Introduction
Embryo implantation is an important step for a
successful pregnancy (1). The
physiological process involves blastocyst migration, positioning,
adhesion, implantation and a series of complex events (2). The interaction between the
blastocyst and the receptive endometrium is essential during
implantation. This complex and delicate process is regulated by
various factors, including the mutual recognition between the
trophocyte and the endometrium, the invasion of the trophocyte,
early proliferation and differentiation of the embryo and the
signal transduction throughout the entire process (3).
RhoA, RhoB and RhoC, Rho subfamily members, present
an abnormal expression in a variety of malignant tumors and are
thus involved in tumorigenesis (4), affecting the polarities and
morphologies of the cells by regulating the aggregation of the
actin filament skeleton. They play a role in the migration,
invasion and proliferation of tumor cells by affectting cell
movement and adhesion in the cell-cell or cell-matrix, and the
reconstruction of the extracellular matrix (5,6).
Rho subfamily members play an important role in the migration,
invasion and proliferation of tumor cells, while the behavior of
the embryo implanted in the endometrium is similar with the
behavior of tumor cells (7).
Thus, RhoA may play an important role in embryo implantation;
however, the exact signaling pathway which regulates RhoA remains
to be identified.
The phosphatidylinositoi 3-kinase (PI3K)/Akt
signaling pathway regulates and controls a variety of cellular
functions, such as proliferation, growth and survival (8) Under normal circumstances, the
activated PI3K products, phosphatidylinositol (3,4)-bisphosphate [PI(3,4)P2]
and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], activated the downstream signaling
protein, Akt, as a second messenger. Activated Akt regulates a
variety of cellular functions by phosphorylation (9,10).
In this process, the activity of the PI3K/Akt signaling pathway is
negatively regulated by the lipid-phosphatase, phosphatase and
tensin homolog (PTEN), which removes the phosphoric acid from the
3′ and 5′ of PIP3 and converts it into PI(4,5)P2
and PI(3,4)P2 and degrades it (11,12). The aim of this study was to
investigate whether the PI3K/Akt signaling pathway plays an
important role during embyro implantation, and whether the PI3K/Akt
signaling pathway affects embyro implantation by regulating the
expression of RhoA. We wished to determine whether the PI3K/Akt
signaling pathway mediates signal transduction at the implantation
site and the inter-implantation site of the pregnant mice by
affecting the expression of RhoA.
Materials and methods
Materials
Experimental SPF level Kunming mice (aged 8–10
weeks, weighing 25–30 g) were purchased from the Experimental
Animal Center, Chongqing Medical University (Chongqing, China). All
animal experiments were approved by the Ethics Committee of
Chongqing Medical University. The mice were bred under controlled
environmental conditions (light, 14 h; dark, 10 h; temperature
range, 22–25°C). The female and male mice were mated at the ratio
of 2:1, and a vaginal plug was used to mark the first day of
pregnancy (D1). Normal adult male mice were fed for 14 days
following a vasectomy (model of pseudopregnancy) and were mated
with the female mice at a ratio of 1:2; a vaginal plug was used to
mark the first day of pseudopregnancy (PD1). The mice were randomly
divided into 3 groups: the first group was the pregnant group, the
second group was the pseudopregnant group, and the third group was
the experimental group administered the intrauterine injection of
the PI3K inhibitor, LY294002. There were 20 mice in each group. The
mice were sacrificed by decapitation and the uterus was then
removed following the injection of 0.4% trypan blue (0.15 ml)
between 8:00–9:00 a.m. on day 5. In each group, 10 mice were used
for quantitative reverse transcription polymerase chain reaction
(qRT-PCR) and western blot analysis and 10 mice for
immunohistochemistry with 4% paraformaldehyde.
qRT-PCR
A total of 50–100 mg of endometrial tissues (from
implantation and inter-implantation sites) obtained (on day 5) from
normal pregnant mice, pseudopregnant mice and mice injected with
the PI3K/Akt inhibitor was placed in a homogenizer. Total RNA was
extracted in accordance with the instructions provided with the
TRlzol reagent (Invitrogen Corp., Carlsbad, CA, USA). The D260/D280
ratio was measured using a spectrophotometer to determine the
purity and concentration of the RNA. RNA was reverse transcribed
into cDNA under the following reaction conditions: 37°C for 15 min
and 85°C for 5 sec. Quantitative fluorescence PCR was used to
amplify the PI3K, Akt, PTEN and RhoA gene products in a 25 μl
reaction system, with the use of SYBR-Green mix (12.5 μl), 1 μl of
upstream and downstream primers, cDNA (2 μl) and RNase-free
H2O (8.5 μl). The reaction conditions were as follows:
95°C for 3 min, 95°C for 10 sec, 60°C for 30 sec, 40 cycles; the
detection of the dissolution curve was carried out at 65–95°C. The
primer sequences used are presented in Table I. The experiment was repeated 3
times. β-actin was used as the reference gene to determine a
normalized arbitrary value for each gene. Relative expression was
calculated according to the equation 2−ΔΔCt and
statistically analyzed using a t-test.
 | Table IPrimer sequences used for qRT-PCR. |
Table I
Primer sequences used for qRT-PCR.
| Gene | Primer sequence |
|---|
| PI3K | F:
5′-CTCTCCTGTGCTGGCTACTGT-3′
R: 5′-GCTCTCGGTTGATTCCAAACT-3′ |
| Akt | F:
5′-ATCCCCTCAACAACTTCTCAGT-3′
R: 5′-CTTCCGTCCACTCTTCTCTTTC-3′ |
| PTEN | F:
5′-CATTGCCTGTGTGTGGTGATA-3′
R: 5′-AGGTTTCCTCTGGTCCTGGTA-3′ |
| RhoA | F:
5′-AGCTTGTGGTAAGACATGCTTG-3
R: 5′-GTGTCCCATAAAGCCAACTCTAC -3′ |
| β-actin | F:
5′-CCTGAGGCTCTTTTCCAGCC-3′
R: 5′-TAGAGGTCTTTACGGATGTCA ACGT-3′ |
Immunohistochemistry
Immunohistochemical measurements of the expression
of PI3K, Akt, PTEN and RhoA in the endometrial tissue of the mice
in the pregnant group, the pseudopregnant group and the group
injected with the PI3K inhibitor were carried out on day 5. The
uterus was fixed with 4% paraformaldehyde, dehyderated with graded
ethanol, wrapped with xylene transparent paraffin and sliced into
sections (5-μm-thick) prior to immunohistochemical staining.
Immunohistochemistry was performed using the SP-9001 Reagent kit
(Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China).
The sections were then stained with primary antibodies to PI3K
(1:50; PI3-kinase p110 α antibody, BSA1236; Bioworld Technology,
Inc.), Akt [1:80; Akt (P125) antibody, BS3987; Bioworld Technology,
Inc.], phosphorylated (p-)Akt [1:80; p-Akt (S473) pAb antibody,
BS4006; Bioworld Technology, Inc.], PTEN [1:80; PTEN (D386)
antibody, BS5534; Bioworld Technology, Inc.], RhoA [1:50; RhoA
(R182) antibody, BS1782; Bioworld Technology, Inc.]. The sections
were then examined under a microscope (Olympus BX51) and images
were acquired. The intensity of positive expression was determined
using Image Pro®Plus v. 6.0 software. Phosphate-buffered
saline (PBS) was used instead of the primary antibody for the
negative control.
Western blot analysis
Pregnant mice [day 5 of pregnancy (D5)] were
injected with 0.4% trypan blue (0.15 ml) into the vena caudalis,
and then sacrificed following anesthesia for 10 min. The uterus
were isolated to obtain the implantation site and the
inter-implantation site in the endometrium. Approximately 100 mg
endometrial tissue was obtained from the mice at the implantation
site and inter-implantation site; 200 μl protein lysate (Beyotime
Institute of Biotechnology, Shanghai, China) and 2 μl of the
protease inhibitor, PMSF (100 mmol/l), were then added to the
sections after being milled by liquid nitrogen; the sections were
then ice bath shock cracked for 20 min at 4°C, and oscillated every
5 min. The tissues were centrifuged at 12,000 rpm for 15 min, and
the BCA kit was used to measure the protein content. Protein sample
extracts (each approximately 50 μg) were dissolved in 5× loading
buffer at a ratio of 4:1, mixed and boiled in water for 10 min;
they were then subjected to 10% SDS-PAGE gel electrophoresis, at a
constant voltage of 80 V for 2 h; the separated protein was
electrically transferred onto PVDF membranes (0.45 μm), and the
membranes were incubated for 1 h at room temperature with 3% BSA,
and then incubated overnight with primary antibody (the antibody
and the proportion of BSA was 1:1,000); the membranes were then
washed 3 times with PBST, each time for 5 min. The bands were
incubated for 1 h with 3% BSA diluted second antibody at room
temperature and washed 3 times with PBST. Finally, the
chemiluminescence ECL method was used to develop the observed strip
with β-actin as the internal control. Densitometric analysis of
gene expression was performed using Quantity One software version
4.6.7.
