Introduction
Pancreatic carcinoma (PC) is an aggressive and
lethal malignancy. The median overall survival is <6 months,
with a 5-year survival rate of 5% (1,2).
Vascular invasion, perineural invasion and distant metastasis are
the critical features in the aggressive phenotype of PC, and
contribute to the lost opportunities for surgical resection
(3,4). Thus, new therapeutic strategies
should be based on a better understanding of biomarkers and their
association with invasion and metastasis.
Rho GTPases, including Rac1, Cdc42 and RhoC, are
important regulators of cell migration, cell motility, cell cycle
progression and cytoskeleton organization (5,6).
The biological activities of Rho GTPases are regulated by guanine
nucleotide exchange factors (GEFs), GTPase-activating proteins
(GAPs) and Rho GDP dissociation inhibitors (RhoGDIs) (7). Rho GDP dissociation inhibitor 2
(RhoGDI2), also known as D4-GDI or LyGDI, belongs to a family of
RhoGDIs. RhoGDI2 was originally expressed in hematopoietic cells,
predominantly in B and T cells (8,9).
However, it has been shown that RhoGDI2 is also abnormally
expressed in tumors. The role of RhoGDI2 in tumor progression
remains controversial. RhoGDI2 may function as a positive regulator
of tumor progression in gastric, colorectal, and breast cancer
(10–12). The role of RhoGDI2 as a metastasis
suppressor gene was also validated in bladder cancer and Hodgkin’s
lymphoma (13,14). However, the expression and role of
RhoGDI2 in the progression of PC remains to be determined.
In this study, we examined the expression of RhoGDI2
in PC tissues and cell lines. Moreover, using small interfering RNA
(siRNA) to silence RhoGDI2 expression, we investigated the effect
of RhoGDI2 in the regulation of invasion and metastasis. These
findings indicated that RhoGDI2 is a potential target for the gene
therapy of PC.
Materials and methods
Clinical samples
Tissue samples from 60 PC patients were collected
during surgical resections performed at the First Affiliated
Hospital of Soochow University between January 2010 and December
2012. Tumorous and adjacent non-tumorous tissues were frozen
immediately after surgical removal in liquid nitrogen and stored at
−80°C. The patients did not receive any preoperative chemotherapy,
radiotherapy or immunotherapy. The samples were obtained following
written patient consent. Study approval was obtained from the local
ethics committee of Soochow University.
Cell culture and transfection
Human PC cell lines, PANC-1, SW1990, Patu8988 and
BxPC-3 were obtained from the Shanghai Institute of Biochemistry
and Cell Biology (Shanghai, China) and maintained in DMEM,
supplemented with 10% fetal bovine serum (both from Gibco, Grand
Island, NY, USA) and 100 μg/ml each of penicillin and streptomycin
(Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37°C.
SiRNA transfection
Small hairpin RNA (shRNA) of human RhoGDI2
(NM_001175; GeneBank) transfer vector encoding the green
flourescent protein (GFP) was constructed by GeneChem Co., Ltd.
(Shanghai, China). The siRNA sequence targeting RhoGDI2
(siRNA-RhoGDI2) was 5′-GGAAGGUUC UGAAUAUAGA-3′, as confirmed by
sequencing. The negative control of siRNA (siRNA-NC) was designed
and the sequence was without obvious homology to the human gene.
Cells (1×104/well) were cultured in 6-well plates
overnight and reached 60–70% confluence. To determine the different
expressions of RhoGDI2, PANC-1 and Patu8988 cells were transfected
with siRNA-RhoGDI2 or siRNA-NC using Lipofectamine 2000
(Invitrogen) as the transfection reagent. Non-transfected cells
were used as the blank control (control).
Quantitative PCR (qPCR)
Total RNA from tissues and cells was extracted using
TRIzol (Invitrogen). Single-strand cDNA for a PCR template was
synthesized from 10 μg of total RNA using random primers and M-MLV
reverse transcriptase (Takara Bio, Dalian, China). The relative
levels of target gene mRNA transcripts to that of the control
(β-actin) were determined by qPCR. The primers used were (forward
and reverse): 5′-ATGACTGAAAAAGCCCCA-3′ and 5′-TCATTC
TGTCCACTCCTT-3′ for RhoGDI2 (606 bp); 5′-GTGCTGAAG
GACACACTAAAGAAGA-3′ and 5′-TTGCCATCCTTCTCA AAGTTGTAGG-3′ for matrix
metalloproteinase 2 (MMP2) (605 bp); 5′-AGCGGGAAATCGTGCGTG-3′ and
5′-CAGGGT ACATGGTGGTGCTGCC-3′ for β-actin (308 bp). The PCR
reactions were 40 cycles (95°C for 15 sec, 62°C for 45 sec, and
72°C for 30 sec). The amplified segments were analyzed by 2.5%
agarose gels.
Western blotting
Cells were collected and lysed in lysis buffer on
ice. Total proteins were separated by 10% SDS-PAGE and blotted on
PVDF membrane. Membranes were blocked with 10% non-fat milk powder
at room temperature for 2 h and incubated with primary antibodies:
anti-RhoGDI2 antibody (1:200), anti-MMP2 antibody (1:200) (both
from Abcam, Cambridge, UK) and anti-β-actin antibody (1:200; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA) at 4°C overnight. After
three washes, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-mouse IgG (1:2,000; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. Reactive bands
were detected using ECL western blotting detection reagent.
Cell proliferation assay
CCK8 assay was performed to evaluate cell
proliferation. Following transfection, the cells were seeded in
96-well plates at 5×103 cells/well, followed by the
addition of 20 μl CCK8 (Dojindo Laboratories, Kumamoto, Japan) and
incubation at 37°C for an additional 2 h. An ultraviolet
spectrophotometer (Implen, Munich, Germany) was used to measure the
absorbance of each well at 450 nm, and each experiment was
performed in triplicate and repeated five times.
In vitro wound scrape assay
Cells from each group were incubated in 6-well
plates. A small wound area was made in the confluent monolayer with
a 200 μl pipette tip in a lengthwise stripe. The cells were washed
twice with PBS and incubated at 37°C. Images were captured at 0 and
48 h. Wound width was measured at a magnification of ×100 using a
microscope (DM2500, Leica Microsystems, Mannheim, Germany) with a
calibrated eyepiece grid (1 mm/100 μm graduation). Ten measurements
were determined at random intervals along the wound length.
