Introduction
Prostate cancer (PCa) can be treated by the removal
of testicular androgens; however, androgen depletion is frequently
associated with the recurrence of malignant neoplasms, defined as
castration-resistant prostate cancer (CRPC), which has a poor
prognosis (1). The enhancer of
zeste homolog 2 (EZH2) belongs to the catalytic subunit of polycomb
repressive complex 2 (PRC2) and primarily functions by silenc
transcription through the trimethylation of histone H3 lysine 27
(H3K27me3) (2). EZH2 is
frequently elevated in many types of cancer, including PCa
(3). EZH2 overexpression has also
been linked to PCa cell invasion and metastasis and poor survival
(4,5). The expression of EZH2 has been
implicated in the progression of human PCa, particularly to lethal
CRPC (6). A recent study reported
that the blockade of EZH2 derepression during androgen deprivation
therapy may represent an effective strategy for the treatment of
androgen-refractory PCa (7). Xu
et al (8) demonstrated
that in patients with CRPC overexpressing EZH2, EZH2-stimulated
solo genes are frequently upregulated, and their higher expression
levels correlate with poor survival. Therefore, the suppression of
EZH2 expression may be a novel strategy for the treatment of
metastatic, hormone-refractory PCa.
Gene therapy is a promising strategy for the
treatment of various genetic and acquired diseases. The use of
small interfering RNA (siRNA) is a novel and promising therapeutic
approach, as it can be used to suppress gene expression in a highly
specific manner for the treatment of genetic diseases (9). Although highly attractive as a
cancer therapeutic strategy, a number of physiological obstacles
need to be overcome to successfully introduce RNAi-based therapies
into clinical practice. Therefore, there is an urgent need to
discover new treatment methods and to find more effective vectors
which may improve the efficiency of target gene expression in order
to achieve greater success with gene therapy.
Non-viral gene delivery systems can be mainly
divided into lipids, polymers and nanomaterials, and have been
developed for siRNA delivery (10,11). Among these delivery systems, the
polymer or lipid-based ones have shown much improvement in
delivering therapeutic genes. However, complex chemical synthetic
procedures and harsh chemical reactions limit their application
(12). Nanoparticle vectors have
been extensively investigated for siRNA delivery due to their ease
of production, safety, lower immune response, target tissue and
cell specificity, facilitatation of cellular entry, ease of
chemical modification and their ability to transfer larger pDNA
molecules (13). Polyethylenimine
(PEI) is a highly efficient carrier for siRNA delivery; however,
the high density of positive charge on the surface of PEI molecules
results in severe cytotoxicity, which has become a limiting factor
for the application of PEI (14).
Polyethylene glycol (PEG) moieties are engrafted to PEI polymers
with the aim of reducing cytotoxicity and enhancing stability
(15).
In the present study, we used the non-viral cationic
polymer vector mPEG-PEI nanoparticles as a carrier and rebuilt the
shRNA plasmid; we evaluated the gene-silencing efficiency in PCa
cells. The results substantiated that the mPEG-PEI/EZH2-shRNA
nanoparticle complexes effectively suppressed EZH2 mRNA expression,
thus rendering these complexes as a promising candidate for the
treatment of advanced PCa and providing the basis for future
research in clinical translation.
Materials and methods
Cells and cell culture
The PC3 human prostate carcinoma cell line was
obtained from the Shanghai Cell Bank, Chinese Academy of Sciences,
Shanghai, China and maintained in RPMI-1640 culture medium
supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C in an atmosphere of
5% CO2.
Synthesis of mPEG-PEI
mPEG-PEI was synthesized according to previously
described methods (16–18). First, 7.9 g of mPEG-1900 and 1.6
ml pyridine were dissolved in dichloromethane.
p-Nitrophenoxycarbonyl chloride (p-NPC) (3.2 g) was dissolved in
dichloromethane. The mPEG solution was then added to the p-NPC
solution drop by drop and continuously stirred for 24 h at room
temperature to complete the reaction. The mixture product was then
precipitated with petroleum ether, frozen, filtrated and
vacuum-dried. Subsequently, 1.03 g of the above product and 3.72 g
PEI (MW 25,000) were added to 50 ml anhydrous chloroform, and the
mixture was continuously stirred for 48 h at room temperature. The
sample was subsequently dialyzed against distilled water in a
dialysis tube (MWCO, 3,500 kDa) for 3 days and lyophilized to
obtain mPEG-PEI. mPEG-PEI was stored at −20°C for further use.
Characterization of mPEG-PEI
To characterize the chemical composition and confirm
that mPEG-PEI was successfully synthesized, 1H nuclear
magnetic resonance (1H-NMR) spectra were recorded using
a Varian 400 spectrometer (Varian, Palo Alto, CA, USA) at 400 mHz
in CDCl3 with tetramethylsilane as an internal
reference. The prepared nanoparticles were dispersed in deionized
water and examined at various temperatures. All experimental groups
were examined 3 times. The chemical shift was expressed in parts
per million (δ) by using the proton peak of H2O (set as
4.7 pm) as an internal reference, as previously described (16).
Preparation of mPEG-PEI/pDNA
complexes
The plasmid pcDNA3.1/EGFP expression vector with
ampicillin resistance and EGFP report was obtained from GenePharma
(Shanghai, China). EZH2 short hairpin RNA (shRNA) sequences and the
EZH2-shRNA negative control sequence were synthesized and cloned
into the pcDNA3.1/EGFP expression vector. We manipulated the charge
ratio between the amino groups of mPEG-PEI and the
pcDNA3.1/EGFP/EZH2-shRNA (N/P) as 20. The appropriate volumes of
mPEG-PEI and pcDNA3.1/EGFP/EZH2-shRNA solutions were quickly mixed
together (mPEG-PEI/pDNA) and vortexed at the speed of 2,500 rpm for
30 sec and then incubated at room temperature for 30 min prior to
use.
Measurement of particle size and zeta
potential of mPEG-PEI/pDNA complexes
The mPEG-PEI/pDNA complexes were prepared at
different N/P ratios (5–50) and then diluted in ultra-pure water.
The particle size and zeta potential of the mPEG-PEI/pDNA complexes
were examined using a Zeta-Plus Instrument (Malvern Instruments,
Ltd., Worcestershire, UK) with an angle of detection of 90°C at
room temperature. The experiment was carried out using a Zetasizer
3000 HSa particle sizer (Malvern Instruments, Ltd.) with an angle
of detection of 90°C at room temperature.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cytotoxicity assays
The cytotoxicity associated with mPEG-PEI was
determined by MTT assay according to a previously described method
(19). The PC3 cells were plated
with 100 μl culture mediun in 96-well plates at a density of 5,000
cells/well. Different mPEG-PEI concentrations and different N/P
ratios were added to each well followed by incubation for 48 h. MTT
assay was performed and the absorbance of each well, which
identifies the quantity of viable cells, was read at 590 nm on a
microplate reader. Each assay was performed in triplicate with 3
independent replicates.