Intrauterine injection of the PI3K
inhibitor, LY294002
Pregnant mice from the third group were narcotized
with 3% sodium pentobarbital on day 2 of pregnancy (D2), and were
then administered an intrauterine injection of LY294002, as
previously described (13)
(concentration, 40 μM; volume, 10 μl), or a contralateral
intrauterine injection of isometrical PBS. The mice were then
injected with 0.4% trypan blue (0.15 ml) into the vena caudalis on
day 5 of pregnancy (D5), and were then sacrificed following
anesthesia for 10 min after the injection.
Statistical analysis
All the experimental data were analyzed using
SPSS19.0 statistical software, and a t-test. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
qRT-PCR analysis of mRNA expression of
the PI3K, Akt, PTEN and RhoA genes at the implantation and
inter-implantation sites in the endometrium of pregnant mice on day
5
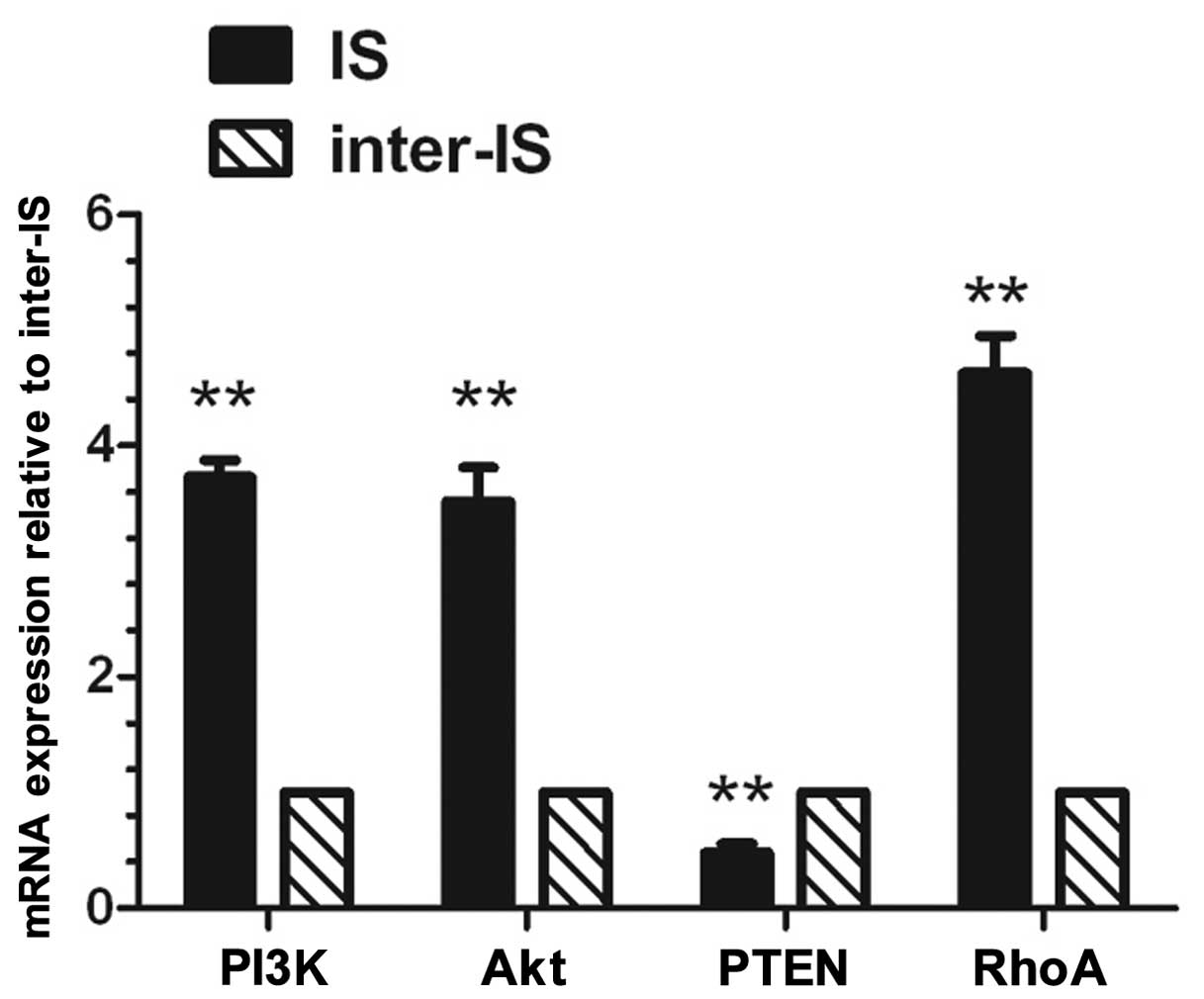
qRT-PCR (Fig. 1)
revealed that the mRNA expression levels of PI3K, Akt and RhoA at
the implantation site in the endometrium on day 5 of pregnancy were
significantly higher than those at the inter-implantation site
(P<0.01). However, the mRNA expression level of PTEN at the
implantation site was significantly lower than that at the
inter-implantation site (P<0.01).
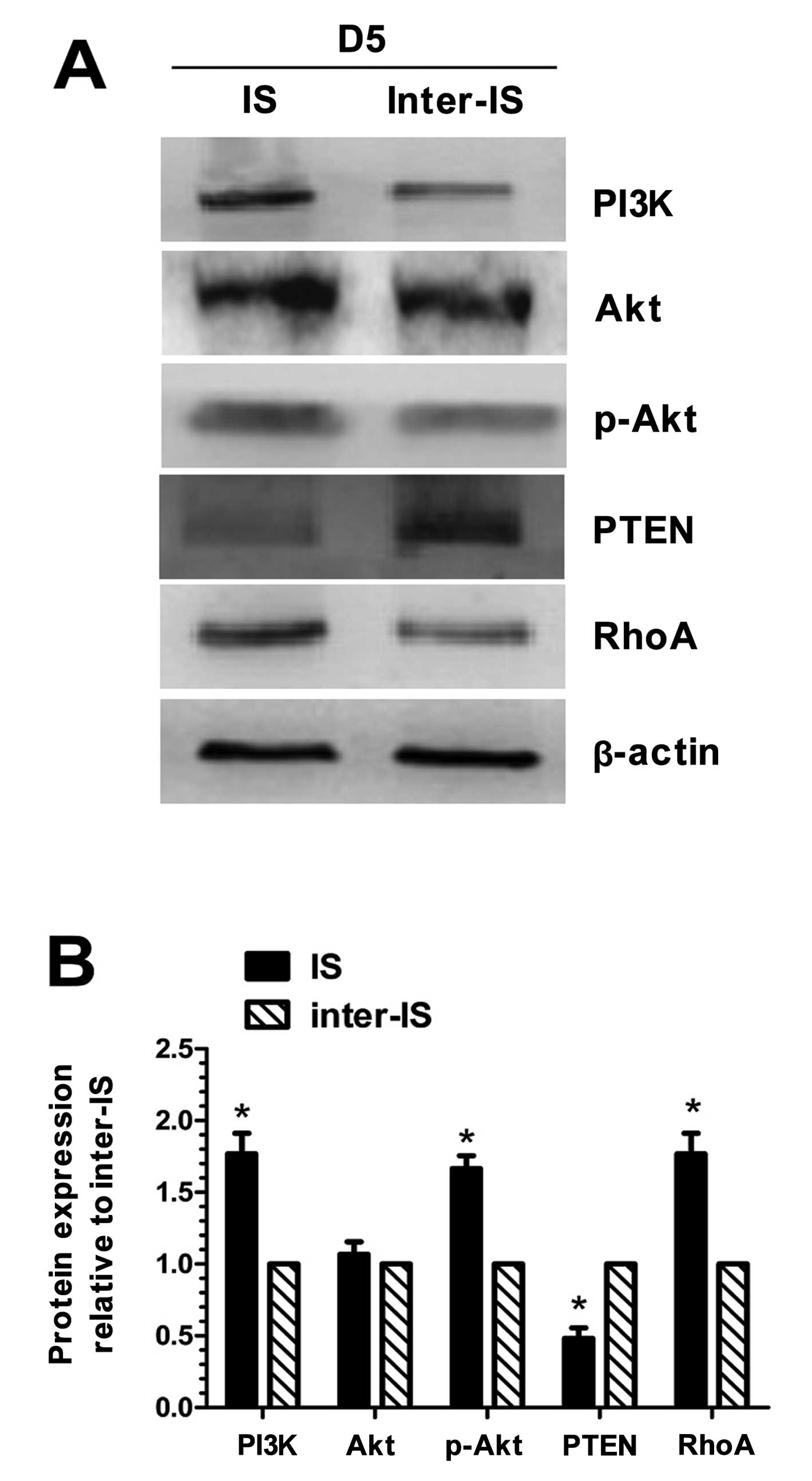
Western blot analysis of PI3K, Akt,
p-Akt, PTEN and RhoA at the implantation and inter-implantation
sites in the endometrium of pregnant mice on day 5
Western blot analysis (Fig. 2) revealed that the protein
expression of PI3K, p-Akt and RhoA at the implantation site in the
endometrium of the mice was significantly higher than that at the
inter-implantation site (P<0.05). As regards the expression of
Akt, no significant difference was observed between the
implantation site and the inter-implantation site in the
endometrium. The expresion of PTEN at the implantation site in the
endometrium was lower than that at the inter-implantation site
(P<0.05).