Invasion assay
Cell invasion was determined using Matrigel-coated
invasion Boyden chambers using a Transwell kit, according to the
manufacturer’s instructions. Briefly, 600 μl DMEM medium containing
10% FBS was added to the bottom chamber. After 12 h of serum
starvation, the cells were collected and placed in the upper
invasion chamber (lx105 cells/well). After incubation at
37°C for 48 h, the non-invasive cells in the upper chamber were
removed with a cotton swab. The insert membranes were fixed with
methanol for 15 min and stained with 0.1% crystal violet. The
stained cells that penetrated through the Matrigel were counted
under the inverted microscope.
Immunohistochemistry (IHC)
Serial sections (4 μm) were prepared for
immunohistological staining. Tissue sections were quenched for
endogenous peroxidase with freshly prepared 3%
H2O2 with 0.1% sodium azide and then placed
in an antigen retrieval solution for 15 min. After incubation in a
casein block, primary anti-RhoGDI2 monoclonal antibody (1:50;
Abcam) was applied to the sections for 1 h at room temperature,
followed by incubation with the secondary antibody and
extravidin-conjugated horseradish peroxidase. The staining
intensity was scored as 0 (negative), 1 (weak), 2 (medium) and 3
(strong). The extent of staining was scored as 0 (0%), 1 (1–25%), 2
(26–50%), 3 (51–75%) and 4 (>76%). The final score was obtained
by the sum of the intensity score and the quantity score. A score
≥3 was considered as a positive expression.
Statistical analysis
SPSS version 17.0 was used for statistical analysis.
Data were expressed as mean ± SD. One-way analysis of variance
(one-way ANOVA), t-test and Chi-square test were performed for
inter-group comparison. P<0.05 was considered statistically
significant.
Results
Overexpression of RhoGDI2 protein in
PC
In 60 patients who recently underwent surgery for
PC, paired tumor samples and non-tumorous tissues were subjected to
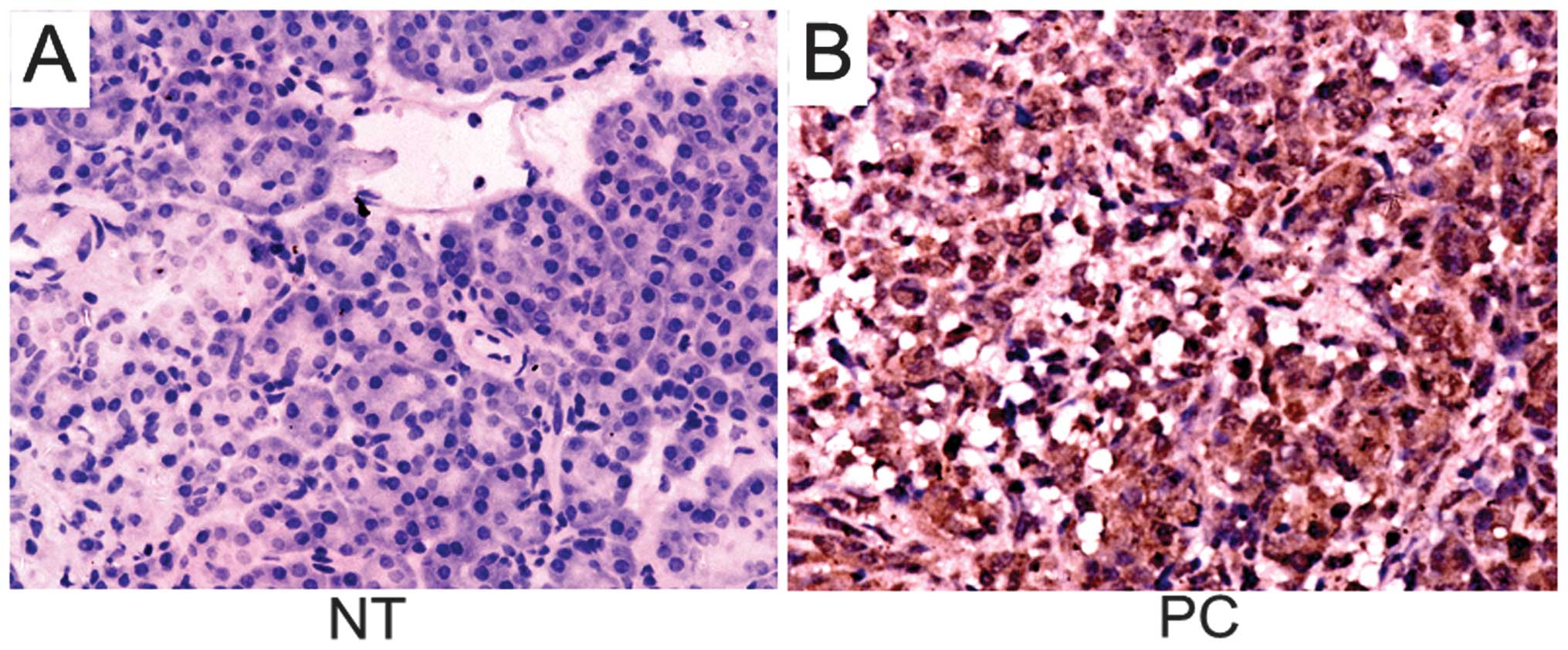
IHC. The subcellular expression pattern of RhoGDI2 was mainly
diffuse cytoplasmic in PC and the non-tumorous tissues were
negative or weekly positive for RhoGDI2 (Fig. 1). The positive rate of RhoGDI2 in
tumor tissues was 73.3% (44/60) while that in non-tumorous tissues
was 41.7% (25/60), showing a significant difference
(χ2=12.310, P=0.001).
