Introduction
Colon cancer is the third most common cancer. A
recent decline in the incidence of colon cancer has been largely
attributed to an increase in screening for the disease, allowing
for the detection and removal of precancerous polyps. However, the
mortality rate associated with colon cancer remains high (1). Establishing treatment strategies
that reduce unfavorable effects and increase the overall prognosis
of colon cancer is challenging. An inverse correlation between a
high intake of fruits, vegetables, and phytochemicals and a reduced
risk of colon cancer has been reported (2). The chemopreventive agents and their
constituent phytochemicals derived from plants have been known to
interfere with various molecular pathways involved in colon cancer
initiation and progression (3).
Therefore, there is considerable interest in developing preventive
and therapeutic agents for cancer from natural products (4).
Sophora alopecuroides (S.
alopecuroides) is a traditional Chinese herb that has been used
clinically for acute bacillary dysentery and enteritis. Its main
bioactive components are quinolizidine alkaloids, including >20
types of alkaloids (5). Most of
the components, such as matrine, oxymatrine and sophoridine,
possess antitumor activities (6–11).
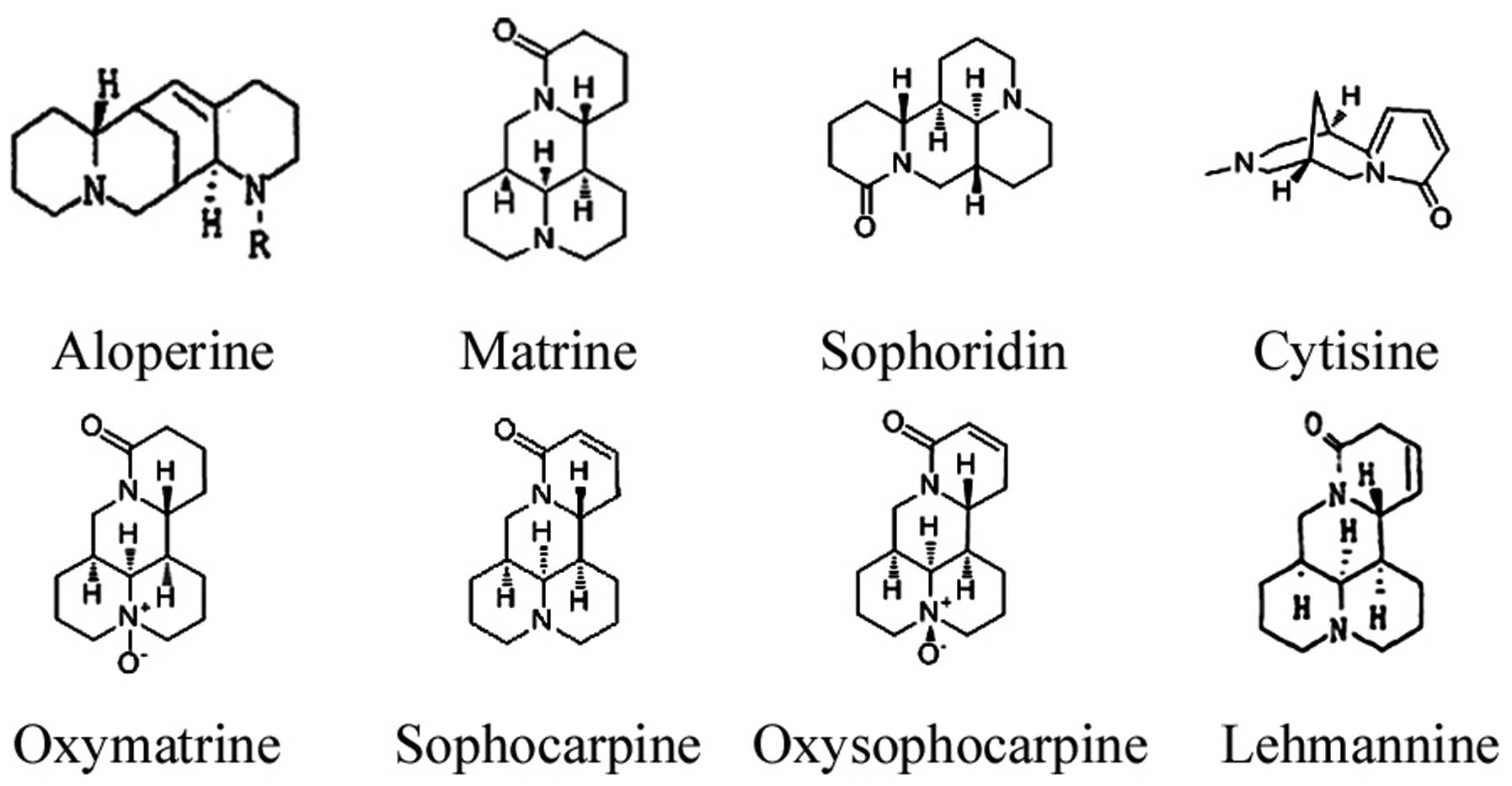
Aloperine (ALO) (Fig.
1) is a quinolizidine alkaloid that is extracted from S.
alopecuroides (12). ALO
exerts anti-inflammatory, anti-allergenic, antitumor and antiviral
effects (12–15). Of six alkaloids derived from S.
alopecuroides, ALO was found to exert the most potent cytotoxic
activity against several human cancer cells of differing tissue
origins (13). However, little is
known with regard to the effect of ALO against colon cancer and the
mechanisms associated with its activity.
