Introduction
Vulvar melanoma is the second most common vulvar
cancer with an incidence of 0.1 per 100,000 individuals, presenting
typically in post-menopausal women (1,2).
Patients with vulvar melanoma, due to the lack of body awareness,
false modesty and neglect, usually present with the disease at a
late stage and have a poor overall prognosis, with reported 5-year
survival rates ranging from 8 to 61% (3–10).
This is unlike cutaneous melanomas where, as a result of increased
public and clinical awareness, many patients are now diagnosed at
an early stage.
The aetiology of vulvar melanoma is poorly
understood but is believed to arise de novo, that is, to
develop from the malignant transformation of a single junctional
melanocyte in situ (4). A
genetic basis is suspected, which may explain why caucasions have a
3-fold higher incidence rate than individuals of African descent
(11) and why individuals of
African descent have a 2-fold shorter median survival than
caucasions (12). Activating
c-KIT mutations have been found in patients with vulvar melanoma
(13,14). By contrast, as previously
demonstrated, gene mutations for cutaneous melanomas were
irrelevant in vulvar melanomas (BRAF, NRAS), indicating that these
two diseases have a different origin (13,15).
Lichen sclerosus (LS) is also suspected to cause
vulvar melanoma, and LS has indeed been reported with vulvar
melanoma in 6 cases (17–20). Whereas 5 childhood vulvar
melanomas cases have been reported, only 1 among thousands of adult
vulvar melanoma patients have been reported (18). The mechanisms involved, however,
are not clear. LS, itself an inflammatory dermatosis of unknown
origin presenting with whitening of the skin and pruritus (21), is the most common precursor of
HPV-negative squamous cell carcinoma of the vulva, where the
postulated pathogenesis is a ‘scar cancer’ similar to Margolin’s
ulcer (22,23). The difficulty of distinguishing
vulvar melanoma in the setting of LS from benign pigmented lesions
of the genitals is acknowledged (24).
There is no consensus regarding the adequate staging
system and the treatment for vulvar melanomas. The standard FIGO
staging, as used for squamous cell carcinoma, is not satisfactory.
In vulvar melanomas, lesions are usually much smaller and prognosis
is related to depth rather than diameter. For this reason, Breslow
staging, which takes into account the depth of tumour rather than
its size, seems to be most adequate and until today the best
predictor of prognosis (25–29). This was also confirmed by a recent
American study with 85 cases of melanomas of the female genitalia
(30): a higher Breslow depth was
associated with declining survival, whereas other histopathological
features, such as ulcerations, increasing mitotic index and the
presence of atypical melanocytic hyperplasia were not associated
with a significant survival difference. Three basic histotypes
(superficial spreading melanoma, mucosal lentiginous melanoma and
nodular melanoma) have been described with varying incidence rates
(5,6,9,31).
It is an ongoing search for reliable histological
features that allow the prognosis of vulvar melanoma; however, the
majority of studies (small case series and retrospective reviews)
have delivered inconclusive results. Thus, we performed a) a
comprehensive literature review, b) a clinicopathological review of
33 vulvar melanoma cases of an Australian cohort to identify
potential histopathological predictors of outcome, and c)
immunohistochemistry for c-KIT expression in a respective tissue
microarray.
Patients and methods
Comprehensive literature review
The systematic literature review was performed using
the online websites, PubMed, Medline and Cochrane for the key words
‘vulva melanoma’, ‘mucosal melanoma’, ‘melanoma’, ‘melanomas’,
‘vulvar’, ‘vulva’ and ‘vulvar neoplasm’. The retrieval was limited
from 1990 to 2012 and included epidemiological studies, literature
reviews, retrospective series, prospective series, meta-analyses
and molecular analyses. Studies with <10 patients were excluded,
as were individual case reports. Studies were reported as to their
year of publication, the number of enrolled patients, type of
study, mean age of patients, 5-year overall survival, and study
results and clinicopathological predictors of outcome (Table I).
 | Table ILiterature review. |
Table I
Literature review.
| Author/(Refs.) | Year | Vulvar melanoma
patients (n) | Melanoma
location | Study type | Mean patient age
(years) | 5-year OS (%) | Results and
predictors of outcome |
|---|
| Bradgate et
al (3) | 1990 | 50 | Vulva | Retrospective
series | N/A | 35 | Clinical stage,
patient age, ulceration, cell type, mitotic rate |
| Trimble et
al (32) | 1992 | 80 | Vulva | Retrospective
series | 58.5 | 60 | Extent of vulva
surgery not relevant |
| Tasseron et
al (33) | 1992 | 30 | Vulva | Retrospective
series | 63.8 | 56 | Ulceration |
| Piura et al
(34) | 1992 | 18 | Vulva | Retrospective
series | N/A | 28.6 | Positive inguinal
lymph nodes not relevant |
| Ragnarsson-Olding
et al (35) | 1993 | 219 | Vulva and other
mucosal melanoma | Epidemiological
study | 75 | 35 | Decrease in
age-standardized incidence in Sweden |
| Look et al
(36) | 1993 | 16 | Vulva | Retrospective
series | 59 | N/A | Depth >1.75 mm
predicts recurrence within 24 months |
| Phillips et
al (37) | 1994 | 71 | Vulva | Prospective
series | 71–80 | 43.9 | AJCC staging,
Breslow’s depth |
| Dunton et al
(38) | 1995 | N/A | N/A | Literature
review | N/A | N/A | Breslow’s depth,
lymph node dissection |
| Scheistroen et
al (31) | 1995 | 75 | Vulva | Retrospective
series | 67 | 46 | Tumour localization
clitoris, DNA ploidy, positive inguinal lymph nodes |
| Raber et al
(39) | 1996 | 89 | Vulva | Retrospective
series | 55.4 | 36.7 | Breslow’s depth,
Clark’s level, lymph node status |
| Trimble (40) | 1996 | N/A | N/A | Literature
review | N/A | N/A | Breslow’s
depth |
| Scheistroen et
al (41) | 1996 | 43 | Vulva | Retrospective
series | 64 | 63 | Angioinvasion, DNA
ploidy |
| Strauss (42) | 1997 | N/A | Melanomas | Molecular
analysis | N/A | N/A | p53 mutations |
| Jiveskog et
al (15) | 1998 | N/A | Cutaneous vs.
