPSS 20.0 software
(SPSS Inc., Chicago, IL, USA). The data are expressed as the means
± standard error of the mean (SEM). For the comparison among
groups, one-way ANOVA and Duncan’s post-hoc tests were performed,
with P<0.05 as the threshold p-value for statistical
significance.
Results
Effects of berberine on nigrostriatal
dopaminergic neurons, motor balance and coordination
The photomicrographs of TH-positive cells in the
SNpc and striatum which were obtained are presented in Fig. 1A–E. The number of the
TH-immunoreactive neurons in the SNpc was 109.87±7.22/section in
the control group, 53.50±4.88/section in the MPTP/P-injected group,
55.29±4.57/section in the MPTP/P-injected and 20 mg/kg
berberine-treated group, 75.89±4.49/section in the MPTP/P-injected
and 50 mg/kg berberine-treated group, and 83.75±6.49/section in the
MPTP/P-injected and 80 mg/kg berberine-treated group (Fig. 1F). These results demonstrated that
berberine treatment alleviated the MPTP/P-induced neuronal cell
loss in the SNpc (P<0.05).
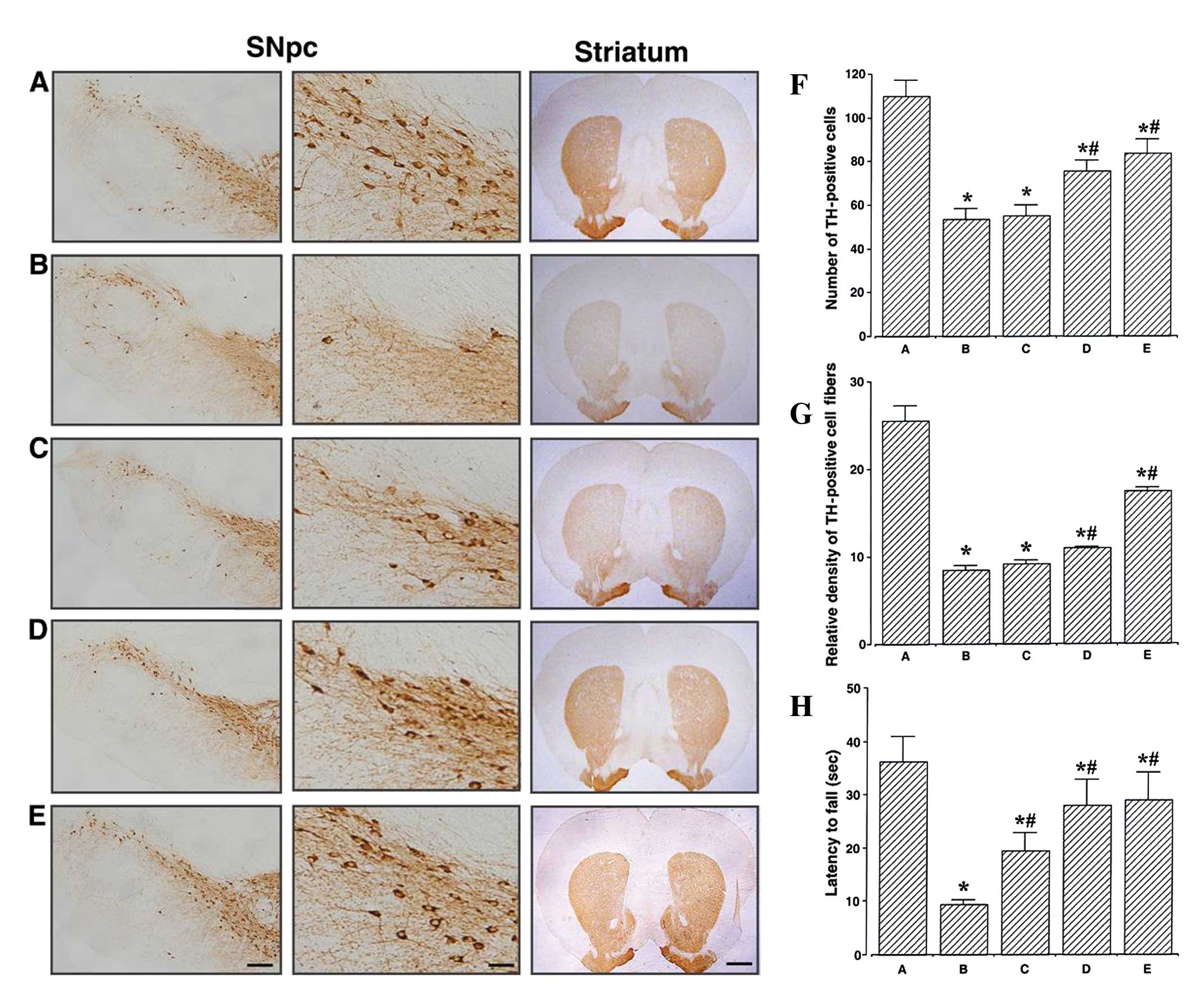 | Figure 1Effects of berberine on tyrosine
hydroxylase (TH) expression in the substantia nigra pars compacta
(SNpc) and the striatum. (A–E) Photomicrographs showing TH in the
SNpc at low (left panel scale bar, 200 μm) and high magnification
(middle panel scale bar, 50 μm) and in the striatum (right panel
scale bar, 800 μm). (A) Control group, (B)
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid
(MPTP/P)-injected group, (C) MPTP/P-injected and 20 mg/kg
berberine-treated group, (D) MPTP/P-injected and 50 mg/kg
berberine-treated group, and (E) MPTP/P-injected and 80 mg/kg
berberine-treated group. (F) Number of TH-positive cells in SNpc,
(G) relative density of TH-positive fibers in the striatum, and (H)
motor balance and coordination skills of mice from groups A–E.