The aim of the present study was to assess the
antitumor efficacy of ALO and other alkaloids isolated from S.
alopecuroides, including matrine, oxymatrine, sophoridine,
sophocarpine, oxysophocarpine, lehmannine and cytisine (Fig. 1) against HCT116 colon cancer cells
and to investigate the mechanism of ALO resistance of colon cancer
cells to provide some basis for the development of ALO as a new
drug against colon cancer. The data showed that the series of
alkaloids isolated from S. alopecuroides inhibited the
growth of HCT116 cells and that ALO possesses the strongest
cytotoxic activity against these cells compared with the other
alkaloids. In HCT116 human colon cancer cells, ALO also produced
long-term clonogenic effects. To identify the molecular mechanisms
underlying these responses to ALO, we investigated the effects of
ALO on cell cycle progression and apoptosis.
Materials and methods
Materials
Matrine, oxymatrine, sophoridine and oxysophocarpine
were purchased from the National Institutes for Food and Drug
Control (Beijing, China). ALO, sophocarpine, lehmannine and
cytisine were generously provided by the Ningxia Bauhinia
Pharmaceutical Co., Ltd. (Ningxia, China). All of the compounds
were dissolved in phosphate-buffered saline (PBS; Boster, Wuhan,
China) at a concentration of 20 mM and stored at 4°C.
Cell culture
HCT116 human colon cancer cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in RPMI-1640 medium (HyClone, Thermo Fisher
Biotechnology, Beijing, China) supplemented with 10% fetal bovine
serum (FBS; Tianhang, Hangzhou, China), 100 U/ml penicillin and 10
mg/ml streptomycin (Gibco, Life Technologies Biotechnology,
Shanghai, China) and grown in a 37°C incubator with 5%
CO2. The medium was replaced at 2–3 day intervals.
Subconfluent cells were routinely harvested with 0.05%
trypsin/0.02% EDTA (Gibco, Life Technologies Biotechnology).
MTT assay
HCT116 cells were seeded at 1×104
cells/well in 180 μl of complete culture medium in 96-well plates
and cultured for 24 h. The medium was then replaced with fresh
RPMI-1640 or with fresh RPMI-1640 containing various concentrations
of ALO (0, 0.3125, 0.625, 1.25, 2.5 and 5 mM) and the other
alkaloids (0, 1.25, 2.5, 5, 7.5 and 10 mM). Each alkaloid
concentration was repeated in 5 wells. After incubating for 24, 48
or 72 h, MTT (5 mg/ml, 20 μl) was added to each well and incubated
for 4 h. The medium was aspirated from the wells and 150 μl of
dimethyl sulfoxide (DMSO; Fuyu Chemical, Tianjin, China) was added
into each well and agitated for 10 min. The optical density (OD) at
570 nm was recorded using a microplate reader (Thermo Scientific,
Multiskan GO, Waltham, MA, USA). The inhibition rate was calculated
using the formula: inhibition rate (%) = (1 -
Atreated/Acontrol) ×100%.
Observation of morphologic changes
HCT116 cells were seeded into 6-well plates and
treated with various concentrations (0, 0.25, 0.5 and 1 mM) of ALO
for 24 h. Cell morphology was observed using a fluorescence
microscope (Eclipse T1; Nikon, Tokyo, Japan).
Clonogenic survival assay
HCT116 cells were counted and seeded into 6-well
tissue culture plates (3×102 cells/well) in RPMI-1640
supplemented with 10% FBS. After the cells adhered, they were
treated with different doses (0, 0.1, 0.2 and 0.3 mM) of ALO for 7
days. The cells were then fixed with 4% paraformaldehyde (PFA) and
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology, Jiangsu, China) (16). Representative images were captured
using a digital camera (Olympus, Tokyo, Japan).
Flow cytometric (FACS) analysis
HCT116 cells were seeded into 6-well plates at a
density of 1×106 cells/well and were treated with a
series of ALO concentrations (0, 0.25 and 0.5 mM) for 24 h. The
cells were collected, washed with PBS and fixed in 70% ethanol, and
then stored at 4°C overnight. The cells were washed with PBS again
and stained with 5 μg/ml RNase and 20 μg/ml of propidium iodide
(PI) (Beyotime Institute of Biotechnology) in the dark at 37°C for
30 min and analyzed using a flow cytometer (FACScan; BD
Biosciences, Franklin Lakes, NJ, USA). The cellular DNA contents
were identified for detection of the cell cycle distribution. At
least 10,000 events were counted for each sample.
Apoptosis was measured using the Annexin V-FITC/PI
Apoptosis Detection kit (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions. HCT116 cells (1×106
cells/well) were treated with a series of ALO concentrations (0,
0.25, 0.5 and 1 mM) for 24 h. The cells were then collected by
centrifugation and washed with PBS. The cells were centrifuged and
resuspended in 100 μl of binding buffer to which 5 μl of Annexin V
and 1 μl of PI were added. The mixture was incubated at room
temperature in the dark for 15 min. The cells were analyzed using a
flow cytometer. For each measurement, at least 10,000 cells were
counted.
Western blot analysis
The cells were incubated with a series of ALO
concentrations (0, 0.25, 0.5 and 1 mM) for 24 h and harvested. The
cells were lysed in RIPA lysis buffer (Beyotime Institute of
Biotechnology) on ice for 30 min. The proteins contained in the
lysates were quantified using the BCA Protein assay kit (Beyotime
Institute of Biotechnology). Total protein (30 μg) was subjected to
10% SDS polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology) and transferred onto PVDF membranes. The membranes
were blocked with 5% non-fat milk at room temperature for 2 h with
rocking and then incubated with specific primary antibodies against
p53, Bax, Bcl-2, cyclin D1, p21, cyclin B1, JAK1, Stat3, p101
(PI3KC3), Akt and β-actin (Cell Signaling Technology, Beverly, MA,
USA) overnight at 4°C. After washing the membranes with TBS (PBS
with 0.05% Tween-20) three times for 15 min each, the membranes
were incubated with secondary antibodies at room temperature for 2
h. After washing three times in TBS for 15 min, the specific
protein bands were detected using the Kodak Image Station (Eastman
Kodak, Rochester, NY, USA). The data were normalized to β-actin for
analyses and plotting.