Correlation between RhoGDI2 protein and
clinicopathological characteristics
The relationship between the expression of RhoGDI2
and the clinicopathological characteristics of PC are shown in
Table I. RhoGDI2 expression was
markedly correlated with tumor size (χ2=11.027,
P=0.003), differentiation (χ2=7.172, P=0.028), clinical
stage (χ2=12.273, P=0.001), lymph node metastasis
(χ2=9.586, P=0.004) and vascular invasion
(χ2=5.860, P=0.023), but did not show a statistically
significant association with gender (χ2=0.007, P=
1.000), age (χ2=4.105, P=0.072) and tumor location
(χ2=4.105, P=0.072).
 | Table IRelationship between RhoGDI2
expression and clinicopathological characteristics of PC
patients. |
Table I
Relationship between RhoGDI2
expression and clinicopathological characteristics of PC
patients.
| | RhoGDI2 | | |
|---|
| |
| | |
|---|
| Characteristics | Cases | Negative | Positive | χ2 | P-value |
|---|
| Gender |
| Male | 38 | 10 | 28 | 0.007 | 1.000 |
| Female | 22 | 6 | 16 | | |
| Age (years) |
| ≤65 | 36 | 13 | 23 | 4.105 | 0.072 |
| >65 | 24 | 3 | 21 | | |
| Tumor location |
| Head | 32 | 7 | 25 | 4.105 | 0.072 |
| Body and tail | 28 | 9 | 19 | | |
| Tumor size
(cm) |
| ≤2 | 8 | 6 | 2 | 11.027 | 0.003a |
| >2 | 52 | 10 | 42 | | |
|
Differentiation |
| Well | 13 | 6 | 7 | 7.172 | 0.028a |
| Moderate | 15 | 6 | 9 | | |
| Poor | 32 | 4 | 28 | | |
| Clinical stage |
| I | 12 | 8 | 4 | 12.273 | 0.001a |
| II | 48 | 8 | 40 | | |
| Lymph node
metastasis |
| Y | 41 | 6 | 35 | 9.586 | 0.004a |
| N | 19 | 10 | 9 | | |
| Vascular
invasion |
| Y | 18 | 1 | 17 | 5.860 | 0.023a |
| N | 42 | 15 | 27 | | |
Depletion of RhoGDI2 by siRNA in PC
cells
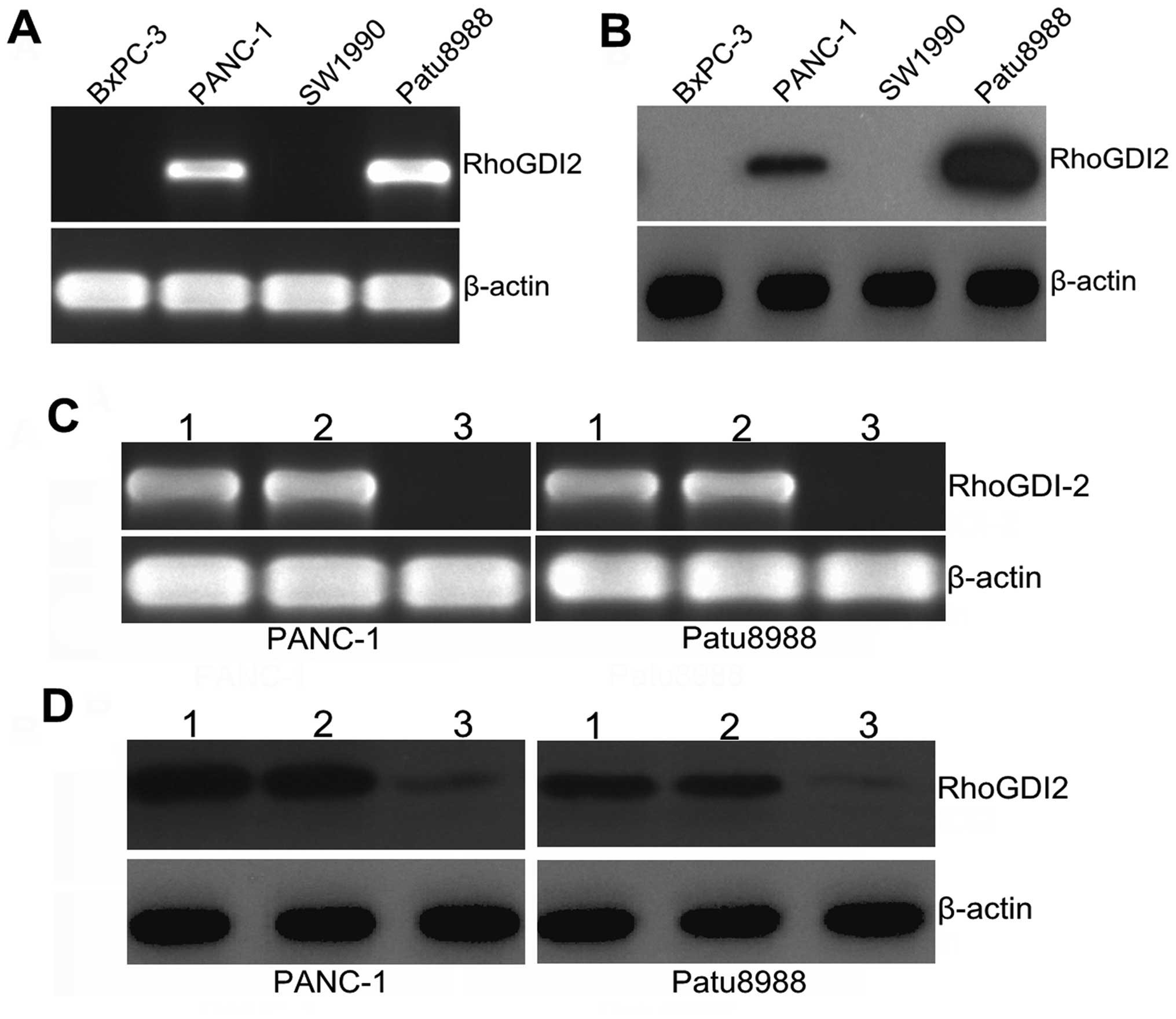
First, we examined the relative expression of
RhoGDI2 at the mRNA and protein levels in the PANC-1, SW1990,
Patu8988 and BxPC-3 cell lines, with PANC-1 and Patu8988 cells
being selected as a high expression of RhoGDI2 (Fig. 2A and B). To investigate the
function of RhoGDI2 in PC cells, we depleted RhoGDI2 expression in
PANC-1 and Patu8988 cells using the RNAi method. As shown in
Fig. 2C, the expression of
RhoGDI2 mRNA was markedly decreased in the RNAi-RhoGDI2 group
compared with the RNAi-NC group in PANC-1 and Patu8988 cells (both
P<0.01). A similar decrease was found at the protein level by
western blotting (Fig. 2D), and
the mean inhibition rate (the RNAi-RhoGDI2 group vs. the RNAi-NC
group) was 78.3% in PANC-1 and 82.4% in Patu8988 (both P<0.05).
