Introduction
Colistin, a polymyxin E, is a cyclic cationic
polypeptide antibiotic that was initially introduced in 1952 to
manage infections. Although its use was discontinued in the 1970s,
colistin was used to treat infections caused by gram-negative
bacteria in the early 1980s, particularly since these type of
bacteria could resist almost all classes of commercially available
antibiotics (1–4). Through alteration of the outer
membrane permeability barrier, antibiotics facilitate their own
uptake and subsequently disrupt the bacterial cytoplasm membrane.
However, colistin resistance rarely occurred (5,6).
Multidrug-resistant (MDR) bacterial infections are on the increase
in hospitals, resulting in the use of colistin. However, colistin
may cause renal and neurological toxicity, as reported in the
1970s. Nephrotoxicity is considered the most common adverse effect
of colistin (9–50%) (7–10). Neurological toxicity has also been
reported previously (11).
Although the abovementioned data are useful in gaining a better
understanding of the effects of colistin on neurons and the
mechanisms of colistin-induced neurotoxicity, the signaling
pathways involved and the identity of its molecular targets remain
to be determined.
Apoptosis is a morphological and biochemical
description of a physiological cell death mechanism that is
commonly associated with programmed events necessary for the
differentiation and development of individuals and organs (12,13). Apoptotic cell death is
characterized by chromatin condensation, DNA fragmentation,
cellular shrinkage, and membrane blebbing resulting in the
formation of apoptotic bodies (12). Apoptosis is regulated and executed
by the major protein families, Bcl-2 and caspase, which are highly
conserved from worms to humans (14,15). Many important observations on
various signaling pathways mediating apoptotic cell death have been
demonstrated. However, the mechanism of apoptosis induced by
colistin remains to be elucidated.
The PC12 cell line is derived from a tumor in the
rat adrenal gland and is the most widely used neuronal cell line
for studying mechanisms associated with neurodegenerative disorders
(16,17). Over the past 30 years, PC12 cells
have become a suitable model to study neuronal function and
differentiation (18–20) as demonstrated in a study that used
PC12 cells to study manganese-induced apoptosis in order to
understand the mechanism of neurological disorders that is similar
to parkinsonism (18).
Consequently, PC12 cells are considered a useful model for studying
the mechanism of apoptosis induced by methyl mercury, paraquat and
manganese (21–23).
The present study was designed to determine whether
colistin could induce apoptosis in PC12 cells as well as to examine
the colistin-induced apoptotic pathway in the PC12 cells model
system. First, cell viability of PC12 cells exposed to colistin was
measured by MTT assay. Subsequently, analysis of DNA fragmentation
and reactive oxygen species (ROS) level in PC12 cells treated with
colistin was carried out. In addition, to determine the apoptotic
pathway initiated by colistin treatment, changes of apoptotic
factors such as Bax, Bcl-2, Fas, Fas-L and the caspase family in
PC12 cells treated with different doses of colistin were measured
by western blot analysis. The results are likely to provide
information pertaining to the reduction and inhibition of the
neurotoxicity of colistin for the wide application of MDR bacterial
infections.
Materials and methods
Materials
Colistin sulfate (20195 U/mg),
2,7-dichlorofluorescein diacetate (DCFH-DA), anhydrous dimethyl
sulfoxide (DMSO), and MTT were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The polyclonal goat anti-rabbit antibody against
p53, cytochrome c, Bax, Bcl-2, Fas, Fas-L, caspase-3,
caspase-8, caspase-9, IgG biotinylated antibody, bicinchoninic acid
assay kit and ECL chemiluminescence detection kit were purchased
from Boster Biotechnology (Wuhan, China). Dulbecco’s modified
Eagle’s medium (DMEM) was purchased from Gibco (Scotland, UK).
Fetal calf serum (FCS) was purchased from Sijiqing Biological
Engineering Material (Hangzhou, China). Hoechst 33258 staining kit,
lysis buffer and homogenization buffer were purchased from KeyGen
Biotechnology (Nanjing, China). All other chemicals were of the
highest grade and available from commercial sources. Flasks, 6-well
and 96-well plates were from Costar, Corning Inc. (Corning, New
York, USA).
Cell culture
The PC12 cell line was obtained from the Chinese
type culture collection (CTCC, Shanghai, China). It was grown in
DMEM (Life Technologies) containing 15% FBS at 37°C in 5%
CO2 under 85–95% humidity. The cells were preincubated
in 25 cm2 flasks overnight, and the medium was replaced
with DMEM containing 10% FBS every two days. Once confluent, the
PC12 cells were collected with 0.025% trypsin and 0.02% EDTA
(dissolved in PBS), passaged at a 1:3 dilution. Cells in the
logarithmic growth phase were included for subsequent experimental
procedures.
Cell viability assay
The MTT assay method was used to assess the cell
viability (24,25). Briefly, ~1×104 cells
were plated in each well of 96-well plates. After 24 h incubation,
the cells were treated with colistin sulfate at 0, 31.25, 62.5,
125, 250 and 500 μg/ml for 24 h. Subsequently, the supernatant was
removed, and 180 μl DMEM and 20 μl of 5 mg/ml of MTT (dissolved in
PBS) were added to each well and incubated at 37°C for 4 h. The
supernatant was discarded and the purple formazan crystals were
dissolved in 150 μl of DMSO. The absorbance was measured at 570 nm
with a microplate reader (Bio-Rad, Hercules, CA, USA). The
viability of colistin-treated cells was expressed as a percentage
compared with the non-colistin treated group (control group): Cell
viability = (average absorbance value of colistin-treated
group/average absorbance value of control group) ×100%. Each
experiment was repeated at least three times.
