Introduction
Prostate cancer (PCa) is one of the most frequently
diagnosed tumor types and its incidence increases with age in males
worldwide. According to the latest evidence, among American males,
the incidence rate of three types of cancer is considered to reach
up to almost half that of all newly diagnosed cancers. These three
types of cancer are prostate, lung and bronchus and colorectal
cancer. The number of patients who suffer from PCa is 238,590,
which accounts for 28% of incident cases in males (1). As one of the critical mechanisms of
tumor metastasis, epithelial-mesenchymal transition (EMT) is
becoming a focus of research. EMT is characterized by the loss of
epithelial markers and is accompanied by the increased expression
of mesenchymal genes. The most important role of EMT in cancer
cells is increased cell motility. It has been previously reported
that in clinical prostate tumor specimens, EMT is not only a
morphological change, but also a behavioral change (2).
Forkhead Box M1 (FoxM1) is a member of the large
family of forkhead box (Fox) transcription factors which share a
conserved winged helix DNA binding domain (3). A great deal of evidence has
confirmed that FoxM1 is upregulated in various human malignancies,
including breast cancer, lung cancer, ovarian carcinoma,
hepatocellular carcinoma (HCC), pancreatic cancer, stomach cancer,
non-Hodgkin’s lymphoma, melanoma and colorectal cancer (4–12).
In addition, according to recent evidence, the overexpression of
FoxM1 correlates with poor prognosis in breast cancer, HCC and lung
cancer (13–15). FoxM1 plays crucial roles in cell
proliferation, cell cycle regulation, angiogenesis, invasion and
metastasis (3,6,16–21). Recently, two studies indicated
that FoxM1 is associated with EMT in lung fibrosis and breast
cancer (22,23). However, the differential
expression of FoxM1 in benign and malignant prostate tissues and
the role of FoxM1 in EMT in PCa cells have not yet been
elucidated.
Metformin has been widely used for the treatment of
type 2 diabetic patients from the 1970’s in Europe and from 1995 in
the United States. In recent years, clinical and epidemiological
evidence indicates that metformin is a novel anticancer agent
(24). The potential mechanisms
of action of metformin in cancer include the regulation of the cell
cycle through the activation of AMP-activated protein kinase
(AMPK), as well as its effects on tyrosine kinases and insulin
(25). However, whether metformin
can suppress EMT by regulating FoxM1 remains an unresolved
issue.
In this study, we investigated the expression of
FoxM1 in benign and malignant prostate tissues, as well as its
correlation with clinicopathological characteristics. We further
demonstrate that metformin inhibits EMT in PCa by downregulating
FoxM1.
Materials and methods
Human tissue specimens and
immunohistochemical analysis
The expression of FoxM1 was analyzed in a total of
101 human PCa and benign prostate hyperplasia (BPH) specimens (62
PCa and 39 BPH) from patients who were scheduled for prostate
biopsy at the General Hospital of The Chinese People’s Liberation
Army (PLA; Beijing, China) between January 2009 and December 2013.
The use of the tissue specimens was approved by the Ethics
Committee of the Chinese PLA General Hospital. A histopathological
analysis of the specimens was performed by pathologists at the
Department of Pathology, General Hospital of The Chinese PLA. The
results from immunohistochemistry were analyzed by two independent
pathologists blinded to the clinical parameters. The scores of the
staining results were based on the following criteria as described
in a previous study (26): i)
percentage of positive tumor cells in the tumor tissue: 0 (0%), 1
(1–10%), 2 (11–50%), 3 (51–70%) and 4 (71–100%); ii) staining
intensity: 0 (none), 1 (weak), 2 (moderate) and 3 (strong). The
staining index was calculated as follows: staining intensity score
× proportion of positive tumor cells. A final score of ≥5 was
considered as a high expression.
Cell culture
The human LNCaP PCa cell line was purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA). The
human DU145 PCa cell line was a gift from Professor J.G. Zhou from
the Beijing Institute of Biotechnology (Beijing, China). The human
PCa cell lines, PC3 and PC3M, were provided by Dr Wang Yu from the
Department of Stomatology (Chinese PLA General Hospital). The DU145
and LNCaP cells were maintained in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) supplemented with 10% FBS (HyClone Laboratories,
Inc., Logan, UT, USA); the PC3 and PC3M cells were cultured in DMEM
medium (Gibco) supplemented with 10% FBS. All the cells were
maintained in a humidified atmosphere of 95% air plus 5%
CO2 at 37°C.
Transforming growth factor
(TGF)-β-induced EMT
For the induction of EMT, the cells were plated on
the previous day. Following starvation (in serum-free medium)
overnight, 10 ng/ml TGF-β1 (R&D Systems, Inc., Minneapolis, MN,
USA) were added; the cells were then examined at various time
points following treatment.
Drug treatments and shRNA plasmid
transfection
The cells were treated with metformin
(Sigma-Aldrich, St. Louis, MO, USA). The cells were transfected
with a FoxM1 shRNA plasmid (provided by Dr Wang Yu) (15) using Lipofectamine™ 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA).