Measurements of latency time until fall are from the beam walking
test. Data are presented as the means ± standard error of the mean
(SEM). *P<0.05 compared to the control group;
#P<0.05 compared to the MPTP/P-injected group.
P-values were obtained by Duncan’s tests. |
The optical density of the TH-immunoreactive fibers
in the striatum was 25.52±1.74 in the control group, 8.46±0.54 in
the MPTP/P-injected group, 9.18±0.44 in the MPTP/P-injected and 20
mg/kg berberine-treated group, 10.99±0.15 in the MPTP/P-injected
and 50 mg/kg berberine-treated group, and 17.49±0.34 in the
MPTP/P-injected and 80 mg/kg berberine-treated group (Fig. 1G). These results indicated that
berberine treatment alleviated the MPTP/P-induced dopaminergic
fiber loss in the striatum (P<0.05).
The results of the beam walking test, performed to
evaluate the motor balance and coordination of the mice in all the
groups, are presented in Fig. 1H.
The latency until fall in this test was found to be 36.20±4.67 sec
in the control group, 9.37±0.89 sec in the MPTP/P-injected group,
19.40±3.44 sec in the MPTP/P-injected and 20 mg/kg
berberine-treated group, 28.00±4.81 sec in the MPTP/P-injected and
50 mg/kg berberine-treated group, and 29.00±5.28 sec in the
MPTP/P-injected and 80 mg/kg berberine-treated group. These results
demonstrated that treatment with berberine improved the
MPTP/P-induced decrease in motor balance and coordination
(P<0.05).
Effects of berberine on short-term memory
and neurogenesis in the dentate gyrus
The photomicrographs of BrdU/NeuN-positive cells in
the hippocampal dentate gyrus are presented in Fig. 2A–E. The number of
BrdU/NeuN-positive cells was counted to determine the level of
neurogenesis (Fig. 2F). This
number was 95.86±7.66 mm2 in the control group,
142.80±8.19 mm2 in the MPTP/P-injected group,
135.76±3.55 mm2 in the MPTP/P-injected and 20 mg/kg
berberine-treated group, 107.46±38.81 mm2 in the
MPTP/P-injected and 50 mg/kg berberine-treated group, and
99.10±6.77 mm2 in the MPTP/P-injected and 80 mg/kg
berberine-treated group. These results indicated that neurogenesis
in the dentate gyrus was increased in the mice in the
MPTP/P-injected group (P<0.05); however, treatment with
berberine significantly inhibited this effect (P<0.05).
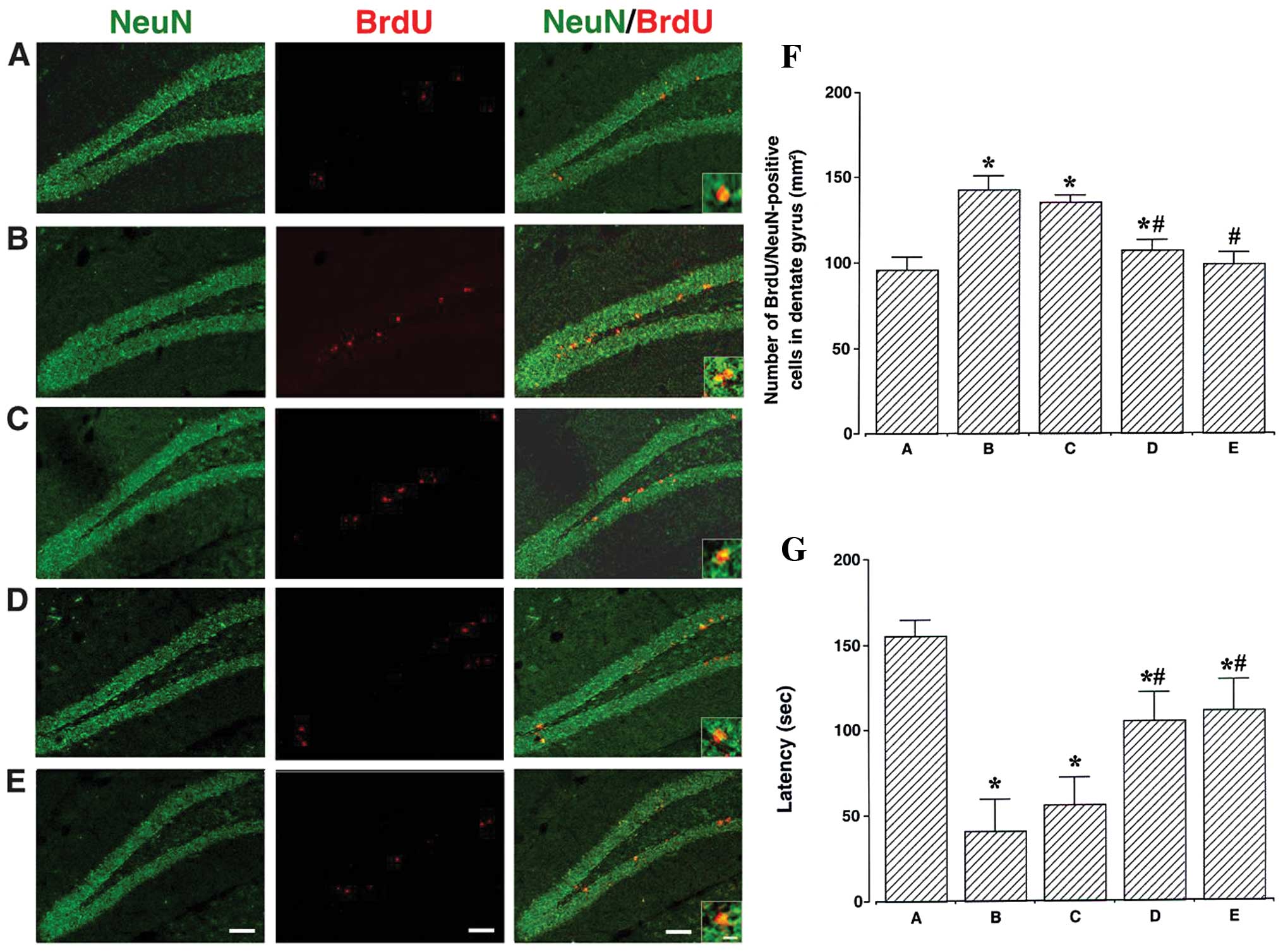 | Figure 2Effects of berberine on neurogenesis