Gel retardation assay
The attachment of the mPEG-PEI/pDNA complexes was
assessed using gel retardation assays. The mPEG-PEI/pDNA complexes
formed at various N/P ratios (0, 1, 2, 3, 5, 10 and 20) and was
placed on 1% agarose gels containing 0.5 mg/ml ethidium bromide in
1X TBE buffer. mPEG-PEI/pDNA binding was analyzed by gel
electrophoresis at 80 V for 40 min. DNA bands in the gel imaging
system were observed and photographed.
Hemagglutination assay
The agglutinating activity of mPEG-PEI/pDNA at
different N/P ratios was examined in a 24-well plate. Briefly,
fresh mouse blood was centrifuged at 2,000 rpm for 20 min and the
plasma and the buffy coat were discarded. Erythrocytes were washed
3 times by centrifugation at 2,000 rpm and were diluted in
phosphate-buffered saline (PBS) to a final concentration of 1%
(v/v). Various N/P ratios were added to the erythrocyte suspension
at ratio of 10, 20 and 50 in a 24-well plate and incubated for 15
min at room temperature. The sample was placed on a microscope
slide and hemagglutination was observed under an optical
microscope.
In vitro transfection experiments
The day prior to transfection, the PC3 cells were
seeded into a 24-well plate at density of 0.5×105
cells/well. mPEG-PEI/pDNA transfection complexes with different N/P
ratios (3, 5, 10 and 20) were prepared and added into the well in a
dropwise manner. After 6 h of incubation, the medium was replaced
with fresh complete medium and the cells were incubated for an
additional 48 h prior to analysis. EGFP expression was examined and
photographed under a fluorescent microscope (Olympus Axiovert S100;
Olympus, Tokyo, Japan) after an additional 48 h of incubation. The
number of EGFP-positive cells and total cells in 5 randomly
selected sections was counted, and the transfection efficiency was
calculated by the ratio between the EGFP-positive cells and the
total number of cells, as previously described (16).
Quantitative reverse transcription PCR
(qRT-PCR)
Total RNA was isolated from the PC3 cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Reverse transcription and quantitative
PCR were performed using the PrimeScript Reverse Transcription
system and the SYBR Premix Ex Taq™ II kit (Takara, Dalian, China)
according to the manufacturer’s instructions. The relative mRNA
expression compared to that of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was calculated using the 2−ΔCt
method. The primers used were as follows: EZH2 forward,
5′-TGCAGTTGCTTCAGTACCCATAAT-3′ and reverse, 5′-ATC
CCCGTGTACTTTCCCATCATAAT-3′; GAPDH forward,
5′-TCGACAGTCAGCCGCATCTTCTTT-3′ and reverse,
5′-ACCAAATCCGTTGACTCCGACCTT-3′. The quantitative PCR reactions were
performed under the following conditions: 95°C for 2 min, followed