RNA isolation, reverse-transcription and
quantitative PCR
The DU145 cells were treated with the plasmid or
metformin, followed by exposure to TGF-β1. Subsequently, total RNA
was prepared using TRIzol reagent (Invitrogen). Quantitative PCR
was carried out as previously described (27). The primers were used for
quantitative PCR are presented in Table I. They were synthesized by Sangon
Biotech (Shanghai, China).
 | Table IPrimers for quantitative PCR. |
Table I
Primers for quantitative PCR.
| Gene | Primers |
|---|
| E-cadherin | F
5′-CGGGAATGCAGTTGAGGATC-3′
R 5′-AGGATGGTGTAAGCGATGGC-3′ |
| Vimentin | F
5′-GAGAACTTTGCCGTTGAAGC-3′
R 5′-GCTTCCTGTAGGTGGCAATC-3′ |
| Snail1 | F
5′-TCGGAAGCCTAACTACAGCGA-3′
R 5′-AGATGAGCATTGGCAGCGAG-3′ |
| Snail2 (Slug) | F
5′-GATGCCGCGCTCCTTCCTGG-3′
R 5′-GGGGGACTCACTCGCCCCAA-3′ |
| Zeb1 | F
5′-GATGATGAATGCGAGTCAGATGC-3′
R 5′-ACAGCAGTGTCTTGTTGTTGT-3′ |
| Zeb2 | F
5′-CAAGAGGCGCAAACAAGCC-3′
R 5′-GGTTGGCAATACCGTCATCC-3′ |
| FoxM1 | F
5′-TTGGACCAGGTGTTTAAGCAGCAG-3′
R 5′-GAGGAGTCTGCTGGGAACGGGAG-3′ |
| GAPDH | F
5′-GTCATCCATGA-CAACTTTGG-3′
R 5′-GAGCTTGACAAAGTGGTCGT-3′ |
MTS assay
The cells were seeded at 1,000–5,000 cells per well
(triplicates) in 96-well plates. After 24 h, the cells were treated
with metformin. A total of 20 μl of MTS solution (Promega Corp.,
Madison, WI, USA) was added to each well, and the cells were
incubated at 37°C with 5% CO2 for 4 h. Cell viability
was detected by scanning with a microplate reader (Bio-Rad,
Hercules, CA, USA) at 490 nm.
Wound-healing assay
The cells were plated in 12-well culture plates in
complete culture medium and grown to confluence. A wound was
created by scraping with a sterilized 10 μl pipette tip in the
middle of the cell monolayer. The cells were then cultured with
fresh complete culture medium containing 10 ng/ml TGF-β1 with or
without metformin treatment for 24 h. Subsequently, the ability of
the cells to migrate into the cleared section was observed and
photographed using a microscope (Nikon Eclipse TS100; Nikon, Tokyo,
Japan).
Transwell assay
The migration ability of the cells was detected
using 24-well plates with 8-μm pore size inserts (Corning Life
Sciences, Oneonta, NY, USA). Following starvation overnight,
1×105 cells were added to the upper well in 200 ml
RPMI-1640 medium without FBS and allowed to migrate to the bottom
compartment containing RPMI-1640 medium with 10% FBS for 24 h,
followed by wiping off the non-migrated cells with a cotton swab.
For the quantification of migration, Transwell filters were fixed
in methanol for 10 min and stained with 0.1% crystal violet in 20%
(v/v) methanol for 20 min and mounted on a glass slide. The
evaluation of the completed transmigration was performed under a
microscope (x200 magnification). An equal volume of 10% acetic acid
was then added to each well to completely dissolve the stained
crystal violet. The OD value was detected by scanning with a
microplate reader (Bio-Rad) at 570 nm to quantify the percentage of
migrated cells. The experiments were performed in triplicate wells.
Data are presented as the means ± SD from three independent
experiments.
Western blot analysis and reagents
Protein samples were size fractionated by SDS-PAGE
and transferred onto PVDF membranes (Millipore UK Ltd., Consett,
UK). The blots were blocked for 1 h in 5% milk/0.1% Tween-20 in TBS
(TBS-T) and then incubated with primary antibodies at 4°C
overnight. Western bolt analysis was conducted using
anti-E-cadherin, anti-vimentin (Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-FoxM1, anti-Slug (Abcam, Cambridge, UK) and
anti-p-AMPKα antibodies (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA).
Statistical analysis
The correlations between the expression levels of
FoxM1 and clinical parameters were evaluated by the
χ2-test. Statistical analyses were performed using ANOVA
or the Student’s t-test. All analyses were performed using SPSS
12.0 software for Windows. P-values <0.05 were considered to
indicate statistically significant differences.
Results
FoxM1 protein is upregulated in PCa
tissues
To investigate the role of FoxM1 in the progression
of PCa, 39 BPH specimens and 62 PCa specimens were collected in
this study (Table II). The mean
age of the patients with BPH was 61.4±13.4 years. All BPH specimens
showed histologically epithelial and stromal cell hyperplasia.