Reactive oxygen species assay
To measure ROS generation, a fluorometric assay
using intracellular oxidation of DCFH-DA was performed as reported
previously, with slight modification (26). Following exposure to colistin
sulfate at 0, 62.5, 125 and 250 μg/ml for 24 h, PC12 cells were
incubated with 40 μM DCFH-DA dissolved in DMEM in the dark for 30
min at 37°C in a 5% CO2 atmosphere. DCFH-DA is a
nonfluorescent compound, and can be enzymatically converted to the
highly fluorescent compound, DCF, in the presence of ROS. After
loading, the cells were washed three times with DMEM and DCF
fluorescence was measured using the microplate spectrofluorometer
(Molecular Devices Co., Sunnyvale, CA, USA) with excitation and
emission wavelengths of 485 and 530 nm, respectively.
Nuclear morphology detected by Hoechst
33258
Hoechst 33258 was employed to label both intact and
apoptotic nuclei (27,28). Cells were seeded in 96-well plates
at a density of 1×105 cells/well, followed by different
concentrations of colistin sulfate (0, 62.5, 125 and 250 mg/ml)
treatment for 24 h. Following treatment, the PC12 cells were washed
in ice-cold PBS buffer (pH 7.4), fixed with 4% p-formaldehyde and
incubated with 1 μg/ml Hoechst 33258 for 3 min at room temperature.
Condensed and fragmented nuclei were evaluated by intercalation of
the fluorescent probe Hoechst 33258 into nuclear DNA. Visualization
was conducted at an excitation and emission wavelengths of 480 and
520 nm, respectively, by Olympus IMT-2 fluorescence microscopy
(Tokyo, Japan).
Western blotting determination
Following treatment with 0, 62.5, 125, 250 μg/ml
colistin sulfate for 24 h, the cellular proteins of PC12 cells were
extracted from the 6-well tissue culture plates via the addition of
100 μl of lysis buffer and incubated on ice for 60 min. Lysates
were centrifuged at 10,000 × g for 10 min at 4°C, and the
supernatant was stored at −80°C prior to electrophoresis. The
protein concentration was determined by bicinchoninic acid assay.
To analyze cytochrome c in the cytosol, the PC12 cells were
resuspended in homogenization buffer and broken by 40 strokes with
a pestle in a glass homogenizer on ice. Nuclei were removed by
centrifugation at 800 × g for 10 min. The supernatant was
centrifuged at 10,000 × g for 20 min and collected as a cytosolic
fraction. The samples (40 μg of protein) were mixed with 2X sample
buffer containing 100 mM Tris-HCl (pH 6.8), 200 mM dithiothreitol
(DTT), 4% sodium dodecyl sulfate (SDS), 20% glycerol and 0.2%
bromophenol blue. The mixtures were boiled at 100°C for 10 min and
were subjected to 12% (p53, Bax, Bcl-2, Fas, Fas-L, caspase-8 and
-9, β-actin as parameter) and 15% (caspase-3 and cytochrome
c, β-actin as parameter) SDS-polyacrylamide minigel
electrophoresis at a constant pressure of 100 V.
Subsequently, proteins on the gel were
electrotransferred onto an immobile nitrocellulose membrane (NC;
Millipore, Bedford, MA, USA) with transfer buffer [25 mM Tris-HCl
(pH 8.3), 192 mM glycine and 25% methanol]. The membranes were
blocked with blocking solution containing TBST [10 mmol/l Tris-HCl
(pH 7.6), 100 mmol/l NaCl, 0.1% Tween-20], 5% skimmed milk powder
at room temperature for 2 h, and then incubated with primary
antibodies including anti-p53, anti-Bcl-2, anti-Bax,
anti-cytochrome c, anti-caspase-9, anti-caspase-3,
anti-caspase-8, anti-Fas and anti-Fas-L antibodies (1:200 dilution)
at 4°C overnight. The membranes were washed three times (3×10 min)
in TBST and incubated with goat-anti-rabbit HRP polyclonal antibody
(1:1,000 dilution) for 1 h at room temperature. The membranes were
washed three times (3×10 min) with TBST and exposed to ECL
chemiluminescence reagents for 1–5 min. Detection was achieved by
measuring the chemiluminescence of blotting agent (ECL) following
exposure of the filters to X-Omat Kodak films. The autoradiogram
was scanned and the protein bands were quantified by densitometry
using Image J 1.42 software.
Statistical analysis
Data were expressed as means ± SEM. Statistical
significance was determined by one-way ANOVA, followed by the
Student-Newman-Keuls test for multigroup comparisons. Differences
were considered significant when P<0.05 or P<0.01.
Results
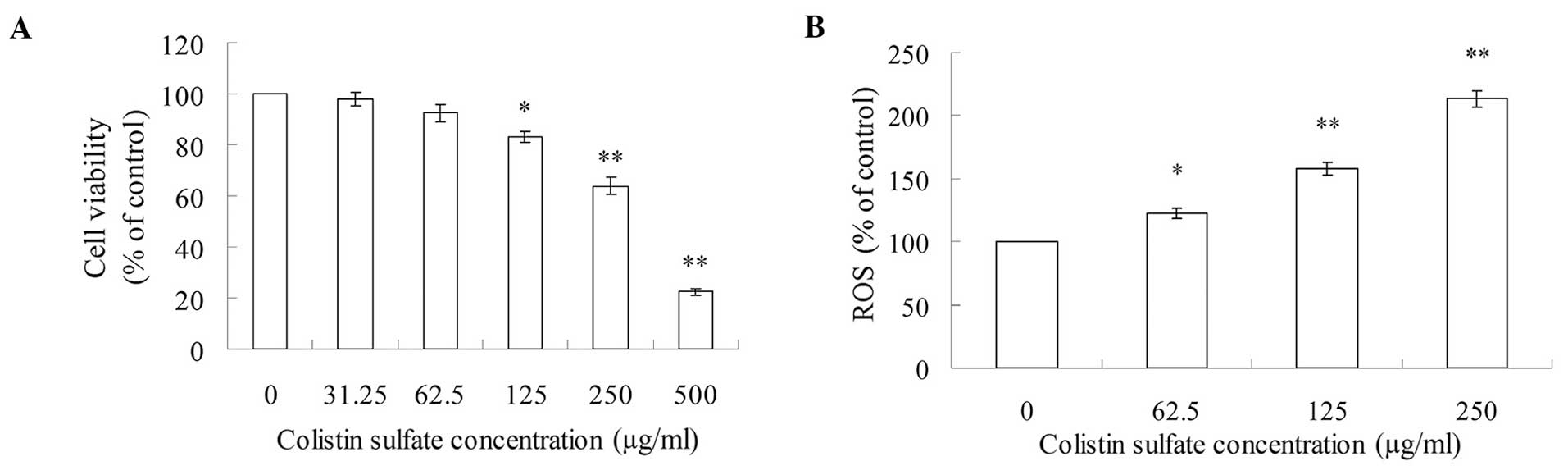
Cell viability evaluation
The PC12 cell viability of control cells was
designated as 100%. Compared with the control group, cell viability
treated with colistin sulfate from 31.25 to 500 μg/ml was reduced
from 97.7 to 22.5% in a concentration-dependent manner (Fig. 1A). When PC12 cells were exposed to
≥125 μg/ml concentration of colistin sulfate, cell viability
decreased significantly (P<0.05 or P<0.01).