in the hippocampal dentate gyrus. (A–E) Photomicrographs showing
immunofluorescence of neuronal nuclear antigen (NeuN, green),
5-bromo-2′-deoxyuridine (BrdU, red), or their superposition
(NeuN/BrdU) at low (scale bar, 50 μm) and high magnification (scale
bar, 10 μm, enclosed images). (A) Control group, (B)
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid
(MPTP/P)-injected group, (C) MPTP/P-injected and 20 mg/kg
berberine-treated group, (D) MPTP/P-injected and 50 mg/kg
berberine-treated group and (E) MPTP/P-injected and 80 mg/kg
berberine-treated group. (F) Number of BrdU/NeuN-positive cells in
the hippocampal dentate gyrus and (G) latency (from step-down
avoidance task) of mice from groups A–E. Data are presented as the
means ± standard error of the mean (SEM). *P<0.05
compared to the control group; #P<0.05 compared to
the MPTP/P-injected group. P-values were obtained by Duncan’s
tests. |
Latency time was measured to evaluate the short-term
memory of the mice (Fig. 2G). It
was 155.33±9.14 sec in the control group, 41.10±18.46 sec in the
MPTP/P-injected group, 56.00±16.03 sec in the MPTP/P-injected and
20 mg/kg berberine-treated group, 105.20±16.29 sec in the
MPTP/P-injected and 50 mg/kg berberine-treated group, and
110.98±18.07 sec in the MPTP/P-injected and 80 mg/kg
berberine-treated group. These results indicated that short-term
memory was impaired in the MPTP/P-injected mice (P<0.05);
however, treatment with berberine alleviated this phenotype
(P<0.05).
Effects of berberine on cleaved caspase-3
expression and DNA fragmentation in the hippocampal dentate
gyrus
The photomicrographs of cleaved caspase-3-positive
and TUNEL-positive cells in the hippocampal dentate gyrus are
presented in Fig. 3A–E. The
number of cleaved caspase-3-positive cells in the hippocampal
dentate gyrus was also counted (Fig.
3F). This number was 54.46±4.75 mm2 in the control
group, 163.95±13.08 mm2 in the MPTP/P-injected group,
152.84±13.07 mm2 in the MPTP/P-injected and 20 mg/kg
berberine-treated group, 123.46±3.51 mm2 in the
MPTP/P-injected and 50 mg/kg berberine-treated group, and
111.59±5.04 mm2 in the MPTP/P-injected and 80 mg/kg
berberine-treated group. These results revealed that the expression
of cleaved caspase-3 in the hippocampal dentate gyrus was enhanced
in the MPTP/P-injected mice (P<0.05); however, treatment with
berberine reduced its expression (P<0.05).