There were also three cases accompanied with prostatic
intraepithelial neoplasia (PIN) of grade I and four cases with PIN
of grade II–III. Among the 62 patients suffering from PCa, the mean
age was 68.6±9.7 years. The numbers of cases with Gleason scores of
<7 and ≥7 were 16 and 46, respectively. By immunohistochemical
analysis, the expression levels of FoxM1 were mainly observed in
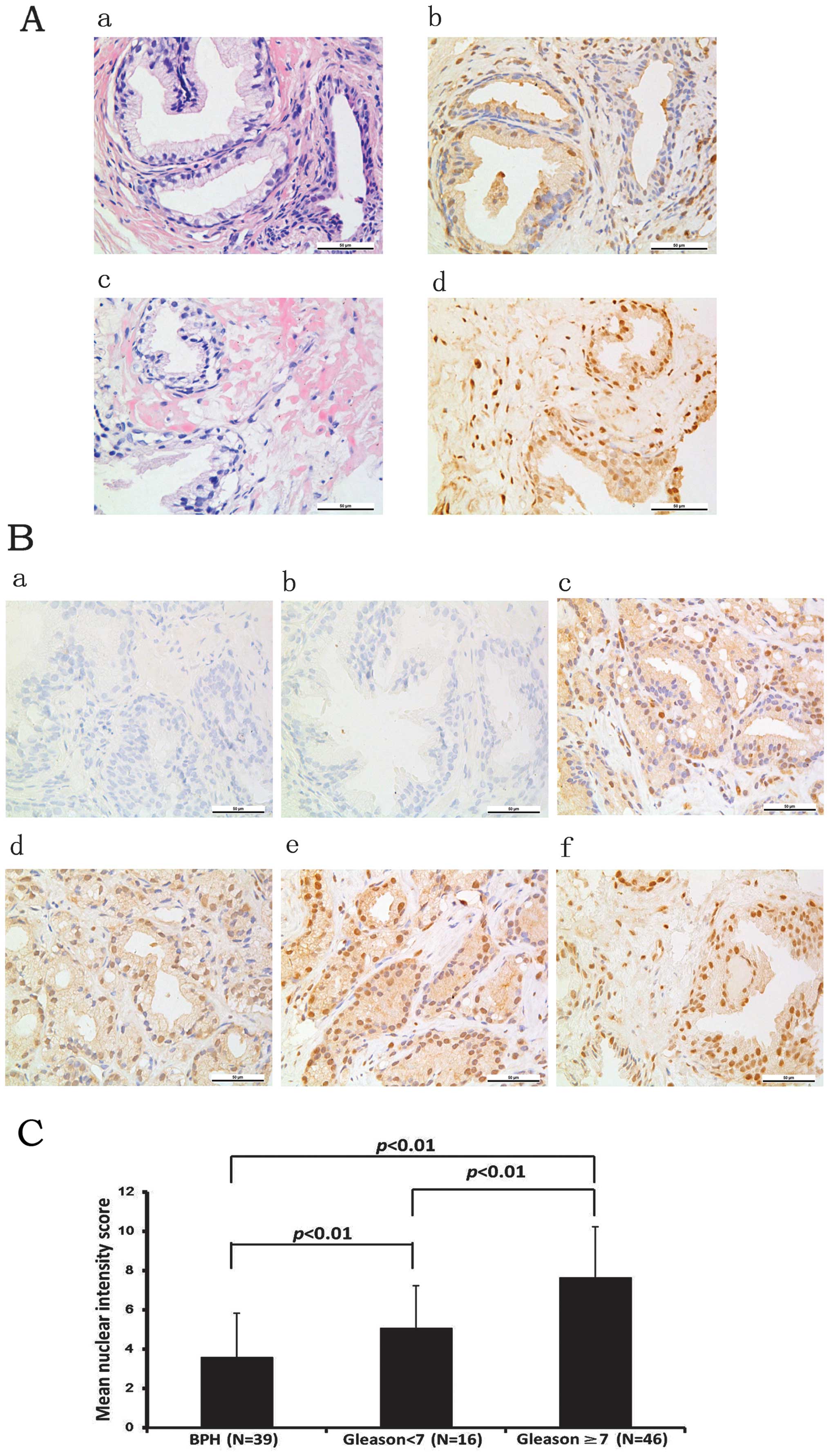
the cytoplasm and the nucleus of the cells (Fig. 1A). The differential expression of
FoxM1 was observed between the BPH and PCa tissues (Fig. 1B). The intensity scores of FoxM1
expression in the PCa tissues were markedly higher than those in
the BPH tissues. In the three PIN cases of grade I and the four PIN
cases of grade II–III, the number of cases with high levels of
FoxM1 expression was one and three, respectively. Moreover, there
was a positive correlation between the expression levels of FoxM1
and the Gleason score. The results indicated that FoxM1 expression
levels were higher in the tissues with a Gleason score ≥7 compared
with those with a Gleason score <7. These results indicate that
FoxM1 plays a crucial role in the progression of PCa (Fig. 1C).
 | Table IICorrelation between the protein
expression of FoxM1 and clinicopathological parameters of the
patients with prostate cancer. |
Table II
Correlation between the protein
expression of FoxM1 and clinicopathological parameters of the
patients with prostate cancer.
| | FoxM1
expression | |
|---|
| |
| |
|---|
| Characteristic | No. | Low | High | P-value |
|---|
| BPH | 39 | 28 (71.8%) | 11 (28.2%) | 0.0005a |
| Age | | | | 0.768 |
| <60 | 12 | 9 | 3 | |
| ≥60 | 27 | 19 | 8 | |
| PIN | | | | |
| Grade I | 3 | 2 | 1 | |
| Grade II–III | 4 | 1 | 3 | |
| PCa | 62 | 21 (33.9%) | 41 (66.1%) | |
| Age | | | | 0.694 |
| <60 | 13 | 5 | 8 | |
| ≥60 | 49 | 16 | 33 | |
| Gleason
scores | | | | 0.0123 |
| <7 | 16 | 10 | 6 | |
| ≥7 | 46 | 11 | 35 | |
Elevated endogenous expression of FoxM1
protein in PCa cell lines
To explore the endogenous expression of FoxM1 in PCa
cells, the expression of FoxM1 in four human PCa cell lines was
examined by quantitative RT-PCR and western blot analysis. The PCa
cell lines (LNCaP, DU-145, PC-3 and PC-3M) showed a positive
expression of FoxM1 by western blot analysis (Fig. 2A). The mRNA levels of FoxM1 were
detected in the aforementioned cell lines by quantitative PCR
(Fig. 2B). Furthermore, a FoxM1
plasmid was transfected into the LNCaP PCa cells and a FoxM1 shRNA
plasmid was transfected into the DU-145 PCa cells (Fig. 2C). The results revealed good
transfection efficiencies.
