Introduction
Colon cancer is a common malignancy worldwide with a
high incidence of tumor recurrence and metastasis, resulting in
cancer-related mortalities (1).
Despite advances in therapy, including surgery and chemotherapy,
tumor recurrence and metastasis cannot be effectively prevented
(2).
Accumulating evidence suggests that a small
subpopulation of cancer cells known as cancer stem cells (CSCs),
which exist in various types of cancer and are characterized by
extensive ability of self-renewal and differentiation, and have a
high potential for tumor propagation and therapy-resistance, may
contribute to tumor progression, recurrence and metastasis
(3,4).
Targeting therapy towards colon CSCs may be a
promising approach to eradicating colon cancer more efficiently.
Several molecular markers have been previously employed to identify
CCSCs, such as CD133, CD44, ALDH and EpCAM (5–8).
However, the specificity of these markers are questionable
(9). LGR5, a Wnt target gene, was
initially identified as a marker of intestinal stem cells (ISCs).
LGR5+ cells are located at the crypt base and generate
various types of differentiated epithelial cells in the intestine
to maintain the self-renewal and homeostasis of intestinal mucosa
(10–12). CCSCs are considered to originate
from normal ISCs (13–15). Conditional deletion of APC
exclusively in the LGR5+ ISCs of a murine model led to
the rapid growth and spread of large adenomas in the small
intestine and colon, whereas deletion of Apc in the non-stem cell
compartment, resulted in the growth inhibition of adenomas
(15). Therefore, LGR5 may also
serve as a marker for CCSCs (16). A higher expression of LGR5 has
been found in colon cancer and adenomas relative to matched normal
mucosa, and is associated with malignant clinicopathological
characteristics, suggesting that LGR5 is involved in tumor
development and progression (17,18).
Although LGR5 is regarded as a potential marker for
CCSCs, little is known concerning its function. We previously
reported that colon cancer spheroid cells derived from serum-free
culture possessed stem-like properties, including higher
proliferative, migratory, invasive, and metastatic ability, and
were thus considered CCSCs-enriched models (19). In this study, we detected the
expression of LGR5 in these spheroid cells, and assessed its role
in CCSCs.
Materials and methods
Cell culture
The HT29 human colon cancer cell line (ATCC, HTB-38)
was maintained in DMEM/F12 with 10% fetal bovine serum (FBS). For
the sphere culture, HT29 cells were grown at a density of
2×106 cells/ml in 100 mm ultra-low attachment dishes
(Corning Life Sciences, Oneonta, NY, USA) in serum-free DMEM/F12
medium (SFM) containing 2% B27 (Invitrogen, Carlsbad, CA, USA), 20
ng/ml epidermal growth factor (EGF), 10 ng/ml basic fibroblast
growth factor (bFGF) (both from Peprotech Inc., Rocky Hill, NJ,
USA), 5 μg/ml routine insulin (Invitrogen). The cells were
incubated in a humidified atmosphere at 37°C with 5%
CO2. To induced differentiation in vitro,
spheroid cells were cultured in DMEM/F12 supplemented with 10% FBS
for 48 h and then harvested for assays.
Immunofluorescent staining
Spheroid cells were cytospun onto glass slides,
fixed with 4% paraformaldehyde for 10 min, and permeablized with
0.1% Triton X-100 for 15 min. Adherent and differentiated cells
were cultured on sterile cover slips in 6-well plates for 48 h and
fixed as described above. The cells were incubated with the primary
anti-LGR5 Ab antibody (Abcam, Cambridge, UK) at 4°C overnight,
followed by incubation with secondary DyLight-conjugated
anti-rabbit Ab antibody (Abcam) for 1 h at room temperature. DAPI
(Invitrogen) was used to counterstain the nuclei. Fluorescent
images were captured using a Zeiss confocal microscope (LSM-710;
Zeiss, Jena, Germany).
Small interfering RNA (siRNA)
transfection
siRNA were obtained from GenePharma Co., Ltd.
(Suzhou, China). Primer sequences of LGR5-siRNA were: forward,
5′-GCUCCA GCAUCACUUAUGATT-3′; and reverse, 5′-UCAUAAGUG
AUGCUGGAGCTT-3′. Dissociated HT29 spheroid cells (5×105)
were seeded in 6-well plates in SFM. Twenty-four hours later, siRNA
were transfected into spheroid cells at a final concentration of
100 nM using Lipofectamine RNAiMAX reagent (Invitrogen) according
to the manufacturer’s instructions. Spheroid cells were either
transfected or not transfected with scrambled siRNA and used as the
blank or negative control (NC). The cells were collected for a
series of experiments at 48 h after transfection.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using RNAiso Plus (Takara
Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. cDNAs were synthesized using PrimeScript RT reagent
kit (Takara) with 1 μg total RNA for each sample. The RT-PCR
reaction was performed using ABI 7500 Fast (Applied Biosystems,
Foster City, CA, USA) with SYBR-Green I reagents (Takara). The
cycle condition were: denaturation at 95°C for 30 sec, 40
amplification cycles at 95°C for 3 sec and 60°C for 30 sec. β-actin
was used as the control. Primer sequences used were: LGR5 forward,
5′-GAGGATCTGGTGAGCCTGAGAA-3′; and reverse,
5′-CATAAGTGATGCTGGAGCTGGTAA-3′; β-actin forward,
5′-CAACTGGGACGACATGGAGAAA-3′; and reverse,
5′-GATAGCAACGTACATGGCTGGG-3′. The results were analyzed by the
2−ΔΔct method.