Location of PI3K, Akt, p-Akt, PTEN and
RhoA expression at the implantation and inter-implantation sites in
the endometrium of pregnant mice on day 5
Immunohistochemistry (Fig. 3) revealed that the gene expression
of PI3K, Akt, p-Akt and RhoA at the implantation site in the uterus
of pregnant mice on day 5 was significantly higher than that at the
inter-implantation site; however, the opposite effect was observed
for PTEN. In the endometrium of pregnant mice on day 5, PI3K
expression was strongly positive in the glandular epithelium and
stromal cells at the implantation site, while at the
inter-implantation site, PI3K protein was significantly expressed
in the glandular epithelium and weakly expressed in the stromal
cells. Akt protein was significantly expressed in the stromal cells
and luminal epithelium of the uterine implantation site, while it
was weakly expressed in the luminal epithelium of the
inter-implantation site. p-Akt protein was strongly expressed in
the stromal cells of the uterine implantation site, and weakly
expressed in the luminal epithelium of the uterine
inter-implantation site. PTEN protein was expressed in the stromal
cells of the implantation site in the endometrium of the pregnant
mice on day 5, and weakly expressed in the stromal cells and
luminal epithelium of the mouse endometrium inter-implantation
site. RhoA was highly expressed in the stromal cells and glandular
epithelium at the implantation site in the endometrium of mice, and
significantly expressed in the luminal epithelium at the
inter-implantation.
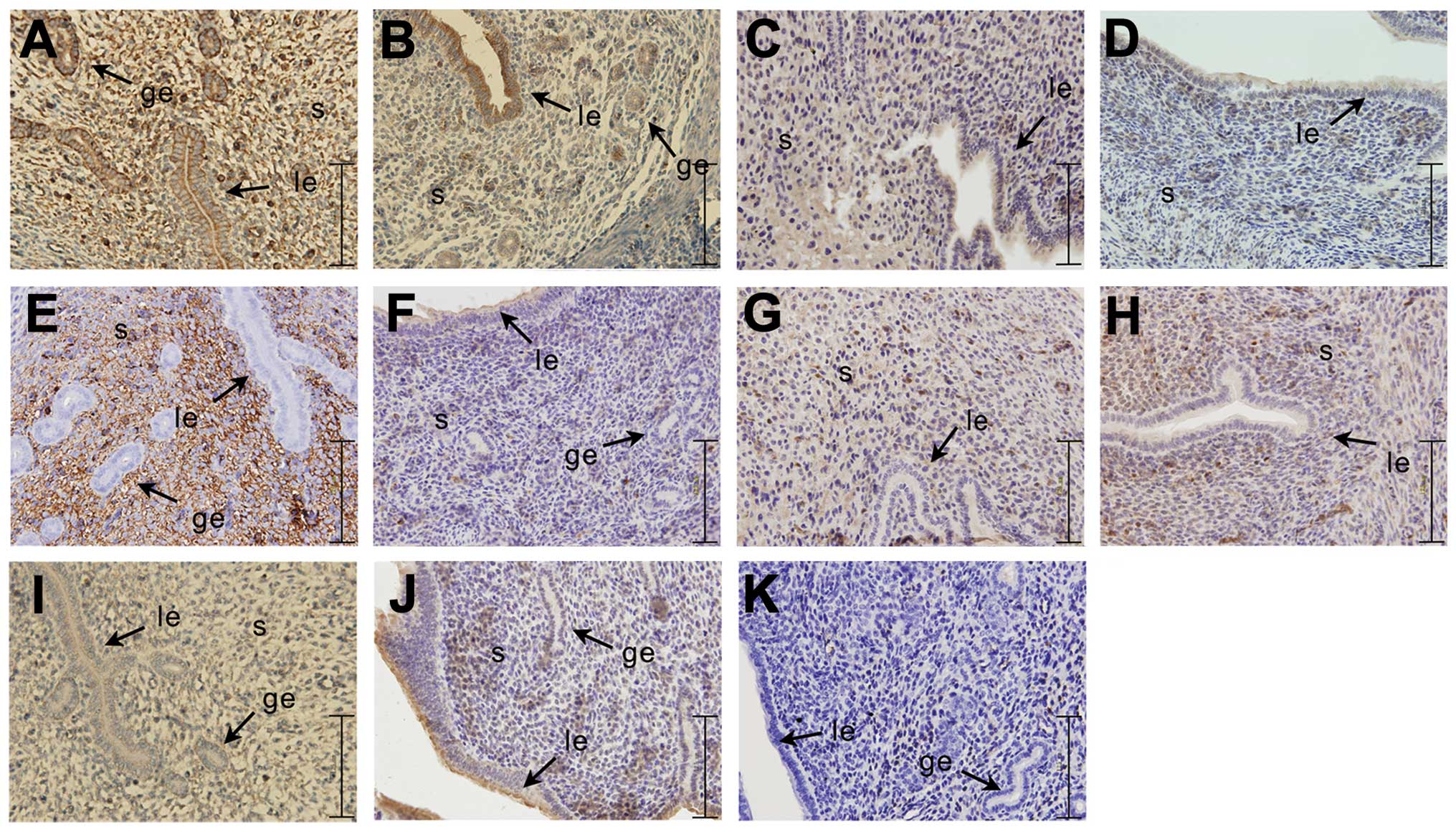 | Figure 3Immunohistochemical analysis of the
expression of PI3K/Akt signaling pathway-related genes and RhoA at