Quantitative RT-PCR (qRT-PCR) assays
Total RNA was isolated from the cells using a TRIzol
RNA isolation kit (Invitrogen). The cDNAs were synthesized using
the PrimeScript™ II first-strand cDNA synthesis kit (Takara,
Dalian, China) according to the manufacturer’s instructions. The
qRT-PCR reactions were performed in triplicate on an ABI PRISM 7300
(Applied Biosystems, Foster City, CA, USA) using the C1000 thermal
cycler PCR (Bio-Rad, Hercules, CA, USA). Primer sequences used in
this study are provided in Table
I. The PCR conditions included an initial denaturation step at
93°C for 2 min, followed by 40 cycles of a denaturation step at
93°C for 15 sec and an annealing/extension step at 55°C for 25 sec
and 72°C for 25 sec. The relative expression values of the
different genes were calculated using the 2−ΔΔCt
method.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | Primer
sequences | Amplied fragment
length (bp) |
|---|
| Bax | F:
5′-GCTGGACATTGGACTTCCTC-3′
R: 5′-CTCAGCCCATCTTCTTCCAG-3′ | 129 |
| Bcl-2 | F:
5′-ATGTGTGTGGAGAGCGTCAA-3′
R: 5′-ACAGTTCCACAAAGGCATCC-3′ | 136 |
| Tp53 | F:
5′-CCAGCCAAAGAAGAAACCAC-3′
R: 5′-CCTCATTCAGCTCTCGGAAC-3′ | 92 |
| JAK1 | F:
5′-TGCCATGATGAAGAAGATGC-3′
R: 5′-GACACGCTGCTGTCACAAAT-3′ | 186 |
| Stat3 | F:
5′-AGTATAGCCGCTTCCTGCAA-3′
R: 5′-GCAATCTCCATTGGCTTCTC-3′ | 106 |
| AKT1 | F:
5′-AGAAGCAGGAGGAGGAGGAG-3′
R: 5′-CCCAGCAGCTTCAGGTACTC-3′ | 139 |
| PI3K | F:
5′-AAGCAGTGCCTGTAGGAGGA-3′
R: 5′-TGTCGATGAGCTTTGGTGAG-3′ | 199 |
| Cyclin | B1 F:
5′-TTGATACTGCCTCTCCAAGCC-3′
R: 5′-AGCTCCATCTTCTGCATCCAC-3′ | 122 |
| H-P21 | F:
5′-TTGTACCCTTGTGCCTCGCT-3′
R: 5′-AATCTGTCATGCTGGTCTGCC-3′ | 101 |
| Cyclin D1 | F:
5′-ACCTGAGGAGCCCCAACAAC-3′
R: 5′-GCTTCGATCTGCTCCTGGC-3′ | 112 |
| H-β-actin | F:
5′-GCATGGGTCAGAAGGATTCCT-3′
R: 5′-TCGTCCCAGTTGGTGACGAT-3′ | 106 |
Statistical analysis
Data are presented as the mean ± SD of at least
three independent experiments. The Student’s t-test and ANOVA were
used to identify statistically significant differences with SPSS
13.0 software. Differences were considered significant when
P<0.05. The IC50 values were calculated with SPSS
13.0 software using Probit analysis.
Results
ALO inhibits the proliferation and
clonogenic survival of HCT116 colon cancer cells
To assess the activity of ALO as a therapeutic agent
against colon cancer, we examined the effects of ALO and other
alkaloids on the proliferation of HCT116 colon cancer cells.
Exponentially increasing HCT116 cells were treated with various
concentrations of ALO and other alkaloids for 24, 48 and 72 h.
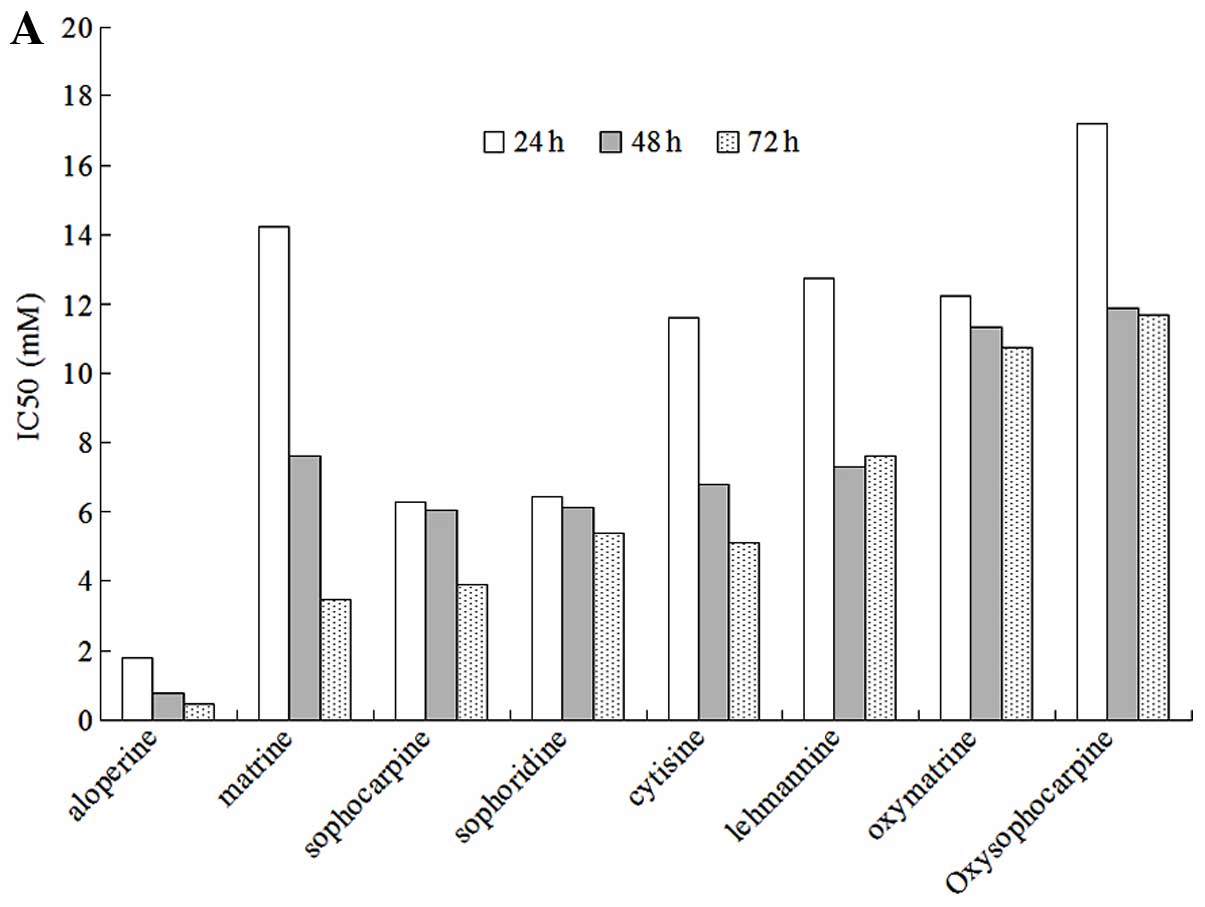
These drugs notably decreased the viability of HCT116 cells as
detected by the MTT assay (Fig.