These findings indicated that downregulation of the RhoGDI2 gene
was specific and efficient.
Silencing RhoGDI2 inhibits invasion in PC
cells
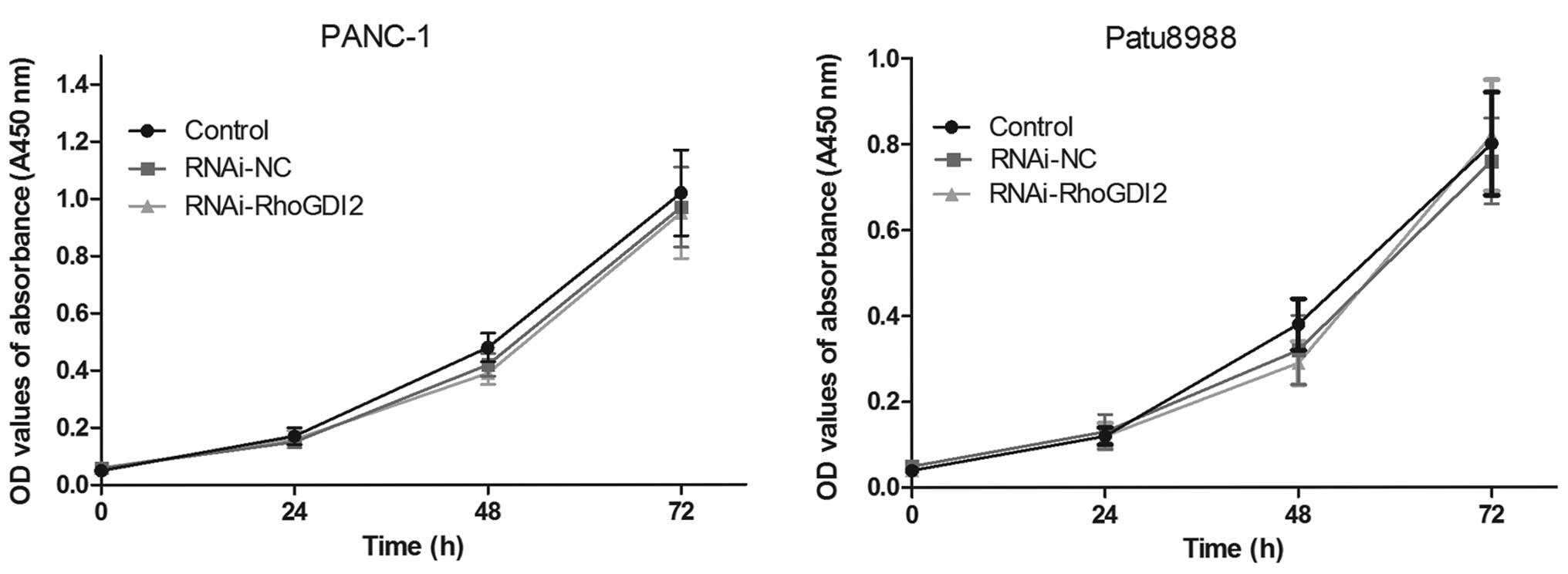
Using the transfected cells, CCK8 assay was
performed to examine the effect of RhoGDI2 on PC cell
proliferation. As shown in Fig.
3, there was no statistical significance in cell proliferation
between the RNAi-RhoGDI2 and RNAi-NC groups (P>0.05).
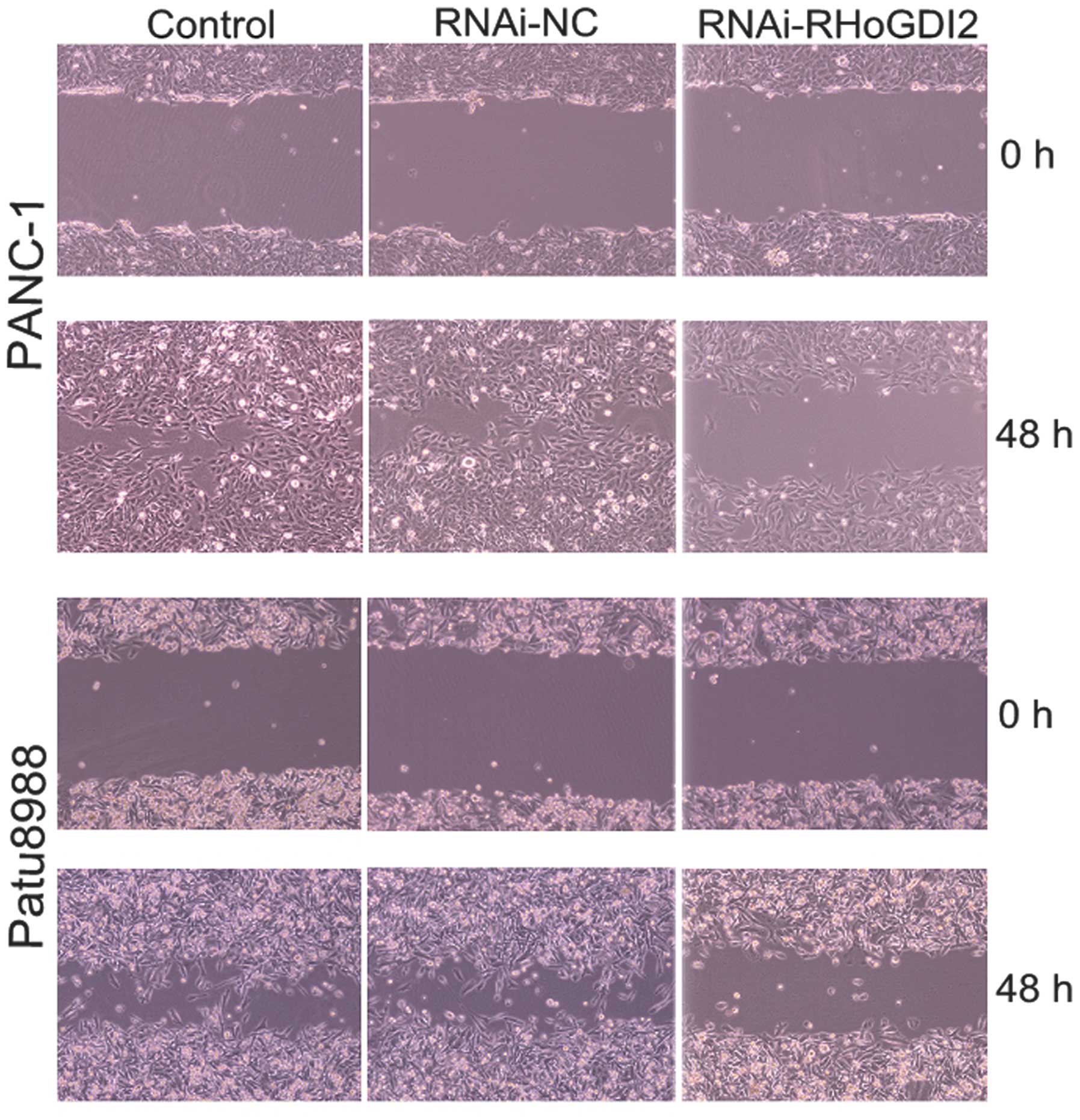
The effect of RhoGDI2 on cell motility and invasion
was assessed using wound scrape and Transwell assays. The wound
scrape assay was used to evaluate the effect of RhoGDI2 depletion