mucosal melanomas | Molecular
analysis | N/A | N/A | NRAS mutations not
relevant |
| De Matos et
al (43) | 1998 | 30 | Vulva | Retrospective
series | 66 | 59 | Regional
metastases |
| Ragnarsson-Olding
et al (5) | 1999 | 219 | Vulva | Epidemiological
study | N/A | 47 | Breslow’s depth,
ulceration, amelanosis |
| Larsson et
al (44) | 1999 | 19 | Vulva | Retrospective
series | N/A | 23 | Stage |
| Creasman et
al (45) | 1999 | 569 | Vulva | Retrospective
series | 66 | 62 | AJCC stage |
| Raspagliesi et
al (46) | 2000 | 40 | Vulva | Retrospective
series | 58 | 48 | Positive inguinal
lymph nodes |
| Verschraegen et
al (9) | 2001 | 51 | Vulva | Retrospective
series | 54 | 27 | AJCC stage,
Breslow’s depth |
| Irvin et al
(8) | 2001 | 14 | Vulva | Retrospective
series | 58 | 42 | Margins, inguinal
lymphadenectomy |
| Ragnarsson-Olding
et al (47) | 2002 | 22 | Vulva | Molecular
analysis | | | p53 mutations |
| De Hullu et
al (48) | 2002 | 33 | Vulva | Retrospective
series | 69 | 52 | Sentinel
lymphadenectomy |
| Finan and Barre
(49) | 2003 | N/A | N/A | Literature
review | N/A | N/A | Age, AJCC
stage |
| Ragnarsson-Olding
(2) | 2004 | 1,442 | Vulva | Meta-analysis | N/A | N/A | Breslow’s depth,
ulceration, amelanosis, angioinvasion, DNA ploidy |
| Ragnarsson-Olding
et al (7) | 2004 | 17 | Vulva | Molecular
analysis | N/A | N/A | p53 protein levels
not relevant |
| Harting and Kim
(50) | 2004 | 11 | Vulva | Retrospective
series | 59 | 10 | Chemotherapy |
| Wechter et
al (51) | 2004 | 21 | Vulva | Retrospective
series | 58 | N/A | Sentinel
lymphadenectomy |
| Edwards et
al (52) | 2004 | 8 | Vulva | Molecular
analysis | N/A | N/A | BRAF mutations not
relevant |
| Dahlgren et
al (53) | 2005 | 7 | Vulva | Molecular
analysis | N/A | N/A | HPV not
relevant |
| Stang et al
(54) | 2005 | 102 | Vulva | Epidemiological
study | 70 | N/A | No change in
incidence rate |
| Rouzier et
al (55) | 2005 | N/A | N/A | Literature
review | N/A | N/A | Wide local excision
with tumour-free margins, sentinel lymphadenectomy |
| Lundberg et
al (56) | 2006 | 7 | Vulva | Molecular
analysis | N/A | N/A | HSV not
relevant |
| Sugiyama et
al (10) | 2007 | 644 | Vulva | Retrospective
series | 68 | 61 | Age, stage,
positive inguinal lymph nodes |
| Dhar et al
(57) | 2007 | 26 | Vulva | Literature
review | N/A | N/A | Sentinel
lymphadenectomy |
| Giraud et al
(58) | 2008 | 7 | Vulva | Molecular
analysis | N/A | N/A | Polyomaviruses not
relevant |
| De Simone et
al (59) | 2008 | 11 | Vulva | Retrospective
series | 53 | 50 | N/A |
| Hu et al
(11) | 2010 | 324 | Vulva | Retrospective
series | N/A | N/A | Ethnicity |
| Moan et al
(60) | 2010 | N/A | Vulva | Literature
review | N/A | N/A | Sun exposure not
relevant |
| Trifiro et
al (61) | 2010 | 12 | Vulva | Prospective
study | 59 | N/A | Sentinel
lymphadenectomy not relevant |
| Terlou et al
(62) | 2010 | N/A | N/A | Literature
review | N/A | N/A | ABCDE and punch
biopsy are useful in diagnosis |
| Baiocchi et
al (63) | 2010 | 11 | Vulva | Retrospective
series | 64.8 | 10 | Sentinel
lymphadenectomy not relevant |
| Ragnarsson-Olding
(64) | 2011 | N/A | N/A | Literature
review | N/A | N/A | Sun exposure not
relevant |
| Omholt et al
(13) | 2011 | 23 | Vulva | Molecular
analysis | N/A | N/A | KIT mutations,
RAF/MEK/ERK and PI3K/AKT pathways activated |
| Tcheung et
al (30) | 2012 | 85 | Genitals/vulva | Retrospective
series | 60.5 | 50.7 | N/A |
| Schoenewolf et
al (14) | 2012 | 16 | Genitals/vulva | Retrospective
series/molecular analysis | 61.9 | N/A | c-KIT expression
and mutations; pERK |
| Heinzelmann-Schwarz
(this study) | | 33 | Vulva | Literature review,
retrospective series, molecular analysis | 67.5 | N/A | At least one of
these pathological features: satellitosis, in-transit metastases,
dermal mitosis, LVSI; strong c-KIT expression, lateral margin >1
cm |
Clinicopathological review of our study
cohort
Upon obtaining ethical approval and informed
consent, we identified and enrolled 33 patients with vulvar
melanoma at the Gynaecological Cancer Centre of the Royal Hospital
for Women, Sydney (20 patients, incepted 1987) and the
Gynaecological Cancer Centre of John Hunter Hospital, Newcastle (13
patients, incepted 1991). The following information was retrieved
from the charts of the patients: age
(diagnosis/menopause/relapse/death), duration of symptoms (months),
menopausal status, family history of melanoma, location of
melanoma, mode of detection, palpable groin nodes, lymph nodes
removed/positive, CT scan results, Breslow’s depth, type of
surgery, chemotherapy/immunotherapy/radiotherapy (number of
sessions and dose), treatment side-effects, site/location of
recurrence, cause of death, and relapse-free and overall
survival.
Characteristics which were assessed included
diagnosis, pathogenic type (superficial spreading, mucosal
lentiginous melanoma, other), predominant cell type (epithelioid,
spindle or other), ulcerations, Breslow’s depth, tumour
infiltrating lymphocytes (TILS), regression (dermal fibrosis,
lymphocytic infiltrate and, in cases of pigmented melanomas,
melanophages), lymphovascular space invasion (LVSI), satellitosis
(discrete tumour nests >0.05 mm in diameter, separated from
invasive tumour by ≥0.3 mm) and in-transit metastases (>20 mm
from invasive tumour), margins (involved by in situ or
invasive melanoma), adjacent abnormal melanocytic proliferation and
LS.
c-KIT immunohistochemistry of tissue
microarrays
An independent, blinded pathological review of all
haematoxylin and eosin slides was performed by a pathologist
specialised in vulvar pathology (Dr J.P. Scurry). These slides
where marked for vulvar melanoma and two 1-mm cores were
transferred onto a tissue microarray, using the ATA-100 Advancer
Tissue Arrayer (Chemicon International, Temecula, CA, USA). Cores
also included control tissues from negative inguinal lymph nodes
that were surgically sampled. Immunohistochemistry was performed in
the Bond™-X System (Leica Biosystems, Wetzlar, Germany) using the
polyclonal rabbit Anti-human CD117 antibody (c-KIT; Dako,
Carpinteria, CA, USA) at a 1:400 dilution followed by secondary
detection with the Bond™ Polymer Refine Detection kit combining
anti-mouse and anti-rabbit antibodies (Leica Biosystems). Prior to
staining, antigen retrieval was performed at 95°C for 15 min in the
PT Link (Dako) using the EnVision™ FLEX target retrieval solution,
low pH (50×; Dako), followed by a water wash. Evaluation of the
intensity of c-KIT cytoplasmic and membrane protein expression was
performed by two researchers independently and consensus was
reached. For the purpose of this analysis either cytoplasmic or
membrane staining of 3+ intensity was taken as strong c-KIT
expression.
Statistical analysis
The clinicopathological data were collected in an
in-house research database based on ACCESS (Microsoft Windows, USA)
and analysed with SAS statistical software (SAS Institute Inc.,
Cary, NC, USA). Mean values with standard deviation and range were
generated for longitudinal datasets and nominal data were presented
as percentages. Potential risk factors for relapse and mortality
were assessed through Kaplan-Meier curves and Cox proportional
hazards models. As the number of cases was limited, the
significance of each hazard ratio (HR) was primarily assessed by
their effect size as p-values alone were likely to miss important
results.
Results
Comprehensive literature review:
predictors of outcome and molecular targets for vulvar
melanoma
Our literature review revealed 46 studies with
>10 patients enrolled with vulvar melanoma (Table I). These studies often combined
both mucosal (including those of the vulva) and cutaneous
melanomas. Out of these 46 studies, 23 were retrospective studies,
with 48.9% comprising the majority of publications in vulvar
melanoma research, 8 studies were literature reviews, 11 comprised
analyses of molecular targets, and 1 was a meta-analysis. No
Cochrane review has been performed to date.
The unequivocal clinical predictors of patient
outcome that were identified were inguinal lymph node status
(either via sentinel or standard lymphadenectomy; 11 studies) and
Breslow’s depth (9 studies). Ambiguous clinical predictors included
tumour ulceration (4 studies), age at diagnosis (3 studies) and DNA
ploidy (3 studies).
Molecular targets suspected to be relevant in
mucosal melanomas have been investigated in 11 studies. Mutations
in mucosal melanomas were found in p53 (3 studies), in
c-KIT (2 studies) and in key kinases of the PI3K/AKT/mTOR-
(1 study) and RAS/RAF/MEK/ERK-pathways (1 study). Mutations in
BRAF or NRAS in mucosal melanomas were not found (1
study each) and evidence of the involvement of viral infections
(HPV, HSV, polyomaviruses) in vulvar melanoma was not found either.
Notably, high-throughput transcription profiling experiments on
vulvar melanomas have not been performed to date.