2A). Compared with the other alkaloids, ALO exhibited the most
potent cytotoxic properties. Oxymatrine and oxysophocarpine
produced the lowest cytotoxic properties of all the alkaloids
tested, while sophoridine, sophocarpine, lehmannine, cytosine and
matrine exhibited similar functions. ALO decreased the percentage
of adherent HCT116 cells in a concentration- and time-dependent
manner (Fig. 2B). Additionally,
the ALO treatment induced obvious morphological changes. Compared
with the control group, the treated cells were more round and
exhibited more floating dead cells (Fig. 2C). To investigate the anticancer
effect of ALO in HCT116 human colon cancer cells, the colony
formation assay was used. After long-term ALO treatment,
clonogenicity was markedly reduced in the ALO-treated cells
compared with the control group (Fig.
2D). The clonogenic number of HCT116 cells decreased after the
treatment with different ALO concentrations, which is consistent
with the results of the morphological changes and MTT assay. Thus,
the results indicated that ALO induces anti-proliferative activity
in HCT116 colon cancer cells.
ALO inhibits cell cycle progression in
HCT116 cells
Cell cycle arrest and apoptosis induction are two
major causes of cell proliferative inhibition. To establish whether
ALO influences cell cycle progression, we determined the
distribution of ALO-treated HCT116 cells in various cell cycle
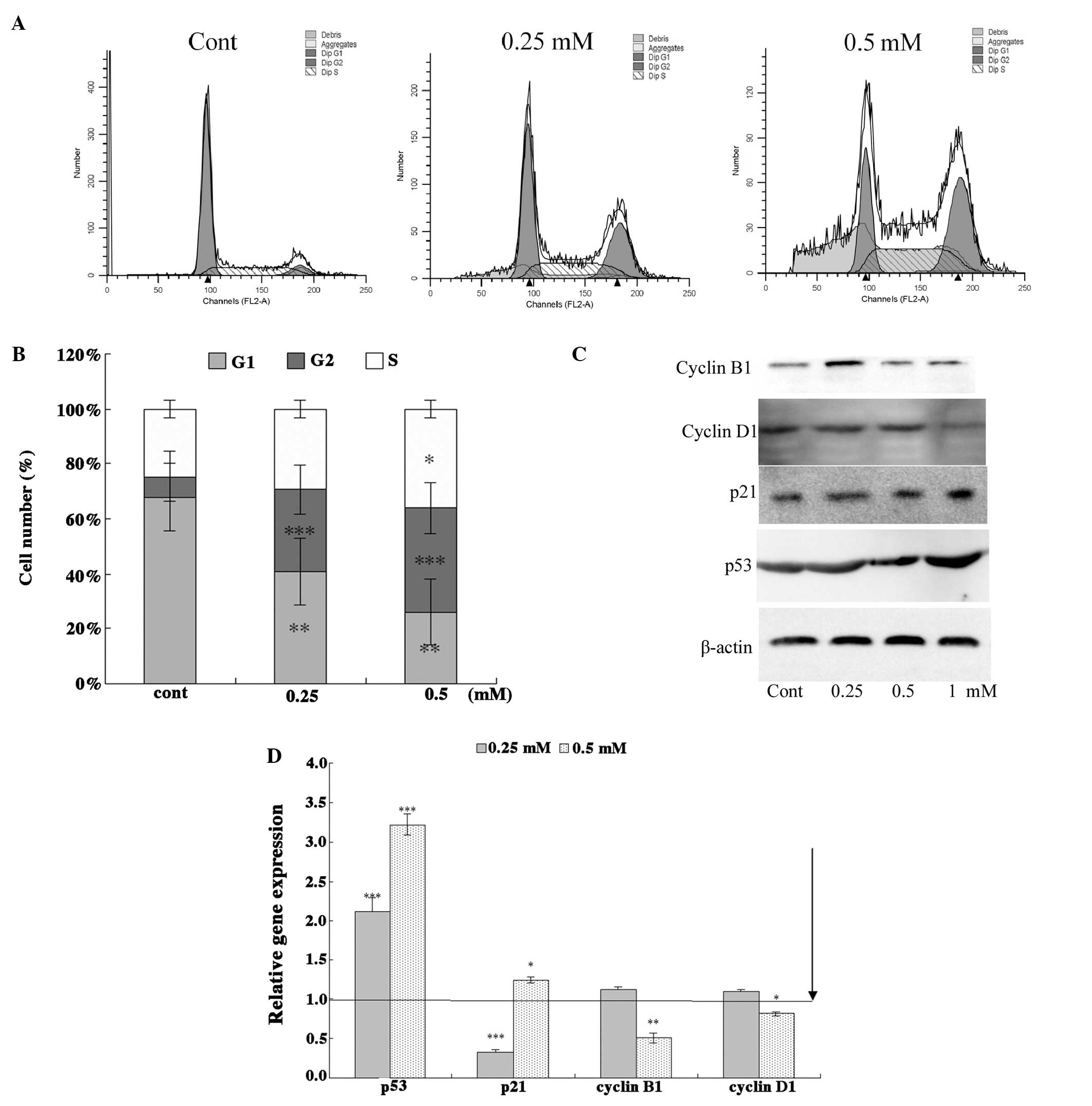
stages using flow cytometry. ALO treatment (24 h) effectively
mediated G2 cell cycle arrest in HCT116 cells (Fig. 3A). As expected, the number of G2
and S phase cells was increased after 24 h ALO treatment (Fig. 3B). The cell cycle analysis of the
HCT116 cells treated with different concentrations of ALO revealed