Western blotting
Cells were lysed in RIPA buffer with 10%
phenylmethylsulfonyl fluoride. The cell extracts were loaded on 10%
SDS-polyacrylamide gels and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked for 1 h at room
temperature with 5% non-fat milk in TBST, and then incubated with
anti-LGR5 (diluted at 1:100; Abgent, San Diego, CA, USA),
anti-Bcl-2 (diluted at 1:1,000), anti-Bcl-xL (diluted at 1:1,000),
anti-Bax (diluted at 1:1,000) (all from Cell Signaling Technology,
Inc., Danvers, MA, USA) at 4°C overnight. Following incubation with
HRP-conjugated secondary antibody (diluted at 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA ), immuno-complexes were
visualized by an enhanced chemiluminescence detection system
(Millipore Corp., Billerica, MA, USA). Endogenous GADPH was used
for normalization.
Detection of surface markers LGR5, CD133
and CD44 by flow cytometry
Cells were dissociated and washed twice in PBS.
Subsequently, cell suspensions were incubated with 1:50
PE-conjugated mouse anti-LGR5 Ab (OriGene, Rockville, MD, USA), or
1:10 PE-conjugated mouse anti-CD133 (Miltenyi Biotec, Bergisch
Gladbach, Germany) and 1:10 FITC-conjugated mouse anti-CD44 (BD
Biosciences, Franklin Lakes, NJ, USA) antibodies for 20 min in the
dark. The cells were then washed twice in cold PBS with 1% BSA and
resuspended in 300 μl cold PBS with 1% BSA for flow cytometric
analysis within 1 h.
Proliferation and chemo-sensitivity
assays
Cell proliferation and chemosensitivity assays were
determined using Cell Counting kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan). Spheroid cells were dissociated and seeded in
96-well plates at a density of 2×103 cells/well in 100
μl SFM overnight, and then transfected with 100 nM of indicated
siRNA. At 0, 1, 2, 3 and 4 days after transfection, 10 μl CCK-8
reagent was added to each well and the culture was incubated for a
further 4 h. The optical density (OD) value in each well was
measured by a microplate reader at a wavelength of 450 nm. For
chemosensitivity assay, cells (4×103) were seeded in
each well and treated with various concentrations of 5-Fu (0, 50,
100 and 200 μg/ml) or cisplatin (0, 12, 24 and 48 μg/ml) (Sigma,
St. Louis, MO, USA) 48 h post-transfection. After incubation for 48
h, a CCK-8 assay was performed and the survival rate of cells was
calculated as: ODtreatment/ODcontrol × 100%.
Experiments were performed in triplicate.
Sphere formation assay
After 48 h transfection, spheroid cells were
dissociated and seeded in 24-well ultra-low attachment plates
(Corning Life Sciences) at a density of 2×103 cells/well
in 500 μl SFM and then grown for a further 7 days. Spheres >50
μm were counted by microscope.
Apoptosis assay
Apoptotic cell rates were measured by Annexin V and
propidium iodide (PI) double staining using an Annexin V/FITC kit
(KeyGen, Nanjing, China). Spheroid cells were collected and
dissociated 48 h after transfection, washed with cold PBS
containing 2% BSA, resuspended in binding buffer, and then
incubated with Annexin V-FITC and PI for 30 min in the dark at room
temperature. The staining cells were analyzed using FACSCanto II
flow cytometer (BD Biosciences).
Cell cycle assay
Spheroid cells were collected 48 h after
transfection. Cells (1×106) were fixed with cold 70%
ethanol at 4°C overnight, washed with cold PBS, and stained with PI
(50 μg/ml) in PBS containing RNase (50 μg/ml) (both from Sigma) in
the dark for 30 min. Analyses were performed by FACSCanto II flow
cytometer (BD Biosciences).
Invasion assay
Invasion assay was performed using 24-well plate
Transwell chambers with 8 μm-pore polycarbonate filter inserts
(Corning Life Sciences). A total of 48 h post-transfection,
spheroid cells were detached and 1×105 cells in 100 μl
DMEM/F12 without FBS were seeded onto the upper chamber coated with
Matrigel (BD Biosciences). Then, 600 μl DMEM/F12 medium containing
10% FBS was added into the lower chamber. After incubation for 48
h, the cells on the upper side of the membrane were removed and the
cells that migrated to the underside were fixed in 4%
paraformaldehyde, stained with 0.1% crystal violet and counted in
five random fields under microscopy.
In vivo tumorigenesis
Spheroid cells were detached and resuspended in PBS.
Cells (2×106) in 100 μl PBS were injected subcutaneously
into the flanks of 5-week-old male BALB/C-nu mice (Beijing HFK
Bioscience, Beijing, China). When the tumor volume reached 80–100
mm3, the mice were randomly divided into the NC and
LGR5-siRNA groups (n=4 mice per group). The NC and LGR5-siRNA
groups were administered intratumoral injection of 50 μg scrambled
siRNA and LGR5-siRNA, respectively, each week. The diameters of
subcutaneous tumors were measured with a caliper every 4 days, and
the tumor volume was calculated using the formula: volume =
width2 × length × 0.5. Four weeks later, the mice were
sacrificed and tumors were extracted and weighed. The experiments
were performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of
Health.
Statistical analysis
Results were presented as means ± SD. Statistical
analysis was performed using one-way ANOVA or the Student’s t-test.
The LSD method was used for multiple comparisons. P<0.05 was
considered to be statistically significant.