the implantation site and the inter-implantation site on day 5 of
pregnant (D5) mice. The experiment was performed 3 times. Three
mice were used in each experiment. (A, C, E, G and I)
Representative images of implantation site; (B, D, F, H and J)
representative images of inter-implantation site; (K) negative
control. (A and B) PI3K, (C and D) Akt, (E and F) p-Akt, (G and H)
PTEN, (I and J) RhoA. Yellow-brown color represents positive
staining. IS, implantation site; inter-IS, inter-implantation site;
le, luminal epithelium; ge, glandular epithelium; s, stromal cells;
bar, 125 μm. |
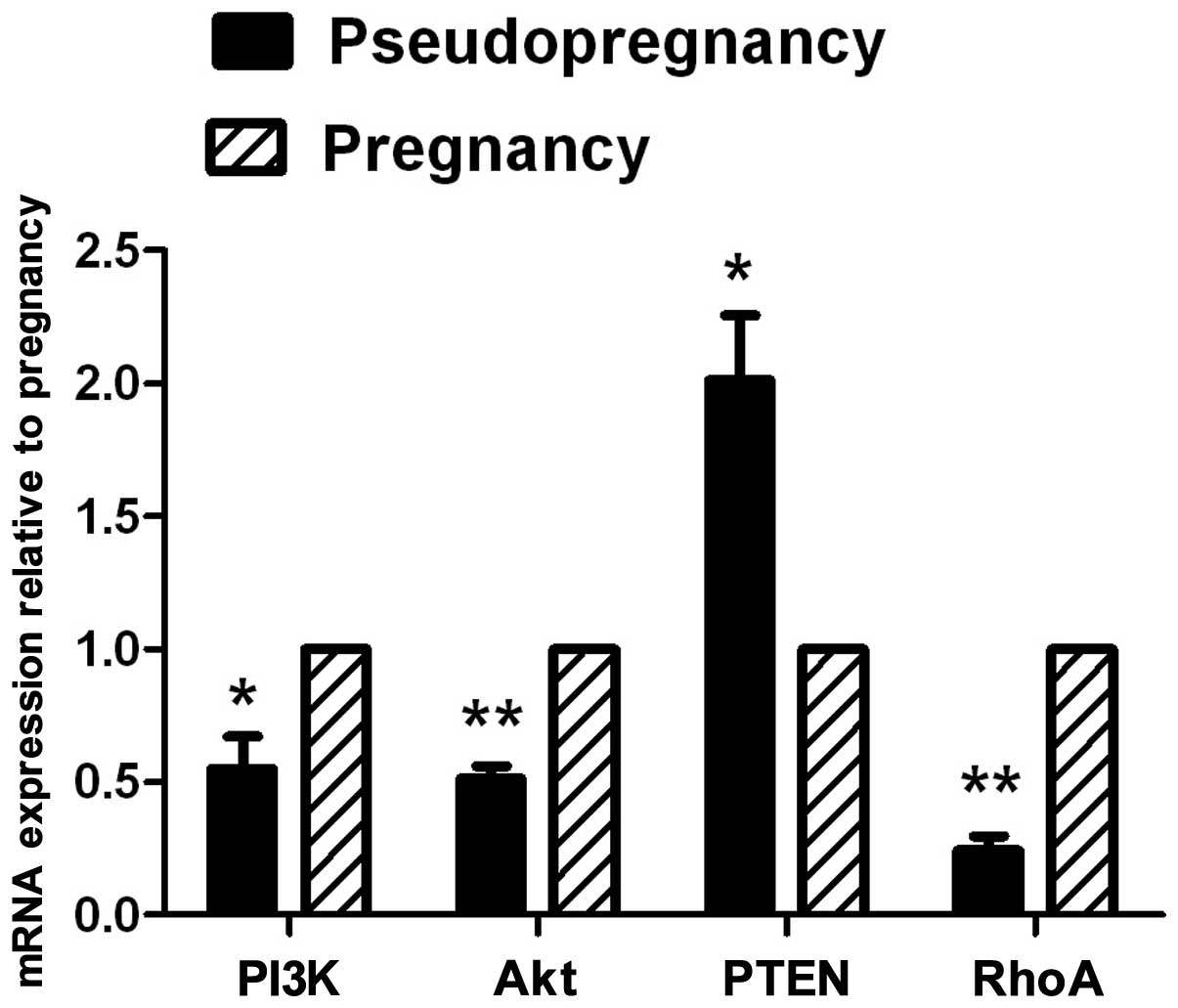
qRT-PCR of PI3K/Akt and RhoA expression
in endometrial tissue from pseudopregnant mice on day 5
The mRNA expression of PI3K, Akt and RhoA in the
endometrial tissue from pseudopregnant mice (Fig. 4) was lower than that in the
pregnant group (P<0.01); the expression of the PTEN gene in the
endometrial tissue from pseudopregnant mice was higher than that in
the pregnant group (P<0.05).
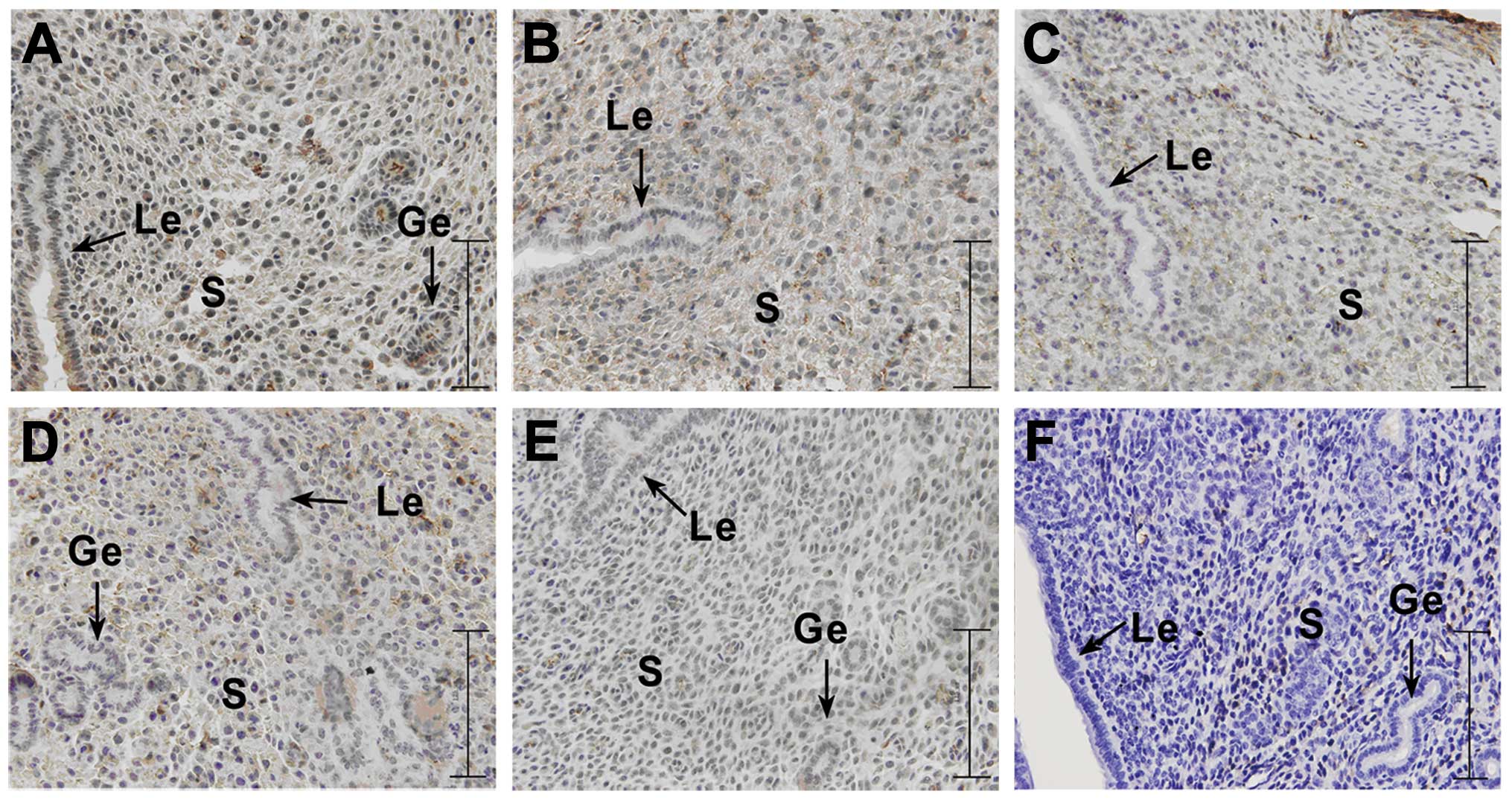
Immunohistochemistry of PI3K, Akt, p-Akt,
PTEN and RhoA expression in endometrial tissue from pseudopregnant
mice
In the pseudopregnant mice on day 5, (PD5) PI3K was
weakly expressed in the luminal epithelium, glandular epithelium
and stromal cells, and Akt was weakly expressed in the luminal
epithelium, glandular epithelium and stromal cells. p-Akt was
weakly expressed in stromal cells and PTEN was weakly expressed in
the stromal cells and luminal epithelium, as well as in the
glandular epithelium. RhoA was not expressed in the endometrium of
the pseudopregnant mice on day 5 (Fig. 5).