Clinicopathological and immunological
characteristics of our cohort
The clinicopathological and immunological
characteristics of the 33 patients of our cohort are summarized in
Table II. The mean age at
diagnosis was 67.5 years (range, 34–95 years) and was higher
compared to that of the 47 literature review studies (62.2 years;
range, 53–80 years). Patients presented with symptoms for an
average of 28.2 months (range, 2–112 months) and in 72.2% of the
cases detected the lesion themselves. By virtue of the advanced
mean age at diagnosis almost three quarters (73.5%) of our patients
were post-menopausal. The vast majority of the patients (93.8%) did
not have a family history of melanoma. The most common location of
vulvar melanomas was at the labia minora (31.6%) and was multifocal
(26.3%).
 | Table IIClinicopathological and
immunohistochemical patient characteristics. |
Table II
Clinicopathological and
immunohistochemical patient characteristics.
| No. | Age | BD | ULC | DM | SAT | ITM | LVSI | LNP | IHC_C | IHC_M | Status |
|---|
| 1 | 51 | 10.5 | Yes | Neg | No | No | No | 0 | 0 | 0 | Alive |
| 2 | 74 | 1.5 | Yes | Neg | No | No | No | 0 | 1.4 | 0.2 | Alive |
| 3 | 53 | 1.15 | No | Neg | No | No | No | N/A | N/A | N/A | Alive |
| 4 | 69 | 5 | Yes | Neg | No | No | No | 0 | 0 | 0 | Alive |
| 5 | 84 | 3.5 | Yes | Pos | Yes | No | No | 0 | 2.2 | 2.75 | Alive |
| 6 | 46 | 1 | Yes | Neg | No | No | No | 0 | N/A | N/A | Alive |
| 7 | 62 | 3.1 | Yes | Neg | No | No | No | 0 | 1 | 1 | Alive |
| 8 | 60 | 4 | Yes | Neg | Yes | No | No | 0 | 0.27 | 1.5 | Deceased |
| 9 | 68 | 4.2 | Yes | Pos | No | No | No | 0 | 0 | 0 | Deceased |
| 10 | 43 | 14 | Yes | Pos | No | No | No | 1 | N/A | N/A | Alive |
| 11 | 96 | 6 | Yes | Pos | No | No | No | 0 | 2.25 | 1.89 | Deceased |
| 12 | 91 | 7.5 | Yes | Pos | No | No | No | 0 | 2.11 | 2.11 | Deceased |
| 13 | 83 | 0 | No | N/A | No | Yes | No | 0 | 0.5 | 0.75 | Deceased |
| 14 | 84 | N/A | N/A | N/A | N/A | N/A | N/A | 3 | N/A | N/A | Deceased |
| 15 | 44 | 6 | Yes | Pos | No | No | No | N/A | 0.9 | 0.86 | Deceased |
| 16 | 71 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | N/A | N/A | Deceased |
| 17 | 68 | 3.3 | Yes | Neg | No | No | Yes | 0 | 1.67 | 2.13 | Deceased |
| 18 | 76 | 5.2 | Yes | Neg | No | Yes | No | 0 | 0 | 0 | Deceased |
| 19 | 50 | 11 | Yes | N/A | No | No | No | 6 | 1 | 1.18 | Alive |
| 20 | 89 | 19.5 | Yes | Pos | No | Yes | Yes | 0 | N/A | N/A | Alive |
| 21 | 73 | 1 | No | Neg | No | No | No | N/A | 1.5 | 2 | Alive |
| 22 | 64 | 28 | Yes | Pos | No | No | Yes | 0 | 0 | 0 | Deceased |
| 23 | 68 | 7 | Yes | Pos | N/A | N/A | N/A | N/A | 0.75 | 0 | Deceased |
| 24 | 82 | 7 | N/A | Pos | N/A | N/A | N/A | N/A | 1 | 0 | Deceased |
| 25 | 67 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 0 | Deceased |
| 26 | 70 | 10 | Yes | Pos | No | No | No | N/A | 1 | 1.78 | Alive |
| 27 | 80 | 3.2 | Yes | Pos | No | No | No | N/A | 1.75 | 2.38 | Alive |
| 28 | 67 | 1 | No | Neg | No | No | No | N/A | 1 | 0.8 | N/A |
| 29 | 94 | 1.7 | Yes | Neg | No | No | No | N/A | 1.83 | 2.17 | Deceased |
| 30 | 34 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1.09 | 1.36 | Deceased |
| 31 | 81 | N/A | No | N/A | N/A | N/A | N/A | 0 | 2.08 | 0.89 | Alive |
| 32 | 58 | 10 | Yes | Pos | Yes | N/A | No | 0 | 1.42 | 2 | Alive |
| 33 | 48 | 2 | Yes | Pos | No | N/A | No | 1 | 1.6 | 2.44 | Alive |
The majority of patients presented with an advanced
Breslow stage of V (56.3%) and had a mean tumour size of 21.9 mm
(range, 5–50 mm), deep margin of 5.20 mm (range, 0–22 mm) and