Results
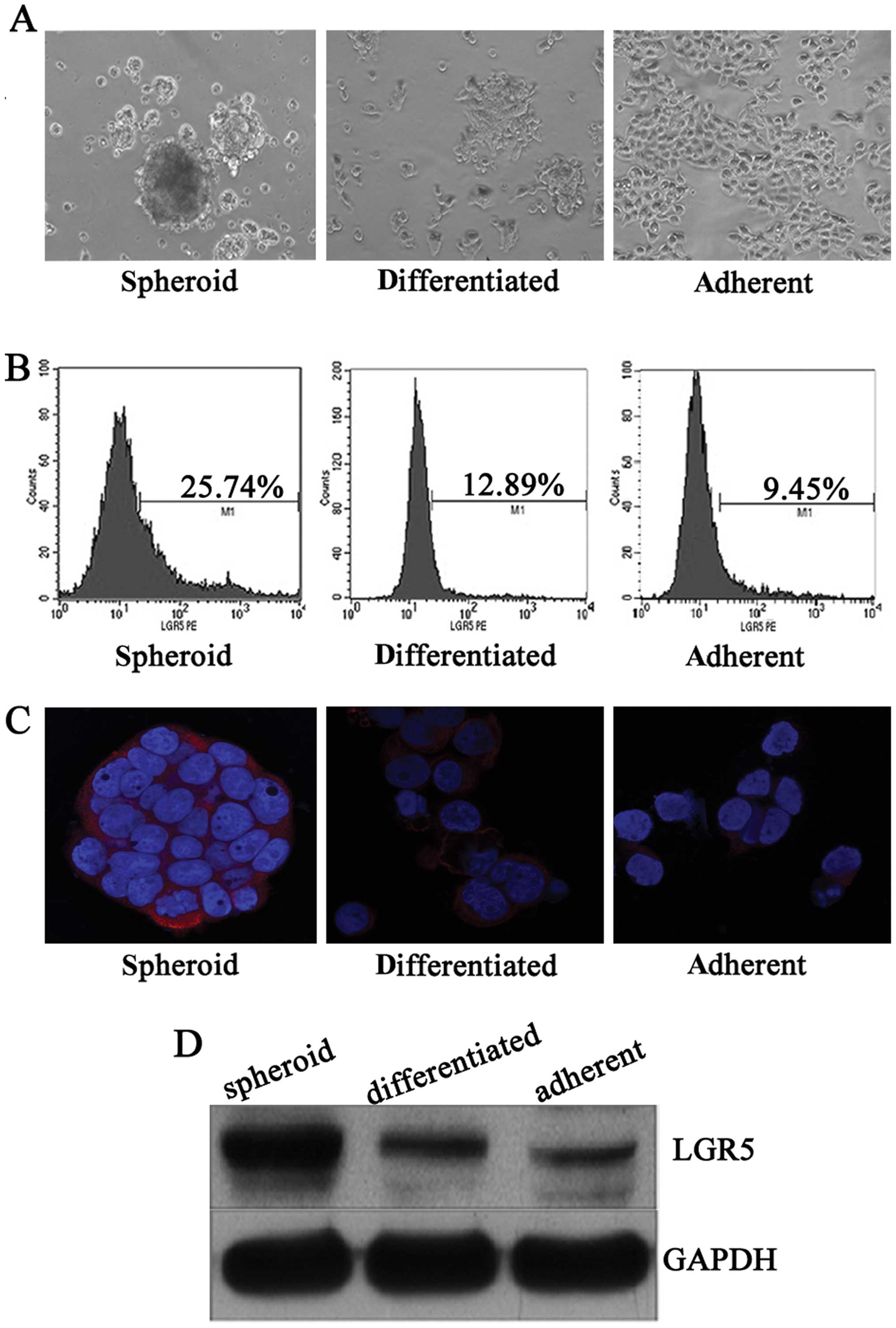
LGR5 is overexpressed in HT29 spheroid
cells
LGR5, also known as GPR49, is a member of the G
protein-coupled receptor (GPCR) family of proteins, and enhances
Wnt signaling by binding with R-spondin (10,20). Therefore, to obtain comprehensive
expression profile in CCSCs, we detected the extra- and
intracellular expression of LGR5 in HT29 spheroid cells and their
counterparts, which were differentiated in vitro and
cultured in monolayer (Fig. 1A).
Flow cytometric analysis revealed that HT29 spheroid cells
contained a high proportion of LGR5+ cells, while
differentiated and adherent cells had a smaller LGR5+
fraction (Fig. 1B). Similarly,
compared with adherent counterparts, stronger cytoplastic staining
and a higher LGR5 protein level was confirmed by immunofluorescent
staining and western blotting in HT29 spheroid cells, respectively,
which were significantly attenuated after inducing differentiation
in vitro (Fig. 1C and D).
These results suggest that LGR5 is associated with
dedifferentiation of CCSCs.
