Introduction
Asthma is a chronic inflammatory disorder of the
airways and is characterized by airway inflammation, airway
hyperresponsiveness (AHR) to non-specific stimuli, as well as
airway remodeling. Th2 cells are classically thought to drive the
development of asthma through the release of Th2 cytokines, which
are indispensable for the synthesis of immunoglobulin E (IgE),
mucus production and AHR (1–3).
In addition to Th2 cell-mediated adaptive immune response, asthma
is strongly influenced by the innate immune responses from airway
cells, such as epithelial cells, mast cells, natural killer T cells
and dendritic cells (DCs) (4,5).
Adenosine 5′-triphosphate (ATP), as an endogenous
danger signal, has unique features. It is found at high
concentrations in the intracellular cytoplasm, at low levels in the
extracellular or pericellular space in healthy tissue, is rapidly
released upon cell damage and is inactivated by the powerful
ubiquitous ectonucleoside triphosphate diphosphohydrolase
(E-NTPDase) (6,7). Extracellular ATP (eATP) and other
nucleotides [e.g., adenosine diphosphate (ADP), uridine
5′-triphosphate (UTP) and uridine diphosphate (UDP)] exert their
effects by binding to purinergic P2-receptors (P2R), which can be
subdivided into 2 families: the G-protein-coupled P2YR and the
ligand-gated ion channel, P2XR. Both P2XR and P2YR are present in
the lungs. Recent studies have indicated that eATP contributes to
the pathogenesis of asthma through purinergic receptors, such as
P2Y2, P2Y4, P2Y6 and
P2X7 receptors (8–11).
However, purinergic signaling pathways are regulated
by CD39/E-NTPDase1. CD39 belongs to the E-NTPDase family and is
also known as E-NTPDase1. CD39 is primarily expressed on vascular
endothelial cells and immune cells and hydrolyzes extracellular ATP
and ADP to adenosine monophosphate (AMP). As ATP is typically a
pro-inflammatory mediator and AMP is rapidly converted to the
anti-inflammatory metabolite, adenosine, by ecto-5′-nucleotidase
(also known as CD73), CD39 tends to promote an anti-inflammatory
and immune suppressive milieu (7,12).
Compelling studies have demonstrated the important roles of CD39 in
the immunoregulatory function (13–17).
Given that extracellular nucleotides play important
roles in the pathogenesis of asthma, little is known about the role
of associated ectonucleotidases, such as CD39 in allergic asthma.
Thereby, we hypothesized that CD39 expression is abnormal in
allergic asthma and that the exogenous supplementation of apyrase
may attenuate allergic airway inflammation. To confirm these
assumptions, we first analyzed CD39 expression levels in the lungs
of asthmatic mice. Second, airway inflammation was evaluated in
ovalbumin (OVA)-sensitized mice treated with apyrase prior to each
OVA challenge. Third, the expression of GATA binding protein
(GATA3), which controls Th2 cell differentiation, was measured.
Finally, we attempted to explore whether apyrase affects the
chemotactic migration of DCs towards eATP.
Materials and methods
Animals
Female 6- to 8-week old C57BL/6 mice were obtained
from the Center for Animal Experiment of Wuhan University (Wuhan,
China) and maintained in the Animal Biosafety Level 3 Laboratory of
the university. All animals were bred at the animal facilities
under specific pathogen-free conditions. Animal experiments were
conducted under the approval of the Institutional Animal Care and
Use Committee of Wuhan University.
Murine model of allergic asthma
The mice were immunized intraperitoneally with 20 μg
OVA (Sigma-Aldrich, St. Louis, MO, USA) adsorbed to 2 mg aluminum
hydroxide (Thermo Fisher Scientific Inc., Rockford, IL, USA) in 200
μl PBS on days 0 and 14 and challenged with 100 μg OVA in 50 μl PBS
by intranasal (i.n.) administration for over 3 consecutive days
(days 25–27). Age- and gender-matched control mice were treated in
the same manner with PBS as a substitute for OVA. Apyrase (diluted
with PBS, 0.2 IU/g body weight; Sigma-Aldrich) or PBS was
intraperitoneally administered to the OVA-sensitized mice prior to
each OVA challenge.