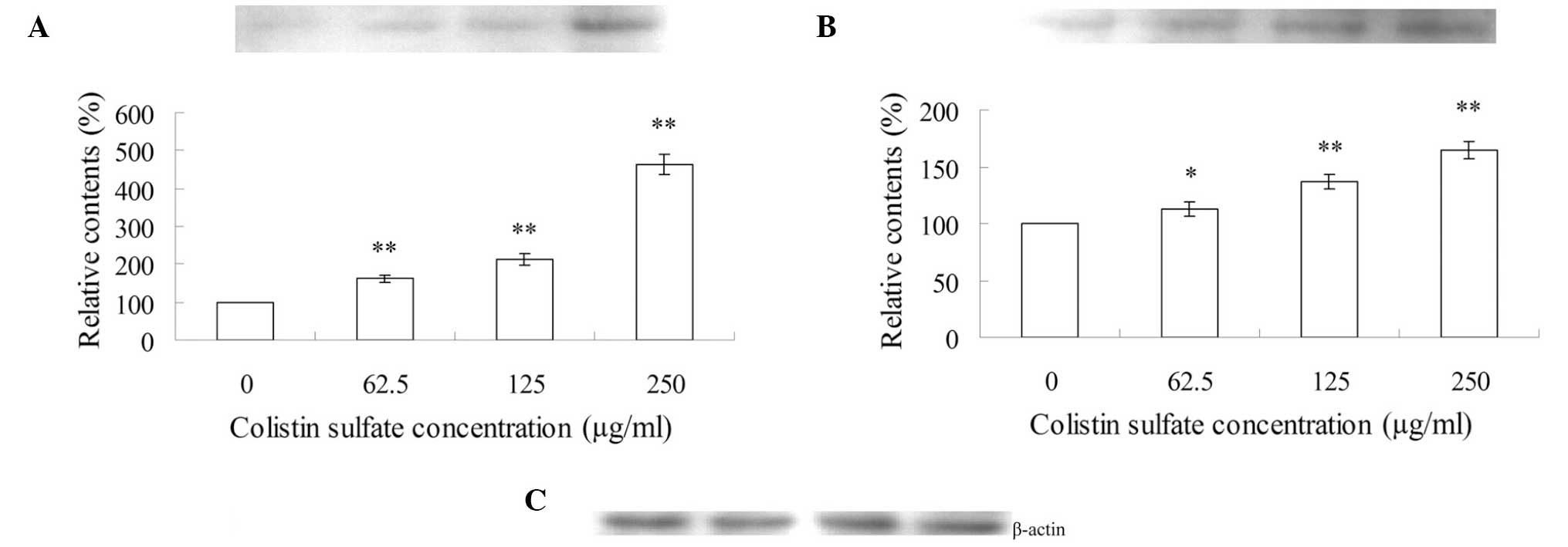
ROS level in PC12 cells
The accumulation of oxygen-free radicals was
estimated by fluorescence assay. ROS production was calculated as a
percentage of the untreated control cells. As shown in Fig. 1B, the ROS level significantly
(P<0.01) increased and had concentration-dependent changes in
PC12 cells after exposure to 62.5, 125 and 250 μg/ml colistin
sulfate for 24 h.