by 40 cycles of 95°C for 15 sec, and 60°C for 1 min.
Western blot analysis
Protein samples were prepared by lysing the cells in
modified RIPA buffer and protease inhibitor [phenylmethylsulfonyl
fluoride (PMSF); Beyotime, Jiangsu, China]. Cell lysates were
subjected to 10% SDS-polyacrylamide gel electrophoresis followed by
electroblotting onto PVDF membranes. After blocking in 5% skim
milk, the membranes were probed with the specific primary antibody
(anti-EZH2 or anti-GAPDH; Cell Signaling Technology, Beverly, MA,
USA) and then incubated with a HRP-conjugated secondary antibody.
Signals were visualized using the ECL chemiluminescence kit
(Boster, Wuhan, China) and exposured to X-ray films.
Statistical analysis
Statistical analysis was performed using SPSS
software version 17.0. The results are presented as the means ±
standard deviation (SD). The Student’s t-test was used to assess
statistically significant differences. A value of P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate.
Results
Synthesis and characterization of
mPEG-PEI
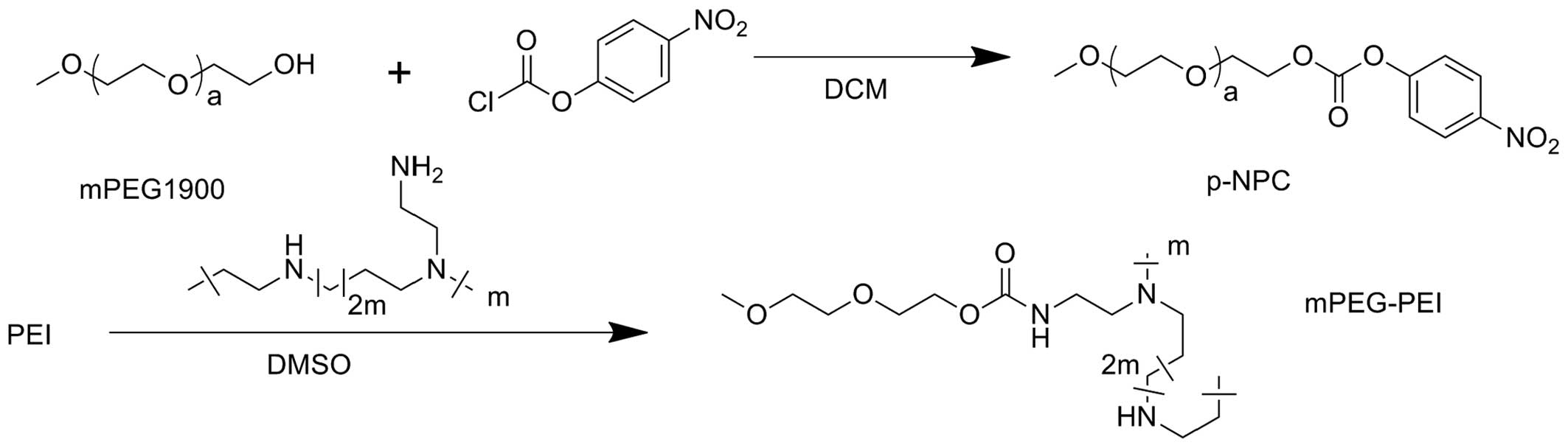
The reaction scheme of mPEG-PEI is shown in Fig. 1. mPEG-PEI was prepared by the
conjugation of mPEG-1900 to PEI. The molecular structure of
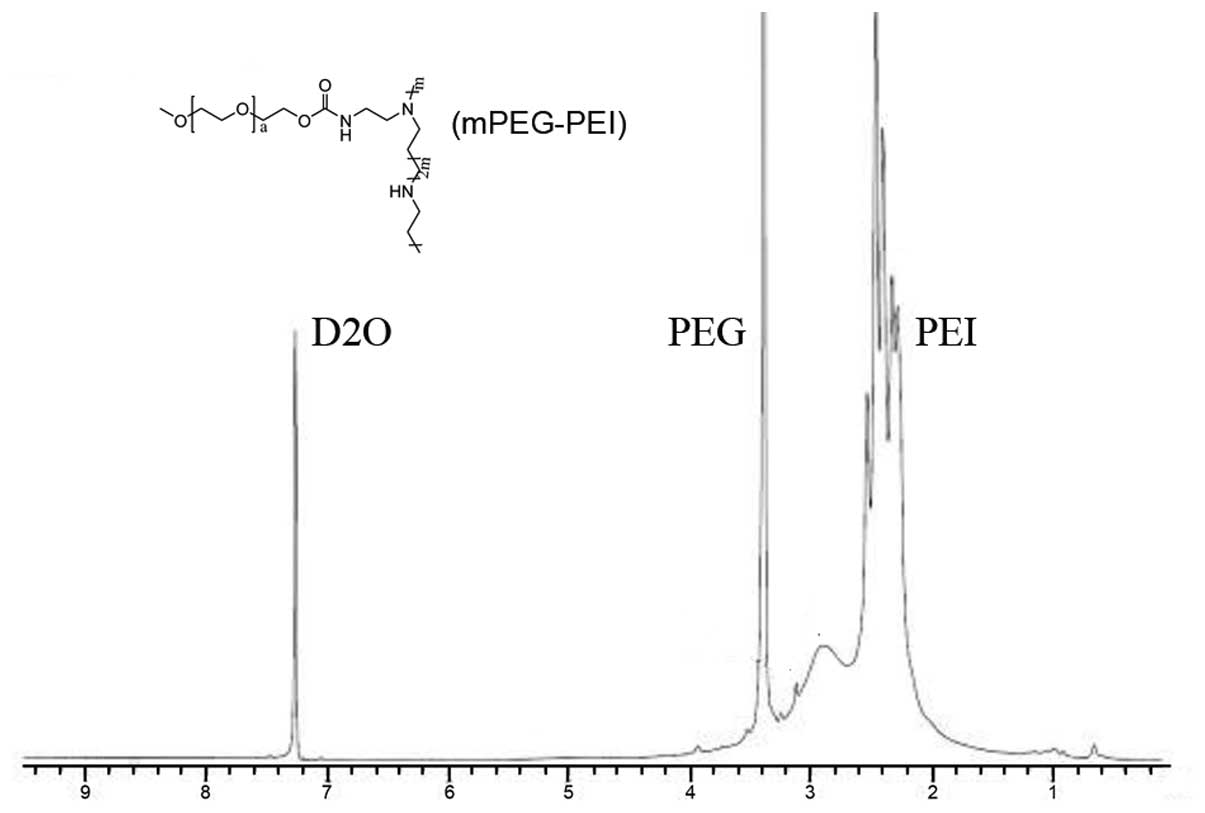
mPEG-PEI was characterized by measuring 1H-NMR in
deuterium oxide. Fig. 2
illustrates that the proton peak appearing at 2.5–3.0 ppm was
attributed to -CH2CH2NH- in PEI. The peaks at
3.6 ppm were assigned to the protons of
-CH2CH2O- in PEG. The peak at 7.25 was the
chemical shifts of protons from solvent D2O. This
copolymer has both the characteristic absorption peaks of the
proton in PEG and characteristic absorption peak in PEI, and it can
be identified as PEG copolymer with PEI. These results indicated
that mPEG-PEI was successfully synthesized.
Particle size and zeta potential
characterization
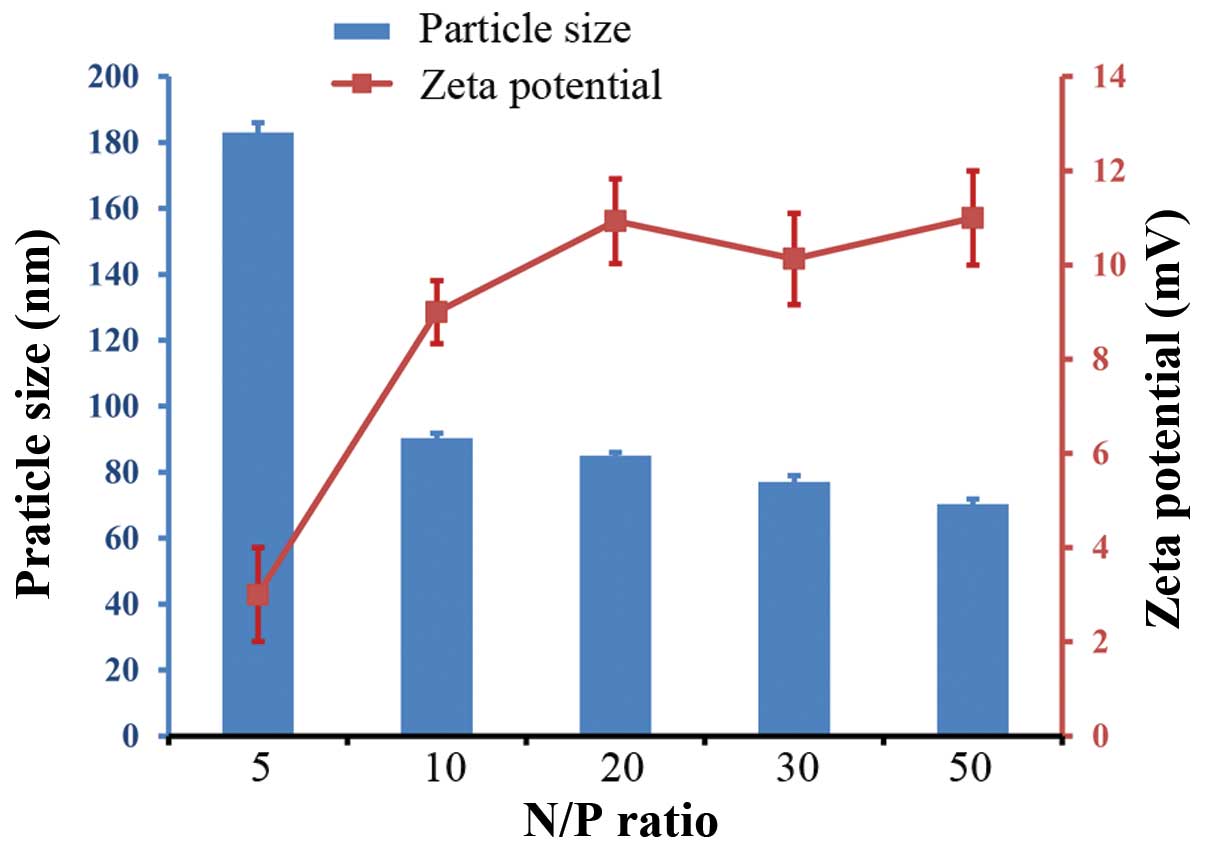
The mPEG-PEI/pDNA complexes were evaluated for
particle size and zeta potential. The particle size and zeta
potential of the mPEG-PEI/pDNA complexes varied at the different
N/P rations (Fig. 3). Dynamic
light scattering (DLS) experiments using different mPEG-PEI/pDNA
(N/P) ratios revealed that the particle size of the mPEG-PEI/DNA
complexes decreased with the increasing N/P ratios. At an N/P ratio
of 5, the particle size was approximately 183 nm. The particle size
reached approximately 90 nm at an N/P ratio of 10, and then
remained relatively constant between 70 and 90 nm. When the N/P
ratio increased, the size of the PEG-PEI/EZH2-shRNA complexes
decreased, but the zeta potential increased. The zeta potential
increased with the increased N/P ratio, but the particle size of
the mPEG-PEI/pDNA complexes decreased.