on cell motility in PANC-1 and Patu8988 cells. Time course analysis
of the wound closure showed that the re-established period of the
monolayer was significantly shorter in the RNAi-NC group than that
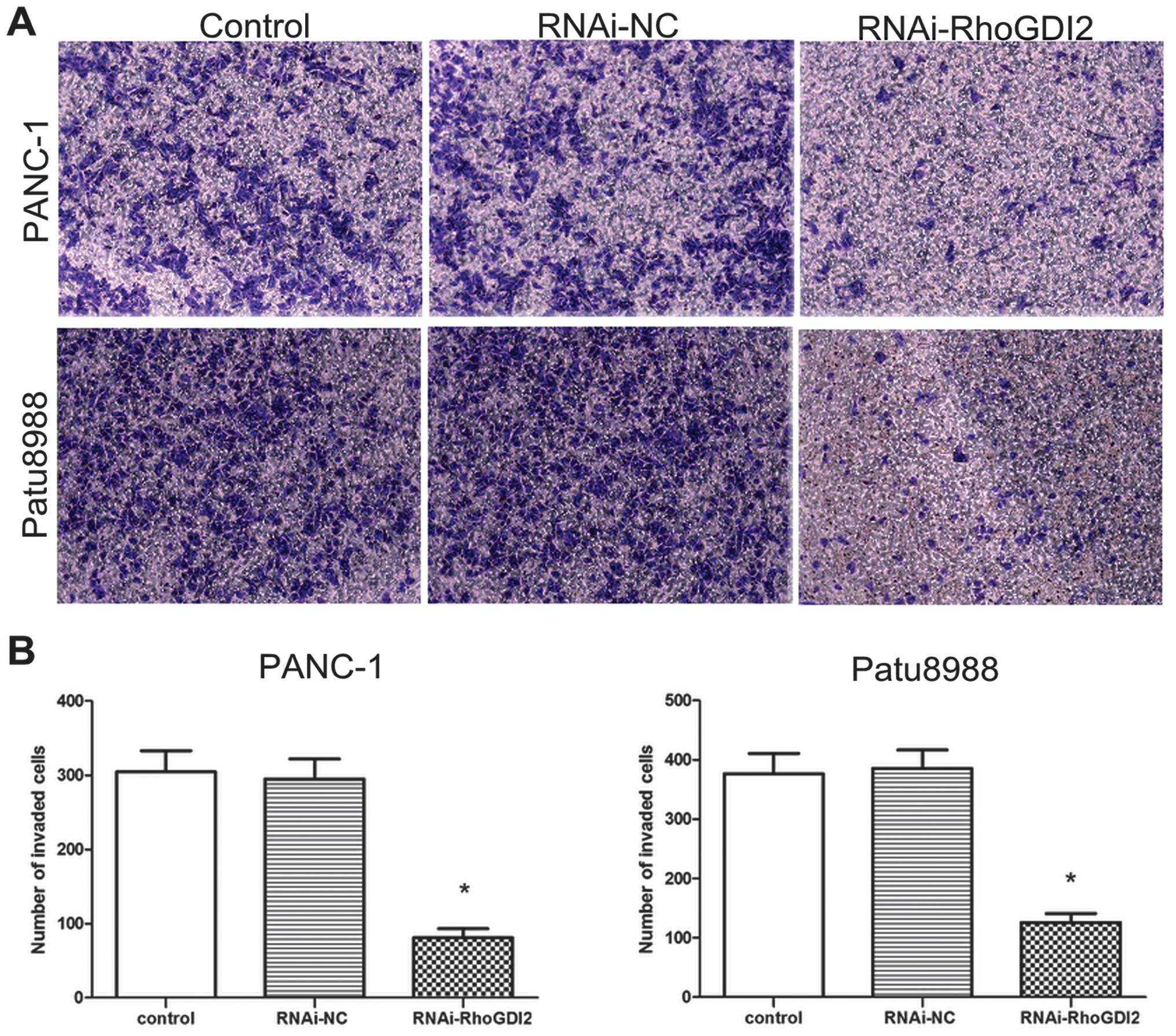
in the RNAi-RhoGDI2 group (both P<0.05) (Fig. 4). Invasion assays were performed
to determine whether RhoGDI2 is required for PC cell invasion based
on the Boyden chamber assay. As shown in Fig. 5, depletion of RhoGDI2
significantly reduced cell invasion in the RNAi-RhoGDI2 group
(81±12 cells per field for PANC-1 and 126±15 cells per field for
Patu8988) compared to the RNAi-NC group (295±27 cells per field for
PANC-1 and 386±31 cells per field for Patu8988) (both
P<0.05).