Histological analysis and
immunofluorescence
At 48 h after the final challenge, the mice were
sacrificed and the left lungs were excised. The lungs were fixed in
4% paraformaldehyde (PFA) buffer, dehydrated, embedded and
sectioned. Paraffin-embedded 5-μm sections were stained with
hematoxylin and eosin (H&E) to evaluate airway inflammation and
periodic acid-Schiff (PAS) to evaluate goblet cell hyperplasia and
mucus secretion. A total of 4–5 sections were evaluated per lung
under a magnification of ×200. The inflammation degree of
peribronchial and perivascular regions was assessed on a subjective
scale of 0–3, as previously described (18). A value of 0 was regarded as
undetectable inflammation, a value of 1 as occasional cuffing with
inflammatory cells, a value of 2 as bronchi or vessels surrounded
by a thin layer of inflammatory cells (1–5 layer cells), and a
value of 3 was regarded as bronchi or vessels surrounded by a thick
layer of inflammatory cells (>5 layers of cells).
The location of CD39 in the lungs obtained from the
mice was detected by immunofluorescence. Briefly, 5-μm-thick
sections were incubated with a rabbit anti-mouse CD39 antibody
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
For detection, Rhodamine-labeled goat anti-rabbit secondary
antibody (Santa Cruz Biotechnology, Inc.) was used. The nuclei were
stained with 4,6-diamidino-2-phenylindole dihydrochloride hydrate
(DAPI; Sigma-Aldrich).
Collection of cells and supernatants in
the bronchoalveolar lavage fluid (BALF)
At 48 h after the final challenge, the mice were
sacrificed and bronchoalveolar lavage was performed 3 times with
0.5-ml aliquots of PBS containing 1 mM sodium EDTA. Cells in the
BALF were collected by centrifugation and were counted with a
hemocytometer. Smears of cells were prepared by cytospin and
stained with Wright-Giemsa in order to differentiate the
inflammatory cells (eosinophils, neutrophils and lymphocytes).
Approximately 400 cells were counted in each random location. The
supernatants in the BALF were collected and stored at −70°C until
use.
RNA extraction and quantitative PCR
(qPCR)
The right upper lung lobes were measured for gene
transcript levels. Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized using
ReverTra Ace qPCR RT Master Mix (Toyobo, Tokyo, Japan) according to
the manufacturer’s instructions. qPCR was conducted in triplicate
using SYBR Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan). The
primer sequences are presented in Table I. The thermal cycling conditions
were as follows: denaturation at 95°C for 1 min, followed by 40
cycles of denaturation at 95°C for 10 sec, annealing at 61°C for 10
sec and extension at 72°C for 10 sec. The data were normalized to
the internal reference gene, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). The relative expression of the target gene
was calculated using the comparative 2−ΔΔCt method.
 | Table IPrimer sequences and product
sizes. |
Table I
Primer sequences and product
sizes.
| Gene name | Primer sequences
(5′→3′) | Product size
(bp) |
|---|
| CD39/ENTPD-1 |
| Sense |
CATCCAAGCATCACCAGACT | 154 |
| Antisense | ATGAT
CTTGGCACCCTGGAA | |
| GATA3 |
| Sense |
AGGGACATCCTGCGCGAACTGT | 166 |
| Antisense |
CATCTTCCGGTTTCGGGTCTGG | |
| GAPDH |
| Sense |
TGTGTCCGTCGTGGATCTGA | 150 |
| Antisense |
TTGCTGTTGAAGTCGCAGGAG | |
Western blot analysis
Protein was extracted from the right median lobes
using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Total protein (60 μg)
was separated by electrophoresis on SDS-PAGE gels, transferred onto
PVDF membranes (Millipore Corp., Billerica, MA, USA), blocked with
TBST-containing 5% non-fat dried milk at room temperature for 1 h
and probed with rabbit anti-mouse CD39 or GAPDH (1:200, Santa Cruz
Biotechnology, Inc.). The membranes were incubated with primary
antibody overnight at 4°C and then with horseradish peroxidase
(HRP)-conjugated anti-rabbit secondary antibody (1:5,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h.