Agarose gel retardation assay
An agarose gel retardation assay was performed to
measure the ability of mPEG-PEI to bind DNA. mPEG-PEI/pDNA
complexes formed at various N/P ratios in a gel retardation assay
(Fig. 4). This phenomenon can be
explained by the fact that the positive charges of mPEG-PEI were
able to neutralize the negative charges of the phosphate groups in
EZH2-shRNA, thus retarding in gel. mPEG-PEI combine with pDNA,
which hinder the EB insert double-stranded DNA. mPEG-PEI completely
retarded the migration of DNA when the N/P ratio was 10, indicating
that the mPEG-PEI/pDNA complexes were fully formed at this N/P
ratio.
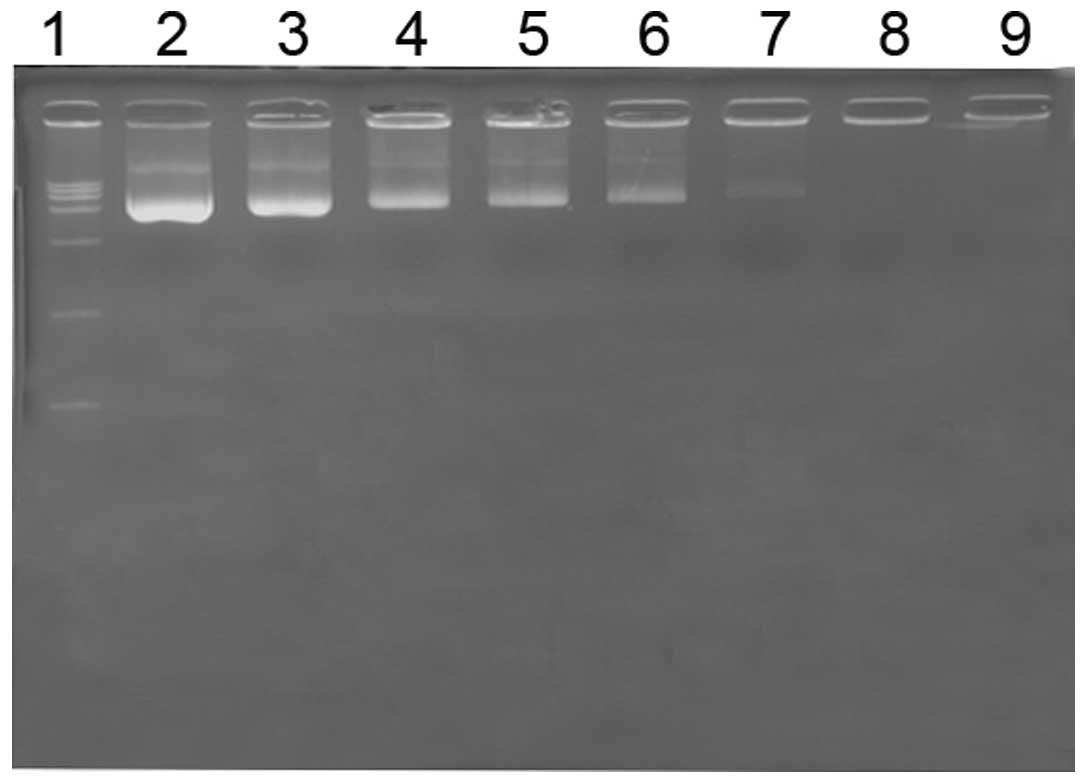 | Figure 4Agarose gel electrophoresis of the
mPEG-PEI/pDNA complexes at different N/P ratios. Agarose gel
electrophoresis of the mPEG-PEI/pDNA complexes at various w/w
ratios. Lane 1, marker; lane 2, naked pDNA; lanes 3–9, complexes at
N/P ratios of 0.1, 0.5, 1, 3, 5, 10 and 20, respectively. PEG,
polyethylene glycol; PEI, polyethylenimine. |
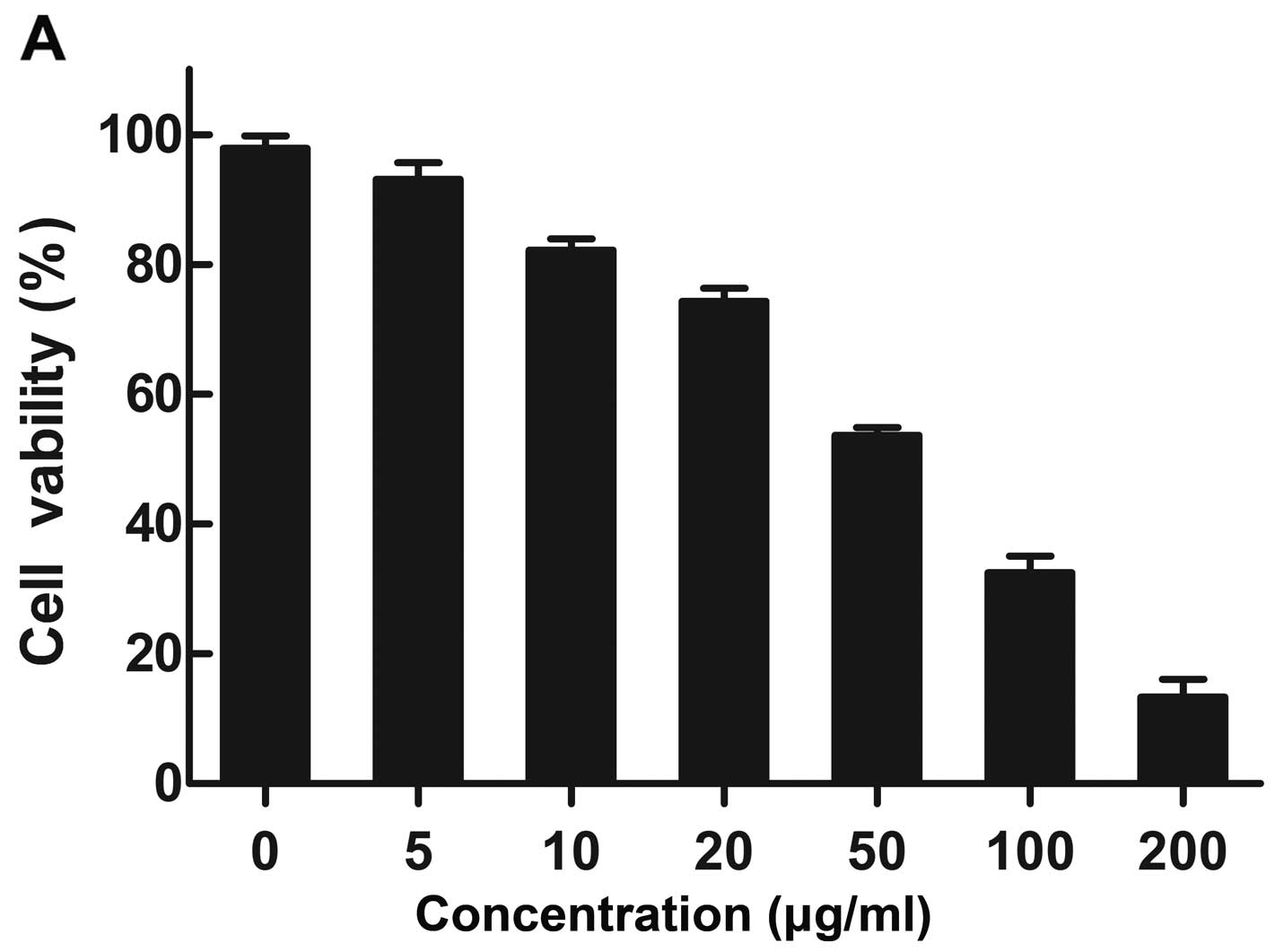
Cytotoxicity assay
PC3 cells were used to determine the cytotoxic
effects of the nanoparticles by MTT assay. Cytotoxicity was
measured at various concentrations of the mPEG-PEI nanoparticles