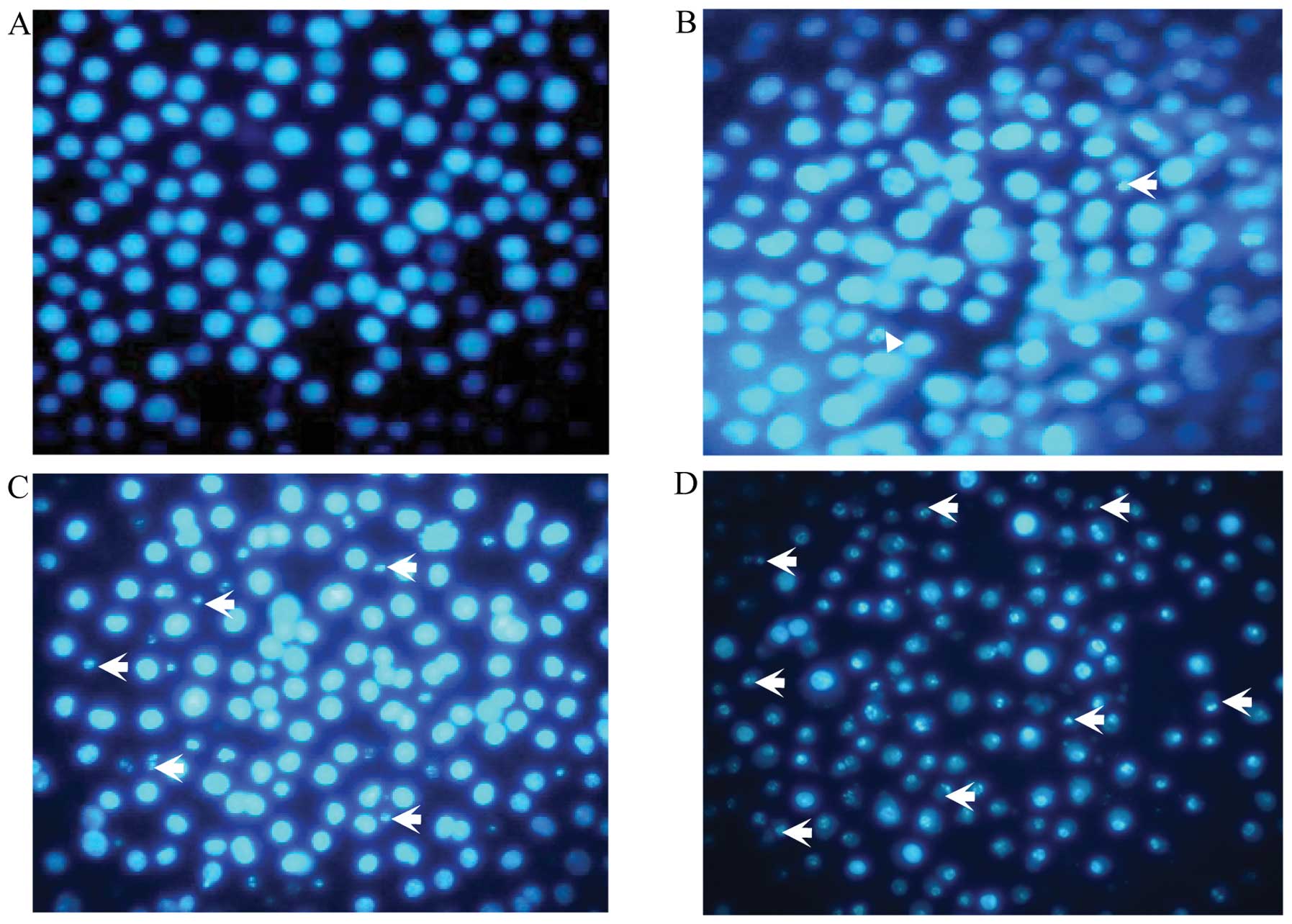
Colistin-induced DNA fragmentation in
PC12 cells
Colistin induced internucleosomal DNA fragmentation,
which is associated with chromatin condensation. Apoptotic
morphological evaluation of PC12 by Hoechst 33258 staining in
inverted fluorescence microscopy was observed. As shown in Fig. 2, the increased dose of colistin
sulfate, led to condensed and fragmented nuclei, which were
gradually increased compared with the control group in a
dose-dependent manner. These results indicated that apoptosis was
induced by colistin.
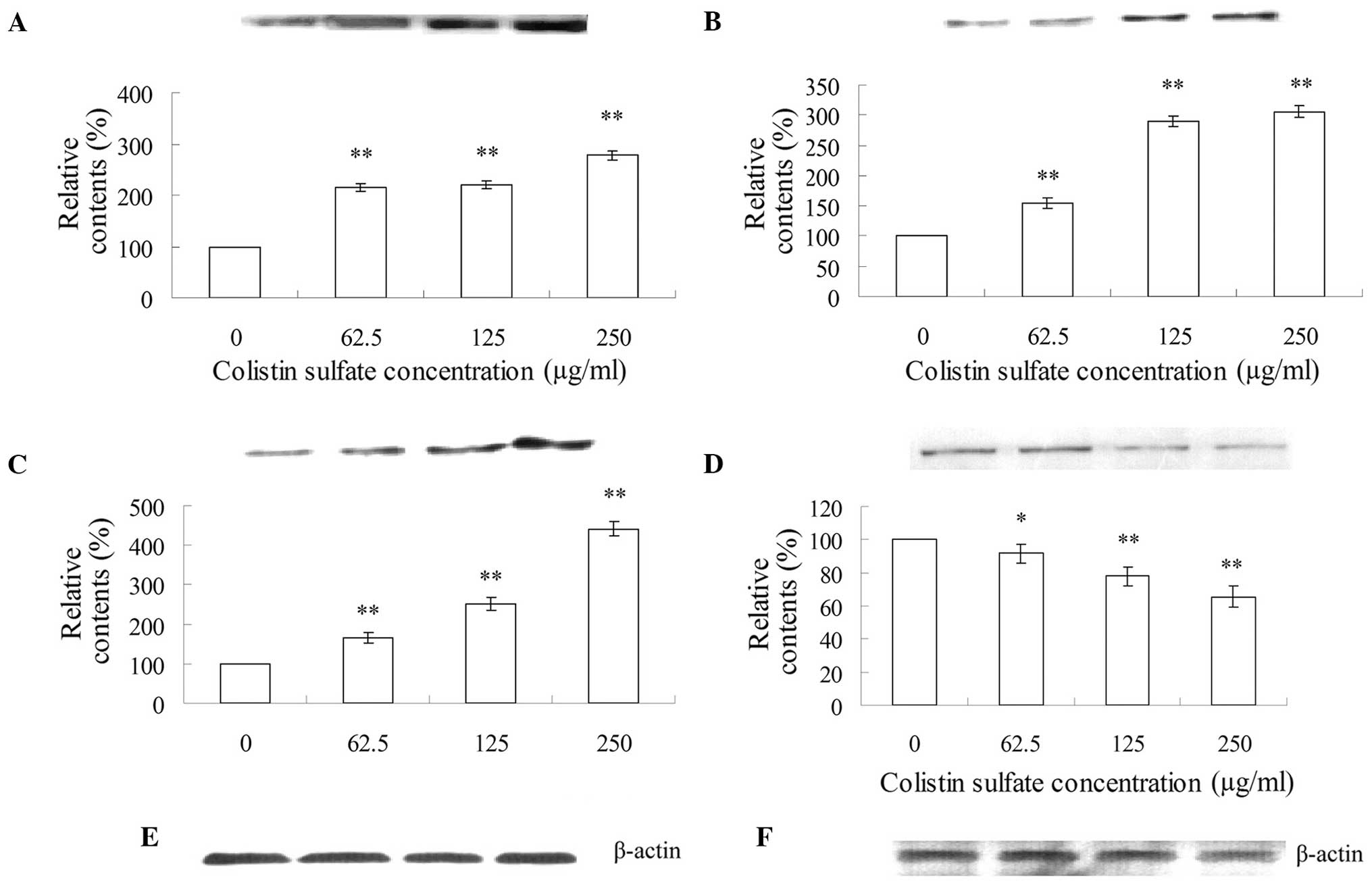
Analyses of apoptosis-related
factors
The effects of colistin sulfate treatment (0–250
μg/ml) for 24 h on the expression of apoptosis-related factors in
PC12 cells is shown in Figs.