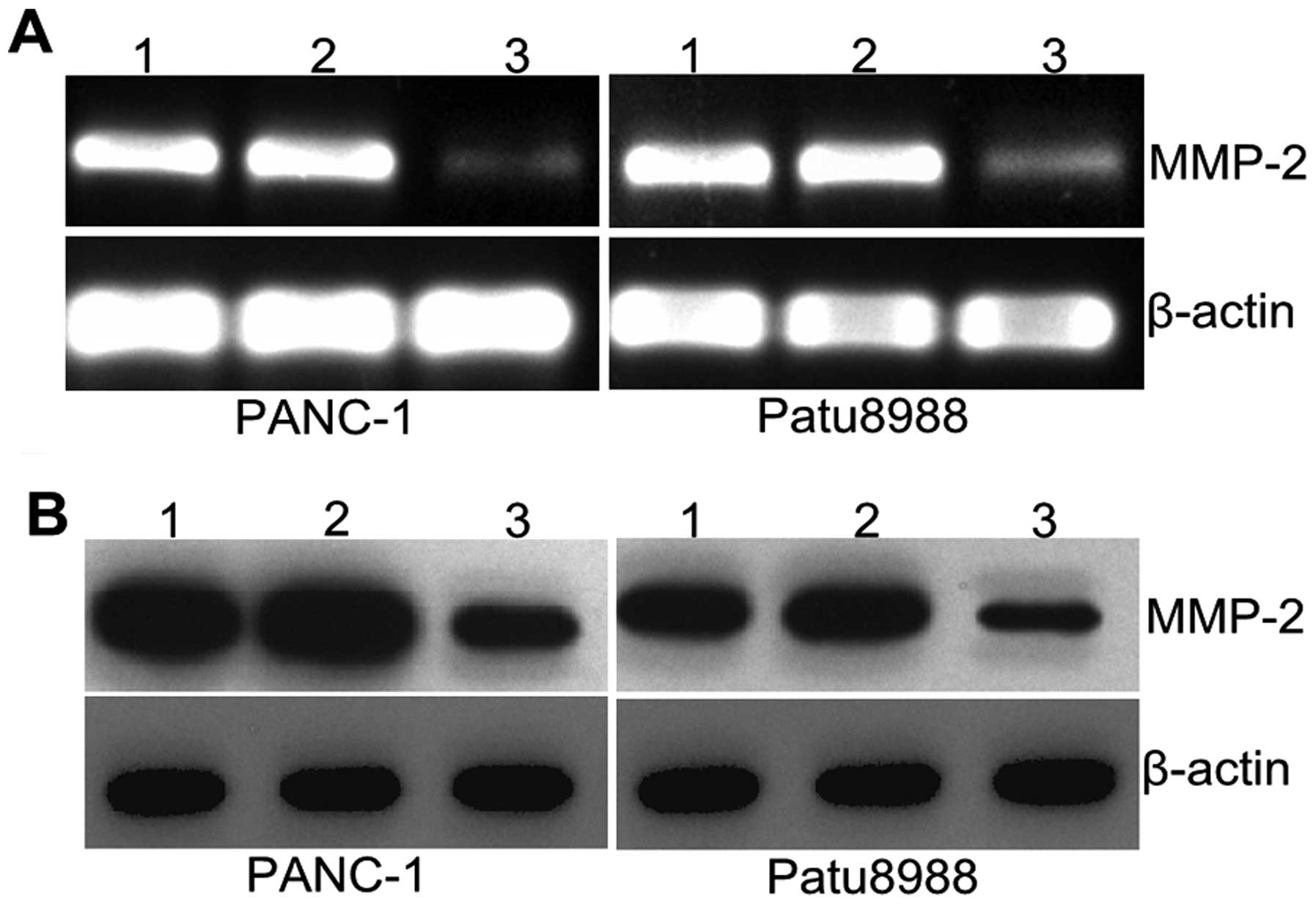
Silencing RhoGDI2 affects MMP2
expression
MMPs are involved in the degradation of
extracellular matrix (ECM) and basement membranes. To determine
whether RhoGDI2 promoted cancer cell invasion through MMPs, the
expression of MMP2 was determined by qPCR and western blotting.
Fig. 6A shows that MMP2 mRNA
expression was significantly decreased in the RNAi-RhoGDI2 group
compared with the RNAi-NC group in PANC-1 and Patu8988 cells (both
P<0.05). Results of western blotting revealed a similar decrease
at the protein level (both P<0.05) (Fig. 6A). The mean inhibition rate was
62.6% vs. the RNAi-NC group in PANC-1 and 60.7% in Patu8988. These
results indicated that RhoGDI2 contributed to the expression of
MMP2 in PC cells.