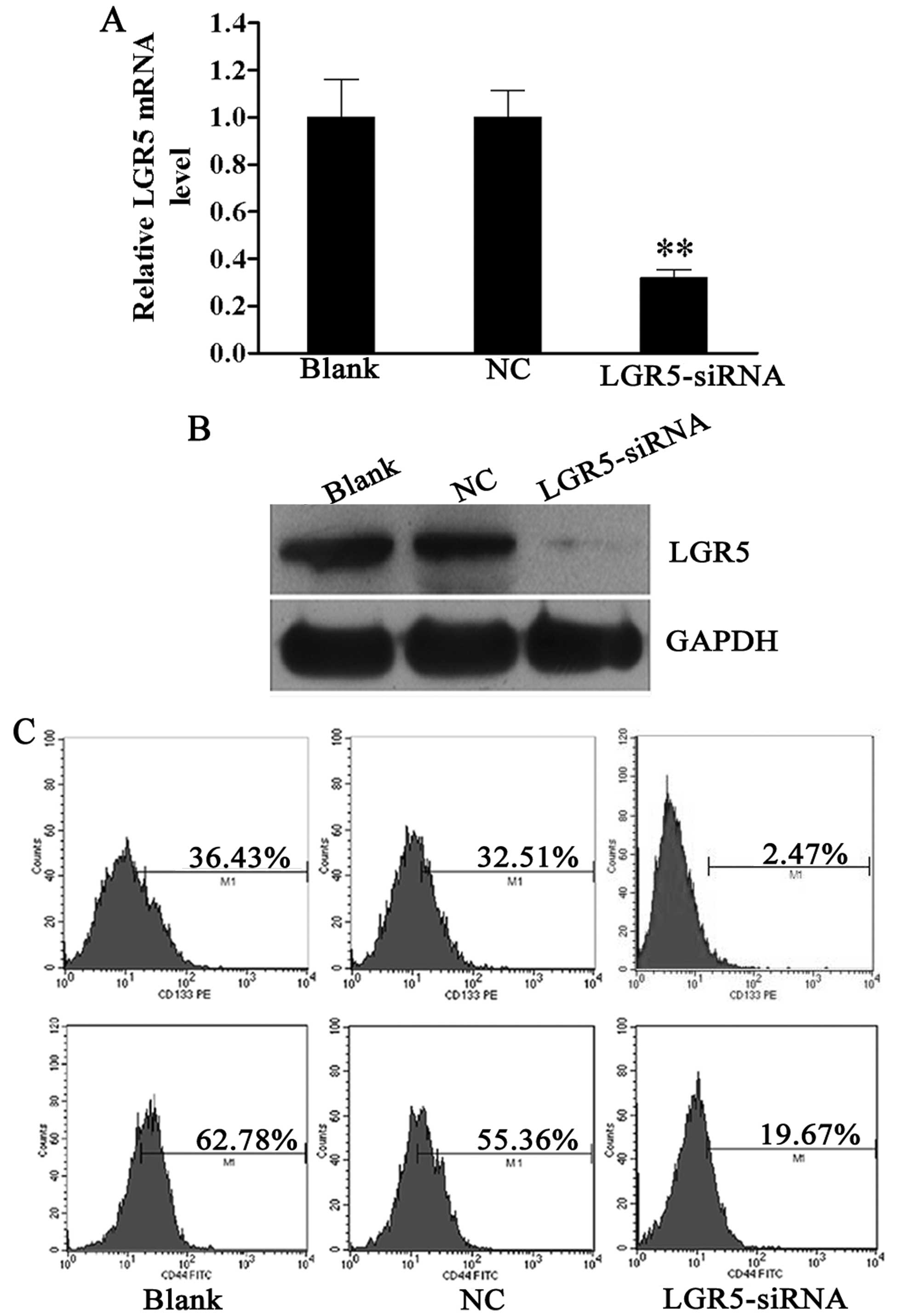
Expression of stem cell markers CD133 and
CD44 is decreased following siRNA-mediated LGR5 knockdown in HT29
spheroid cells
Since a high expression of LGR5 was detected in HT29
spheroid cells, we investigated the biological function of LGR5 in
these cells. siRNA was used to knock down the expression of LGR5 in
spheroid cells. LGR5 mRNA was downregulated by 68.2% in HT29
spheroid cells at 48 h after LGR5-siRNA transfection compared to
the blank control (Fig. 2A).
Moreover, western blotting confirmed that the LGR5 protein
expression was also markedly reduced in HT29 spheroid cells
transfected with LGR5-siRNA compared with the NC and blank controls
(Fig. 2B).
In a previous study, we confirmed that HT29 spheroid
cells were rich in CD133+ and CD44+ cells,
which represented the subpopulation with stem-like properties
(19). The effect of LGR5 on
these cell populations was then examined. Forty-eight hours
post-transfection, flow cytometric analysis revealed that the
percentages of CD133+ and CD44+ cells were
decreased in the LGR5-siRNA group (2.47 and 19.67%), as compared to
the NC group (32.51 and 55.36%) and blank group (36.43 and 62.78%)
(all P<0.01, Fig. 2C). These
data reveal that LGR5 plays a key role in sustaining the stemness
property of CCSCs.
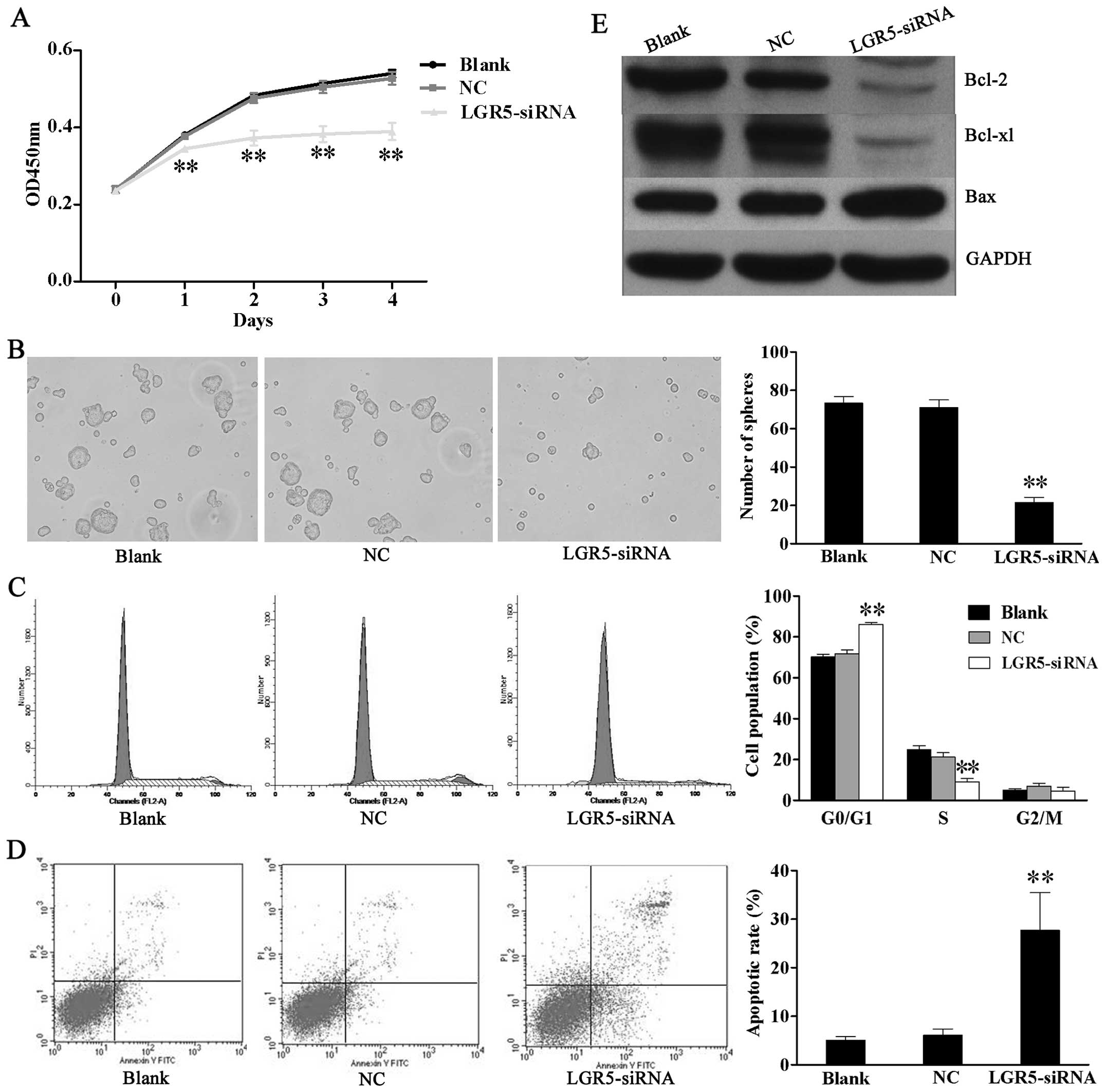
Downregulation of LGR5 expression impairs
survival of HT29 spheroid cells in vitro
In order to investigate the effect of LGR5 on
survival of CCSCs, proliferation, tumor sphere formation, cell
cycle and apoptosis assays were performed in LGR5-siRNA transfected
HT29 spheroid cells. CCK-8 assay showed that LGR5-siRNA cell growth
was slower than the NC and blank control cells (Fig. 3A). In addition, LGR5 silencing
suppressed the self-renewal of HT29 spheroid cells. LGR5-siRNA
cells formed smaller and fewer secondary tumor spheres than the NC
and blank control cells (Fig.
3B). The cell cycle assay revealed that the percentage of cells
at the G0/G1 phase were significantly increased in the LGR5-siRNA
group (86.23±0.85%) compared to the NC group (70.19±1.35%) and
blank group (71.84 ± 1.78%), while those at S phase were markedly