Metformin suppresses the proliferation of
PCa cells and downregulates the protein expression of FoxM1
To determine whether metformin inhibits the
proliferation of PCa cells and in particular, the expression of
FoxM1 in PCa cells, the growth curves and expression of FoxM1 were
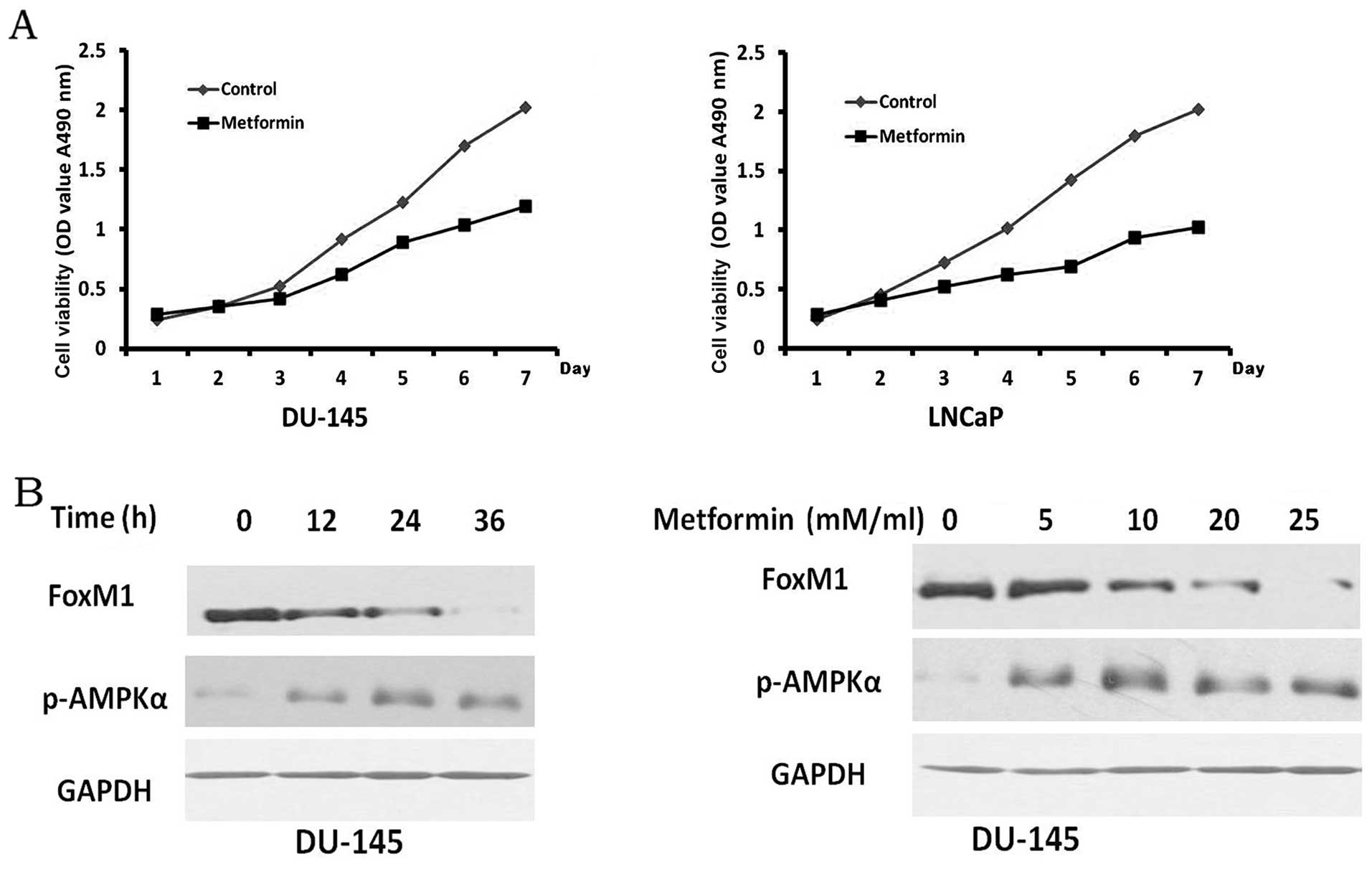
observed in the PCa cells treated with metformin. In the DU-145 and
LNCaP cells, cell viability was markedly inhibited by metformin
(Fig. 3A). The expression levels
of FoxM1 protein in the DU-145 cells markedly decreased when the
cells were treated with metformin in a time- and dose-dependent
manner (Fig. 3B). Consistent with
previous findings (28),
metformin as an AMPK activator, decreased the expression of FoxM1.
Thus, these data demonstrate that metformin suppresses the
proliferation and downregulates the expression of FoxM1 in PCa
cells.