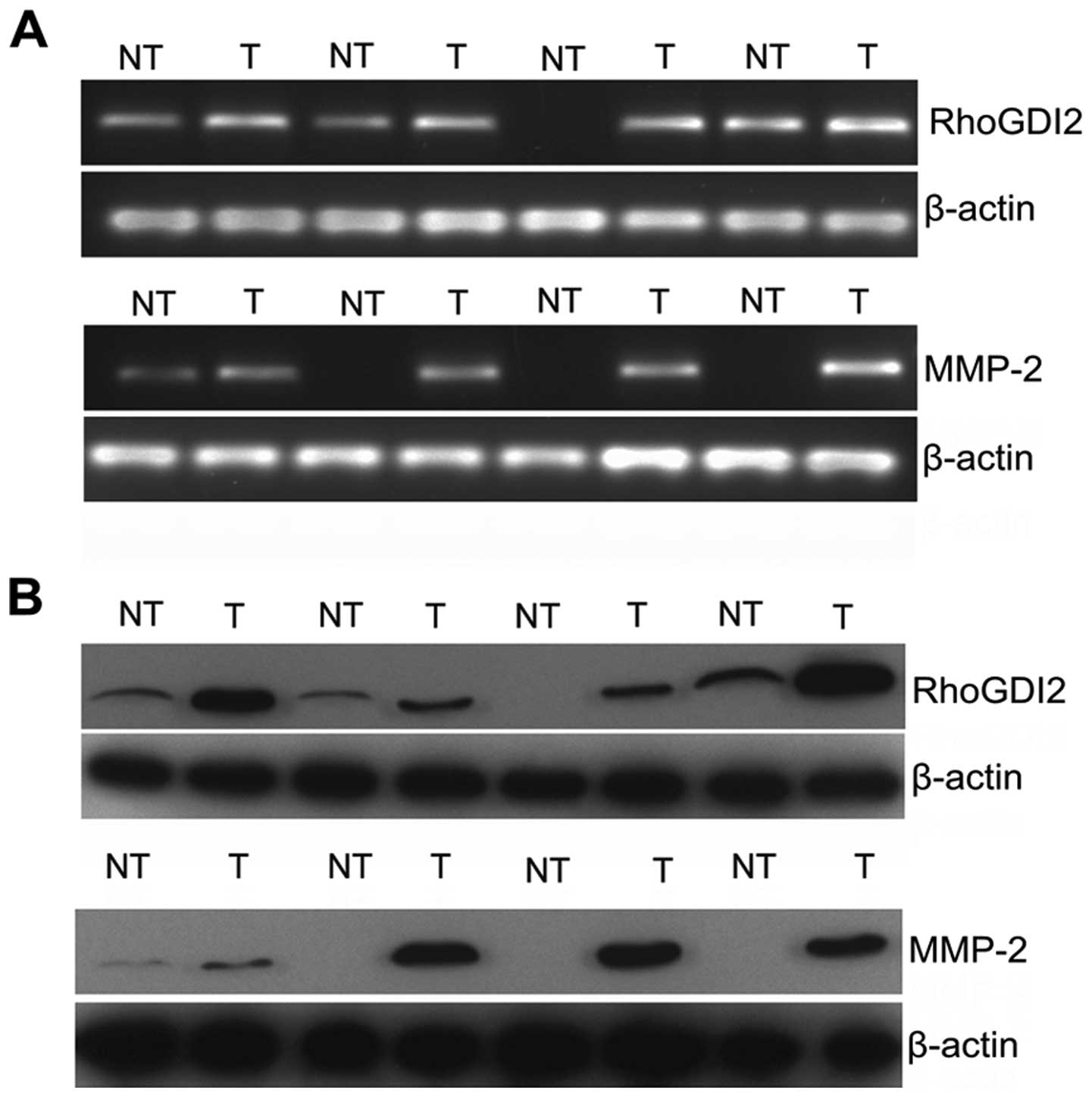
RhoGDI2 is positively correlated with
MMP2 in PC
To obtain clinical evidence of a positive correction
between RhoGDI2 and MMP2, the expression of 30 pairs of fresh PC
and NT tissues were assessed by qPCR (Fig. 7A) and western blotting (Fig. 7B). The majority of PC exhibited
overexpression of RhoGDI2 compared with NT at the mRNA and protein
levels (both P<0.01). Similarly, MMP2 was overexpressed in PC
compared with NT at the mRNA and protein level (both P<0.01).
Results of subsequent analysis revealed that RhoGDI2 expression was
positively correlated with MMP2 expression at the mRNA level
(Spearman analysis, r=0.627, P<0.001). Consistent results were
obtained when we compared the data at the protein level (Spearman
analysis, r=0.817, P<0.001). Our clinical study together with
the experimental data confirmed that RhoGDI2 modulated the
expression of MMP2.
Discussion
In this study, the expression of RhoGDI2 in four PC
cell lines, 30 matched clinical fresh PC tissues and 60 pairs of
clinical paraffin-embedded PC with clinical data was identified. We
found a tendency towards the upregulation of RhoGDI2 in PC tissues
compared to adjacent normal ones at the mRNA and protein levels.
IHC results showed that the expression of RhoGDI2 was positively
correlated with tumor size, clinical stage, lymph node metastasis
and vascular invasion. In addition, depletion of RhoGDI2 in PANC-1
and Patu8988 cells by RNAi significantly inhibited cell motility
and invasion in vitro, but had no effect on cell
proliferation.
Three human RhoGDIs have been identified thus far:
RhoGDI1, RhoGDI2 and RhoGDI3 (15–17). The proteins are key regulators of
Rho GTPases function, such as cell motility, polarity and invasion.
In contrast to RhoGDI1 and RhoGDI3, which are expressed
ubiquitously, RhoGDI2 was reported originally to be expressed in
hematopoietic cells, predominantly in B and T cells (18,19). However, accumulating evidence
shows that RhoGDI2 is also differentially expressed in human
cancers (20). In the majority of
studies, RhoGDI2 has been shown to promote tumor cell invasion,
angiogenesis and metastasis, such as in lung and gastric cancer
(21,22). However, it can function as a
metastasis suppressor gene in bladder cancer and Hodgkin’s lymphoma
(14,23). Our results showed that RhoGDI2 was
overexpressed in PC and silencing RhoGDI2 inhibited cell
invasiveness in PC cells. Those studies along with our findings
indicate that the function of RhoGDI2 in cancer progression depends
on different tumor types.
Although the reason for this discrepancy currently
remains unclear, the conflicting role of RhoGDI2 may result from
the dual roles of RhoGDI2 in the regulation of activities of Rho
GTPases during cancer progression. RhoGDI2 bound the majority of
Rho GTPases in the cytoplasm, maintaining Rho in an inactive form
and inducing the disruption of Rho-dependent cell motility
(24,25). By contrast, RhoGDI2 acted as an
escort protein directing Rho GTPases to the membrane and associated
with active forms of Rho, Rac and cdc42, maintaining Rho in an
active form (26,27). Our results indicate that knockdown
of RhoGDI2 expression in PC cells resulted in decreased cell
motility and decreased invasion ability. However, the manner in
which RhoGDI2 impacts on the activation of Rho GTPases requires
further analysis. In addition to Rho GTPases activities, other
mechanisms of RhoGDI2 in cancer have been reported in some studies.
β1-integrin and cyclooxygenase-2, which play key roles in breast
cancer progression, were identified as target genes of RhoGDI2 in
breast cancer cells (12,28). PLCγ were validated to be required
for RhoGDI2-mediated cell invasion and metastasis in gastric cancer
cells (29).
Tumor invasion and metastasis are the critical
characteristics in determining the aggressive phenotype of human
cancers, and require both tumor cell migration and degradation of
matrix barriers (30). MMPs are
involved in the degradation of ECM and basement membranes. Binker
et al found that activation of Rac1 in PANC-1 cells were
responsible for MMP2 secretion and activation (31). In the present study, we observed
that the expression of MMP2 was significantly decreased following
depletion of RhoGDI2 in PANC-1 and Patu8988 cells. Additionally, we
found that MMP2 was positively correlated with RhoGDI2 expression
in clinical PC tissues at the mRNA and protein levels. Thus,
RhoGDI2 contributes to PC cell motility and invasion, at least in
part by activating MMP2.