a higher number of cells in the G2/M and S phases, whereas the
number of cells in G0/G1 phase decreased compared with the
untreated cells. This result indicated that inhibition of the cell
cycle progression is an upstream event leading to apoptosis. To
resolve ALO-mediated G2 cell cycle arrest, the effects of ALO on
the expression of cell cycle regulatory proteins (p53, p21, cyclin
B1 and cyclin D1) were examined by western blot analysis (Fig. 3C). Results of the western blot
analysis indicated that the levels of p53 and p21 increased after
exposure to ALO for 24 h. By contrast, the levels of cyclin D1 and
B1 were significantly reduced at 24 h. The PCR analysis results
concurred with those of the western blot analysis (Fig. 3D). These results suggested that
ALO deregulates cell cycle progression and induces apoptosis.
ALO induces apoptosis in HCT116 cancer
cells
Apoptotic assays were performed to determine whether
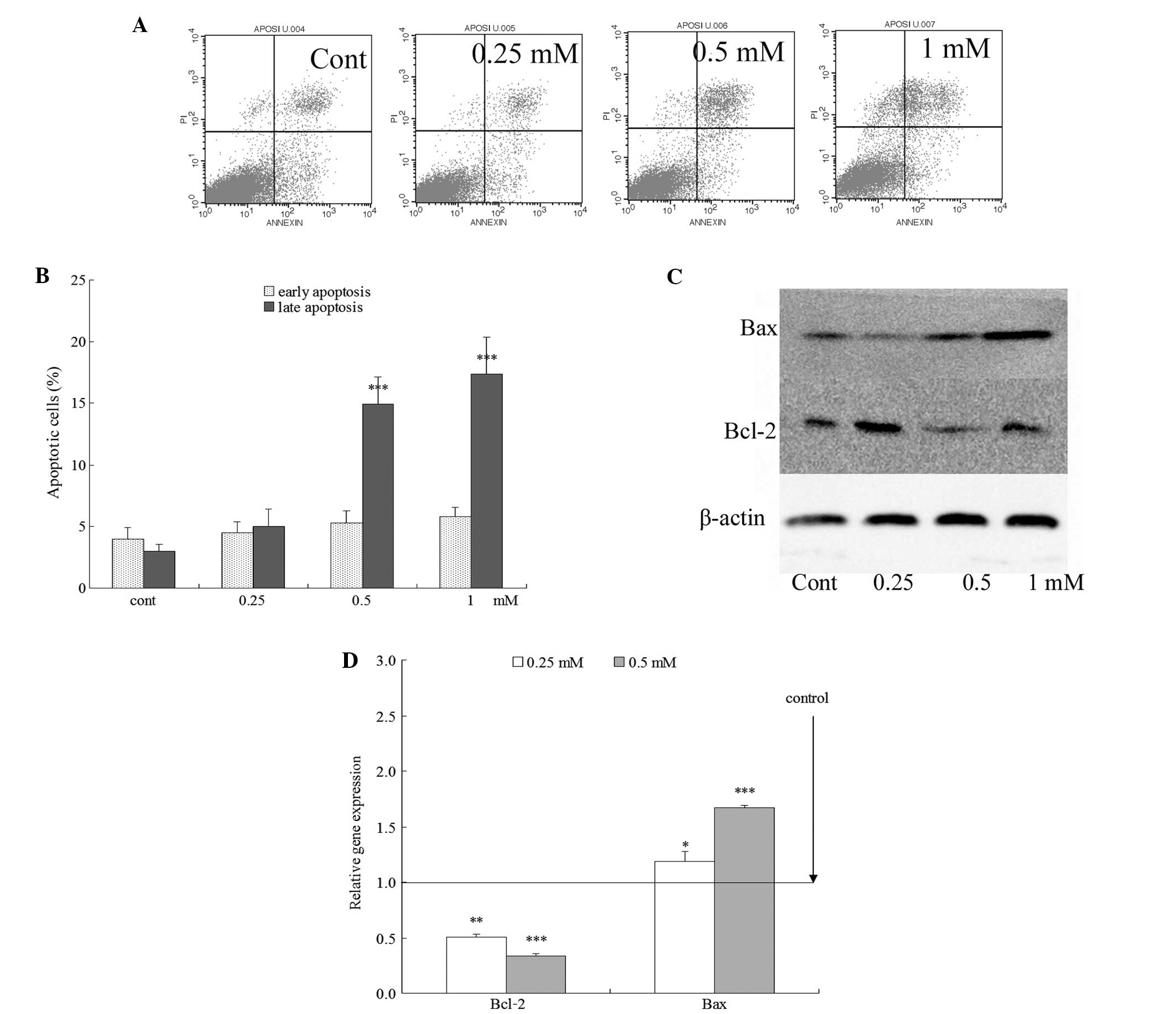
ALO induced apoptosis in HCT116 cells. The cells were stained with
Annexin V and PI and analyzed by flow cytometry. The early
apoptotic death rate in HCT116 cells treated with increasing
concentrations of ALO was not significantly different from the
control group (Fig. 4A). A few
late apoptotic cells were observed in the control group. However,
in the ALO-treated group, large amounts of apoptotic cells were
found. The rate of late apoptotic cells in the control group was
<5.0%, while the treatment with various concentrations of ALO
for 24 h significantly increased the incidence of late apoptotic
cells, and the rate of late apoptosis reached 4.99±1.37, 14.93±2.20
and 17.40±2.93% (P<0.01) with ALO concentrations of 0.25, 0.5
and 1 mM, respectively (Fig. 4B).