Establishment of in vitro model of EMT
using PCa cell lines
In order to examine the association between FoxM1
and EMT in PCa cells, the establishment of an in vitro model
of EMT is essential. TGF-β1 (10 ng/ml) stimulation was used to
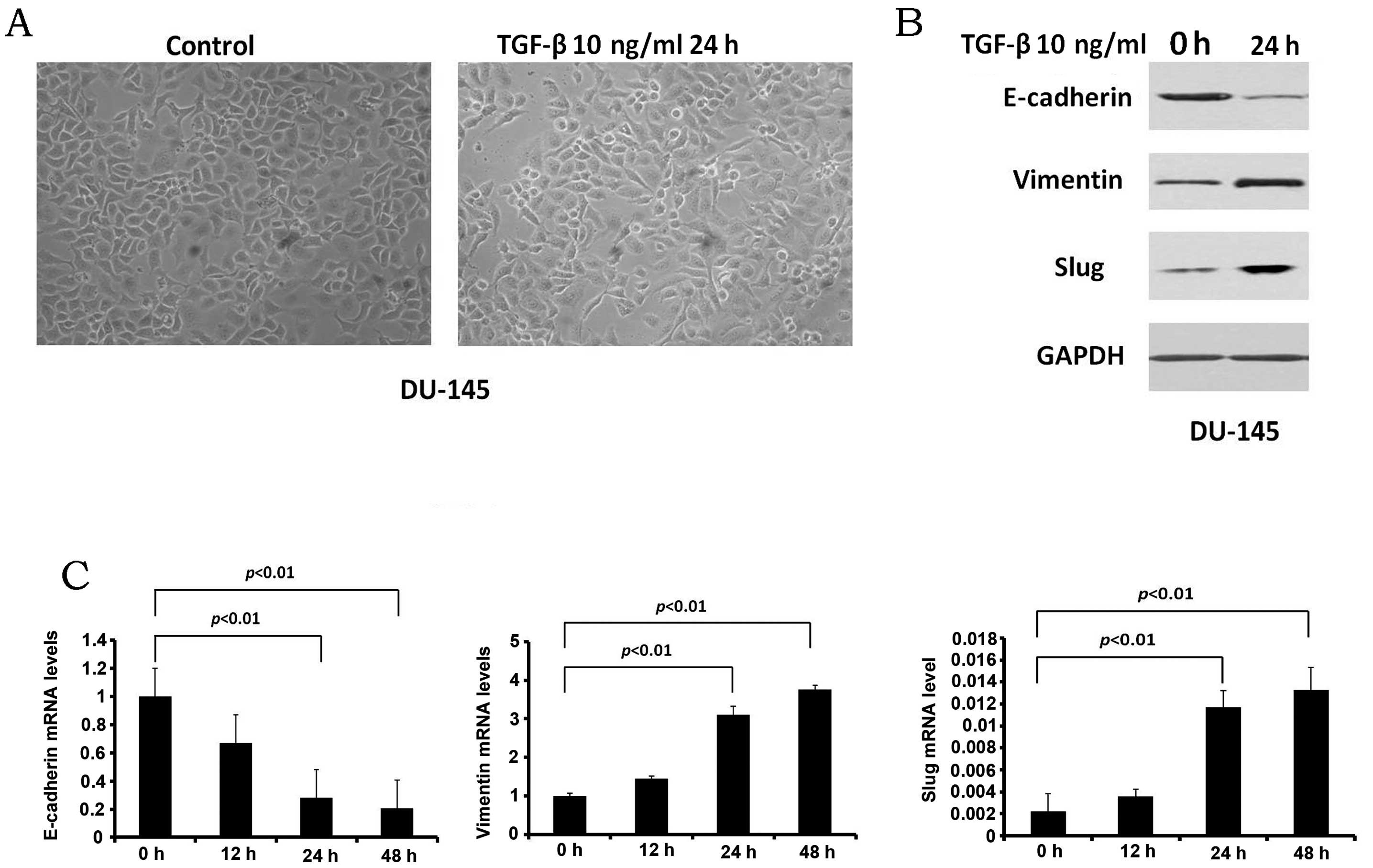
induce EMT in the DU-145 cells in our study. Following treatment
with TGF-β1 for 24 h, the cell morphology was slightly altered
(Fig. 4A). The epithelial cancer
cells were partly transformed into mesenchymal-like cells. The
epithelial marker, E-cadherin, and the mesenchymal markers,
vimentin and Snail2 (Slug), were also examined by western blot
analysis (Fig. 4B). The protein
expression of E-cadherin was downregulated, whereas the expression
of vimentin and Slug was upregulated. The results indicated that
the model of EMT with the DU-145 cells was established. The mRNA
levels of EMT-related genes were also detected (Fig. 4C); the mRNA level of E-cadherin
was decreased, while the mRNA levels of vimentin and Slug were
increased.