lateral margin of 3.9 mm (range, 0–12 mm). Most of our patients
received radical local excision. Of the 4 patients that had
received a radical vulvectomy, 3 had multifocal disease and the
other was treated by an outside consultant. The majority of the
patients (68.8%) underwent at least an unilateral inguino-femoral
lymphadenectomy without any notable side-effects (in particular no
lymphoceles or lymphoedema). For the majority of patients, this was
the only adjuvant treatment received: only 25% received
chemotherapy, 18.2% immunotherapy and 38.9% radiotherapy. Seventy
percent of the patients relapsed, with local and distant metastases
equally common: the most common local recurrence was at the vulva
(30.8%). The median time to relapse was 40 months and to death 44
months. Fifty-five percent of the patients succumbed to the
disease, mostly due to causes related to their disease (90%).
The majority of patients presented with a clinically
or pathologically detected ulceration (53.3% or 88.9%,
respectively) of a large tumour nodule of spindle cell type
(52.9%), with a mean of 7.3 dermal mitoses per mm2
(range, 1–40) and high TILS (58.8%). The majority of the patients
did not have regression (66.5%), satellitosis (88.9%), in-transit
metastases (83.3%), LVSI (88.9%), or LS (88.9%). In our cohort, 3
cases of LS with vulvar melanoma were identified (Table III). In all these patients, LS
was observed with or without melanoma in situ, but always
disappeared beneath the invasive melanoma. No pre-existing nevi
were found, but 2 patients showed large single melanocytes at the
edge of the melanoma in situ. Representative macroscopic
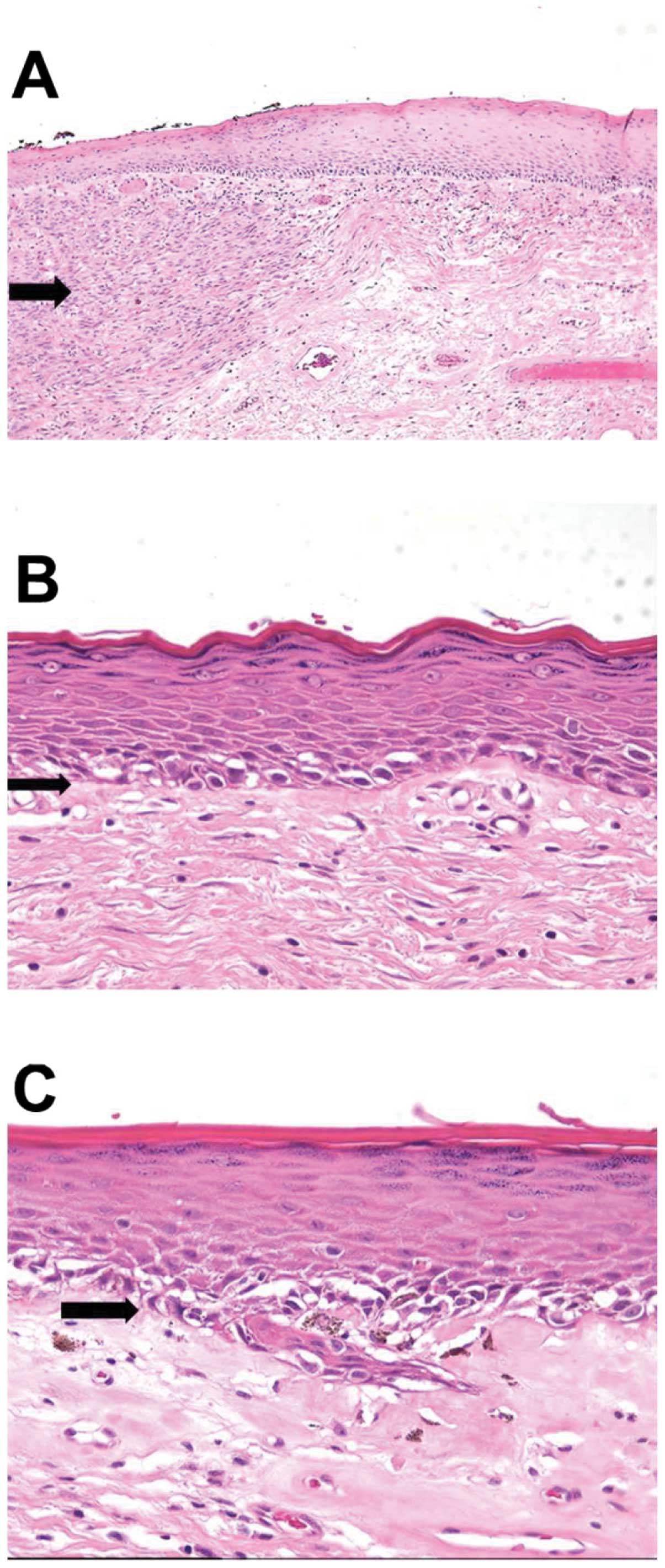
images of a vulvar melanoma specimen and histological examples for
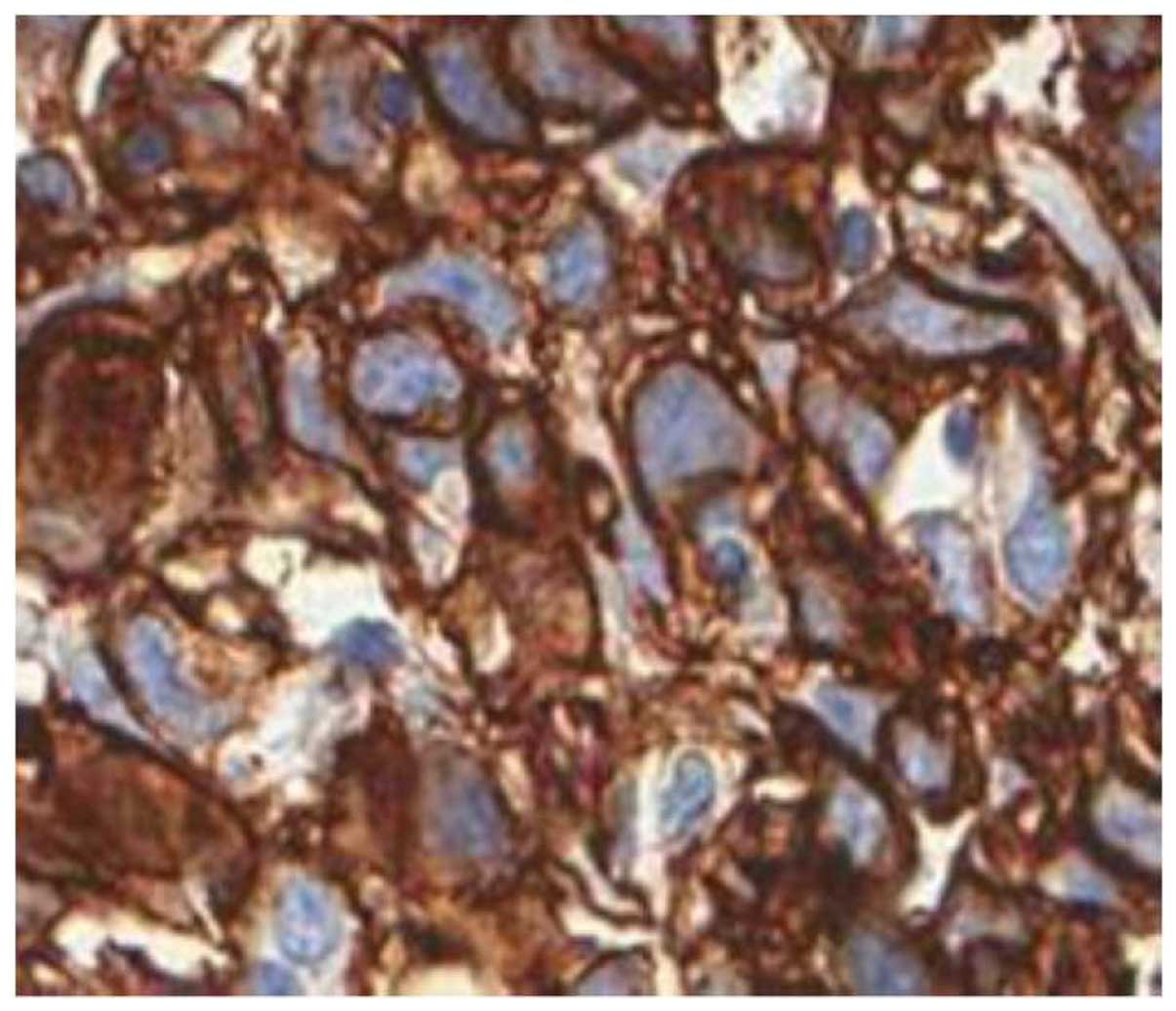
various pathological features are presented in Figs. 1 and 2. An example of a strong c-KIT
expression in an invasive melanoma of the vulva is illustrated in
Fig. 3.
 | Table IIIStudies identified in the literature
documenting synchronous lichen sclerosus and vulvar melanoma. |
Table III
Studies identified in the literature
documenting synchronous lichen sclerosus and vulvar melanoma.
| Author/(Refs.) | Year | Age | Depth (mm) | Lymph nodes | Follow-up
(months) | Status |
|---|
| Friedman et
al (16) | 1984 | 14 | 0.7 | Negative | 12 | NED |
| Egan et al
(17) | 1997 | 9 | In situ | N/A | N/A | N/A |
| Egan et al
(17) | 1997 | 11 | 0.47 | N/A | N/A | N/A |
| Carlson et
al (18) | 2002 | 83 | 2.7 | Negative | 21 | NED |
| Hassanein et
al (19) | 2004 | 10 | 0.44 | Negative | 12 | NED |
| Rosamilia et
al (20) | 2006 | 10 | 1 | Positive | 32 | NED |
| De Simone et
al (59) | 2008 | N/A | N/A | N/A | N/A | N/A |
| This study | | 69 | 1 | Negative | 120 | DOD |
| This study | | 84 | 3.5 | Negative | 12 | DOD |
| This study | | 81 | | Negative | 2 | NED |
Predictors of outcome for vulvar melanoma
identified in our cohort
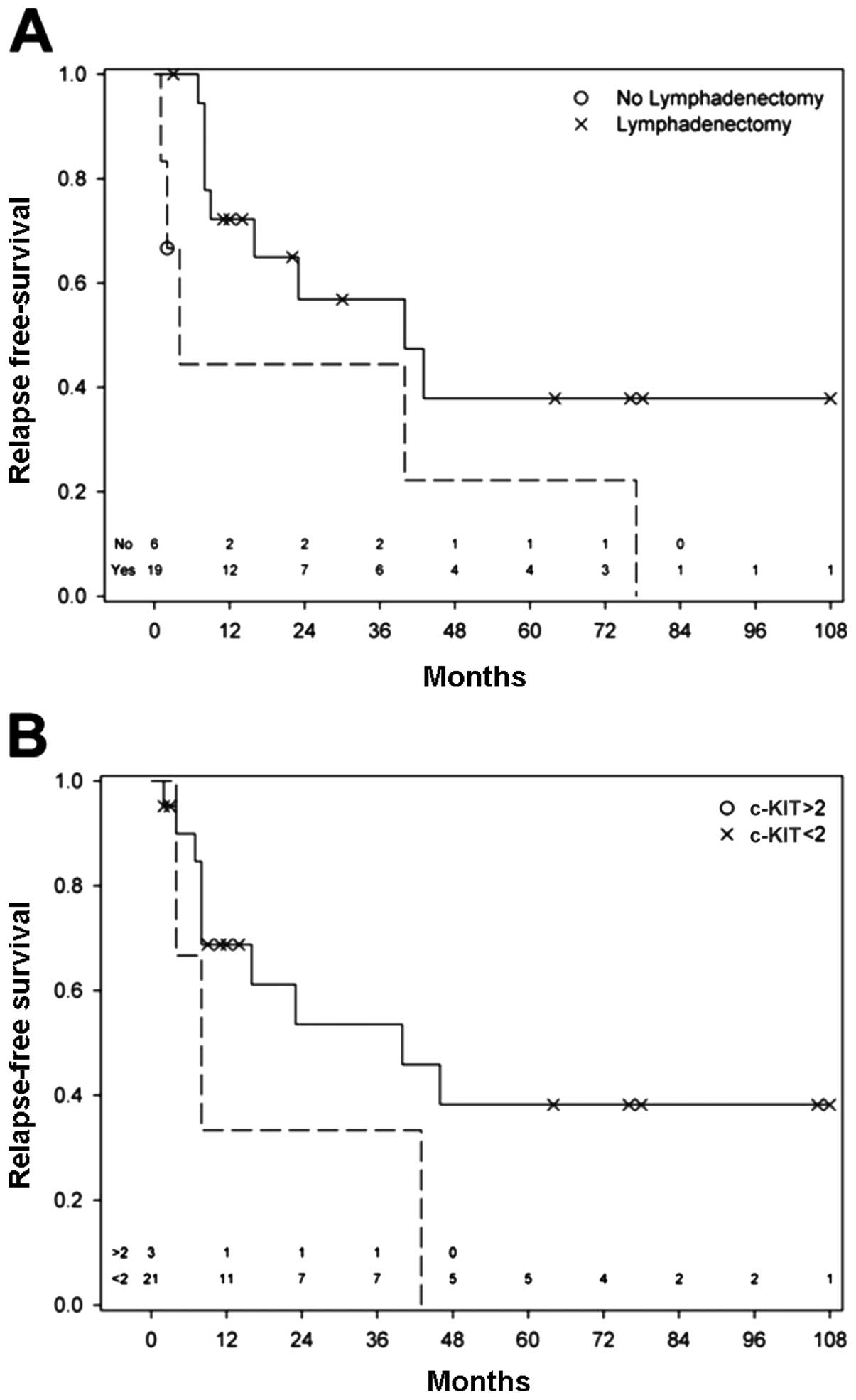
Our study confirmed the known predictive
clinicopathological characteristics Breslow’s depth [relapse-free
survival (RFS): HR=1.08, p=0.049] and lymphadenectomy (RFS:
HR=0.376, p=0.087, Table IV and
Fig. 4A). These were particularly
important in relation to recurrence. No significant results for
positivity of lymph nodes were found in our series, possibly due to
the low numbers of positive lymph nodes. In the presence of a
lateral margin of >10 mm [disease-free survival (DFS): HR=2.7,
p=0.21] and a strong (intensity 3+) c-KIT expression (DFS: HR=1.8,
p=0.49; RFS: HR=3.13, p=0.108; Fig.