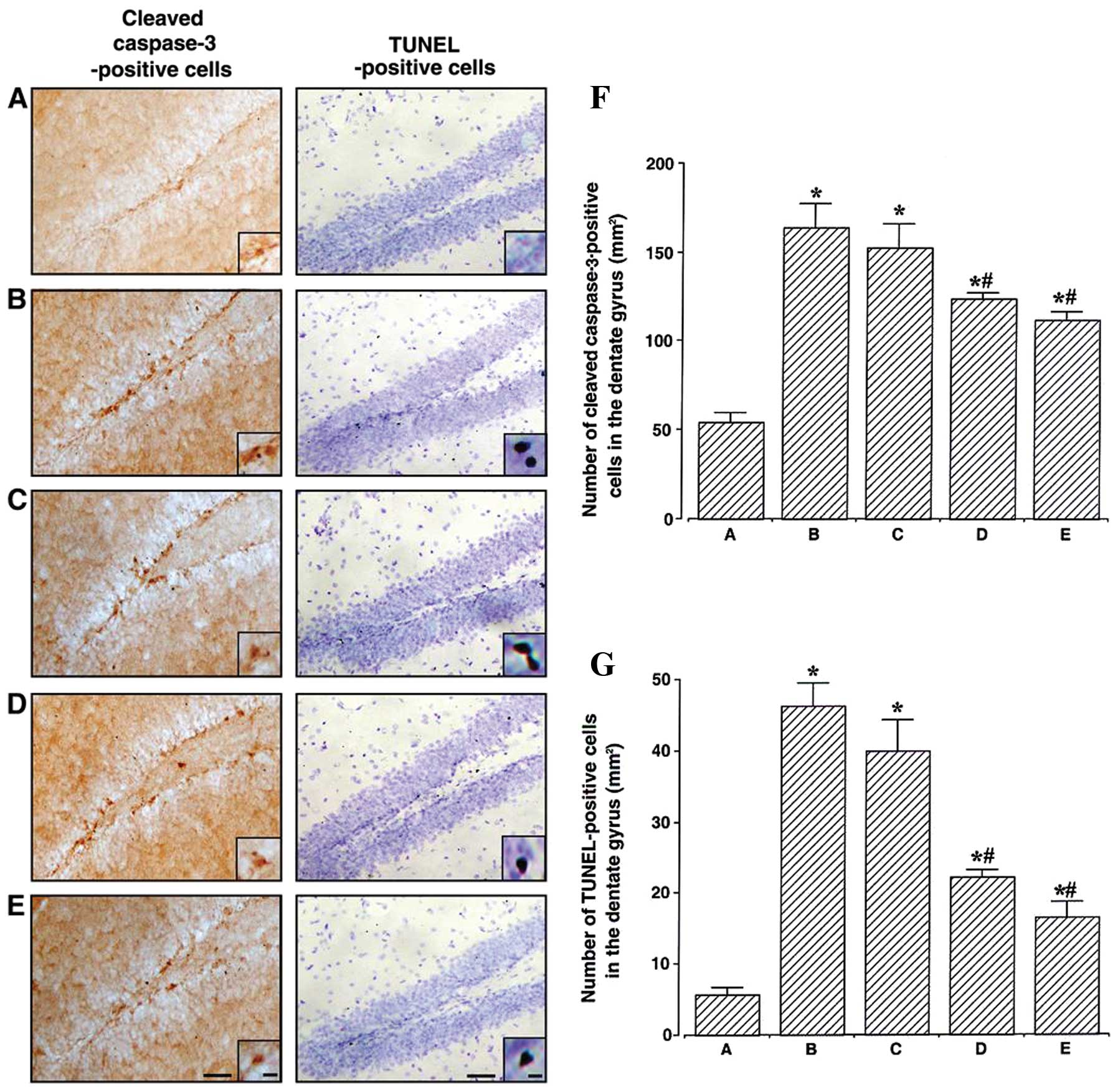 | Figure 3Effect of berberine on the numbers of
cleaved caspase-3-positive and terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL)-positive cells
in the hippocampal dentate gyrus. (A–E) Photomicrographs showing
cleaved caspase-3-positive and TUNEL-positive cells in the
hippocampal dentate gyrus, at low (scale bar, 200 μm) and high
magnification (scale bar, 10 μm, enclosed images). (A) Control
group, (B) 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid
(MPTP/P)-injected group, (C) MPTP/P-injected and 20 mg/kg
berberine-treated group, (D) MPTP/P-injected and 50 mg/kg
berberine-treated group and (E) MPTP/P-injected and 80 mg/kg
berberine-treated group. (F) Number of caspase-3-positive and (G)
TUNEL-positive cells in the hippocampal dentate gyrus of mice from
groups A–E. Data are presented as the means ± standard error of the
mean (SEM). *P<0.05 compared to the control group;
#P<0.05 compared to the MPTP/P-injected group.