Metformin prevents TGF-β1-induced EMT
through FoxM1
In order to examine whether FoxM1 is required for
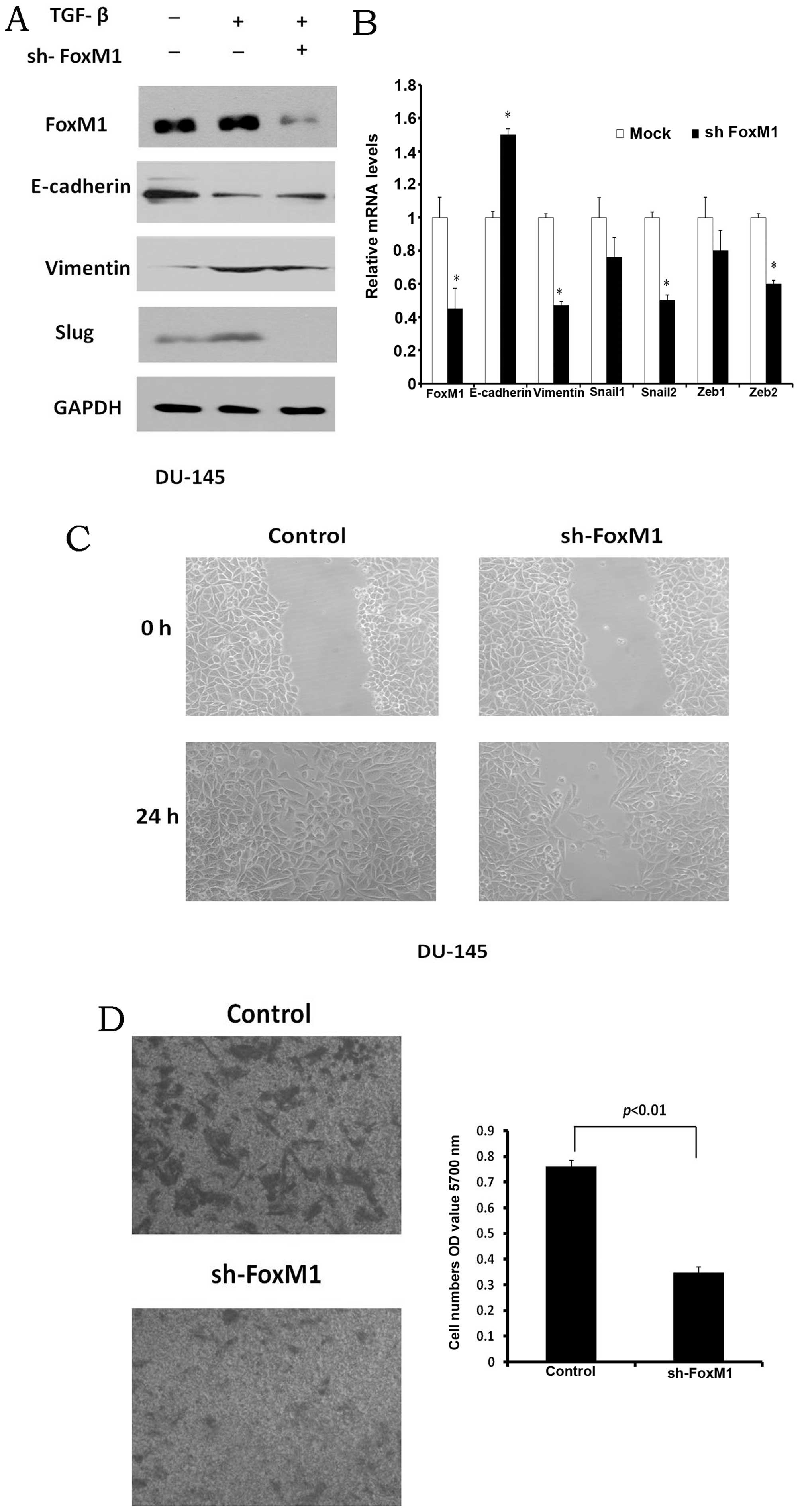
the migration of PCa cells, FoxM1 shRNA was transfected into the
DU-145 cells. After 24 h, TGF-β1 was added to the cells. The
protein levels of E-cadherin, vimentin and Slug were examined by
western blot analysis. The protein level of E-cadherin was
increased partly, whereas the expression levels of vimentin and
Slug in the cells transfected with FoxM1 shRNA were decreased
(Fig. 5A). The mRNA levels of
FoxM1, E-cadherin, vimentin, Snail1, Slug, zinc finger E-box
binding homeobox (Zeb)1 and Zeb2 in the FoxM1-knockdown cells were
also examined by quantitative PCR. The loss of FoxM1 was associated
with the increased E-cadherin mRNA and the decreased vimentin, Slug
and Zeb2 mRNA expression (Fig.
5B).
To confirm that FoxM1 regulates PCa cell migration,
the migration ability of the DU-145 cells was detected following
transfection with FoxM1 shRNA. The wound disappeared after 24 h in
the control cells, while the self-healing ability of the FoxM1
shRNA-transfected cells was poor (Fig. 5C). After plating into the insert
of the Transwell chamber (24 h later), the cell migration ability
was measured. The knockdown of FoxM1 markedly decreased the
migration ability of the DU145 cells (Fig. 5D). These data confirm that FoxM1
is required for the migration of PCa cells and that metformin
regulates the EMT process in PCa cells through FoxM1.
Discussion
PCa is one of the human malignant cancers of which
the incidence and mortality rate are very high. In particular, in
patients with advanced stages of PCa, metastasis will aggravate the
condition and shorten the life span of the patients. In recent
years, the process of EMT in cancer has increasingly become a
research hotspot. As previously demonstrated, in pathological
specimens, the altered expression of various cell lineage markers
supports the hypothesis that the EMT process promotes the
progression of PCa (29).
FoxM1 belongs to the forkhead superfamily of
transcription factors which share an evolutionary conserved ‘winged
helix’ DNA-binding domain. It regulates the expression of
downstream target genes by the consensus binding sequence, TAAACA
(30,31). FoxM1 is expressed in proliferating
cells, but its expression is lost in cells which are in the
stationary phase and are terminally differentiated. It plays
important roles in cell proliferation, cell cycle, cell
differentiation, angiogenesis and metastasis. Previous studies have
demonstrated that FoxM1 is upregulated in diverse human
malignancies and its high expression indicates poor prognosis
(4,8,11,14,15). Although the expression of FoxM1 in
PCa has been previously reported (7), our study compares the expression of
FoxM1 in BPH with that in PCa tissues obtained from the Chinese
population. The results revealed that the expression level of FoxM1
protein was highly elevated in the PCa tissues. Our data also
demonstrated that FoxM1 was expressed in the BPH tissues. However,
the intensity scores of FoxM1 expression were markedly higher in
the PCa tissues and were associated with the Gleason scores.