3), Breslow’s depth becomes less important as regards the
outcome (Table IV, Fig. 4B).
 | Table IVMultivariable analysis of high-risk
features. |
Table IV
Multivariable analysis of high-risk
features.
| A, Relapse-free
survival | | | |
|---|
|
|---|
| Predictor | HR (95% CI) | aHRa (95% CI) | aHRb (95% CI) |
|---|
| Pathological
characteristics | 5.02
(0.62–40.61) | 4.86
(0.58–40.81) | 2.89
(0.35–23.83) |
|
Lymphadenectomy | 0.38
(0.12–1.15) | 0.15
(0.03–0.64) | |
| Cell type | 0.75
(0.26–2.19) | 0.72
(0.23–2.22) | |
| Lateral margin | 1.95
(0.53–7.22) | 1.86
(0.49–7.03) | |
| c-KIT
expression | 2.45
(0.66–9.08) | 3.13
(0.78–12.58) | 2.51
(0.61–10.36) |
| Breslow’s
depth | 1.08
(1.00–1.17) | | 1.12
(1.02–1.22) |
|
| B, Disease-free
survival | | | |
|
| Predictor | HR (95% CI) | aHRa (95% CI) | aHRb (95% CI) |
|
| Pathological
characteristics | | | |
|
Lymphadenectomy | 0.71
(0.19–2.63) | 0.25
(0.06–0.99) | 0.31
(0.04–2.44) |
| Cell type | 0.82
(0.25–2.73) | 0.80
(0.24–2.72) | |
| Lateral margin | 2.72
(0.58–12.88) | 2.67
(0.56–12.76) | 7.32
(0.77–69.86) |
| c-KIT
expression | 1.82
(0.38–8.67) | 1.75
(0.35–8.63) | 3.34
(0.42–26.29) |
| Breslow’s
depth | 1.01
(0.92–1.10) | 0.93
(0.57–1.50) | |
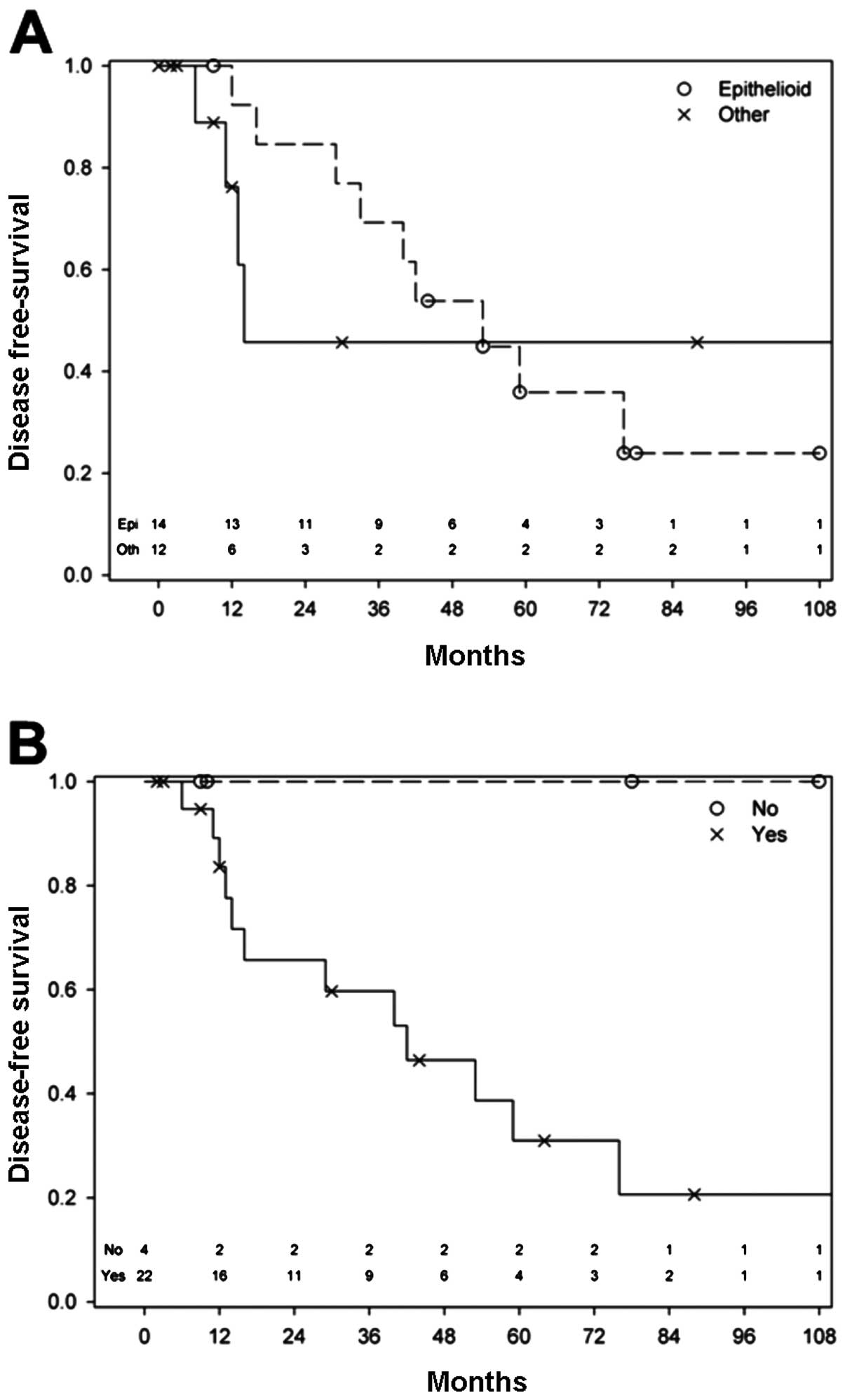
The presence of epithelioid cells within a vulvar
melanoma, even when mixed in combination with spindle or nodular
cells, predicted a better prognosis for these patients (HR=0.82,
p=0.75) (Table IV, Fig. 5A). Of the 3 patients, who
contribute to the plateau in the non-epithelioid curve in the
Kaplan-Meier plot, 2 had a spindle/epithelioid and the other had
another cell type, thus supporting our findings.