P-values were obtained by Duncan’s tests. |
The number of TUNEL-positive cells in the
hippocampal dentate gyrus (Fig.
3G) was determined as 5.72±1.00 mm2 in the control
group, 46.33±3.21 mm2 in the MPTP/P-injected group,
39.92±4.44 mm2 in the MPTP/P-injected and 20 mg/kg
berberine-treated group, 22.20±1.10 mm2 in the
MPTP/P-injected and 50 mg/kg berberine-treated group, and
16.51±2.27 mm2 in the MPTP/P-injected and 80 mg/kg
berberine-treated group. These results indicated that DNA
fragmentation in the hippocampal dentate gyrus was enhanced in the
MPTP/P-injected mice (P<0.05); however, treatment with berberine
reduced DNA fragmentation (P<0.05).
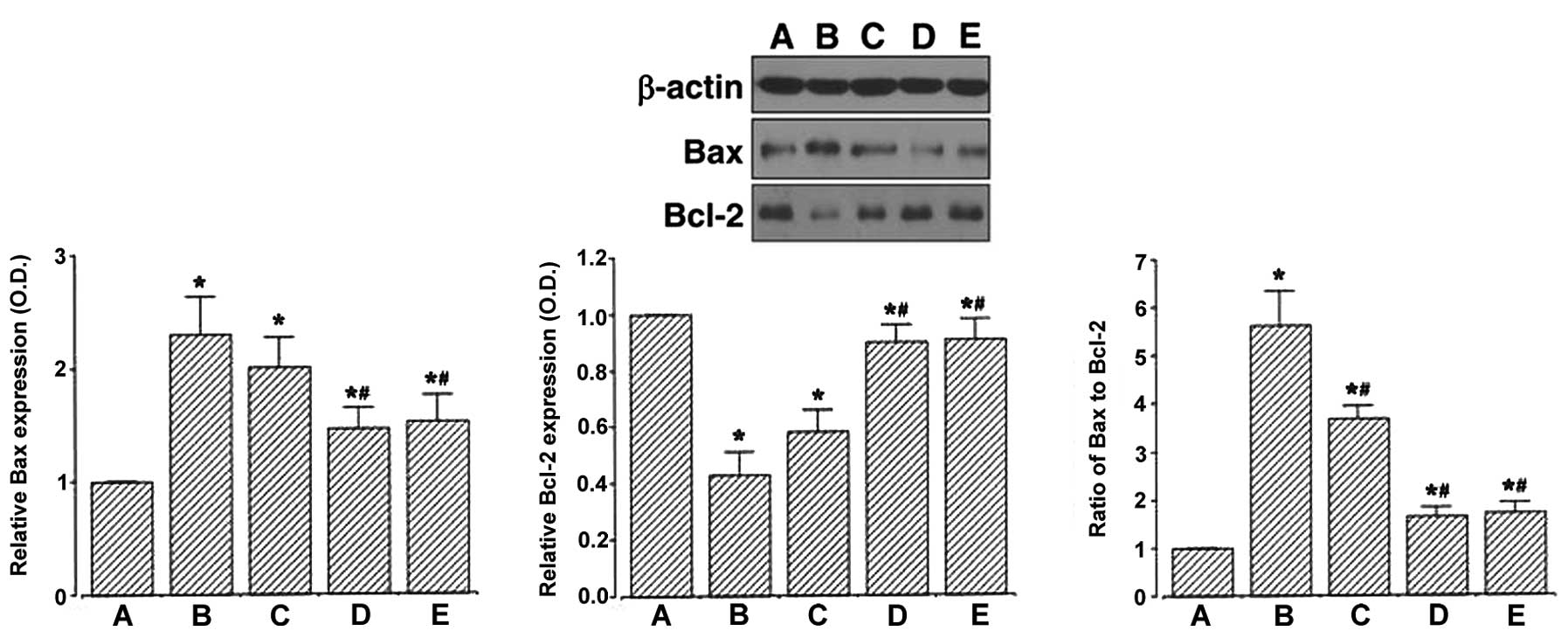
Effect of berberine on Bax and Bcl-2
expression in the hippocampus
We determined the relative expression of Bax and
Bcl-2 proteins in the hippocampus by western blot analysis
(Fig. 4). When the level of Bax
(24 kDa) was set to 1.00 in the control group, the level of Bax was
2.30±0.33 in the MPTP/P-injected group, 2.10±0.26 in the
MPTP/P-injected and 20 mg/kg berberine-treated group, 1.46±0.19 in
the MPTP/P-injected and 50 mg/kg berberine-treated group and
1.53±0.23 in the MPTP/P-injected and 80 mg/kg berberine-treated
group. These results demonstrated that the injection of MPTP/P
increased Bax expression in the hippocampus (P<0.05) and that
treatment with berberine decreased Bax expression in the
MPTP/P-injected mice (P<0.05).