(Fig. 5A) and mPEG-PEI/pDNA
ratios (N/P) from 0–60 (Fig. 5B).
PC3 cell viability displayed a decreasing trend with the increasing
mPEG-PEI polymer concentration; when the mPEG-PEI nanoparticle
concentration was >50 μg/ml, there was a significant increase in
toxicity, and the cell death rate was >50%. The cytotoxicity of
the mPEG-PEI/pDNA complexes increased as the N/P ratio increased.
The mPEG-PEI/pDNA complexes showed very small or negligible
cytotoxicity when the N/P ratio was <50. Cell viability was
94.13±3.42 and 78.6±3.94% at an N/P ratio of 3 and 20,
respectively. However, the cytotoxicity of mPEG-PEI/pDNA markedly
increased at an N/P ratio of >50.
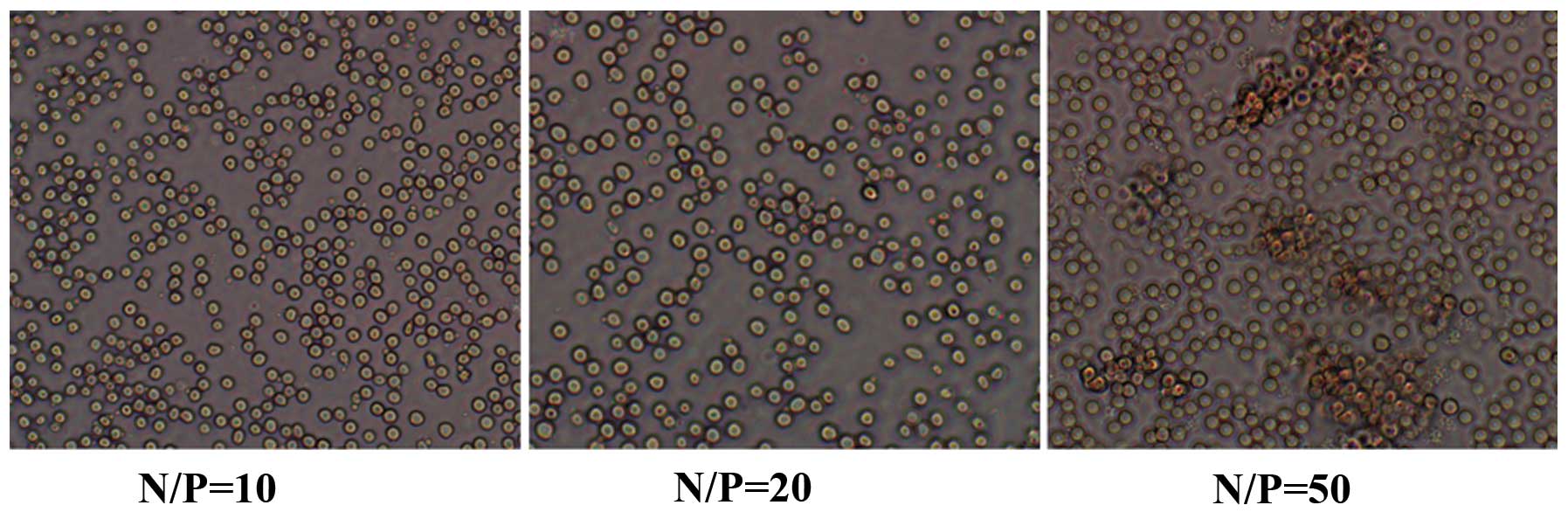
Hemagglutination assay
Hemagglutination assay with mouse erythrocytes was
performed to assess the agglutinating activity of mPEG-PEI/pDNA at
different N/P ratios. Hemagglutination assay for N/P ratios of 10,
20 and 50 is shown in Fig. 6.
When the N/P ratio was 50, the mouse erythrocytes began to
aggregate, whereas no agglutinating ability was observed at an N/P
ratio of 10 and 20. These findings indicate that different N/P
ratios have different hemagglutination abilities. This is a
significantl factor in determining which ratio is suitable for use
in clinical applications.