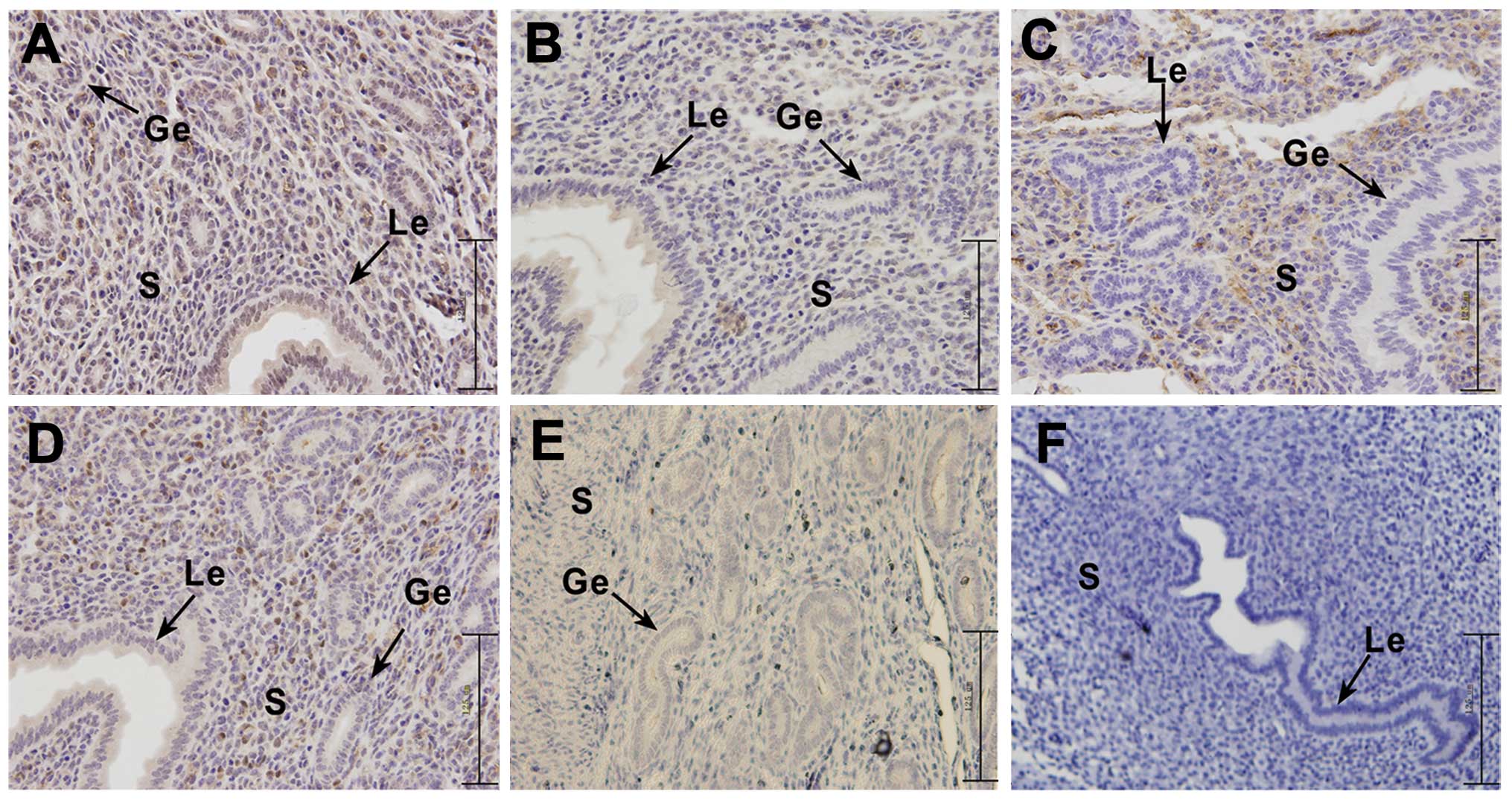 | Figure 5Immunohistochemical analysis of the
expression of PI3K/Akt singnaling pathway-related genes (PI3K, Akt,
p-Akt and PTEN) and RhoA on day 5 in endometrial tissue from
pseudopregnant mice. The experiment was performed 3 times. Three
mice were used in each experiment. (A) Representative image of
PI3K, (B) representative image of Akt, (C) representative image of
p-Akt, (D) representative image of PTEN, (E) representative image
of RhoA, (F) representative image of negetive control. Yellow-brown
color represents positive staining. le, luminal epithelium; ge,
glandular epithelium; s, stromal cells; bar, 125 μm. |
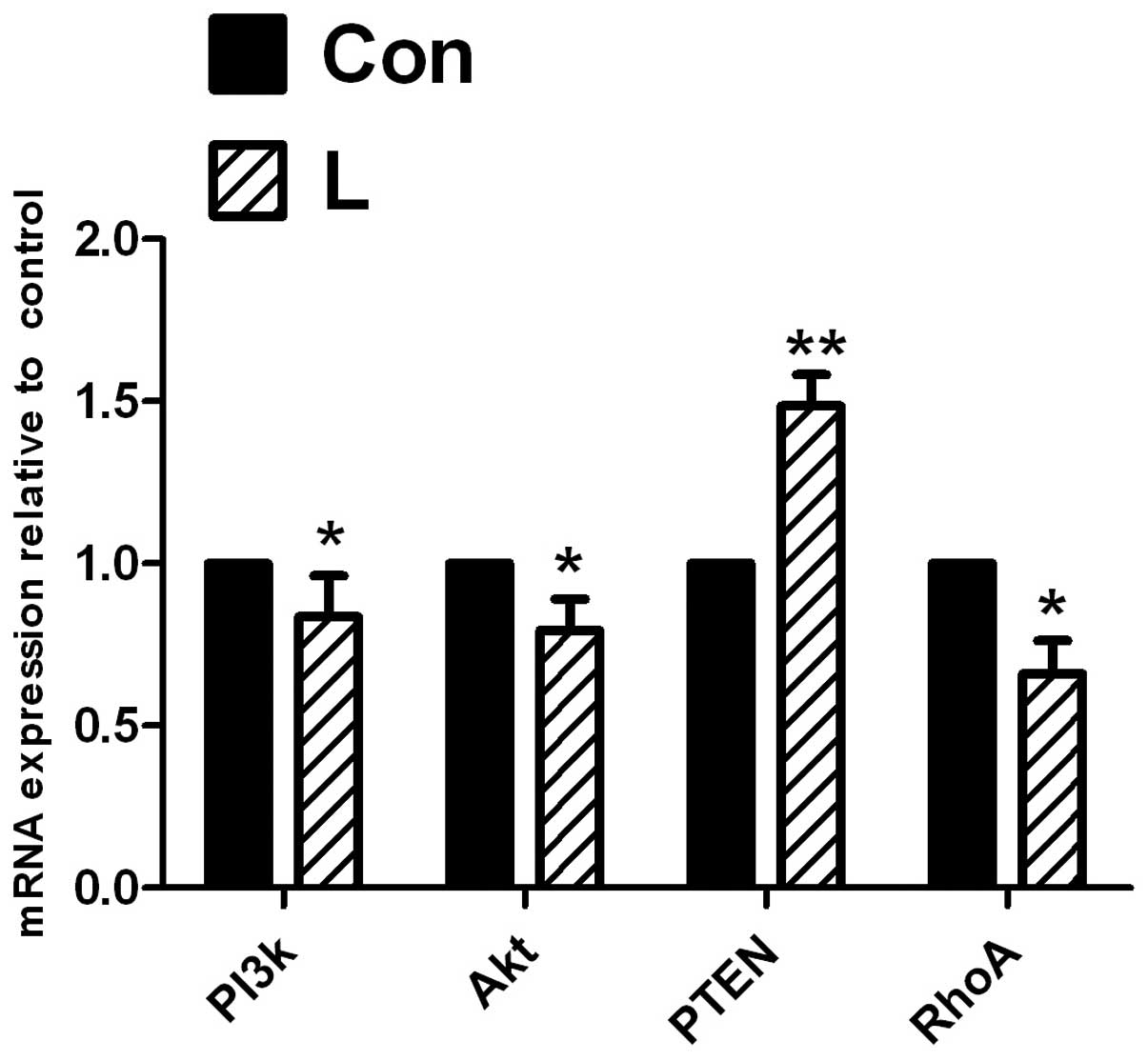
qRT-PCR of PI3K, Akt, p-Akt, PTEN and
RhoA expression at the implantation site in endometrial tissue
following the intrauterine injection of PI3K inhibitor
The expression of PI3K and Akt was significantly
decreased (P<0.05) when comparing the inhibitor group with the
control group (Fig. 6) following
the intrauterine injection of the PI3K inhibitor, LY294002, and
PTEN expression was significantly increased (P<0.01); RhoA
expression was significantly decreased (P<0.05).