When the level of Bcl-2 (26 kDa) was set to 1.00 in
the control group, the level of Bcl-2 was 0.43±0.08 in the
MPTP/P-injected group, 0.58±0.09 in the MPTP/P-injected and 20
mg/kg berberine-treated group, 0.90±0.06 in the MPTP/P-injected and
50 mg/kg berberine-treated group, and 0.91±0.07 in the
MPTP/P-injected and 80 mg/kg berberine-treated group. These results
demonstrated that the injection of MPTP/P decreased Bcl-2
expression in the hippocampus (P<0.05) and that treatment with
berberine increased Bcl-2 expression in the MPTP/P-injected mice
(P<0.05).
The ratio of Bax to Bcl-2 was then calculated. When
the ratio of Bax/Bcl-2 in the control group was set to 1.00, the
ratio of Bax/Bcl-2 was 5.28±0.71 in the MPTP/P-injected group,
3.68±0.27 in the MPTP/P-injected and 20 mg/kg berberine-treated
group, 1.64±0.19 in the MPTP/P-injected and 50 mg/kg
berberine-treated group, and 1.70±0.23 in the MPTP/P-injected and
80 mg/kg berberine-treated group. These results demonstrated that
the injection of MPTP/P increased the Bax/Bcl-2 ratio in the
hippocampus (P<0.05) and that treatment with berberine decreased
the ratio in the MPTP/P-injected mice (P<0.05).
Discussion
The selective degeneration of dopaminergic neurons
and the reduced expression of TH in the SNpc, along with the
decreased levels of dopamine transporter (DAT) and dopamine (DA) in
the striatum are the main pathological hallmarks of PD (31). The decrease in dopamine synthesis
and storage in the dopaminergic nerve endings has been shown to
cause motor dysfunction in PD (32), while MPTP-induced TH reduction in
the nigrostriatal pathway correlates with motor dysfunction
(33). In a previous study, in
MPTP/P-treated mice, latency in the rotarod test was decreased, and
was accompanied by a decreased number of TH-positive cells in the
SNpc and reduced TH-positive fiber density in the striatum
(4). In our study, the MPTP/P
injection decreased the number of TH-positive cells in the SNpc and
reduced the density of TH-positive fibers in the striatum. MPTP/P
treatment also reduced the latency in the beam walking test,
indicating impaired motor balance and coordination skills.
Treatment with berberine enhanced motor balance and coordination by
preventing dopaminergic neuronal damage.
Memory impairment is an important symptom of
patients with PD (34).
MPTP-induced neuronal loss in the substantia nigra has previously
been shown to result in altered working memory and impaired
learning ability (35). In our
study, short-term memory in the step-down avoidance task was
deteriorated in the mice with MPTP/P-induced PD, while treatment
with berberine improved short-term memory. Berberine has been
reported to improve spatial memory impairment in Alzheimer’s
disease (21), and prevent
changes in oxidative stress and choline esterase activity,
consequently alleviating memory impairment in diabetic rats
(20).
The hippocampal dentate gyrus is a site of
continuous production of new neurons in the adult hippocampus, and
receives dopaminergic input from the neurons of the substantia
nigra (2). Generally, the
increase of neurogenesis in the hippocampal dentate gyrus improves
learning ability and memory function (8,9).
However, the ablation of dopaminergic neurons is also known to
induce neural stem/progenitor cell division (36). Increased neurogenesis in the
hippocampal dentate gyrus is observed in various neurodegenerative
diseases, including PD (37,38). It is believed that increased
neurogenesis following brain injuries is a compensatory mechanism
to restore neuronal loss (2,39).
Park and Enikolopov (2) reported
that the damage of dopaminergic neurons transiently induced
hippocampal neurogenesis and they suggested that destruction of
dopaminergic neurons in the substantia nigra may be the main cause
of this induction. In our study, neurogenesis, which is a
compensatory adaptive response to excessive apoptosis, was
increased upon PD induction in the hippocampal dentate gyrus.
Treatment with berberine alleviated the MPTP/P-induced neurogenesis
in the hippocampal dentate gyrus.
Apoptosis is an important event in neurodegenerative
diseases, including PD (4,40).
A previous study reported that the number of TUNEL-positive and
caspase-3 positive cells was increased in the hippocampal dentate
gyrus of aged rats, indicating age-induced apoptosis (8). Increased numbers of TUNEL-positive
and caspase-3-positive cells in the hippocampus are also associated
with short-term memory impairment in rats with intracerebral
hemorrhage, indicating intracerebral hemorrhage-induced apoptosis
in the hippocampus (13).
Decreased numbers of TUNEL-positive and caspase-3-positive cells
indicate the inhibition of apoptosis (9,13).