In summary, results of the present study
demonstrated that the overexpression of RhoGDI2 was associated with
PC progression. Depletion of RhoGDI2 in PC cells inhibited cell
motility and invasion in vitro, at least in part by
affecting the expression of MMP2. Our findings indicate that
targeting RhoGDI2 by a genetic approach may provide a new strategy
for the treatment in PC.
Acknowledgements
This study was supported by Project of Nature
Science Foundation of China (81201905), China Postdoctoral Science
Foundation (2013M540374), Shanghai Postdoctoral Scientific Program
of China (13R21415200) and Project of Medical Research of Jiangsu
Province (H201209).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
3
|
Dai H, Li R, Wheeler T, et al: Enhanced
survival in perineural invasion of pancreatic cancer: an in vitro
approach. Hum Pathol. 38:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleeff J, Beckhove P, Esposito I, et al:
Pancreatic cancer microenvironment. Int J Cancer. 121:699–705.
2007. View Article : Google Scholar
|
|
5
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reymond N, Riou P and Ridley AJ: Rho
GTPases and cancer cell transendothelial migration. Methods Mol
Biol. 827:123–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Mata R, Boulter E and Burridge K:
The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev
Mol Cell Biol. 12:493–504. 2011.
|
|
8
|
Scherle P, Behrens T and Staudt LM:
Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding
protein, is expressed preferentially in lymphocytes. Proc Natl Acad
Sci USA. 90:7568–7572. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho HJ, Baek KE and Yoo J: RhoGDI2 as a
therapeutic target in cancer. Expert Opin Ther Targets. 14:67–75.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho HJ, Baek KE, Park SM, et al: RhoGDI2
expression is associated with tumor growth and malignant
progression of gastric cancer. Clin Cancer Res. 15:2612–2619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Wang J, Zhang X, Zeng Y, Liang L and
Ding Y: Overexpression of RhoGDI2 correlates with tumor progression
and poor prognosis in colorectal carcinoma. Ann Surg Oncol.
19:145–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y and Zhang B: D4-GDI, a Rho GTPase
regulator, promotes breast cancer cell invasiveness. Cancer Res.
66:5592–5598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gildea JJ, Seraj MJ, Oxford G, et al:
RhoGDI2 is an invasion and metastasis suppressor gene in human
cancer. Cancer Res. 62:6418–6423. 2002.PubMed/NCBI
|
|
14
|
Ma L, Xu G, Sotnikova A, et al: Loss of
expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor, in
Hodgkin lymphoma. Br J Haematol. 139:217–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dransart E, Olofsson B and Cherfils J:
RhoGDIs revisited: novel roles in Rho regulation. Traffic.
6:957–966. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boulter E, Garcia-Mata R, Guilluy C, et
al: Regulation of Rho GTPase crosstalk, degradation and activity by
RhoGDI1. Nat Cell Biol. 12:477–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morin A, Cordelières FP, Cherfils J and
Olofsson B: RhoGDI3 and RhoG: vesicular trafficking and
interactions with the Sec3 exocyst subunit. Small GTPases.
1:142–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lelias JM, Adra CN, Wulf GM, et al: cDNA
cloning of a human mRNA preferentially expressed in hematopoietic
cells and with homology to a GDP-dissociation inhibitor for the rho
GTP-binding proteins. Proc Natl Acad Sci USA. 90:1479–1483. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boulter E and Garcia-Mata R: RhoGDI: A
rheostat for the Rho switch. Small GTPases. 1:65–68. 2010.
View Article : Google Scholar
|
|
20
|
Harding MA and Theodorescu D: RhoGDI
signaling provides targets for cancer therapy. Eur J Cancer.
46:1252–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu H, Li H, Xu C and He P: Expression
profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung
cancer metastasis. Oncol Rep. 24:465–471. 2010.PubMed/NCBI
|
|
22
|
Cho HJ, Baek KE, Kim IK, et al:
Proteomics-based strategy to delineate the molecular mechanisms of
RhoGDI2-induced metastasis and drug resistance in gastric cancer. J
Proteome Res. 11:2355–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Theodorescu D, Sapinoso LM, Conaway MR,
Oxford G, Hampton GM and Frierson HF Jr: Reduced expression of
metastasis suppressor RhoGDI2 is associated with decreased survival
for patients with bladder cancer. Clin Cancer Res. 10:3800–3806.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dovas A and Couchman JR: RhoGDI: multiple
functions in the regulation of Rho family GTPase activities.
Biochem J. 390:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DerMardirossian C and Bokoch GM: GDIs:
central regulatory molecules in Rho GTPase activation. Trends Cell
Biol. 15:356–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hart MJ, Maru Y, Leonard D, Witte ON,
Evans T and Cerione RA: A GDP dissociation inhibitor that serves as
a GTPase inhibitor for the Ras-like protein CDC42Hs. Science.
258:812–815. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chuang TH, Xu X, Knaus UG, Hart MJ and
Bokoch GM: GDP dissociation inhibitor prevents intrinsic and GTPase
activating protein-stimulated GTP hydrolysis by the Rac GTP-binding
protein. J Biol Chem. 268:775–778. 1993.PubMed/NCBI
|
|
28
|
Schunke D, Span P, Ronneburg H, et al:
Cyclooxygenase-2 is a target gene of rho GDP dissociation inhibitor
beta in breast cancer cells. Cancer Res. 67:10694–10702. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho HJ, Baek KE, Nam IK, et al: PLCγ is
required for RhoGDI2-mediated cisplatin resistance in gastric
cancer. Biochem Biophys Res Commun. 414:575–580. 2011.
|
|
30
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Binker MG, Binker-Cosen AA, Richards D,
Oliver B and Cosen-Binker LI: EGF promotes invasion by PANC-1 cells
through Rac1/ROS-dependent secretion and activation of MMP-2.
Biochem Biophys Res Commun. 379:445–450. 2009. View Article : Google Scholar : PubMed/NCBI
|