According to these results, the high protein expression of FoxM1
may be a potential marker indicating the progression of PCa.
EMT is detected initially in embryonic development
and wound healing in physiological processes. Subsequently, EMT is
known to be associated with fibrotic diseases and cancer (32). EMT is classified into three
subtypes according to the biological features (33). EMT during implantation,
embryogenesis, and organ development is defined as type 1. Type 2
refers to EMT related to tissue regeneration and organ fibrosis. A
previous study reported that EMT found in prostate hyperplasia
cells should belong to this type (34). Type 3, which we payed close
attention to in our study, occurs in the process of cancer
progression and metastasis. The changes that occur in biomarkers in
EMT can be summed up in two parts: loss of epithelial cell markers,
such as E-cadherin and cytokeratins, and the upregulation of
mesenchymal cell markers, including vimentin and N-cadherin
(29). Several transcription
factors have been found to involved in the process of EMT, such as
the Snail family of zinc-finger transcription factors, Snail and
Slug; the two-handed zinc-finger factors of δEF1 family proteins,
Zeb1 and Zeb2 (also known as Smad-interacting protein), and the
basic helix-loop-helix factor, Twist (33,35,36). Further studies have shown that the
Snail transcription factor inhibits E-cadherin expression by
binding several E-boxes located in the promoter region (37). In this study, our data indicated
that FoxM1 was involved in the process of EMT induced by TGF-β in
PCa cells. Our results are in accordance with those of a previous
study, in which Slug was regulated by FoxM1 (23). These results strengthen the
concept that the Snail family plays an important role in EMT in
cancer. FoxM1 is a crucial transcription factor in EMT by
regulating the Snail family.
Evidence from epidemiological, histopathological,
molecular pathological and clinical studies indicates that there is
a correlation between metabolic syndrome (MetS) and the the
development of BPH and PCa (38,39). As the most common therapy to lower
blood glucose concentration in patients with type 2 diabetes and
MetS, metformin notably blocks hepatic glucose production, reduces
insulin resistance and lowers insulin levels. Several studies have
shown that it inhibits human cancer cell proliferation and tumor
growth by decreasing cyclin D1 expression (40). In addition to this, metformin has
been proven to impede the TGF-β-induced loss of the epithelial
marker, E-cadherin, in human breast cancer cells (41). In our study, we found that
metformin inhibited the expression of FoxM1; thus, it participates
in the regulation of EMT in PCa cells. Our results are in
accordance with those presented in the study by Yung et al
(28); thus, the suppression of
FoxM1 by metformin is dependent on the AKT/FOXO3a/FoxM1 signaling
cascade in PCa cells. When FoxM1 was knocked down, the expression
of the gene, Slug, which is crucial for EMT, was markedly
decreased. Thus, these results indicate that metformin inhibits EMT
through the downregulation of FoxM1 expression in PCa. However,
there were some limitations in our study. Firstly, the number of
specimens was limited and a larger sample size is required to
further confirm our results. Secondly, due to the limited research
time, the dynamic tracing data was not sufficient. We aim to
provide further information and discuss the correlation between the
expression of FoxM1 and the outcome of PCa patients in future
studies.
In conclusion, the data in the current study suggest
that the expression levels of FoxM1 are associated with the
progression of PCa. FoxM1 plays an important role in EMT in PCa and
the suppressive effects of metformin on EMT may partly involve the
downregulation of FoxM1. FoxM1 plays a crucial role in the
progression of PCa and the inhibition of FoxM1 may prove to be an
effective therapeutic strategy.
Acknowledgements
We thank Professor J.G. Zhou for providing the
DU-145 cell line. This study was supported in part by a grant from
the National Natural Science Foundation of China (no.
81171357).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Christiansen JJ: Reassessing epithelial to
mesenchymal transition as a prerequisite for carcinoma invasion and
metastasis. Cancer Res. 66:8319–8326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laoukili J, Kooistra MRH, Brás A, et al:
FoxM1 is required for execution of the mitotic programme and
chromosome stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang DK, Son CH, Lee SK, Choi PJ, Lee KE
and Roh MS: Forkhead box M1 expression in pulmonary squamous cell
carcinoma: correlation with clinicopathologic features and its
prognostic significance. Hum Pathol. 40:464–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalin TV, Wang IC, Ackerson TJ, Major ML,
Detrisac CJ, Kalinichenko VV, Lyubimov A and Costa RH: Increased
levels of the FoxM1 transcription factor accelerate development and
progression of prostate carcinomas in both TRAMP and LADY
transgenic mice. Cancer Res. 66:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun HC, Li M, Lu JL, et al: Overexpression
of Forkhead box M1 protein associates with aggressive tumor
features and poor prognosis of hepatocellular carcinoma. Oncol Rep.
25:1533–1539. 2011.PubMed/NCBI
|
|
9
|
Cancer Genome Atlas Research Network.
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar
|
|
10
|
Green MR, Aya-Bonilla C, Gandhi MK, et al:
Integrative genomic profiling reveals conserved genetic mechanisms
for tumorigenesis in common entities of non-Hodgkin’s lymphoma.