Chemoluminescence images were captured with ECL (Beyotime Institute
of Biotechnology) using the Fusion Fx7 image acquisition system
(Vilber Lourmat, Marne La Vallée, France). The quantification of
the bands was performed by densitometry using ImageJ software. The
CD39 protein expression level was calculated by densitometry
relative to GAPDH.
Detection of cytokines
The levels of cytokines were measured by
enzyme-linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions. The lower limit of detection was 4
pg/ml for interleukin (IL)-4 and IL-5. All ELISA kits for cytokines
were purchased from eBioscience, Inc. (San Diego, CA, USA).
Generation of bone marrow-derived DCs
(BMDCs)
Immature DCs were prepared from bone marrow
progenitors. Bone marrow mononuclear cells were prepared from femur
bone marrow suspensions of C57BL/6 male mice (5–6 weeks old) and
then cultured at a density of 2×106 cells/ml in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 10 ng/ml
recombinant murine granulocyte-macrophage colony-stimulating factor
(GM-CSF) and 10 ng/ml recombinant murine IL-4 (both from PeproTech,
Rocky Hill, NJ, USA). Non-adherent cells were gently washed out on
the 3rd day of culture. On days 5 and 7, the medium was refreshed.
The purity of the DCs was at least 90%. On day 8, the BMDCs were
incubated with 1 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich)
overnight and collected for subsequent experiments.
Migration assay of DCs in vitro
A migration assay was performed in 24-well Transwell
chambers with 5 μm pore size polycarbonate filters (Corning Life
Sciences, Tewksbury, MA, USA). Various concentrations of ATP
diluted in RPMI-1640 containing 0.5% bovine serum albumin (BSA)
were added to the lower chambers in a volume of 0.6 ml; 0.1 ml
RPMI-1640 containing DCs (2×105 cells/well) was added to
the upper chambers followed by culture at 37°C for 4 h. In some
experiments, apyrase (1 IU) was added to the medium containing ATP
in the lower chambers. The number of migrated DCs to the lower