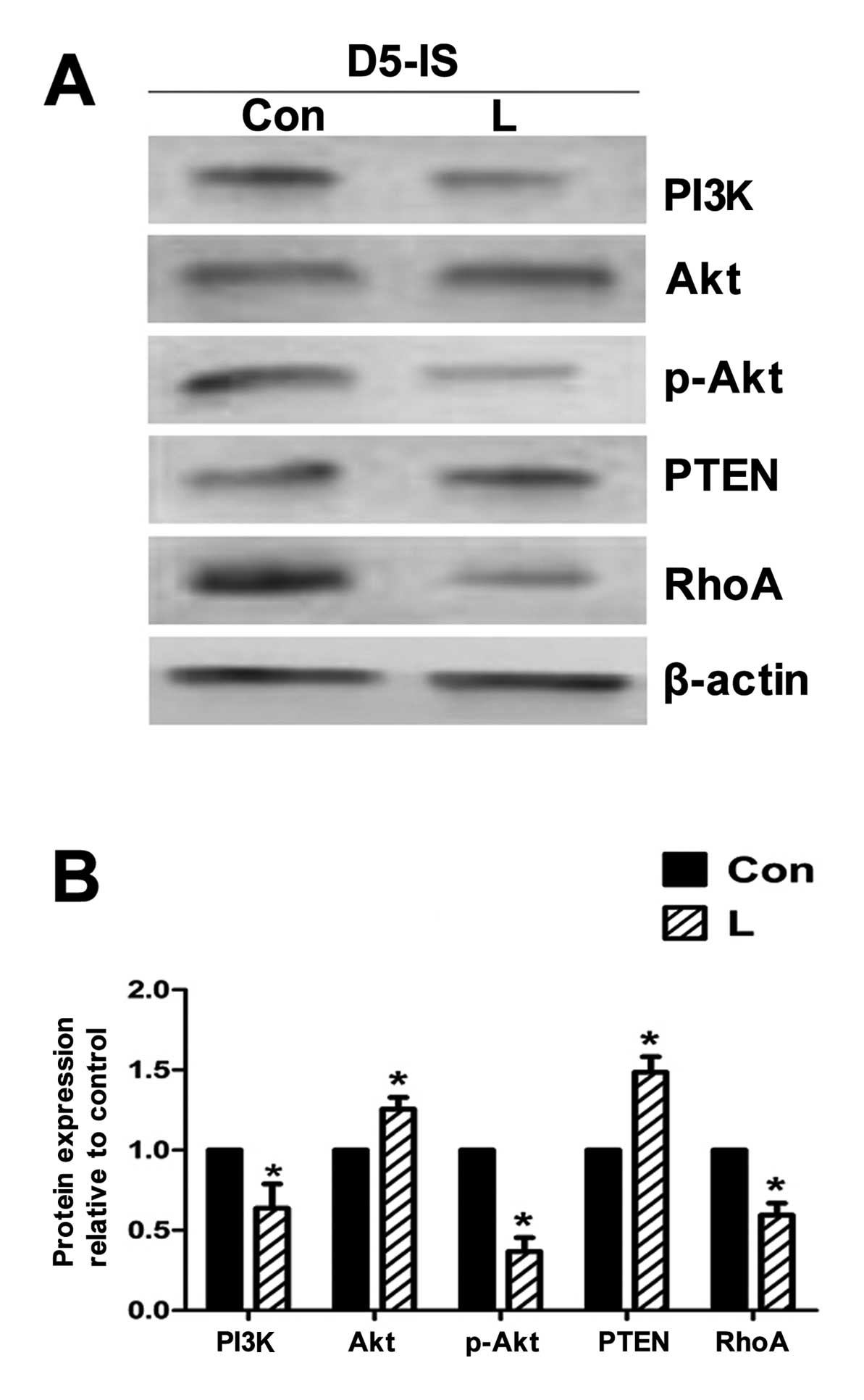
Western blot analysis of PI3K, Akt,
p-Akt, PTEN and RhoA expression at the implantation site in
endometrial tissue following the intrauterine injection of PI3K
inhibitor
The expression of Akt showed no significant
alteration; the expression of PI3K and p-Akt was decreased in the
group injected with the PI3K inhibitor, LY294002, compared with the
control group. The expression of PTEN was significantly increased,
and RhoA expression was significantly decreased (Fig. 7) following the intrauterine
injection of the PI3K inhibitor, LY294002.
Immunohistochemistry of PI3K, Akt, p-Akt,
PTEN and RhoA expression at the implantation site in endometrial
tissue following the intrauterine injection of PI3K inhibitor
The expression of PI3K, Akt and p-Akt was
significantly decreased in the group injected with the inhibitor
compared with that in the non-injected control group. PTEN
expression in the inhibitor group was significantly increased
(Fig. 8) following the
intrauterine injection of the PI3K inhibitor, LY294002. The
expression of RhoA was decreased. In addition, the locations of the
expression of the above genes had not changed, and the genes were
mainly expressed in the stromal cells.
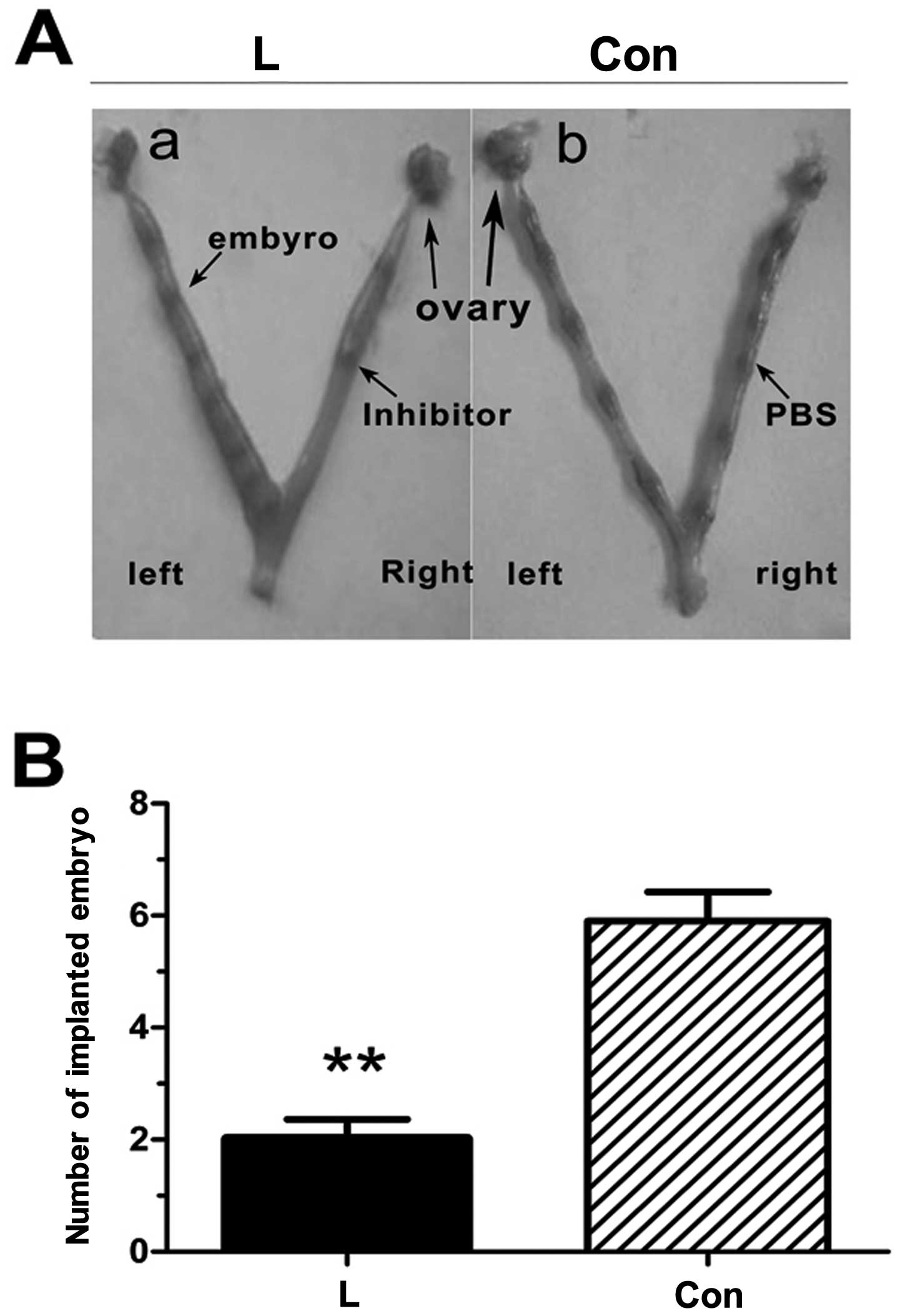
Effect of PI3K/Akt inhibitor on embryo
implantation
The unilateral intrauterine injection of PBS had no
effect on embryo implantation, whereas embryo implantation was
reduced following the contralateral intrauterine injection of the
PI3K inhibitor, LY294002 (Fig.