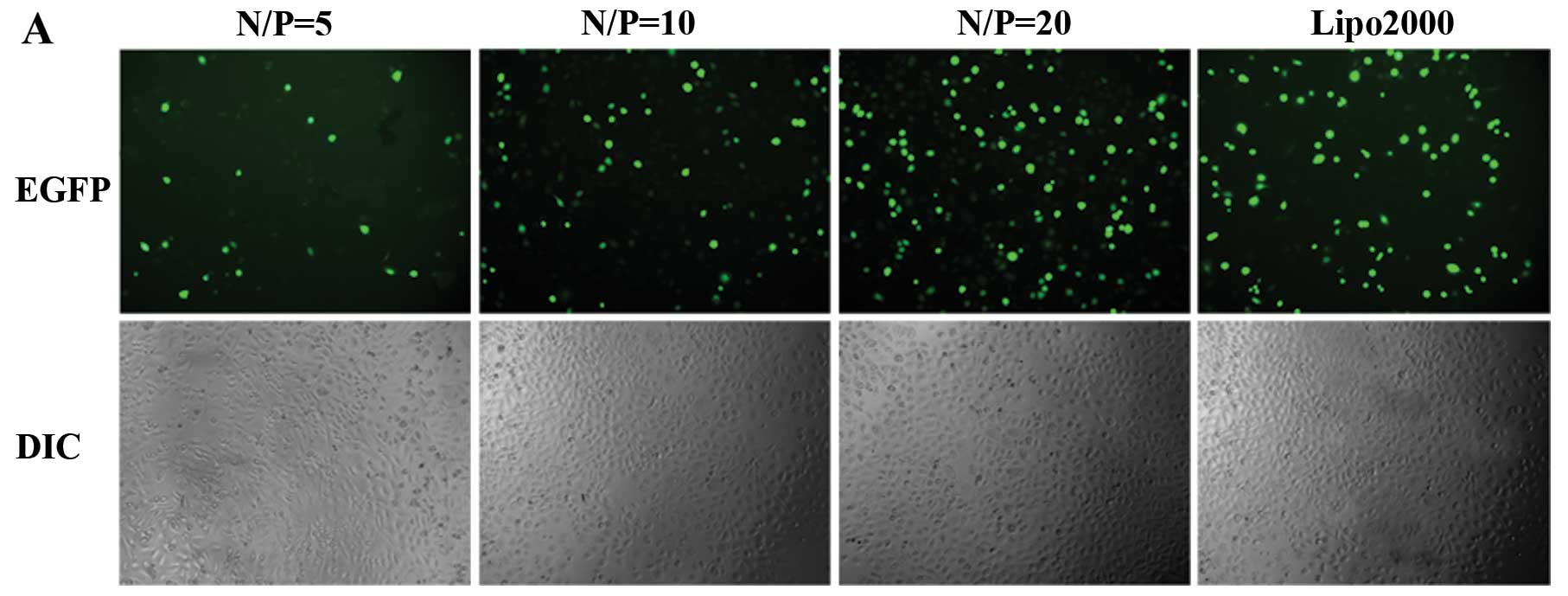
Transfection efficiency assay in
vitro
The transfection efficiency of mPEG-PEI/pDNA at
various N/P ratios was observed 48 h after transfection.
Fluorescence images in the cells were evaluated under a
fluorescence microscope (Fig.
7A). We compared the transfection efficiency of mPEG-PEI/pDNA
with the transfection reagent, Lipofectamine™ 2000. The gene
transfection efficiency correspondingly increased with the
increasing N/P ratio from 3 to 20, and the transfection
efficiencies increased from 12.10±1.61 to 64.67±3.92%. The
transfection efficiency of Lipofectamine™ 2000 was 62.63±3.62%. The
highest transfection activity of mPEG-PEI/pDNA was obtained at an
N/P ratio of 20 in the PC3 cells (Fig. 7B).
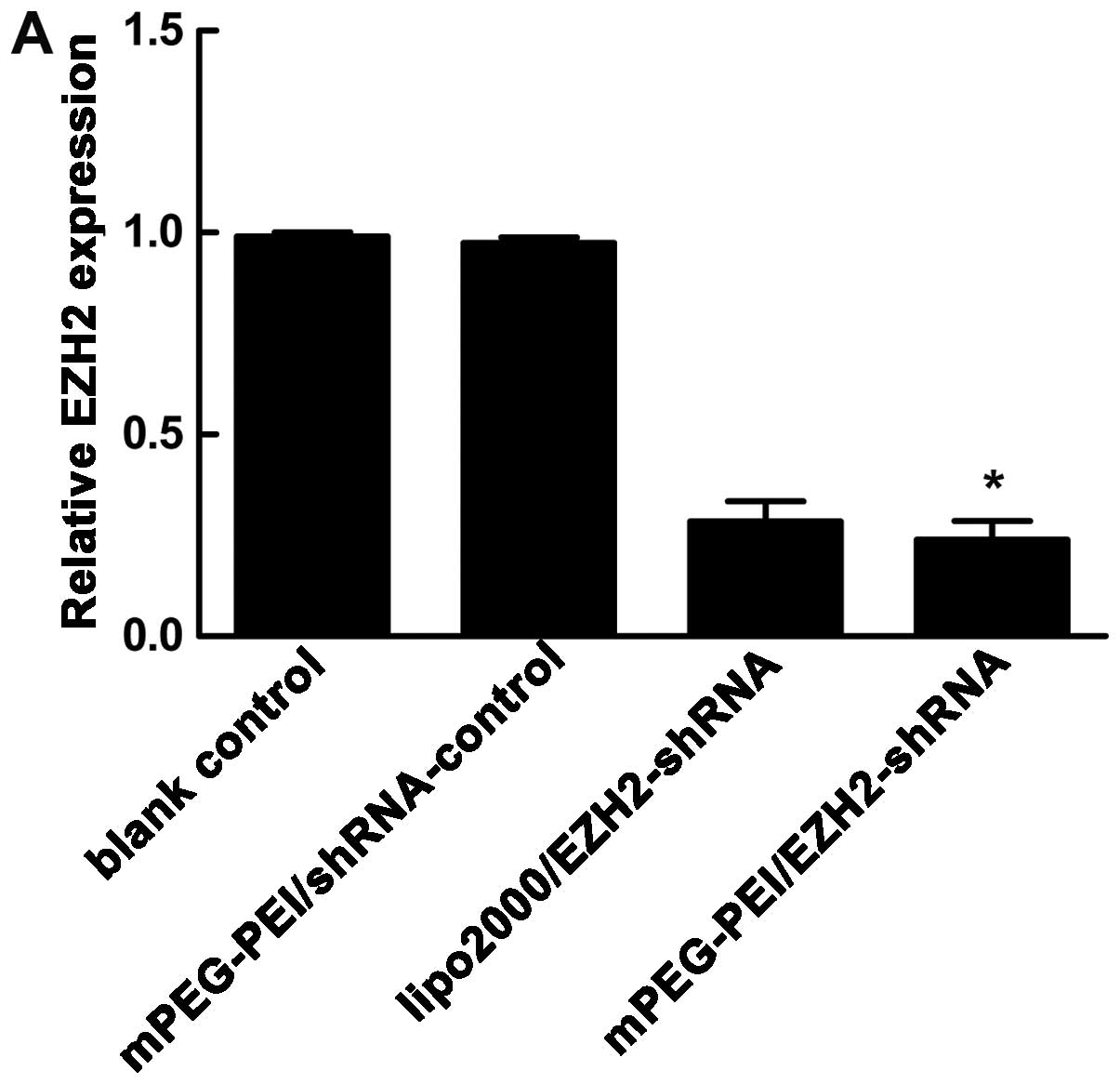
Gene silencing effect of EZH2-shRNA
To determine whether the mPEG-PEI/pDNA particles
knockdown EZH2 mRNA expression in vitro, we investigated the
efficacy of the mPEG-PEI/pDNA particles in the PC3 cells.
mPEG-PEI/pDNA at an N/P ratio of 20 was selected for the
transfection of the PC3 cells. Following 48 h of treatment, EZH2
gene expression was measured by qRT-RCR and western blot analysis.