The rate of late apoptosis was increased in a
concentration-dependent manner.
ALO induces cell death by activating the
mitochondrial apoptotic pathway
The Bcl-2 family of proteins plays a crucial role in
regulating the mitochondria-dependent pathway of apoptosis.
Therefore, we examined whether Bcl-2 and Bax regulation was
involved in ALO-induced apoptosis using RT-PCR and western blotting
to detect their expression. Bcl-2 was downregulated, and Bax
expression was upregulated following ALO treatment (Fig. 4C and D). Therefore, the results
indicated that ALO induced apoptosis, which was accompanied by the
dose-dependent upregulation of Bax and downregulation of Bcl-2
(P<0.05).
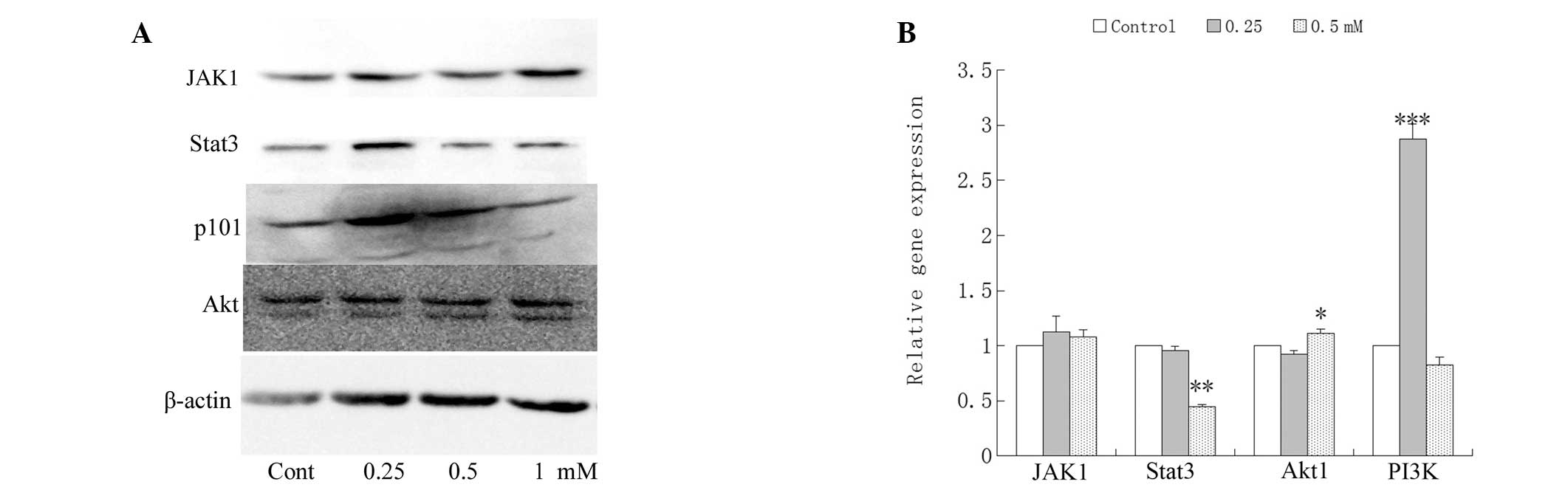
Effects of ALO on JAK/Stat3 and
phosphatidylinositol 3-kinase (PI3K)/Akt signaling
PI3K/Akt and JAK/Stat3 signaling pathways regulate
cell proliferation, survival, and apoptosis (17–19). Therefore, the effects of ALO on
the modification of apoptosis-related proteins and mRNA were
examined. The HCT116 cells treated with ALO for 24 h demonstrated a
dose-dependent decrease in mRNA and protein in Stat3 and PI3KC3. By
contrast, modest dose-dependent decreases were detected in JAK1 and
Akt (Fig. 5).
Discussion
The identification of effective anticancer drugs
from plant-derived natural products plays an important role in
cancer chemotherapy. Alkaloids constitute a rich resource of
compounds for identifying drugs. Most of the alkaloids identified
in S. alopecuroides are quinolizidine alkaloids. Previous
studies have confirmed that some quinolizidine alkaloids, including
matrine, oxymatrine and sophoridine, exert a marked anticancer
effect and are believed to be beneficial for cancer chemoprevention
(6–11). ALO demonstrated a more effective
inhibition of several cancer cell lines than matrine, oxymatrine
and sophoridine (13). However,
there are no reports that address the effect of ALO on colon cancer
cells or whether ALO is more effective than other quinolizidine
alkaloids. The mechanisms underlying the antitumor activity of ALO
have not been studied in detail. In this study, we investigated the
antitumor activities of the alkaloids in vitro. Compared to
seven other alkaloids, ALO produced the most potent time- and
dose-dependent inhibition of proliferation of HCT116 colon cancer
cells. ALO effectively inhibited the long-term clonogenic survival
of HCT116 cells by inhibiting cell cycle progression and inducing
apoptosis. Cell cycle arrest at the G2/M phase correlated with
reduced levels of cyclin B1 and D1 protein and increased levels of
p53 and p21, while the apoptotic effects of ALO were associated
with the upregulation of Bax and a decrease in Bcl-2 protein
levels. Moreover, ALO-induced apoptosis may be involved in the
inhibition of JAK/Stat3 and PI3K/Akt signaling.