3–5. As shown in Fig. 3, after treatment with 62.5, 125
and 250 μg/ml colistin sulfate for 24 h, the expression levels of
p53 (Fig. 3A), Bax (Fig. 3B) and cytochrome c
(Fig. 3C) were increased and were
statistically significant as compared to those of the control. By
contrast, the expression of Bcl-2 decreased significantly (Fig. 3D). Therefore, the ratio of
Bax/Bcl-2 increased in a concentration-dependent manner.
As shown in Fig.
4, Fas-L (Fig. 4A) and Fas
(Fig. 4B) in the cell lysates
released from PC12 cells treated with 62.5, 125 and 250 μg/ml
colistin sulfate for 24 h showed significant increases in
comparison with that in the control cell lysates.
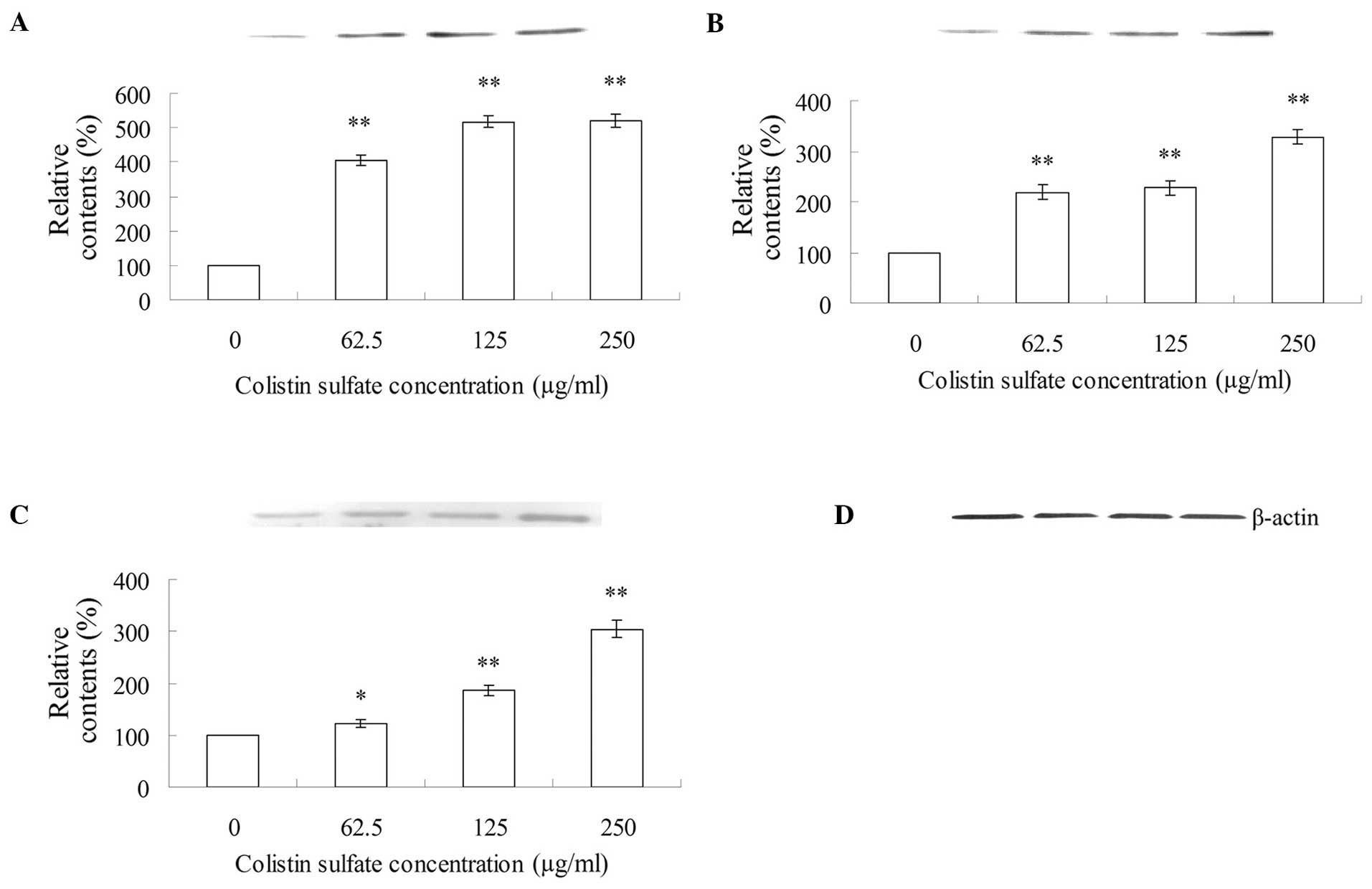
As shown in Fig.
5, the expression levels of caspase-3 (Fig. 5A), -9 (Fig. 5B) and -8 (Fig. 5C) in PC12 cells treated with 62.5,
125 and 250 μg/ml colistin sulfate for 24 h showed a significant
increase compared with those of the control
Discussion
Nosocomial infections are an increasing public
health concern worldwide that are secondary only to pan-resistant
bacterial infections. However, this increase in infections is
coupled with a marked decline of potentially active molecules in
the pharmaceutical pipeline, particularly regarding Gram-negative
infections. Colistin is a type of antibiotic that has been recently
considered as the last therapeutic option for the treatment of
patients with infections caused by multidrug resistant
gram-negative bacteria.