decreased in LGR5-siRNA group (9.16±1.62%) relative to the NC group
(21.21±2.13%) and blank group (24.77±2.02%) (P<0.01, Fig. 3C). On the other hand, the
LGR5-siRNA group had a higher apoptotic rate (27.7±7.74%) than the
NC group (6.06±1.34%) and blank group (5.11±0.77%) (P<0.01,
Fig. 3D). Furthermore, we
analyzed the expression of survival-related genes including Bcl-2,
Bcl-xL and Bax. Western blotting revealed that the expression of
anti-apoptotic Bcl-2 and Bcl-xL genes was downregulated while the
expression of the pro-apoptotic Bax gene was upregulated following
LGR5 knockdown in HT29 spheroid cells (Fig. 3E). These results show that LGR5
may promote the spheroid cells survival by modulating the intrinsic
apoptotic signaling pathway.
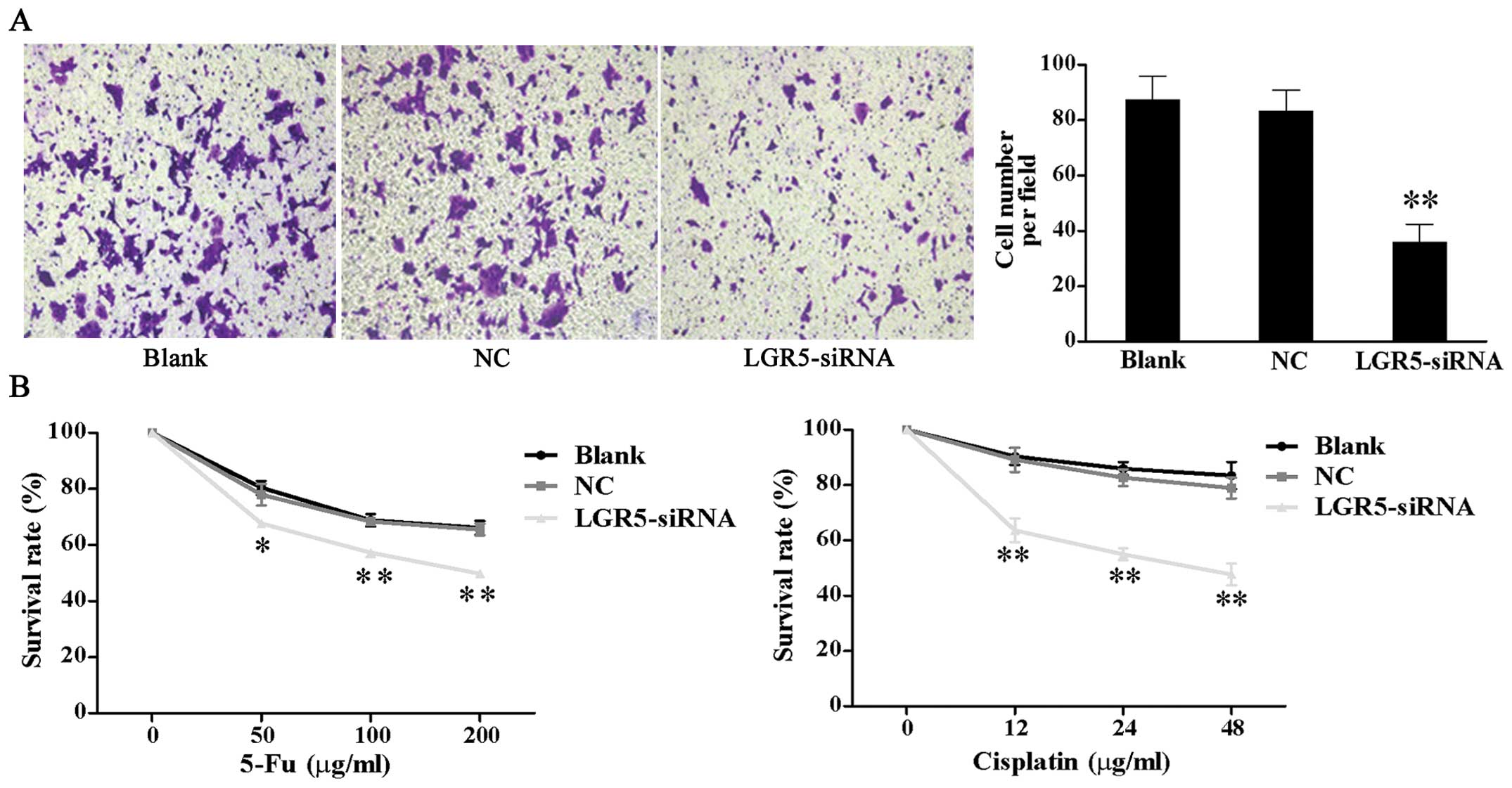
Downregulation of LGR5 expression
suppresses invasion and increases the chemosensitivity of HT29
spheroid cells
As enhanced invasive ability and chemotherapy
resistance are critical features of CSCs, we examined whether LGR5
affected these features in colon cancer. Results of matrigel
invasion assays showed that the number of LGR5-siRNA cells
(35.87±6.59) that invaded the underside of the membrane was
significantly less than that of the NC (83.2±7.71) and blank
control cells (87.27±8.57) (P<0.01, Fig. 4A).
To evaluate the effect of LGR5 on the drug
resistance of spheroid cells, we conducted a chemosensitivity
assay. Spheroid cells were treated with various concentration of
cisplatin and 5-Fu 48 h post-transfection. After incubation for
further 48 h, CCK-8 assay demonstrated that the survival rates of
LGR5-siRNA transfected cells were significantly reduced compared
with the NC and blank control cells at the respective
concentrations (Fig. 4B).
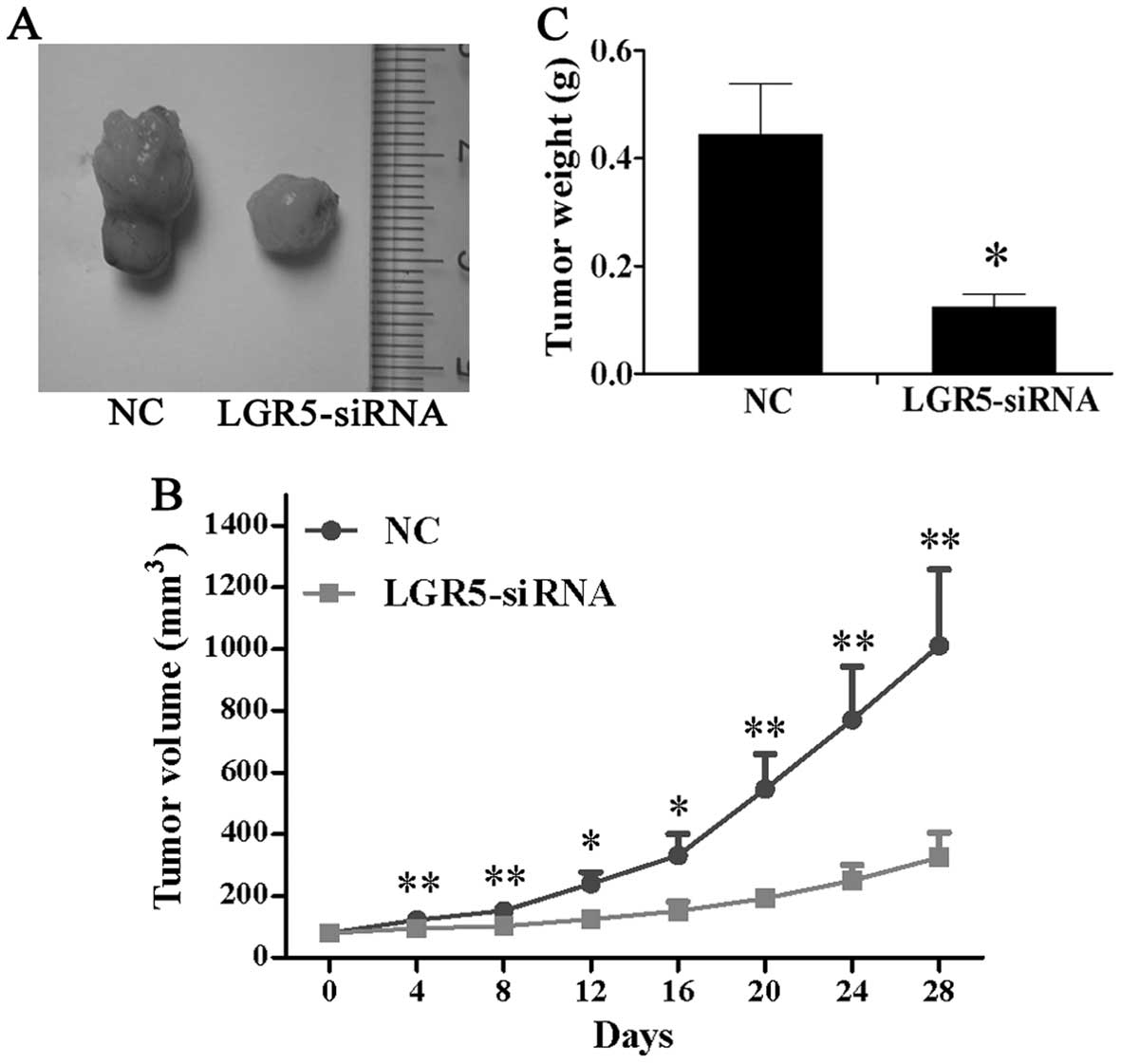
LGR5-siRNA attenuates tumorigenicity of
HT29 spheroid cells in vivo
To assess the function of LGR5 for tumorigenicity
in vivo, LGR5-siRNA were injected into the subcutaneous
tumors in nude mice triggered by HT29 spheroid cells and tumor
volume was measured every 4 days. As shown in Fig. 5A and B, mice in the LGR5-siRNA
group developed much smaller subcutaneous tumors than those in the
NC group. Four weeks after injection, the tumors were excised and