9), and the results were statistically significant
(P<0.01).
Discussion
Embryo implantation is an important event which
relates to the continuation of species. Successful embryo
implantation requires effective maternal-fetal dialogue, namely,
mutual recognition and adhesion between the blastocyst with the
ability to implant and the receptive endometrium (1). Uterine receptivity is considered as
the ‘implantation window’, and the uterine microenvironment at this
time helps to support blastocyst growth, adhesion response and
embryo implantation (14). For
pregnant mice, the 4th to 5th day of pregnancy is considered to be
the ‘implantation window’ (15).
In the process of embryo implantation, all elements in the uterus,
including the luminal epithelium, the glandular epithelium and the
stromal cells will go through the process of continuous
proliferation and differentiation with the embryo adhering to the
luminal epithelium and implanting into the stromal cells (16). The endometrial luminal and
glandular epithelial cells of mice undergo a proliferative state on
days 1 and 2 of pregnancy (D1 and D2). With pregnancy progressing,
they exit from the cell cycle and enter a differentiation program
that allows them to transit a receptive state. The stromal cells
adjacent to the epithelium then begin to proliferate on day 3 and
this proliferation becomes widespread following embryo attachment
to the receptive luminal epithelium on day 4 of pregnancy. As the
embryos invade through the luminal epithelium into the stromal
compartment, the stromal cells differentiate into secretory
decidual cells, which support further growth and development of the
implanted embryos until placentation ensues (16–19).
The endometrium has different adaptive responses in
different periods during the embryo implantation process, and its
changes are regulated by various cytokines and growth factors
(20). During mammalian
preimplantation, the developing embryo is dependent on signals
generated by growth factors which are known to regulate cell
proliferation and differentiation in an autocrine and paracrine
manner by means of the endometrial microenvironment (15,21). A large number of PI3K/Akt
signaling pathway activated receptors are present in the embryo
during the pre-implantation stage (22). In this study, we found that the
activation of the PI3K/Akt signaling pathway during the embryo
‘implantation window’ may be activated by the endometrial signaling
pathway caused by the activated embryo adhesion. Additionally, RhoA
is involved in the regulation of the endometrium. There are a
number of signaling factors and signaling pathways involved in
embryo implantation; however, the molecular mechanisms involved
remain unclear (23,24). As shown in the study by
Vanhaesebroeck et al (25), the p110α isoform of PI3K plays an
important role during embyro implantation; thus, we selected p110α
in our study. We found that PI3K expression was strongly positive
in the glandular epithelium and stromal cells at the implantation
site in the endometrium on day 5 of pregrancy (D5), while PI3K was
significantly expressed in the glandular epithelium and weakly
expressed in the stromal cells at the inter-implantation site. Akt
was significantly expressed in the stromal cells and luminal
epithelium at the implantation site, while it was weakly expressed
in the luminal epithelium at the inter-implantation site. The p-Akt
protein was strongly expressed in the stromal cells at the
implantation site, and weakly expressed in the luminal epithelium
at the inter-implantation site. The PTEN protein was expressed in
the stromal cells at the implantation site in the endometrium on
D5, and weakly expressed in the stromal cells and luminal
epithelium of the endometrium at the inter-implantation site. RhoA
was highly expressed in the stromal cells and glandular epithelium
of the mouse endometrium at the implantation site, and
significantly expressed in the luminal epithelium of the mouse
uterine inter-implantation site. This expression characteristic may
be consistent with its functions.
The PI3K/Akt signaling pathway has frequently
appeared in a variety of human cancers, such as non-small cell lung
cancer (26), breast cancer
(27), prostate cancer (9), ovarian cancer (28) and other physiological processes,
such as epithelial-mesenchymal transition and hippocampal cell
multiplication in tumor development and cancer (26,29). The PI3K/Akt signaling pathway
regulates a variety of critical cellular functions, such as
proliferation, growth, survival, apoptosis, tumor growth and
angiogenesis (8,9). Riley et al (21) found that the activation of the
PI3K signaling pathway plays an important role in glucose
metabolism of the mouse embryo and in embryonic survival; the
PI3K/Akt signaling pathway is also crucial during the
pre-implantation stage (22). Our
study demonstrates that the PI3K/Akt signaling pathway plays an
important role during the ‘implantation window’ in mice.
RhoA belongs to the small molecule G protein
superfamily, which is widely expressed in different types of cells
and tissues. With a variety of biological functions, RhoA plays an
important role in the regulation of the actin cytoskeleton, which
is mainly involved in the reorganization of the cytoskeleton, cell
migration and adhesion, cell polarization and activation and DNA
transcription, as well as other functions (30,31). In the reproductive field, Melendez
et al found that RhoA was essential in mouse embryonic
fibroblastic mitosis (9). In our
study, we found that when the PI3K inhibitor, LY294002, was used,
the expression of RhoA was reduced. At the same, we also used the
inhibitor, wortmannin, in our experiments, and observed a similar
trend. The specificity of LY294002 to PI3K is higher than that of
wortmannin; thus, we only showed the results obtained for LY294002.
When we obtained the above results, we hypothesized that RhoA
expression is involved during the ‘implantation window’, and may be
regulated by the PI3K/Akt signaling pathway.
During the embryo implantation period, embryos which
are capable of implantation by means of the endometrial
microenvironment release a variety of cytokines, such as integrin
and epidermal growth factor (EGF) in an autocrine or paracrine
manner. These cytokines activate the expression of signaling
pathway-related genes. In this study, the expression levels of the
PI3K/Akt signaling pathway-related genes, PI3K, Akt and p-Akt, at
the implantation site in the endometrium were higher than those at
the inter-implantation site, and the location of their expression
was basically the same, mainly strongly positive in the stromal
cells; however, the expression of PTEN showed an opposite trend.
The expression levels and the distribution characteristics of the
PI3K/Akt signaling pathway-related genes at the implantation and
inter-implantation sites in the endometrium suggest that the
PI3K/Akt signaling pathway is involved during early embryo
implantation, particularly during the ‘implantation window’.
In addition, the expression level of RhoA at the
implantation site in the endometrium was higher than that at the
inter-implantation, which was basically consistent with the
expression levels, expression sites and expression time of the PI3K
and Akt genes, suggesting that there may be some type of
association between the PI3K/Akt signaling pathway and RhoA. This
suggests that the mRNA and protein levels of the signaling
pathway-related genes and RhoA may be activated by embryo adhesion.