Due to our cohort size and the low numbers of
certain pathological characteristics, we looked in a combined
approach at high-risk pathological features, such as satellitosis,
in-transit metastases, LVSI and dermal mitosis. This meant that the
presence of any of these 4 features within a cancer was taken for
the purpose of this analysis as the presence of a high-risk
pathological feature. Using this approach, we found that in the
absence of at least one of these features, none of the patients
died (log-rank test, p=0.088), which had a sensitivity of 100%
(Fig. 5B). As regards recurrence,
the presence of at least one of these pathological features
increased the risk of recurrence from the disease by a factor of 5
(HR=5.02).
Independent predictors of outcome and
c-KIT expression
We modelled the identified predictors of prognosis
with each other in order to identify the degree of correlation
between the predictive parameters. In all models, the strongest
predictors for outcome, both in respect of relapse and survival,
was the absence of any of the pathological high-risk
characteristics, such as satellitosis, in-transit metastases, LVSI
or dermal mitosis.
In combination with the pathological high-risk
characteristics, the strongest predictors for earlier relapse were
c-KIT expression [adjusted HR (aHR)=2.51, Table IVA] and Breslow’s depth
(aHR=1.12, Table IVA). With the
increasing depth of the melanoma, lymphadenectomy presents with a
HR of 6.86 (p=0.011) and Breslow’s depth remains statistically
significant (HR=1.13, p=0.0079). Breslow’s depth, in the presence
of some of the other factors, seems to be more important for
recurrence than DFS. None of the classical adjuvant treatment
options, including immunotherapy showed any benefit in our
study.
When modelled together, the strongest predictors of
earlier death were pathologically high-risk characteristics,
followed by lateral margin (>10 mm, aHR=7.32, Table IVB), a strong c-KIT expression
(aHR=3.34, Table IVB) and
lymphadenectomy (aHR=0.3, Table
IVB). For DFS, Breslow’s depth loses its strong predictive
value when compared to a lateral margin of >10 mm and a strong
c-KIT expression. The comparison of the lateral margin to strong
c-KIT expression identified the lateral margin as more important
for survival.
The combined multivariable model for the prediction
of DFS consisted of a) lymphadenectomy, b) absence of any of the
pathological high-risk characteristics, c) strong c-KIT expression,
and d) Breslow’s depth, and was highly statistically significant
(p=0.0004).
Discussion
Whilst in recent years great achievements in disease
awareness, in early diagnosis, and in the treatment of cutaneous
melanomas with subsequent benefits in morbidity and mortality have
been made, no similar development exists for vulvar melanomas.
Patients with vulvar melanomas usually present with the disease at
a late stage and have a poor prognosis. Its aetiology is poorly
understood and the prognostic predictors reported in the literature
are not fully conclusive. Research into vulvar melanoma is also
limited due to the low incidence of cases per centre and low
numbers of international collaborative studies or
meta-analyses.
Our comprehensive literature review of 46 studies
published from 1990 until 2012 identified Breslow’s depth and the
inguinal lymph node status as unequivocal and tumour ulceration,
age at diagnosis, and DNA ploidy as less clear or ambiguous
clinical predictors of outcome. On the molecular/genetic level,
mutations in p53, c-KIT and kinases of the PI3K/AKT/mTOR-
and RAS/RAF/MEK/ERK-pathways have been reported in association with
vulvar melanoma. p53 is a tumour suppressor gene (65), c-KIT is a receptor tyrosine
kinase, mutations of which are integral for tumour growth and
progression (66), and
PI3K/AKT/mTOR- and RAS/RAF/MEK/ERK-pathways regulate growth and
proliferation (67,68). By contrast, neither mutations in
BRAF or NRAS nor an involvement of viral infection
were found. These data may not be conclusive and high-throughput
transcription profiling experiments on vulvar melanomas are likely
to identify additional genes, the mutations of which are associated
with vulvar melanoma.
In our cohort of 33 adult patients, 3 cases (9.1%)
of vulvar melanoma with LS (Table
III) were identified, suggesting an association. This is
noteworthy, as to date, reported cases of LS associated with vulvar
melanomas were mainly limited to juvenile cases (16–20) (Table III). In our 3 cases, the LS was
present in melanoma in situ, but disappeared in the invasive
melanoma, where dermal hyalinisation was replaced by desmoplasia.
The limited number of reports on the association of LS with adult
vulvar melanoma may be due to under-reporting and lack of
recognition.
In our cohort, we also found an increased c-KIT
protein expression in approximately half of the patients,
suggesting a role of c-KIT in vulvar melanoma. In fact, c-KIT
mutations have been shown to be more common in vulvar than
cutaneous melanomas (13,14). c-KIT is a receptor tyrosine kinase
regulating a variety of biological responses, such as chemotaxis,
cell proliferation, apoptosis and adhesion in many cell types,
including melanocytes, and activating KIT mutations are integral
for tumour growth and progression (69); however, their role in vulvar
melanoma is yet not known.
Over the years, a number of histopatological
features have been shown to correlate with adverse prognosis. These
include Breslow’s depth, ulceration, epithelioid cell type,
microsatellitosis, regression, angiolymphatic involvement, high
mitotic rate, amelanosis and association with an existing nevus
(3,5,35,51). An American study demonstrated that
increasing Breslow depth was associated with declining survival,
whereas other histopathological features, such as ulceration,
increasing mitotic index, and the presence of atypical melanocytic
hyperplasia were not associated with a significant difference in
survival (30). A recent Chinese
study revealed that macroscopic tumour growth and treatment method
were independent prognostic factors for overall survival (70). Our study confirmed Breslow’s depth
and lymphadenectomy as strong predictors for recurrence and poorer
DFS.
Our study also identified other predictive features.
Among those was the absence of any of the pathological high-risk
characteristics (satellitosis, in-transit metastases, LVSI or
dermal mitosis) identified in a subset of patients with vulvar
melanoma. These patients survived disease with a prediction of 100%
sensitivity, making the absence of these characteristics strong
predictors for outcome, both in terms of relapse and survival. This
group of patients may qualify for follow-up after surgery,
particularly when an optimal adjuvant therapy is not available. An
increased c-KIT expression was also identified as a strong negative
predictor of DFS and a strong positive predictor of earlier
relapse. By contrast, no significant results for positivity of
lymph nodes were observed in our study, possibly due to the low
numbers of positive lymph nodes.
The identification of mutated genes, such as
c-KIT and p53 or increased levels of c-KIT in vulvar
melanomas seems consistent with the current consensus that vulvar
melanomas arise de novo from the malignant transformation of
a single junctional melanocyte in situ (4). Indeed, we found single large
junctional melanocytes adjacent to melanoma in situ, which
has, to our knowledge, not been reported previously. Mucosal
melanomas arise from an epithelium normally devoid of melanocytes;
the significance of melanocytes in a location where they are not
normally present therefore requires further investigation.
The treatment of vulvar melanomas has thus far been
largely restricted to surgical options, with little prospective
data and no randomised studies available. Following on the trend
from cutaneous melanomas, the surgical approach for vulvar
melanomas has changed from extensive to more limited procedures due
to the recognition that no improvement in overall survival can be
achieved with aggressive surgery despite increasing patient
morbidity (32,71), and no benefit is found from pelvic
lymphadenectomies in the absence of groin node metastases (72,73), similar to squamous cell carcinomas
of the vulva. In the absence of adequate randomized controlled
trials, adjuvant treatments included radiotherapy, chemotherapy,
immunotherapy, and in one case targeted therapy. Immunotherapy
using interferon α2b has shown significantly improved DFS in
randomized controlled trials, but there is significant morbidity
(74–76). The role of adjuvant radiotherapy
is unknown and may only be used in the case of close surgical
margins, whilst recurrent cancer in the absence of metastatic
disease is best managed surgically.
An important development is the evidence that
mucosal (vulvar melanomas are classified as mucosal) and cutaneous
melanomas are distinct genetic entities and should be studied and
treated as such (13,15,77). Gene mutations for cutaneous
melanomas did not prove to be of relevance in vulvar melanomas
(BRAF, NRAS) whilst p53 and c-KIT mutations were identified and may
enable therapeutic options in the future. Pathological classifiers,
such as satellitosis, in-transit metastases, LVSI and dermal
mitosis can stratify patients who would profit from the
investigation into c-KIT expression and the subsequent imatinib
treatment. Imatinib is a targeted oral therapeutic agent against
cutaneous melanomas. An Australian study has shown some efficacy
with the treatment of imatinib in mucosal melanomas, including
vulvar melanomas (78). More
studies into the genetic background, making use of high-throughput
transcription profiling technology increasingly becoming available,
are required to develop targeted treatment options, particularly in
high-risk groups.