weighed. The result showed that the average tumor weight of the
LGR5-siRNA group was significantly lower than that of the NC group
(Fig. 5C). These findings showed
that LGR5-siRNA suppressed tumor growth in vivo in HT29
spheroid cells.
Discussion
In colon cancer, a small fraction of tumor cells
termed CSCs, which possess the ability of multi-differentiation and
self-renewal, are suggested to be responsible for tumor initiation
and growth. Therapies targeting CCSCs would likely result in more
complete tumor degeneration. Identification of CCSCs is therefore a
critical step for these targeting therapies. LGR5, known as an
intestinal stem cell marker, has been considered a marker for CCSCs
(15,16). Previous studies have shown that
LGR5 was overexpressed in colon cancer tissue (17,18). However, the expression of LGR5 in
CCSCs is unclear. In this study, we identified an increased
expression of LGR5 on the surface of spheroid cells by flow
cytometry, indicating that these CSCs-enriched colon cancer cells
contained a high proportion of LGR5+ cells. Compared
with CD133 expression in spheroid cells we reported previously
(19), LGR5 expression may serve
as a marker for a smaller heterogeneous population in spheroid
cells. More importantly, recent studies have demonstrated that
almost all of the LGR5+ cells isolated from xenografts,
generated by the sorted LGR5+ colon cancer cells, were
positive for CD133 and CD166. In addition,
LGR5+/CD133+ cells formed more colonies than
LGR5−/CD133+ cells (21). Findings of recent studies showed
that the overlapping expression of putative stem cell markers
CD133, CD44 and LGR5 in particular areas of gastric and intestinal
mucosa, and concluded that they may be functionally associated
(22,23). Our findings that LGR5 knockdown
resulted in a decreased expression of CD133 and CD44 in spheroid
cells confirm this conclusion, although the underlying molecular
mechanism involved remains to be determined. These observations
suggest that LGR5 may serve as a robust marker for CCSCs.
LGR5 belongs to the GPCR family and is a target gene
of Wnt signaling that regulates tumorigenesis in colon cancer
(24). Transformation exclusively