As shown in Fig. 8A, the EZH2
mRNA expression was knocked down to 24.0±2.64 and 28.47±2.90% by
the mPEG-PEI/pDNA particles and Lipofectamine™ 2000/shRNA-EZH2
complex, respectively. The blank control and mPEG-PEI/shRNA control
particle treatment groups showed no obvious silencing effect on the
expression of EZH2. The cells transfected with the mPEG-PEI/pDNA
particles showed a decreased EZH2 protein expression compared to
the cells transfected with the negative control (Fig. 8B).
Discussion
PCa is the most common malignant disease and the
second leading cause of cancer-related mortality in males in the
Western world (20). The majority
of patients with PCa initially respond to androgen ablation, but
eventually progress to CRPC, which is a lethal form of PCa that is
an aggressive malignancy with a poor prognosis (21). Thus, it is crucial to discover an
novel effective approaches for the treatment of CRPC.
It is mandatory to discover a novel and effective
therapeutic strategy for CRPC. The EZH2 gene, which has been
implicated in metastatic PCa, belongs to an important component of
PRC2 and regulates epigenetic gene silencing (22–24). Numerous studies have indicated
that EZH2 is overexpressed in hormone-refractory, metastatic PCa
(5,6,25).
The downregulation of the expression of EZH2 decreases prostate
cell proliferation and ectopically expressed EZH2 in prostate cells
enhances the proliferation and invasion of PCa cells (26). Since EZH2 is overexpressed and
acts as an oncogene in PCa, it has been suggested to be a
therapeutic target for metastatic, hormone-refractory PCa (8,27).
The silencing of gene expression by siRNA is rapidly
becoming a powerful tool for genetic analysis and represents a
potential strategy for therapeutic product development (28,29). Despite the enormous therapeutic
potential of siRNAs, their delivery remains problematic due to
unfavorable biodistribution profiles and poor intracellular
bioavailability (30). An
increasing number of studies have indicated that nanoparticles have
low cytotoxicity and high gene transfection efficiency and may thus
be potentially applied as a new vector system for gene delivery
(31,32). In our study, the non-viral
vehicle, mPEG-PEI, was applied in the siRNA plasmid delivery to PCa
cells to overcome the obstacles involved with the application of
siRNA. Studies have shown that the adequate size and positive
potential of nanocomplexes are essential for cellular uptake
(33,34).
In this study, the affinity of the mPEG-PEI/pDNA
complexes was evaluated by agarose gel electrophoresis. The plasmid
DNA was completely retarded when the N/P ratios of the
mPEG-PEI/pDNA complexes were 10 and the charge of shRNA was
completely neutralized. Therefore, it may be considered that
mPEG-PEI completely complexed with shRNA at N/P ratios of ≥10.
The relative surface charges of the resulting
complexes were quantified with zeta potential measurements
(16). The particle size and zeta
potential of the mPEG-PEI/pDNA complexes were dependent on the N/P
ratio. We designed different N/P ratios to discover which is the
optimal N/P ratio of the nanoparticles for transfection. At N/P
ratios of ≥10, the size of the nanoparticles was approximately
90.33±1.53 nm and the zeta potential was 9.0±0.67 mV. This can be
partly explained by the fact that the electrostatic interaction of
mPEG-PEI with siRNA becomes stronger when the N/P ratio is enhanced
(35). Therefore, mPEG-PEI/pDNA
complexes with N/P ratio of 10 are suitable for transfection.
The cytotoxicity of the mPEG-PEI/pDNA complexes was
analyzed by MTT assay. Cytotoxicity was induced by mPEG-PEI in a
concentration-dependent manner; these results are consistent with
those of a previous study (36).
When the N/P ratios were >30, the cytotoxicity of PEG-PEI
markedly increased and the cell viability was <70%. The results
of hemagglutination assay also indicated that the mouse
erythrocytes began to agglutinate when the N/P ratio was 50 and was
not suitable for application.
We constructed the plasmid pcDNA3.1/EGFP/EZH2-shRNA
expression vector. This construct carries a coding sequence for the
EGFP protein, providing the extra feature of an EGFP marker for
monitoring the cell distribution of the nanoparticles. The
increased transfection efficiency of mPEG-PEI/pDNA was observed at
higher N/P ratios, reaching the highest transfection efficiency at
an N/P ratio of 20, which displayed the most bright fluorescent
spots. The present study shows that mPEG-PEI/pDNA complexes at an
N/P ratio of <10 cannot achieve excellent cell transfection
efficacy.
Our results revealed that the mRNA and protein
levels of EZH2 were downregulated in the cancer tissues following
transfection with mPEG-PEI/pDNA for 48 h, indicating that mPEG-PEI
can deliver EZH2-specific shRNA into PC3 cells, resulting in the
inhibition of EZH2 expression.
In this study, we developed a novel strategy of
nanoparticles loaded with EZH2-shRNA constructs for PCa gene
therapy. mPEG-PEI/pDNA complexes were successfully synthesized with
suitable physicochemical properties. More importantly, the
mPEG-PEI/pDNA complexes exhibited low cytotoxicity and high
tranfection efficiency. In addition, mPEG-PEI delivered EZH2-shRNA,
effectively inhibiting the expression of EZH2 in the PC3 cells,
which was a key target for the treatment of CRPC. At present, there
are limited therapeutic options for CRPC. Therefore, mPEG-PEI may
be a promising non-viral vector for delivering EZH2-shRNA plasmids
to PCa cells and its application for CRPC therapy in vivo
requires further investigation.