Apoptosis and cell cycle arrest are two important
events involved in anticancer drug treatment. Apoptosis is the
result of a highly complex series of events involving cell
chromatin condensation, DNA fragmentation, and cell shrinkage
(20). Based on this study,
apoptosis may be involved in the inhibition of cell proliferation
by ALO. ALO inhibited cell proliferation, leading to morphological
changes, enhanced loss of plasma membrane polarity, and long-term
clonogenic inhibition.
Cells undergo apoptosis through the extrinsic
pathway (death receptor pathway) or intrinsic pathway (the
mitochondrial pathway) (21).
Mitochondria play a crucial role in apoptotic regulation and the
mitochondrial dysfunction that occurs during apoptosis (22,23). The Bcl-2 family of proteins
promotes cell death by modulating the mitochondrial release of
proapoptotic factors and acts as a critical life-death decision
point within the common pathway of apoptosis (24,25). In this study, we observed the
downregulation of proapoptotic Bax and upregulation of
antiapoptotic Bcl-2 proteins in ALO-treated HCT116 cells (Fig. 4C and D).
Cell growth is defined by several genetically
defined checkpoints to ensure its coordinated progression through
the different stages of the cell cycle and to monitor DNA integrity
(26). Cell-cycle arrest in
response to stress is integral to the maintenance of genomic
integrity. The control mechanisms that restrain cell-cycle
transition or induce apoptotic signaling pathways after cell stress
are known as cell-cycle checkpoints (27). The analysis of the cell cycle with
different concentrations of ALO in HCT116 cells revealed a higher
number of cells in the G2/M and S phase, whereas the number of
cells in the G0/G1 phase decreased compared with the untreated
cells (Fig. 3B). These results
indicate that ALO inhibits cell proliferation via G2/M phase arrest
in a dose-dependent manner. The G2 checkpoint is regulated by the
activation of multiple pathways that act together to inhibit the
activity of the cyclin B1/cdc2 kinase complex. p53 plays an
important role in the regulation of the G2 checkpoint (27). D-type cyclins play an important
role in tumor development and are overexpressed in various types of
cancer, including breast, colon, and thyroid cancer, as well as
melanoma (28). p53 and p21
appear to be essential for maintaining the G2 checkpoint in human
cells (29). The accumulation of
G2/M phase cells resulting from ALO treatment led us to determine
the expression level of cell cycle regulators. ALO induced cell
cycle retardation in the G2/M phase by increasing the expression of
p21 and p53 and by suppressing cyclin B1 and D1 (Fig. 3C). Therefore, our results suggest
that apoptosis induction and G2 phase cell cycle arrest both
contributed to the anticancer activity of ALO.
Stat proteins are involved in the regulation of
fundamental cell processes, such as cell growth, differentiation,
and survival (30). Stat3 is
often activated in many types of human cancer, including colon
cancer. The inhibition of cancer cell growth by blocking the
signaling to Stat3 demonstrated that Stat3 is crucial to the
survival and growth of tumor cells and is an attractive therapeutic
target for cancer (31–33). Unphosphorylated Stat3 activates
the transcription of Stat3 target genes and plays an important role
in oncogenesis (34). The
inhibition of the JAK/Stat3 signaling pathway may be an important
mechanism for apoptosis (18). As
anticipated, in the present study, ALO downregulated Stat3 protein
and mRNA levels in HCT116 cells (Fig.
5).
The PI3K/Akt signaling pathway is an important
regulator of proliferation, differentiation, and metastasis and
plays a significant role in apoptosis (35,36). Additionally, the PI3K/Akt
signaling pathway is a potential therapeutic target of cancer
(37). To elucidate the mechanism
involved in ALO-induced cell death, the effects of ALO on PI3K/Akt
were examined. PI3KC3 protein was largely affected.
Our experiments mainly aimed at studying the
anticancer effect of ALO and the mechanisms of apoptosis and the
cell cycle. Other pathways involved in the anticancer properties of
the alkaloid compounds, including those that mediate DNA damage,
and the relationship between the PI3K/Akt and JAK-Stat3 signaling
pathways, remain to be investigated.
In conclusion, compared with the other tested
alkaloids, ALO had the most potent effects against HCT116 colon
cancer cells. The pure ALO compound exerts an anti-proliferative
effect by inducing G2/M cell cycle arrest and apoptosis as well as
activating the mitochondrial death pathway in HCT116 cells. In
addition, ALO-induced apoptosis may be involved in the inhibition
of the JAK/Stat3 and PI3K/Akt signaling pathways. These findings
suggest that ALO should be studied further as an agent of
chemotherapeutic activity in human colon cancer.
Acknowledgements
This study was partly supported by grants from the
Natural Science Foundation of China (no. 81001701 to Dr L.L.); the
Project of Zhujiang New Star of Guangzhou Science and Technology
Project (no. 2013J2200030 to Dr L.L.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Ali R, Mirza Z, Ashraf GM, et al: New
anticancer agents: recent developments in tumor therapy. Anticancer
Res. 32:2999–3005. 2012.PubMed/NCBI
|
|
3
|
Parkinson DR, Arbuck SG, Moore T, Pluda JM
and Christian MC: Clinical development of anticancer agents from
natural products. Stem Cells. 12:30–43. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukagoshi S: Recent trend on the
development of new anticancer drugs. Gan To Kagaku Ryoho.