occurring in LGR5+ cells could induce growth of colon
adenomas (15). These findings
show that LGR5 may be important in CCSCs. This study aimed to
assess the function of LGR5 in CCSCs. As such, we examined the
intracellular expression of LGR5 in CCSCs-enriched HT29 spheroid
cells. The results showed that a high level of LGR5 was detected in
the cytoplasm of HT29 spheroid cells, but was markedly decreased
when the spheroid cells were induced to differentiate by culturing
in FBS-containing medium. These preferential expression patterns
suggest that LGR5 was correlated with the maintenance of HT29
spheroid cells. We downregulated LGR5 expression in spheroid cells
by siRNA and examined whether it would affect the cells. First, we
observed that LGR5 knockdown significantly inhibited the
proliferation and secondary tumor sphere formation of HT29 spheroid
cells in vitro. In addition, tumor growth in vivo was
also suppressed by LGR5 siRNA. In order to clarify the mechanism of
these effects, apoptosis and cell cycle assays were performed. The
results showed that LGR5 silencing induced apoptosis and G0/G1
phase arrest in spheroid cells. These data show that LGR5 is
essential for survival and the self-renewal of CCSCs, which is
consistent with the findings in brain CSCs (25).
High potential for invasion and drug resistance are
two important properties of CSCs (3,26).
Recent studies have demonstrated that a high LGR5 expression in
colon cancer tissues was closely associated with increased lymph
node invasion, distant metastasis and poor chemotherapy response
(18,27). These findings suggest that LGR5
exerts an effect on the malignant profile of CCSCs. To verify this
hypothesis, invasion and chemosensitivity assays were conducted. As
expected, LGR5 knockdown significantly reduced invasive ability and
increased chemosensitivity in spheroid cells.
The molecular mechanism of LGR5 in modulating CCSCs
properties remains to be determined. Results of a recent study
showed that Rac1-ROS-NF-κB axis is critical for malignant
transformation of LGR5+ ISC (28). Focal adhesion kinase (FAK),
nuclear factor-κB (NF-κB), and c-fos are regulated by LGR5 through
the Rho signaling pathway (29).