Based on the results obtained from the MTT assay,
concentrations of 62.5, 125, and 250 μg/ml colistin sulfate were
used in the subsequent apoptosis-related experiments. According to
the literature, it has been demonstrated that excessive generation
of ROS can cause DNA damage in neurons and initiate various other
effects (29). In the present
study, a marked ROS burst was observed in cells treated with
colistin sulfate (62.5, 125 and 500 μg/ml) (Fig. 1B). The marked ROS burst may
explain the internucleosomal DNA fragmentation. The morphological
changes of apoptotic PC12 cells induced by hydrogen peroxide were
observed by Hoechst 33258 staining (30). Our results indicated that colistin
induced internucleosomal DNA fragmentation (Fig. 2). Notably, we found that colistin
caused PC12 cell death by inducing apoptosis, as revealed by
results of the western blot analysis.
Apoptosis involves two main pathways. The first
pathway of apoptosis involves the participation of mitochondria,
regulated by the anti- and pro-apoptotic members of the Bcl-2
family (31). The Bcl-2 family of
proteins localize (or can be targeted) to mitochondria and regulate
the permeability of the mitochondrial outer membrane to several
apoptotic factors (32). Previous
studies have shown that anti-apoptotic proteins, such as Bcl-2 and
Bcl-xL, on the outer membranes of mitochondria maintain the
integrity of mitochondria (33,34). In response to an apoptotic
stimulus, pro-apoptotic Bcl-2 members, such as Bax and Bad
translocate from cytosol to mitochondria, which leads to the
formation of the membrane pores at the mitochondrial membranes
(35,36). The translocation from the
cytoplasm to mitochondria is one of the crucial steps in the
Bax-mediated apoptotic process (37,38). It has been suggested that Bax may
have induced a decrease in the membrane potential of the
mitochondria, which leads to the release of AIF and cytochrome
c from mitochondria (39,40). While cytochrome c is linked
to the caspase-dependent apoptotic signaling, AIF is involved in
the caspase-independent responses. Mitochondrial AIF translocates
to the cytosol and then into the nucleus, binds to DNA, and induces
chromatin condensation and DNA fragmentation (41,42). To investigate whether the Bcl-2
family and cytochrome c were involved in colistin-induced
apoptosis, western blot analyses of the cell lysates from PC12
cells treated with colistin were performed using antibodies against
Bax, Bcl-2 and cytochrome c. Results of the present study
have shown that mitochondria may be pertinent in mediating
apoptosis, putatively via colistin inducing the increase of ROS.
ROS may trigger the subsequent release of cytochrome c and
DNA damage in PC12 cells, and the activation of p53 (Fig. 3A) by DNA damage, which leads to
the release of cytochrome c from the mitochondria (Fig. 3C). We observed appreciable changes
in the expression of Bcl-2 and Bax by colistin induced by PC12 cell
apoptosis. Thus, the increase of Bax (Fig. 3B) and the decrease of the Bcl-2
activity in our study (Fig. 3D)
may lead to an imbalance of Bax/Bcl-2 and the further release of
cytochrome c from the mitochondria (Fig. 3C).
The second pathway is the interaction of the cell
surface receptors with their ligands, followed by the downstream
activation cascade (31). After
the involvement of Fas and its ligand, Fas-L, on the cell surface,
Bid, a pro-apoptotic Bcl-2 family member, is cleaved by caspase-8.
The cleavage of Bid produces a 15 kDa truncated Bid protein (tBid),
which translocates to the mitochondria membrane and triggers the
release of cytochrome c (39, 43). We found that the expression of Fas
and Fas-L increased in the colistin-treated PC12 cells (Fig. 4). These observations suggest that
the colistin-induced apoptosis in PC12 cells also occurred via the
death receptor-mediated pathway.
To clarify which caspases were stimulated and
increased their expression following administration of colistin,
the activity of caspase-3, -8 and -9 in PC12 cells treated with
0–250 μg/ml colistin was measured by western blotting (Fig. 5). As shown in Fig. 5, caspase-3, -8 and -9 were
activated by colistin in a dose-dependent manner. The release of
cytochrome c contributes to the activation of caspase-9 and
the subsequent activation of caspase-3 (Fig. 5) (44,45). The activation of caspase-8 led to
the subsequent activation of caspase-3. Subsequently, following the
activation of caspase-3, colistin sulfate-induced apoptosis
occurred. In this study, we found that the activity of caspase-3,
-9 and -8 in PC12 cells treated with 250 μg/ml colistin were ~5.2-,
3.3- and 3.0-fold compared with the control, respectively (Fig. 5A–C). Since the activity of
caspase-9 was higher than that of caspase-8, it indicated that
mitochondria were most pertinent in mediating apoptosis.