International trials with imatinib or any other
therapeutic option available in the future in high-risk vulvar
melanomas will be beneficial, but will face all the difficulties
associated with targeting very rare tumours. The centralization of
care for patients with vulvar melanoma is inevitable. Whilst the
surgical part of their treatment is best performed in a
gynaecological cancer centre, ongoing care should best be shared
within a multi-disciplinary approach, involving both gynaecological
oncologists and melanoma centres.
Acknowledgements
This study was supported by the Cancer Institute NSW
(09/CRF/2–02 to V.H.S.); RANZCOG (to V.H-.S.); William Maxwell
Trust (to V.H-.S.) and the Royal Hospital for Women Foundation (to
V.H-.S.).
References
|
1
|
Woolcott RJ, Henry RJ and Houghton CR:
Malignant melanoma of the vulva. Australian experience. J Reprod
Med. 33:699–702. 1988.PubMed/NCBI
|
|
2
|
Ragnarsson-Olding BK: Primary malignant
melanoma of the vulva - an aggressive tumor for modeling the
genesis of non-UV light-associated melanomas. Acta Oncol.
43:421–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradgate MG, Rollason TP, McConkey CC, et
al: Malignant melanoma of the vulva: a clinicopathological study of
50 women. Br J Obstet Gynaecol. 97:124–133. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blessing K, Kernohan NM and Park KG:
Subungual malignant melanoma: clinicopathological features of 100
cases. Histopathology. 19:425–429. 1999. View Article : Google Scholar
|
|
5
|
Ragnarsson-Olding BK, Kanter-Lewensohn LR,
Lagerlof B, et al: Malignant melanoma of the vulva in a nationwide,
25-year study of 219 Swedish females: clinical observations and
histopathologic features. Cancer. 86:1273–1284. 1999.
|
|
6
|
Ragnarsson-Olding BK, Nilsson BR,
Kanter-Lewensohn LR, et al: Malignant melanoma of the vulva in a
nationwide, 25-year study of 219 Swedish females: predictors of
survival. Cancer. 86:1285–1293. 1999.
|
|
7
|
Ragnarsson-Olding B, Platz A, Olding L, et
al: p53 protein expression and TP53 mutations in malignant
melanomas of sun-sheltered mucosal membranes versus chronically
sun-exposed skin. Melanoma Res. 14:395–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irvin WP Jr, Legallo RL, Stoler MH, et al:
Vulvar melanoma: a retrospective analysis and literature review.
Gynecol Oncol. 83:457–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verschraegen CF, Benjapibal M,
Supakarapongkul W, et al: Vulvar melanoma at the M.D. Anderson
Cancer Center: 25 years later. Int J Gynecol Cancer. 11:359–364.
2001.PubMed/NCBI
|
|
10
|
Sugiama VE, Chan JK, Shin JY, et al:
Vulvar melanoma: a multivariable analysis of 644 patients. Obstet
Gynecol. 110:296–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu DN, Yu GP and McCormick SA:
Population-based incidence of vulvar and vaginal melanoma in
various races and ethnic groups with comparisons to other
site-specific melanomas. Melanoma Res. 20:153–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mert I, Semaan A, Winer I, et al:
Vulvar/Vaginal melanoma: an updated surveillance epidemiology and
end results database review, comparison with cutaneous melanoma and
significance of racial disparities. Int J Gynecol Cancer.
23:1118–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omholt K, Grafstrom E, Kanter-Lewensohn L,
et al: KIT pathway alterations in mucosal melanomas of the vulva
and other sites. Clin Cancer Res. 17:3933–3942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schoenewolf NL, Bull C, Belloni B, et al:
Sinonasal, genital and acrolentiginous melanomas show distinct
characteristics of KIT expression and mutations. Eur J Cancer.
48:1842–1852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiveskog S, Ragnarsson-Olding B, Platz A,
et al: N-ras mutations are common in melanomas from sun-exposed
skin of humans but rare in mucosal membranes or unexposed skin. J
Invest Dermatol. 111:757–761. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman RJ, Kopf AW and Jones WB:
Malignant melanoma in association with lichen sclerosus on the
vulva of a 14-year-old. Am J Dermatopathol. 6(Suppl): 253–256.
1984.PubMed/NCBI
|
|
17
|
Egan CA, Bradley RR, Logsdon VK, et al:
Vulvar melanoma in childhood. Arch Dermatol. 133:345–348. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carlson JA, Mu XC, Slominski A, et al:
Melanocytic proliferations associated with lichen sclerosus. Arch
Dermatol. 138:77–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassanein AM, Mrstik ME, Hardt NS, et al:
Malignant melanoma associated with lichen sclerosus in the vulva of
a 10-year-old. Pediatr Dermatol. 21:473–476. 2004.PubMed/NCBI
|
|
20
|
Rosamilia LL, Schwartz JL, Lowe L, et al:
Vulvar melanoma in a 10-year-old girl in association with lichen
sclerosus. J Am Acad Dermatol. 54:S52–S53. 2006.PubMed/NCBI
|
|
21
|
Powell JJ and Wojnarowska F: Lichen
sclerosus. Lancet. 353:1777–1783. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scurry J: Does lichen sclerosus play a
central role in the pathogenesis of human papillomavirus negative
vulvar squamous cell carcinoma? The itch-scratch-lichen sclerosus
hypothesis. Int J Gynecol Cancer. 9:89–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith YR and Haefner HK: Vulvar lichen
sclerosus: pathophysiology and treatment. Am J Clin Dermatol.
5:105–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El Shabrawi-Caelen L, Soyer HP, Schaeppi
H, et al: Genital lentigines and melanocytic nevi with superimposed
lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol.
50:690–694. 2004.PubMed/NCBI
|
|
25
|
Breslow A: Thickness, cross-sectional
areas and depth of invasion in the prognosis of cutaneous melanoma.
Ann Surg. 172:902–908. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung AF, Woodruff JM and Lewis JL Jr:
Malignant melanoma of the vulva: a report of 44 cases. Obstet
Gynecol. 45:638–646. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Podratz KC, Gaffey TA, Symmonds RE, et al:
Melanoma of the vulva: an update. Gynecol Oncol. 16:153–168. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim CJ, Reintgen DS and Balch CM: The new
melanoma staging system. Cancer Control. 9:9–15. 2002.
|
|
29
|
Zambo K, Szabo Z, Schmidt E, et al: Is the
clinical staging system a good choice in the staging of vulvar
malignancies? Eur J Nucl Med Mol Imaging. 34:1878–1879. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tcheung WJ, Selim MA, Herndon JE 2nd, et
al: Clinicopathologic study of 85 cases of melanoma of the female
genitalia. J Am Acad Dermatol. 67:598–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scheistroen M, Trope C, Koern J, et al:
Malignant melanoma of the vulva. Evaluation of prognostic factors
with emphasis on DNA ploidy in 75 patients. Cancer. 75:72–80. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trimble EL, Lewis JL Jr, Williams LL, et
al: Management of vulvar melanoma. Gynecol Oncol. 45:254–258. 1992.
View Article : Google Scholar
|
|
33
|
Tasseron EW, van der Esch EP, Hart AA, et
al: A clinicopathological study of 30 melanomas of the vulva.
Gynecol Oncol. 46:170–175. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piura B, Egan M, Lopes A, et al: Malignant
melanoma of the vulva: a clinicopathologic study of 18 cases. J
Surg Oncol. 50:234–240. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ragnarsson-Olding B, Johansson H, Rutqvist
LE, et al: Malignant melanoma of the vulva and vagina. Trends in
incidence, age distribution, and long-term survival among 245
consecutive cases in Sweden 1960–1984. Cancer. 71:1893–1897.
1993.PubMed/NCBI
|
|
36
|
Look KY, Reisinger M, Stehman FB, et al:
Blood transfusion and the risk of recurrence in squamous carcinoma
of the vulva. Am J Obstet Gynecol. 168:1718–1723. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Phillips GL, Bundy BN, Okagaki T, et al:
Malignant melanoma of the vulva treated by radical hemivulvectomy.
A prospective study of the Gynecologic Oncology Group. Cancer.