These downstream targets, which are key factors for inflammation
and cell adhesion, also play an important role in self-renewal and
differentiation and migration of stem cells (30,31). Therefore, the crosstalk between
LGR5 and other signaling pathways may contribute to maintaining the
function of CCSCs. In this study, we observed that LGR5 silencing
altered the expression of Bcl-2, Bcl-xL and Bax in HT29 spheroid
cells, suggesting LGR5 is a potential regulator of
apoptosis-related genes, although the underlying mechanism involved
remains to be determined.
In conclusion, the present study has shown that LGR5
is overexpressed in spheroid-derived CCSCs. LGR5 knockdown
suppressed growth, self-renewal, invasion and drug resistance, and
induced apoptosis and G0/G1 phase arrest of spheroid cells in
vitro, as well as the expression of stem cell markers CD133 and
CD44, attenuating their tumorigenicity in vivo. LGR5 is
therefore indispensable for the maintenance of CCSCs. Targeting
LGR5 may be an effective therapeutic strategy for eliminating colon
cancer.
Acknowledgements
This study was supported by grants from the Project
of Science and Technology of Guangzhou City (no. 2011J4100105), the
Key Project of Science Foundation of Guangdong Province (no.
10251008901000011), the Project of Science and Technology of
Guangdong Province (no. 2009B030801091), the National Natural
Science Foundation of China (no. 81000960), and the Fundamental
Research Funds for the Central Universities (no. 12ykpy42).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics. CA Cancer J Clin. 57:43–66.
2007.
|
|
2
|
O’Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004.
|
|
3
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
4
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumor
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
6
|
Du L, Wang H, He L, et al: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both CD133+
and CD133− metastatic colon cancer cells initiate tumors. J Clin
Invest. 118:2111–2120. 2008.
|
|
10
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato T, Vries RG, Snippert HJ, et al:
Single Lgr5 stem cells build crypt-villus structures in vitro
without a mesenchymal niche. Nature. 459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snippert HJ, van der Flier LG, Sato T, et
al: Intestinal crypt homeostasis results from neutral competition
between symmetrically dividing Lgr5 stem cells. Cell. 143:134–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sangiorgi E and Capecchi MR: Bmi1 is
expressed in vivo in intestinal stem cells. Nat Genet. 40:915–920.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Gibson P, Currle DS, et al:
Prominin 1 marks intestinal stem cells that are susceptible to
neoplastic transformation. Nature. 457:603–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barker N, Ridgway RA, van Es JH, et al:
Crypt stem cells as the cells-of-origin of intestinal cancer.
Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Merlos-Suárez A, Barriga FM, Jung P, et
al: The intestinal stem cell signature identifies colorectal cancer
stem cells and predicts disease relapse. Cell Stem Cell. 8:511–524.
2011.PubMed/NCBI
|
|
17
|
Uchida H, Yamazaki K, Fukuma M, et al:
Overexpression of leucine-rich repeat-containing G protein-coupled
receptor 5 in colorectal cancer. Cancer Sci. 101:1731–1737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi H, Ishii H, Nishida N, et al:
Significance of Lgr5(+ve) cancer stem cells in the colon and
rectum. Ann Surg Oncol. 18:1166–1174. 2011.
|
|
19
|
Wei B, Han XY, Qi CL, et al: Coaction of
spheroid derived stem-like cells and endothelial progenitor cells
promotes development of colon cancer. PLoS One. 7:e390692012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Lau W, Barker N, Low TY, et al: Lgr5
homologues associate with Wnt receptors and mediate R-spondin
signalling. Nature. 476:293–297. 2011.PubMed/NCBI
|
|
21
|
Kobayashi S, Yamada-Okabe H, Suzuki M, et
al: LGR5-positive colon cancer stem cells interconvert with
drug-resistant LGR5-negative cells and are capable of tumor
reconstitution. Stem Cells. 30:2631–2644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu C, Xie Y, Gao F, et al: Lgr5 expression
as stem cell marker in human gastric gland and its relatedness with
other putative cancer stem cell markers. Gene. 525:18–25. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou NY, Yang K, Chen T, et al: CD133+
CD44+ subgroups may be human small intestinal stem cells. Mol Biol
Rep. 38:997–1004. 2011.
|
|
24
|
Vermeulen L: Wnt activity defines colon
cancer stem cells and is regulated by the microenvironment. Nat
Cell Biol. 12:468–476. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakata S, Campos B, Bageritz J, Bermejo
JL, et al: LGR5 is a marker of poor prognosis in glioblastoma and
is required for survival of brain cancer stem-like cells. Brain
Pathol. 23:60–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu HC, Liu YS, Tseng KC, et al:
Overexpression of Lgr5 correlates with resistance to 5-FU-based
chemotherapy in colorectal cancer. Int J Colorectal Dis.
28:1535–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Myant KB, Cammareri P, McGhee EJ, et al:
ROS production and NF-κB activation triggered by RAC1 facilitate
WNT-driven intestinal stem cell proliferation and colorectal cancer
initiation. Cell Stem Cell. 12:761–773. 2013.
|
|
29
|
Kwon MS, Park BO, Kim HM and Kim S:
Leucine-rich repeat-containing G-protein coupled receptor 5/GPR49
activates G12/13-Rho GTPase pathway. Mol Cells. 36:267–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schugar RC, Robbins PD and Deasy BM: Small
molecules in stem cell self-renewal and differentiation. Gene Ther.
15:126–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang SD, Rath P, Lal B, et al: EphB2
receptor controls proliferation/migration dichotomy of glioblastoma
by interacting with focal adhesion kinase. Oncogene. 31:5132–5143.
2012. View Article : Google Scholar : PubMed/NCBI
|