20:425–431. 1993.(In Japanese).
|
|
5
|
Wang H, Guo S, Qian D, Qian Y and Duan JA:
Comparative analysis of quinolizidine alkaloids from different
parts of Sophora alopecuroides seeds by UPLC-MS/MS. J Pharm
Biomed Anal. 67–68:16–21. 2012.PubMed/NCBI
|
|
6
|
Zhang L, Jiang J and Tan R: Effects of
matrine on telomerase activity and cell cycle in K562 cell.
Zhonghua Zhong Liu Za Zhi. 20:328–329. 1998.(In Chinese).
|
|
7
|
Zhang LP, Jiang JK, Tam JW, et al: Effects
of matrine on proliferation and differentiation in K-562 cells.
Leuk Res. 25:793–800. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Wang X, Wu W, et al: Effects of
matrine on proliferation and apoptosis in gallbladder carcinoma
cells (GBC-SD). Phytother Res. 26:932–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan C, Lu SQ and Yao X: Effects of
oxymatrine on the antitumor activity and toxicity of
cyclophosphamide in mice. Yao Xue Xue Bao. 22:245–249.
1987.PubMed/NCBI
|
|
10
|
Li XM, Wu YG, Chen SL, Pan DX, Wu JN and
Yu YH: Antitumor action of sophoridine. Zhongguo Yao Li Xue Bao.
8:153–158. 1987.(In Chinese).
|
|
11
|
Liang L, Wang XY, Zhang XH, et al:
Sophoridine exerts an anti-colorectal carcinoma effect through
apoptosis induction in vitro and in vivo. Life Sci. 91:1295–1303.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan XY, Liu W, Zhang P, Wang RY and Guo
JY: Effects and mechanisms of aloperine on 2,
4-dinitrofluorobenzene-induced allergic contact dermatitis in
BALB/c mice. Eur J Pharmacol. 629:147–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Z, Huang CF, Liu XS and Jiang J: In
vitro anti-tumour activities of quinolizidine alkaloids derived
from Sophora flavescens Ait. Basic Clin Pharmacol Toxicol.
108:304–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou CC, Gao HB, Sun XB, et al:
Anti-inflammatory and anti-allergic action of aloperine. Zhongguo
Yao Li Xue Bao. 10:360–365. 1989.(In Chinese).
|
|
15
|
Lin WC and Lin JY: Five bitter compounds
display different anti-inflammatory effects through modulating
cytokine secretion using mouse primary splenocytes in vitro. J
Agric Food Chem. 59:184–192. 2011. View Article : Google Scholar
|
|
16
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi S, Gooding WE and Grandis JR: In vivo
antitumor efficacy of STAT3 blockade using a transcription factor
decoy approach: implications for cancer therapy. Oncogene.
24:970–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Z, Li E, Liu Y, et al: Inhibition of
Jak-STAT3 pathway enhances bufalin-induced apoptosis in colon
cancer SW620 cells. World J Surg Oncol. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Y, Zhou J, Xu C, et al: JAK/STAT and
PI3K/AKT pathways form a mutual transactivation loop and afford
resistance to oxidative stress-induced apoptosis in cardiomyocytes.
Cell Physiol Biochem. 21:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Debatin KM and Krammer PH: Death receptors
in chemotherapy and cancer. Oncogene. 23:2950–2966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
22
|
Hu W, Shen T and Wang MH: Cell cycle
arrest and apoptosis induced by methyl 3,5-dicaffeoyl quinate in
human colon cancer cells: Involvement of the PI3K/Akt and MAP
kinase pathways. Chem Biol Interact. 194:48–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh K, Hase H, Kojima H, Saotome K,
Nishioka K and Kobata T: Central role of mitochondria and p53 in
Fas-mediated apoptosis of rheumatoid synovial fibroblasts.
Rheumatology (Oxford). 43:277–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
25
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brooks G and La Thangue NB: The cell cycle
and drug discovery: the promise and the hope. Drug Discov Today.
4:455–464. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flatt PM and Pietenpol JA: Mechanisms of
cell-cycle checkpoints: at the crossroads of carcinogenesis and
drug discovery. Drug Metab Rev. 32:283–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwak YT, Radaideh SM, Ding L, et al: Cells
lacking IKKα show nuclear cyclin D1 overexpression and a neoplastic
phenotype: role of IKKα as a tumor suppressor. Mol Cancer Res.
9:341–349. 2011.
|
|
29
|
Bunz F, Dutriaux A, Lengauer C, et al:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin L, Liu A, Peng Z, et al: STAT3 is
necessary for proliferation and survival in colon cancer-initiating
cells. Cancer Res. 71:7226–7237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishimoto A, Kugimiya N, Hosoyama T, Enoki
T, Li TS and Hamano K: JAB1 regulates unphosphorylated STAT3
DNA-binding activity through protein-protein interaction in human
colon cancer cells. Biochem Biophys Res Commun. 438:513–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia SS, Xi GP, Zhang M, et al: Induction
of apoptosis by D-limonene is mediated by inactivation of Akt in
LS174T human colon cancer cells. Oncol Rep. 29:349–354.
2013.PubMed/NCBI
|
|
36
|
Harashima N, Inao T, Imamura R, Okano S,
Suda T and Harada M: Roles of the PI3K/Akt pathway and autophagy in
TLR3 signaling-induced apoptosis and growth arrest of human
prostate cancer cells. Cancer Immunol Immunother. 61:667–676. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Mei C, Sun L, et al: The PI3K-Akt
pathway regulates calpain 6 expression, proliferation, and
apoptosis. Cell Signal. 23:827–836. 2011. View Article : Google Scholar : PubMed/NCBI
|