The increased expression of Bcl-2 inhibits apoptosis, while the
overexpression of Bax promotes it (41). Enhanced Bax expression with
reduced Bcl-2 expression indicates intracerebral hemorrhage-induced
enhancement of apoptosis in the hippocampus (13). In our experiments, the numbers of
cleaved caspase-3-positive cells and TUNEL-positive cells in the
hippocampal dentate gyrus were increased following MPTP/P
injection, while treatment with berberine reduced these numbers in
the mice with PD. Bcl-2 expression was decreased and Bax expression
was increased, and as a result, the Bax to Bcl-2 ratio was
increased in the hippocampus following MPTP/P injection. Berberine
treatment enhanced Bcl-2 expression and reduced Bax expression, and
as a result, the Bax to Bcl-2 ratio was decreased in the mice with
PD. These results indicate that PD accelerates apoptotic neuronal
death, but berberine treatment attenuates PD-induced apoptosis in
the hippocampus. Berberine has also been shown to exert protective
effects against apoptotic cell death in the hippocampus following
ischemic brain injury (23,42).
In this study, we demonstrate that berberine
enhances motor balance and coordination by preventing dopaminergic
neuronal loss in mice with PD. Berberine further ameliorated
short-term memory impairment through the inhibition of apoptosis in
the hippocampus. These effects of berberine were observed at doses
exceeding 50 mg/kg. Based on the present results, we suggest that
berberine may serve as a therapeutic agent for the alleviation of
memory impairment and motor dysfunction in patients with PD.
References
|
1
|
Dauer W and Przedborski S: Parkinson’s
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
|
|
2
|
Park JH and Enikolopov G: Transient
elevation of adult hippocampal neurogenesis after dopamine
depletion. Exp Neurol. 222:267–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asanuma M, Miyazaki I and Ogawa N:
Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine
quinone formation and tyrosinase in a model of Parkinson’s disease.
Neurotox Res. 5:165–176. 2003.
|
|
4
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson’s disease mice. Life Sci.
91:1309–1316. 2012.PubMed/NCBI
|
|
5
|
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG,
Sung YH, Kim SE, Lee HH, Kim YP and Kim CJ: Treadmill exercise
suppresses nigrostriatal dopaminergic neuronal loss in
6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett.
423:12–17. 2007.PubMed/NCBI
|
|
6
|
Eriksson PS, Perfilieva E, Bjork-Eriksson
T, Alborn AM, Nordborg C, Peterson DA and Gage FH: Neurogenesis in
the adult human hippocampus. Nat Med. 4:1313–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kempermann G, Jessberger S, Steiner B and
Kronenberg G: Milestones of neuronal development in the adult
hippocampus. Trends Neurosci. 27:447–452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim
CJ, Kim SH, Baek SS, Lee EK and Jee YS: Treadmill exercise prevents
aging-induced failure of memory through an increase in neurogenesis
and suppression of apoptosis in rat hippocampus. Exp Gerontol.
45:357–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin MS, Ko IG, Kim SE, Kim BK, Kim TS,
Lee SH, Hwang DS, Kim CJ, Park JK and Lim BV: Treadmill exercise
ameliorates symptoms of methimazole-induced hypothyroidism through
enhancing neurogenesis and suppressing apoptosis in the hippocampus
of rat pups. Int J Dev Neurosci. 31:214–223. 2013. View Article : Google Scholar
|
|
10
|
Dash PK, Mach SA and Moore AN: Enhanced
neurogenesis in the rodent hippocampus following traumatic brain
injury. J Neurosci Res. 63:313–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Felling RJ and Levison SW: Enhanced
neurogenesis following stroke. J Neurosci Res. 73:277–283. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Cui XL, Wang Y, Li XW, Yang F, Wei D
and Jiang W: Aspirin attenuates spontaneous recurrent seizures and
inhibits hippocampal neuronal loss, mossy fiber sprouting and
aberrant neurogenesis following pilocarpine-induced status
epilepticus in rats. Brain Res. 21:103–113. 2012. View Article : Google Scholar
|
|
13
|
Hwang L, Choi IY, Kim SE, Ko IG, Shin MS,
Kim CJ, Kim SH, Jin JJ, Chung JY and Yi JW: Dexmedetomidine
ameliorates intracerebral hemorrhage-induced memory impairment by
inhibiting apoptosis and enhancing brain-derived neurotrophic
factor expression in the rat hippocampus. In J Mol Med.
31:1047–1056. 2013.
|
|
14
|
Savitz SI and Rosenbaum DM: Apoptosis in
neurological disease. Neurosurgery. 42:555–574. 1998. View Article : Google Scholar
|
|
15
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
16
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutic effects of Berberis vulgaris
and its active constituent, berberine. Phytother Res. 22:999–1012.
2008.PubMed/NCBI
|
|
19
|
Kulkarni SK and Dhir A: On the mechanism
of antidepressant-like action of berberine chloride. Eur J
Pharmacol. 589:163–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhutada P, Mundhada Y, Bansod K, Tawari S,
Patil S, Dixit P, Umathe S and Mundhada D: Protection of
cholinergic and antioxidant system contributes to the effect of
berberine ameliorating memory dysfunction in rat model of
streptozotocin-induced diabetes. Behav Brain Res. 220:30–41. 2011.
View Article : Google Scholar
|
|
21
|
Zhu F and Qian C: Berberine chloride can
ameliorate the spatial memory impairment and increase the
expression of interleuin-1beta and inducible nitric oxide synthase
in the rat model of Alzheimer’s disease. BMC Neurosci.