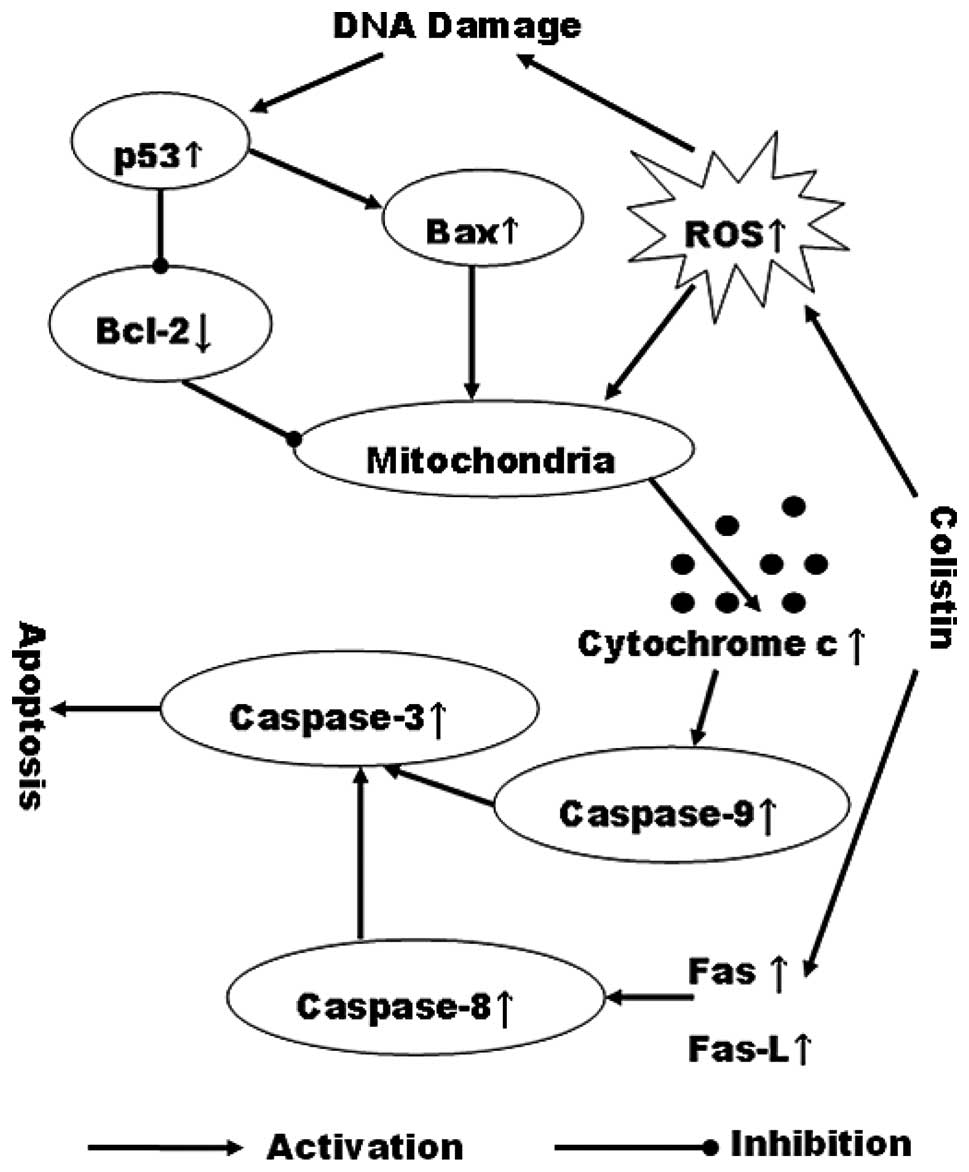
In conclusion, we demonstrated that colistin
treatment can induce PC12 cell apoptosis via the mitochondrial and
death receptor pathways (Fig. 6).
A high content of colistin can increase ROS levels in PC12 cells
and enhance the activation of caspase-3. Additionally, an increase
of Fas and Fas-L induces activating caspase-8 leading to the
activation of caspases-3. Subsequently, after activation of
caspase-3, colistin sulfate-induced apoptosis occurred. These
results have demonstrated that the colistin-induced apoptosis in
PC12 cells involves the complicated regulation of the mitochondrial
apoptotic and death receptor pathways. Thus, our findings may
provide a novel mechanism underlying the neurotoxicity of
colistin.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 31201951 and 31272613), and the
Scientific and Technological Innovation Talent Scientific Research
Foundation for the Returned Overseas Chinese Scholars by State
Education Ministry and Heilongjiang Province in China (nos.
2012RFLXN005 and LC201018), and the Program for Liaoning Excellent
Talents in University (no. LR2013087), and the Youth Science and
Technology Foundation of Liaoning Medical University in China (no.
Y2012Z023), and Liaoning province produce-learn-research projects
(LYHX2012059).
References
|
1
|
Falagas ME and Kasiakou SK: Toxicity of
polymyxins: a systematic review of the evidence from old and recent
studies. Crit Care. 10:R272006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michalopoulos A and Falagas ME: Colistin
and polymyxin B in critical care. Crit Care Clin. 24:377–391. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michalopoulos A, Kasiakou SK, Mastora Z,
Rellos K, Kapaskelis AM and Falagas ME: Aerosolized colistin for
the treatment of nosocomial pneumonia due to multidrug-resistant
Gram-negative bacteria in patients without cystic fibrosis. Crit
Care. 9:R53–R59. 2005. View
Article : Google Scholar
|
|
4
|
Zavascki AP, Goldani LZ, Li J and Nation
RL: Polymyxin B for the treatment of multidrug-resistant pathogens:
a critical review. J Antimicrob Chemother. 60:1206–1215. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conway SP, Brownlee KG, Denton M and
Peckham DG: Antibiotic treatment of multidrug-resistant organisms
in cystic fibrosis. Am J Respir Med. 2:321–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Littlewood JM, Koch C, Lambert PA, et al:
A ten year review of colomycin. Respir Med. 94:632–640.
2000.PubMed/NCBI
|
|
7
|
Betrosian AP, Frantzeskaki F, Xanthaki A
and Douzinas EE: Efficacy and safety of high-dose
ampicillin/sulbactam vs. colistin as monotherapy for the treatment
of multidrug resistant Acinetobacter baumannii
ventilator-associated pneumonia. J Infect. 56:432–436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furtado GH, d’Azevedo PA, Santos AF, Gales
AC, Pignatari AC and Medeiros EA: Intravenous polymyxin B for the
treatment of nosocomial pneumonia caused by multidrug-resistant
Pseudomonas aeruginosa. Int J Antimicrob Agents. 30:315–319.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holloway KP, Rouphael NG, Wells JB, King
MD and Blumberg HM: Polymyxin B and doxycycline use in patients
with multidrug-resistant Acinetobacter baumannii infections
in the intensive care unit. Ann Pharmacother. 40:1939–1945. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oliveira MS, Prado GV, Costa SF, Grinbaum
RS and Levin AS: Ampicillin/sulbactam compared with polymyxins for
the treatment of infections caused by carbapenem-resistant
Acinetobacter spp. J Antimicrob Chemother. 61:1369–1375.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng CY, Sheng WH, Wang JT, Chen YC and
Chang SC: Safety and efficacy of intravenous colistin (colistin
methanesulphonate) for severe multidrug-resistant Gram-negative
bacterial infections. Int J Antimicrob Agents. 35:297–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maroto R and Perez-Polo JR: BCL-2-related
protein expression in apoptosis: oxidative stress versus serum
deprivation in PC12 cells. J Neurochem. 69:514–523. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aravind L, Dixit VM and Koonin EV: The
domains of death: evolution of the apoptosis machinery. Trends
Biochem Sci. 24:47–53. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spanos S, Rice S, Karagiannis P, et al:
Caspase activity and expression of cell death genes during
development of human preimplantation embryos. Reproduction.