References
|
1
|
Cavalli G: Molecular biology. EZH2 goes
solo. Science. 338:1430–1431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao Q, Mani RS, Ateeq B, et al:
Coordinated regulation of polycomb group complexes through
microRNAs in cancer. Cancer Cell. 20:187–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Leenders GJ, Dukers D, Hessels D, et
al: Polycomb-group oncogenes EZH2, BMI1, and RING1 are
overexpressed in prostate cancer with adverse pathologic and
clinical features. Eur Urol. 52:455–463. 2007.PubMed/NCBI
|
|
4
|
Ren G, Baritaki S, Marathe H, et al:
Polycomb protein EZH2 regulates tumor invasion via the
transcriptional repression of the metastasis suppressor RKIP in
breast and prostate cancer. Cancer Res. 72:3091–3104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karanikolas BD, Figueiredo ML and Wu L:
Polycomb group protein enhancer of zeste 2 is an oncogene that
promotes the neoplastic transformation of a benign prostatic
epithelial cell line. Mol Cancer Res. 7:1456–1465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bohrer LR, Chen S, Hallstrom TC and Huang
H: Androgens suppress EZH2 expression via retinoblastoma (RB) and
p130-dependent pathways: a potential mechanism of
androgen-refractory progression of prostate cancer. Endocrinology.
151:5136–5145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu K, Wu ZJ, Groner AC, et al: EZH2
oncogenic activity in castration-resistant prostate cancer cells is
Polycomb-independent. Science. 338:1465–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pecot CV, Calin GA, Coleman RL,
Lopez-Berestein G and Sood AK: RNA interference in the clinic:
challenges and future directions. Nat Rev Cancer. 11:59–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park TG, Jeong JH and Kim SW: Current
status of polymeric gene delivery systems. Adv Drug Deliv Rev.
58:467–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jing GJ, Fu ZG, Dan B, Lin LR, Yang TC and
Shi SL: Development and evaluation of a novel nano-scale vector for
siRNA. J Cell Biochem. 111:881–888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Liu G, Zheng H and Chen X: Rigid
nanoparticle-based delivery of anti-cancer siRNA: Challenges and
opportunities. Biotechnol Adv. Sep 5;S0734-9750(13)00154-7.
2013.(Epub ahead of print).
|
|
13
|
Mintzer MA and Simanek EE: Nonviral
vectors for gene delivery. Chem Rev. 109:259–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang W, Lv M and Gao Z: Polyethylenimine
grafted with diblock copolymers of polyethylene glycol and
polycaprolactone as siRNA delivery vector. J Control Release.
152(Suppl 1): e143–e145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber ND, Merkel OM, Kissel T and
Muñoz-Fernández MA: PEGylated poly(ethylene imine)
copolymer-delivered siRNA inhibits HIV replication in vitro. J
Control Release. 157:55–63. 2012.PubMed/NCBI
|
|
16
|
Xu Z, Jin J, Siu LK, et al: Folic acid
conjugated mPEG-PEI600 as an efficient non-viral vector for
targeted nucleic acid delivery. Int J Pharm. 426:182–192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veiseh O, Kievit FM, Mok H, et al: Cell
transcytosing poly-arginine coated magnetic nanovector for safe and
effective siRNA delivery. Biomaterials. 32:5717–5725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi S, Zhu X, Guo Q, et al: Self-assembled
mPEG-PCL-g-PEI micelles for simultaneous codelivery of
chemotherapeutic drugs and DNA: synthesis and characterization in
vitro. Int J Nanomedicine. 7:1749–1759. 2012.PubMed/NCBI
|
|
19
|
Liu Y, Liu Z, Wang Y, et al: Investigation
of the performance of PEG-PEI/ROCK-II-siRNA complexes for
Alzheimer’s disease in vitro. Brain Res. 1490:43–51.
2013.PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
21
|
Kirby M, Hirst C and Crawford ED:
Characterising the castration-resistant prostate cancer population:
a systematic review. Int J Clin Pract. 65:1180–1192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min J, Zaslavsky A, Fedele G, et al: An
oncogene-tumor suppressor cascade drives metastatic prostate cancer
by coordinately activating Ras and nuclear factor-kappaB. Nat Med.
16:286–294. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan J, Yang X, Zhuang L, et al:
Pharmacologic disruption of Polycomb-repressive complex 2-mediated
gene repression selectively induces apoptosis in cancer cells.
Genes Dev. 21:1050–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao R, Wang L, Wang H, et al: Role of
histone H3 lysine 27 methylation in Polycomb-group silencing.
Science. 298:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu J, Yu J, Rhodes DR, Tomlins SA, et al:
A polycomb repression signature in metastatic prostate cancer
predicts cancer outcome. Cancer Res. 67:10657–10663. 2007.
View Article : Google Scholar
|
|
26
|
Bryant RJ, Cross NA, Eaton CL, Hamdy FC
and Cunliffe VT: EZH2 promotes proliferation and invasiveness of
prostate cancer cells. Prostate. 67:547–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao P, Deng Z, Wan M, et al: MicroRNA-101
negatively regulates Ezh2 and its expression is modulated by
androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 9:1082010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeshita F, Minakuchi Y, Nagahara S, et
al: Efficient delivery of small interfering RNA to bone-metastatic
tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA.
102:12177–12182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Monteagudo S, Pérez-Martínez FC,
Pérez-Carrión MD, et al: Inhibition of p42 MAPK using a nonviral
vector-delivered siRNA potentiates the anti-tumor effect of
metformin in prostate cancer cells. Nanomedicine (Lond). 7:493–506.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giger EV, Castagner B, Räikkönen J,
Mönkkönen J and Leroux JC: siRNA transfection with calcium
phosphate nanoparticles stabilized with PEGylated chelators. Adv
Healthc Mater. 2:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li YH, Shi QS, Du J, et al: Targeted
delivery of biodegradable nanoparticles with ultrasound-targeted
microbubble destruction-mediated hVEGF-siRNA transfection in human
PC-3 cells in vitro. Int J Mol Med. 31:163–171. 2013.
|
|
32
|
Liu XQ, Xiong MH, Shu XT, Tang RZ and Wang
J: Therapeutic delivery of siRNA silencing HIF-1 alpha with
micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm.
9:2863–2874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Su J, Cai W, et al:
Hepatocyte-targeting gene transfer mediated by galactosylated
poly(ethylene glycol)-graft-polyethylenimine derivative. Drug Des
Devel Ther. 7:211–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Wang W, Chen Y, et al: The
investigation of polymer-siRNA nanoparticle for gene therapy of
gastric cancer in vitro. Int J Nanomedicine. 5:129–136. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aigner A: Delivery systems for the direct
application of siRNAs to induce RNA interference (RNAi) in vivo. J
Biomed Biotechnol. 2006:716592006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang B, He ML, Xiao ZP, et al: Synthesis
and characterization of folate-PEG-grafted-hyperbranched-PEI for
tumor-targeted gene delivery. Biochem Biophys Res Commun.
367:874–880. 2008. View Article : Google Scholar : PubMed/NCBI
|