Genes Chromosomes Cancer. 50:313–326. 2011.PubMed/NCBI
|
|
11
|
Huynh KM, Soh JW, Dash R, Sarkar D, Fisher
PB and Kang D: FOXM1 expression mediates growth suppression during
terminal differentiation of HO-1 human metastatic melanoma cells. J
Cell Physiol. 226:194–204. 2011. View Article : Google Scholar
|
|
12
|
Uddin S, Ahmed M, Hussain A, Abubaker J,
Al-Sanea N, AbdulJabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Jehan
Z, Bavi P, Siraj AK and Al-Kuraya KS: Genome-wide expression
analysis of Middle Eastern colorectal cancer reveals FOXM1 as a
novel target for cancer therapy. Am J Pathol. 178:537–547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bektas N1, Haaf At, Veeck J, et al: Tight
correlation between expression of the Forkhead transcription factor
FOXMl and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia L1, Mo P, Huang W, Zhang L, Wang Y,
Zhu H, Tian D, Liu J, Chen Z, Zhang Y, Chen Z, Hu H, Fan D, Nie Y
and Wu K: The TNF-α/ROS/HIF-1-induced upregulation of FoxMI
expression promotes HCC proliferation and resistance to apoptosis.
Carcinogenesis. 33:2250–2259. 2012.
|
|
15
|
Wang Y1, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang IC, Chen YJ, Hughes D, et al:
Forkhead box M1 regulates the transcriptional network of genes
essential for mitotic progression and genes encoding the SCF
(Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad A, Wang Z, Kong D, et al: FoxM1
down-regulation leads to inhibition of proliferation, migration and
invasion of breast cancer cells through the modulation of
extra-cellular matrix degrading factors. Breast Cancer Res Treat.
122:337–346. 2010. View Article : Google Scholar
|
|
18
|
Priller M, Pöschl J, Abrão L, et al:
Expression of FoxM1 is required for the proliferation of
medulloblastoma cells and indicates worse survival of patients.
Clin Cancer Res. 17:6791–6801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Zhang N, Jia Z, et al: Critical role
and regulation of transcription factor FoxM1 in human gastric
cancer angiogenesis and progression. Cancer Res. 69:3501–3509.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karadedou CT, Gomes AR, Chen J, et al:
FOXO3a represses VEGF expression through FOXM1-dependent and
-independent mechanisms in breast cancer. Oncogene. 31:1845–1858.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai B, Kang SH, Gong W, et al: Aberrant
FoxM1B expression increases matrix metalloproteinase-2
transcription and enhances the invasion of glioma cells. Oncogene.
26:6212–6219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balli D, Ustiyan V, Zhang Y, et al: Foxm1
transcription factor is required for lung fibrosis and
epithelial-to-mesenchymal transition. EMBO J. 32:231–244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang C, Chen H, Tan G, et al: FOXM1
promotes the epithelial to mesenchymal transition by stimulating
the transcription of Slug in human breast cancer. Cancer Lett.
340:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Del Barco S, Vazquez-Martin A, Cufi S, et
al: Metformin: multi-faceted protection against cancer. Oncotarget.
2:896–917. 2011.PubMed/NCBI
|
|
25
|
Hadad SM, Hardie DG, Appleyard V and
Thompson AM: Effects of metformin on breast cancer cell
proliferation, the AMPK pathway and the cell cycle. Clin Transl
Oncol. Dec 12–2013.(Epub ahead of print).
|
|
26
|
He SY, Shen HW, Xu L, et al: FOXM1
promotes tumor cell invasion and correlates with poor prognosis in
early-stage cervical cancer. Gynecol Oncol. 127:601–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu K, Yin X, Weng T, et al: Targeting WW
domains linker of HECT-type ubiquitin ligase Smurf1 for activation
by CKIP-1. Nat Cell Biol. 10:994–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yung MMCD, Liu VW, Yao KM and Ngan HY:
Activation of AMPK inhibits cervical cancer cell growth through AKT
FOXO3a FOXM1 signaling cascade. BMC Cancer. 13:3272013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial-mesenchymal and mesenchymal-epithelial transitions in
carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korver W, Roose J, Heinen K, et al: The
human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and
promoter characterization. Genomics. 46:435–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laoukili J, Stahl M and Medema RH: FoxM1:
at the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
32
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slabáková E, Pernicová Z, Slavíčková E,
Staršíchová A, Kozubík A and Souček K: TGF-β1-induced EMT of
non-transformed prostate hyperplasia cells is characterized by
early induction of SNAI2/Slug. Prostate. 71:1332–1343. 2011.
|
|
35
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013.
|
|
36
|
Saitoh M and Miyazawa K: Transcriptional
and post-transcriptional regulation in TGF-β-mediated
epithelial-mesenchymal transition. J Biochem. 151:563–571.
2012.
|
|
37
|
Lin T, Ponn A, Hu X, Law BK and Lu J:
Requirement of the histone demethylase LSD1 in Snai1-mediated
transcriptional repression during epithelial-mesenchymal
transition. Oncogene. 29:4896–4904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorbachinsky I, Akpinar H and Assimos DG:
Metabolic syndrome and urologic diseases. Rev Urol. 12:e157–e180.
2010.PubMed/NCBI
|
|
39
|
Parsons JK, Carter HB, Partin AW, et al:
Metabolic factors associated with benign prostatic hyperplasia. J
Clin Endocrinol Metab. 91:2562–2568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jalving M, Gietema JA, Lefrandt JD, et al:
Metformin: taking away the candy for cancer? Eur J Cancer.
46:2369–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cufi S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFβ-induced epithelial-to-mesenchymal transition
(EMT): from cancer stem cells to aging-associated fibrosis. Cell
Cycle. 9:4461–4468. 2010.
|