124:353–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng H, Li C, Feng L, et al: Effects of
Ginkgolide B on 6-OHDA-induced apoptosis and calcium over load in
cultured PC12. Int J Dev Neurosci. 25:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki N, Toda T, Kaneko T, Baba N and
Matsuo M: Flavonoids suppress the cytotoxicity of linoleic acid
hydroperoxide toward PC12 cells. Biol Pharm Bull. 25:1093–1096.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirata Y: Manganese-induced apoptosis in
PC12 cells. Neurotoxicol Teratol. 24:639–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravni A, Bourgault S, Lebon A, et al: The
neurotrophic effects of PACAP in PC12 cells: control by multiple
transduction pathways. J Neurochem. 98:321–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vaudry D, Chen Y, Hsu CM and Eiden LE:
PC12 cells as a model to study the neurotrophic activities of
PACAP. Ann N Y Acad Sci. 971:491–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou RR, Chen JZ, Chen H, Kang XG, Li MG
and Wang BR: Neuroprotective effects of
(−)-epigallocatechin-3-gallate (EGCG) on paraquat-induced apoptosis
in PC12 cells. Cell Biol Int. 32:22–30. 2008.
|
|
22
|
Li Y, Shi W, Zhou Y, et al:
Neuroprotective effects of chlorogenic acid against apoptosis of
PC12 cells induced by methylmercury. Environ Toxicol Pharmacol.
26:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibata S, Maeda M, Furuta K, et al:
Neuroprotective effects of (arylthio)cyclopentenone derivatives on
manganese-induced apoptosis in PC12 cells. Brain Res. 1294:218–225.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmadian S, Barar J, Saei AA, Fakhree MA
and Omidi Y: Cellular toxicity of nanogenomedicine in MCF-7 cell
line: MTT assay. J Vis Exp. 11912009.PubMed/NCBI
|
|
25
|
Peng Q, Wei Z and Lau BH: Fructus corni
attenuates oxidative stress in macrophages and endothelial cells.
Am J Chin Med. 26:291–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YM, Park SH, Shin DI, et al: Oxidative
modification of peroxiredoxin is associated with drug-induced
apoptotic signaling in experimental models of Parkinson disease. J
Biol Chem. 283:9986–9998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng AC, Lee MF, Tsai ML, et al: Rosmanol
potently induces apoptosis through both the mitochondrial apoptotic
pathway and death receptor pathway in human colon adenocarcinoma
COLO 205 cells. Food Chem Toxicol. 49:485–493. 2011. View Article : Google Scholar
|
|
28
|
Pan MH, Chang WL, Lin-Shiau SY, Ho CT and
Lin JK: Induction of apoptosis by garcinol and curcumin through
cytochrome c release and activation of caspases in human leukemia
HL-60 cells. J Agric Food Chem. 49:1464–1474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami M, Inagawa R, Hosokawa T, Saito T
and Kurasaki M: Mechanism of apoptosis induced by copper in PC12
cells. Food Chem Toxicol. 46:2157–2164. 2008. View Article : Google Scholar
|
|
30
|
Woodgate A, MacGibbon G, Walton M and
Dragunow M: The toxicity of 6-hydroxydopamine on PC12 and P19
cells. Brain Res Mol Brain Res. 69:84–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin SJ and Green DR: Protease
activation during apoptosis: death by a thousand cuts? Cell.
82:349–352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang X, Wang Y, Luo J, Liu S and Yang Z:
Protective effects of YC-1 against glutamate induced PC12 cell
apoptosis. Cell Mol Neurobiol. 31:303–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Basanez G, Sharpe JC, Galanis J, Brandt
TB, Hardwick JM and Zimmerberg J: Bax-type apoptotic proteins
porate pure lipid bilayers through a mechanism sensitive to
intrinsic monolayer curvature. J Biol Chem. 277:49360–49365. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goping IS, Gross A, Lavoie JN, et al:
Regulated targeting of BAX to mitochondria. J Cell Biol.
143:207–215. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gross A, Jockel J, Wei MC and Korsmeyer
SJ: Enforced dimerization of BAX results in its translocation,
mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outer mitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Narita M, Shimizu S, Ito T, et al: Bax
interacts with the permeability transition pore to induce
permeability transition and cytochrome c release in isolated
mitochondria. Proc Natl Acad Sci USA. 95:14681–14686. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Candé C, Cecconi F, Dessen P and Kroemer
G: Apoptosis-inducing factor (AIF): key to the conserved
caspase-independent pathways of cell death? J Cell Sci.
115:4727–4734. 2002.PubMed/NCBI
|
|
42
|
Candé C, Cohen I, Daugas E, et al:
Apoptosis-inducing factor (AIF): a novel caspase-independent death
effector released from mitochondria. Biochimie. 84:215–222.
2002.PubMed/NCBI
|
|
43
|
Wei MC, Lindsten T, Mootha VK, et al:
tBID, a membrane-targeted death ligand, oligomerizes BAK to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
44
|
Csokay B, Prajda N, Weber G and Olah E:
Molecular mechanisms in the antiproliferative action of quercetin.
Life Sci. 60:2157–2163. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zimmermann KC and Green DR: How cells die:
apoptosis pathways. J Allergy Clin Immunol. 108:S99–S103. 2001.
View Article : Google Scholar : PubMed/NCBI
|