73:2626–2632. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dunton CJ, Kautzky M and Hanau C:
Malignant melanoma of the vulva: a review. Obstet Gynecol Surv.
50:739–746. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raber G, Mempel V, Jackisch C, et al:
Malignant melanoma of the vulva. Report of 89 patients. Cancer.
78:2353–2358. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trimble EL: Melanomas of the vulva and
vagina. Oncology (Williston Park). 10:1017–1024. 1996.PubMed/NCBI
|
|
41
|
Scheistroen M, Trope C, Kaern J, et al:
Malignant melanoma of the vulva FIGO stage I: evaluation of
prognostic factors in 43 patients with emphasis on DNA ploidy and
surgical treatment. Gynecol Oncol. 61:253–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Strauss BS: Silent and multiple mutations
in p53 and the question of the hypermutability of tumors.
Carcinogenesis. 18:1445–1452. 1997.PubMed/NCBI
|
|
43
|
De Matos P, Tyler D and Seigler HF:
Mucosal melanoma of the female genitalia: a clinicopathologic study
of forty-three cases at Duke University Medical Center. Surgery.
124:38–48. 1998.PubMed/NCBI
|
|
44
|
Larsson KB, Shaw HM, Thompson JF, et al:
Primary mucosal and glans penis melanomas: the Sydney Melanoma Unit
experience. Aust N Z J Surg. 69:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Creasman WT, Phillips JL and Menck HR: A
survey of hospital management practices for vulvar melanoma. J Am
Coll Surg. 188:670–675. 1999.PubMed/NCBI
|
|
46
|
Raspagliesi F, Ditto A, Paladini D, et al:
Prognostic indicators in melanoma of the vulva. Ann Surg Oncol.
7:738–742. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ragnarsson-Olding BK, Karsberg S, Platz A,
et al: Mutations in the TP53 gene in human malignant melanomas
derived from sun-exposed skin and unexposed mucosal membranes.
Melanoma Res. 12:453–463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
De Hullu JA, Hollema H, Hoekstra HJ, et
al: Vulvar melanoma: is there a role for sentinel lymph node
biopsy? Cancer. 94:486–491. 2002.
|
|
49
|
Finan MA and Barre G: Bartholin’s gland
carcinoma, malignant melanoma and other rare tumours of the vulva.
Best Pract Res Clin Obstet Gynaecol. 17:609–633. 2003.
|
|
50
|
Harting MS and Kim KB: Biochemotherapy in
patients with advanced vulvovaginal mucosal melanoma. Melanoma Res.
14:517–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wechter ME, Gruber SB, Haefner HK, et al:
Vulvar melanoma: a report of 20 cases and review of the literature.
J Am Acad Dermatol. 50:554–562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Edwards RH, Ward MR, Wu H, et al: Absence
of BRAF mutations in UV-protected mucosal melanomas. J Med Genet.
41:270–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dahlgren L, Schedvins K, Kanter-Lewensohn
L, et al: Human papilloma virus (HPV) is rarely detected in
malignant melanomas of sun sheltered mucosal membranes. Acta Oncol.
44:694–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Stang A, Streller B, Eisinger B, et al:
Population-based incidence rates of malignant melanoma of the vulva
in Germany. Gynecol Oncol. 96:216–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rouzier R, Haddad B, Atallah D, et al:
Surgery for vulvar cancer. Clin Obstet Gynecol. 48:869–878. 2005.
View Article : Google Scholar
|
|
56
|
Lundberg R, Brytting M, Dahlgren L, et al:
Human herpes virus DNA is rarely detected in non-UV
light-associated primary malignant melanomas of mucous membranes.
Anticancer Res. 26:3627–3631. 2006.PubMed/NCBI
|
|
57
|
Dhar KK, Das N, Brinkman DA, et al:
Utility of sentinel node biopsy in vulvar and vaginal melanoma:
report of two cases and review of the literature. Int J Gynecol
Cancer. 17:720–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Giraud G, Ramqvist T, Ragnarsson-Olding B,
et al: DNA from BK virus and JC virus and from KI, WU, and MC
polyomaviruses as well as from simian virus 40 is not detected in
non-UV-light-associated primary malignant melanomas of mucous
membranes. J Clin Microbiol. 46:3595–3598. 2008. View Article : Google Scholar
|
|
59
|
De Simone P, Silipo V, Buccini P, et al:
Vulvar melanoma: a report of 10 cases and review of the literature.
Melanoma Res. 18:127–133. 2008.PubMed/NCBI
|
|
60
|
Moan J, Porojnicu AC, Dahlback A, et al:
Where the sun does not shine: is sunshine protective against
melanoma of the vulva? J Photochem Photobiol B. 101:179–183. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Trifiro G, Travaini LL, Sanvito F, et al:
Sentinel node detection by lymphoscintigraphy and sentinel lymph
node biopsy in vulvar melanoma. Eur J Nucl Med Mol Imaging.
37:736–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Terlou A, Blok LJ, Helmerhorst TJ, et al:
Premalignant epithelial disorders of the vulva: squamous vulvar
intraepithelial neoplasia, vulvar Paget’s disease and melanoma in
situ. Acta Obstet Gynecol Scand. 89:741–748. 2010.
|
|
63
|
Baiocchi G, Duprat JP, Neves RI, et al:
Vulvar melanoma: report on eleven cases and review of the
literature. Sao Paulo Med J. 128:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ragnarsson-Olding BK: Spatial density of
primary malignant melanoma in sun-shielded body sites: a potential
guide to melanoma genesis. Acta Oncol. 50:323–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar
|
|
66
|
Postow MA and Carvajal RD: Therapeutic
implications of KIT in melanoma. Cancer J. 18:137–141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Markman B, Dienstmann R and Tabernero J:
Targeting the PI3K/Akt/mTOR pathway-beyond rapalogs. Oncotarget.
1:530–543. 2010.PubMed/NCBI
|
|
68
|
De Luca A, Maiello MR, D’Alessio A, et al:
The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in
cancer pathogenesis and implications for therapeutic approaches.
Expert Opin Ther Targets. 16(Suppl 2): S17–S27. 2012.PubMed/NCBI
|
|
69
|
Mehnert JM and Kluger HM: Driver mutations
in melanoma: lessons learned from bench-to-bedside studies. Curr
Oncol Rep. 14:449–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang Q, Huang H, Wan T, Deng T and Liu J:
Clinical outcome of 31 patients with primary malignant melanoma of
the vagina. J Gynecol Oncol. 24:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kornberg R, Harris M and Ackerman AB:
Epidermotropically metastatic malignant melanoma. Differentiating
malignant melanoma metastatic to the epidermis from malignant
melanoma primary in the epidermis. Arch Dermatol. 114:67–69. 1978.
View Article : Google Scholar
|
|
72
|
Jaramillo BA, Ganjei P, Averette HE, et
al: Malignant melanoma of the vulva. Obstet Gynecol. 66:398–401.
1985.PubMed/NCBI
|
|
73
|
Beller U, Demopoulos RI and Beckman EM:
Vulvovaginal melanoma. A clinicopathologic study. J Reprod Med.
31:315–319. 1986.PubMed/NCBI
|
|
74
|
Kirkwood JM, Strawderman MH, Ernstoff MS,
et al: Interferon alfa-2b adjuvant therapy of high-risk resected
cutaneous melanoma: the Eastern Cooperative Oncology Group Trial
EST 1684. J Clin Oncol. 14:7–17. 1996.PubMed/NCBI
|
|
75
|
Kirkwood JM, Ibrahim J, Lawson DH, et al:
High-dose interferon alfa-2b does not diminish antibody response to
GM2 vaccination in patients with resected melanoma: results of the
Multicenter Eastern Cooperative Oncology Group Phase II Trial
E2696. J Clin Oncol. 19:1430–1436. 2001.
|
|
76
|
Gray RJ, Pockaj BA and Kirkwood JM: An
update on adjuvant interferon for melanoma. Cancer Control.
9:16–21. 2002.PubMed/NCBI
|
|
77
|
Dunton CJ and Berd D: Vulvar melanoma,
biologically different from other cutaneous melanomas. Lancet.
354:2013–2014. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Handolias D, Salemi R, Murray W, et al:
Mutations in KIT occur at low frequency in melanomas arising from
anatomical sites associated with chronic and intermittent sun
exposure. Pigment Cell Melanoma Res. 23:210–215. 2010. View Article : Google Scholar : PubMed/NCBI
|