In order to confirm this hypothesis, we designed the pseudopregnant
group. We found that the expressions of PI3K, p-Akt and RhoA in the
pseudopregnant group was lower than that in the pregnant group,
which confirmed our hypothesis. To further confirm the association
between the PI3K/Akt signaling pathway and RhoA in the embryo
‘implantation window’, we used the PI3K/Akt signaling pathway
inhibitor, LY294002. We found that the expression level of RhoA was
significantly decreased, and the number of embryo implantations was
reduced following the application of the inhibitor, which to some
extent reflected that the PI3K/Akt signaling pathway may regulate
RhoA expression in the process of the embryo implantation. This
suggests that the PI3K/Akt signaling pathway at the implantation
site in the endometrium promotes the migration and decidualization
of the stromal cells by regulating the expression of RhoA under
normal circumstances, thereby contributing to the implantation of
the embryo; when the PI3K/Akt signaling pathway was inhibited, the
expression level of RhoA was decreased follwoing the injection of
the inhibitor, LY294002, which was not conducive to the migration
and decidualization of endometrial stromal cells, thereby not
conducive to the implantation of the embryo, thus reducing the
number of embryo implantations. The PI3K/Akt signaling pathway
itself may affect embryo implantation by affecting cell
proliferation (22); RhoA can
also regulate the expression of PI3K/Akt through the RhoA-ROCK-PTEN
signaling pathway in mouse osteoblasts and may affect cell
proliferation (13). This
suggests that a dual-direction regulation may exist between
PI3K/Akt and RhoA in the process of embryo implantation, but this
was not further confirmed in this study. Further studies are
required to determine the mechanisms through which the PI3K/Akt
signaling pathway regulates the expression of RhoA and affects
embryo implantation, and whether there are other genes which can
regulate embryo implantation.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81100443) and Chongqing
Yuzhong District Science and Technology Plan projects
(20120214).
References
|
1
|
Banerjee P, Sapru K, Strakova Z and
Fazleabas AT: Chorionic gonadotropin regulates prostaglandin E
synthase via a phosphatidylinositol 3-kinase-extracellular
regulatory kinase pathway in a human endometrial epithelial cell
line: implications for endometrial responses for embryo
implantation. Endocrinology. 150:4326–4337. 2009. View Article : Google Scholar
|
|
2
|
Sales KJ, Grant V, Catalano RD and Jabbour
HN: Chorionic gonadotrophin regulates CXCR4 expression in human
endometrium via E-series prostanoid receptor 2 signalling to
PI3K-ERK1/2: implications for fetal-maternal crosstalk for embryo
implantation. Mol Hum Reprod. 17:22–32. 2011. View Article : Google Scholar
|
|
3
|
Singh M, Chaudhry P and Asselin E:
Bridging endometrial receptivity and implantation: network of
hormones, cytokines, and growth factors. J Endocrinol. 210:5–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timpson P, McGhee EJ, Morton JP, et al:
Spatial regulation of RhoA activity during pancreatic cancer cell
invasion driven by mutant p53. Cancer Res. 71:747–757. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazarini M, Traina F, Machado-Neto JA, et
al: ARHGAP21 is a RhoGAP for RhoA and RhoC with a role in
proliferation and migration of prostate adenocarcinoma cells.
Biochim Biophys Acta. 1832.365–374. 2012.PubMed/NCBI
|
|
6
|
Nalbant P, Chang YC, Birkenfeld J, Chang
ZF and Bokoch GM: Guanine nucleotide exchange factor-H1 regulates
cell migration via localized activation of RhoA at the leading
edge. Mol Biol Cell. 20:4070–4082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bischof P and Campana A: A model for
implantation of the human blastocyst and early placentation. Hum
Reprod Update. 2:262–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiokawa S, Sakai K, Akimoto Y, et al:
Function of the small guanosine triphosphate-binding protein RhoA
in the process of implantation. J Clin Endocrinol Metab.
85:4742–4749. 2000.PubMed/NCBI
|
|
9
|
Melendez J, Stengel K, Zhou X, et al: RhoA
GTPase is dispensable for actomyosin regulation but is essential
for mitosis in primary mouse embryonic fibroblasts. J Biol Chem.
286:15132–15137. 2011. View Article : Google Scholar
|
|
10
|
Yuan TL, Wulf G, Burga L and Cantley LC:
Cell-to-cell variability in PI3K protein level regulates PI3K-AKT
pathway activity in cell populations. Curr Biol. 21:173–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu LJ, Bagchi MK and Bagchi IC:
Attenuation of calcitonin gene expression in pregnant rat uterus
leads to a block in embryonic implantation. Endocrinology.
139:330–339. 1998.PubMed/NCBI
|
|
14
|
Song H, Han K and Lim H: Progesterone
supplementation extends uterine receptivity for blastocyst
implantation in mice. Reproduction. 133:487–493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Chen X, Ding Y, Liu X, Wang Y and He
J: Expression of translationally controlled tumor protein (TCTP) in
the uterus of mice of early pregnancy and its possible significance
during embryo implantation. Hum Reprod. 26:2972–2980. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nallasamy S, Li Q, Bagchi MK and Bagchi
IC: Msx homeobox genes critically regulate embryo implantation by
controlling paracrine signaling between uterine stroma and
epithelium. PLoS Genet. 8:e10025002012. View Article : Google Scholar
|
|
17
|
Carson DD, Bagchi I, Dey SK, et al: Embryo
implantation. Dev Biol. 223:217–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dey SK, Lim H, Das SK, et al: Molecular
cues to implantation. Endocr Rev. 25:341–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramathal CY, Bagchi IC, Taylor RN and
Bagchi MK: Endometrial decidualization: of mice and men. Semin
Reprod Med. 28:17–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentilini D, Busacca M, Di Francesco S,
Vignali M, Viganò P and Di Blasio AM: PI3K/Akt and ERK1/2
signalling pathways are involved in endometrial cell migration
induced by 17beta- estradiol and growth factors. Mol Hum Reprod.
13:317–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riley JK, Carayannopoulos MO, Wyman AH,
Chi M and Moley KH: Phosphatidylinositol 3-kinase activity is
critical for glucose metabolism and embryo survival in murine
blastocysts. J Biol Chem. 281:6010–6019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riley JK, Carayannopoulos MO, Wyman AH,
Chi M, Ratajczak CK and Moley KH: The PI3K/Akt pathway is present
and functional in the preimplantation mouse embryo. Dev Biol.
284:377–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Q, Zhang Y, Lu J, et al:
Embryo-uterine cross-talk during implantation: the role of Wnt
signaling. Mol Hum Reprod. 15:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamura K, Kawamura N, Sato W, Fukuda J,
Kumagai J and Tanaka T: Brain-derived neurotrophic factor promotes
implantation and subsequent placental development by stimulating
trophoblast cell growth and survival. Endocrinology. 150:3774–3782.
2009. View Article : Google Scholar
|
|
25
|
Vanhaesebroeck B, Ali K, Bilancio A,
Geering B and Foukas LC: Signalling by PI3K isoforms: insights from
gene-targeted mice. Trends Biochem Sci. 30:194–204. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
27
|
Zuo T, Liu TM, Lan X, et al: Epigenetic
silencing mediated through activated PI3K/AKT signaling in breast
cancer. Cancer Res. 71:1752–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu R, Hu TC, Rehemtulla A, Fearon ER and
Cho KR: Preclinical testing of PI3K/AKT/mTOR signaling inhibitors
in a mouse model of ovarian endometrioid adenocarcinoma. Clin
Cancer Res. 17:7359–7372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fournier NM, Lee B, Banasr M, Elsayed M
and Duman RS: Vascular endothelial growth factor regulates adult
hippocampal cell proliferation through MEK/ERK- and
PI3K/Akt-dependent signaling. Neuropharmacology. 63:642–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou TC, Lin JJ, Wen HC, Chen LC, Hsu SP
and Lee WS: Folic acid inhibits endothelial cell migration through
inhibiting the RhoA activity mediated by activating the folic acid
receptor/cSrc/p190RhoGAP-signaling pathway. Biochem Pharmacol.
85:376–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato K, Yazawa T, Taki K, et al: The
inositol 5-phosphatase SHIP2 is an effector of RhoA and is involved
in cell polarity and migration. Mol Biol Cell. 23:2593–2604. 2012.
View Article : Google Scholar : PubMed/NCBI
|