7:782006.PubMed/NCBI
|
|
22
|
Hung TM, Dang NH, Kim JC, Jang HS, Ryoo
SW, Lee JH, Choi JS, Bae K and Min BS: Alkaloids from roots of
Stephania rotunda and their cholinesterase inhibitory
activity. Planta Med. 76:1762–1764. 2010.PubMed/NCBI
|
|
23
|
Zhang Q, Qian Z, Pan L, Li H and Zhu H:
Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine
in neurons during hypoxia/ischemia. Acta Physiol Hung. 99:311–323.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae J, Lee D, Kim YK, Gil M, Lee JY and
Lee KJ: Berberine protects 6-hydroxydopamine-induced human
dopaminergic neuronal cell death through the induction of heme
oxygenase-1. Mol Cells. 35:151–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon IH, Choi HS, Shin KS, Lee BK, Lee CK,
Hwang BY, Lim SC and Lee MK: Effects of berberine on
6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat
model of Parkinson’s disease. Neurosci Lett. 486:29–33.
2010.PubMed/NCBI
|
|
26
|
Miyoshi E, Wietzikoski S, Camplessei M,
Silveira R, Takahashi RN and Da Cunha C: Impaired learning in a
spatial working memory version and in a cued version of the water
maze in rats with MPTP-induced mesencephalic dopaminergic lesions.
Brain Res Bull. 58:41–47. 2002. View Article : Google Scholar
|
|
27
|
Schneider JS, Tinker JP, VanVelson M and
Giardiniere M: Effects of the partial glycine agonist D-cycloserine
on cognitive functioning in chronic low dose MPTP-treated monkeys.
Brain Res. 860:190–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meredith GE, Totterdell S, Potashkin JA
and Surmeier DJ: Modeling PD pathogenesis in mice: advantages of a
chronic MPTP protocol. Parkinsonism Relat Disord. 14(Suppl 2):
S112–S115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lau YS, Trobough KL, Crampton JM and
Wilson JA: Effects of probenecid on striatal dopamine depletion in
acute and long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(MPTP)-treated mice. Gen Pharmacol. 21:181–187. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng XH, Liu P, Wang H, Zhao XF, Xu ZM,
Chen GH and Xu DX: Gender-specific impairments on cognitive and
behavioral development in mice exposed to fenvalerate during
puberty. Toxicol Lett. 203:245–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nutt JG, Carter JH and Sexton GJ: The
dopamine transporter: importance in Parkinson’s disease. Ann
Neurol. 55:766–773. 2004.
|
|
32
|
Von Bohlen and Halbach O: Modeling
neurodegenerative diseases in vivo review. Neurodegener Dis.
2:313–320. 2005.
|
|
33
|
Schintu N, Frau L, Ibba M, Garau A,
Carboni E and Carta AR: Progressive dopaminergic degeneration in
the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res.
16:127–139. 2009.
|
|
34
|
Emre M: Dementia associated with
Parkinson’s disease. Lancet Neurol. 2:229–237. 2003.
|
|
35
|
Ho YJ, Ho SC, Pawlak CR and Yeh KY:
Effects of D-cycloserine on MPTP-induced behavioral and
neurological changes: potential for treatment of Parkinson’s
disease dementia. Behav Brain Res. 219:280–290. 2011.PubMed/NCBI
|
|
36
|
Peng J, Xie L, Jin K, Greenberg DA and
Andersen JK: Fibroblast growth factor 2 enhances striatal and
nigral neurogenesis in the acute
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s
disease. Neuroscience. 153:664–570. 2008.PubMed/NCBI
|
|
37
|
Kim SE, Ko IG, Park CY, Shin MS, Kim CJ
and Jee YS: Treadmill and wheel exercise alleviate
lipopolysaccharide-induced short-term memory impairment by
enhancing neuronal maturation in rats. Mol Med Rep. 7:31–36.
2013.PubMed/NCBI
|
|
38
|
Lesemann A, Reinel C, Hühnchen P,
Pilhatsch M, Hellweg R, Klaissle P, Winter C and Steiner B:
MPTP-induced hippocampal effects on serotonin, dopamine,
neurotrophins, adult neurogenesis and depression-like behavior are
partially influenced by fluoxetine in adult mice. Brain Res.
1457:51–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parent JM: Injury-induced neurogenesis in
the adult mammalian brain. Neuroscientist. 9:261–272. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liedhegner EA, Steller KM and Mieyal JJ:
Levodopa activates apoptosis signaling kinase 1 (ASK1) and promotes
apoptosis in a neuronal model: implications for the treatment of
Parkinson’s disease. Chem Res Toxicol. 24:1644–1652.
2011.PubMed/NCBI
|
|
41
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou XQ, Zeng XN, Kong H and Sun XL:
Neuroprotective effects of berberine on stroke models in vitro and
in vivo. Neurosci Lett. 447:31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|