chambers was calculated using a hemocytometer. The results are
presented as the chemotactic index and calculated as the number of
cells in the lower chamber containing the different stimuli divided
by the number of cells in the chamber containing medium alone.
Statistical analysis
The data are expressed as the means ± SD,
representing at least 2 independent experiments with consistent
results (n=4–6 mice per group). The data from 1 representative
experiment of 3 are shown. The differences were compared using the
unpaired Student’s t-test for 2 groups or one-way ANOVA analysis
for 3 groups. Statistical analysis was performed using SPSS 17.0
software (IBM SPSS, Chicago, IL, USA). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
OVA-induced airway inflammation leads to
a reduced expression of CD39 in lung tissue
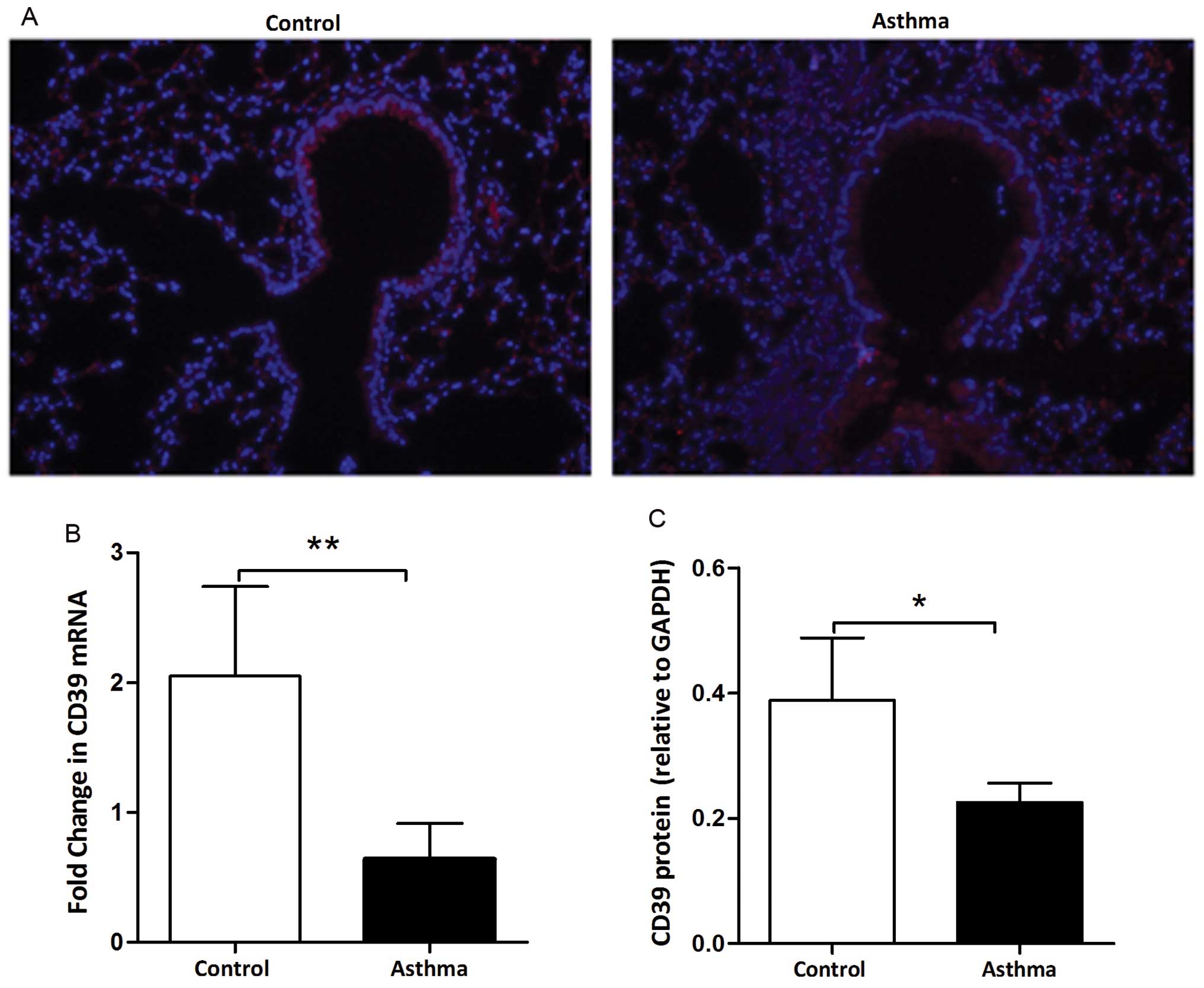
We investigated the distribution of CD39 in the
lungs obtained from mice by immunofluorescence assay. As shown in
Fig. 1A, CD39 was located at the
cytomembrane and cytoplasm of bronchial epithelial cells (red area
indicates the positive location). The fluorescence intensity was
reduced in the lungs from OVA-sensitized and challenged (asthma)
mice compared to that in the lungs from PBS-sensitized and
challenged (control) mice. We further assessed the expression
levels of CD39 in the lungs from the control and asthmatic mice. At
the mRNA level, CD39 expression was decreased in the asthmatic
mice, as shown by qPCR (Fig. 1B).
A marked decrease in CD39 protein expression was also observed in
the asthmatic mice, as shown by western blot analysis (Fig. 1C). These data indicate that a
reduction in CD39 expression is associated with OVA-induced
allergic airway inflammation.
Treatment with apyrase attenuates
OVA-induced tissue eosinophilia, mucus production and airway
inflammation
Having shown that a reduced CD39 expression is
associated with OVA-induced allergic airway inflammation, we
exogenously supplemented apyrase, an eATP scavenger, to investigate
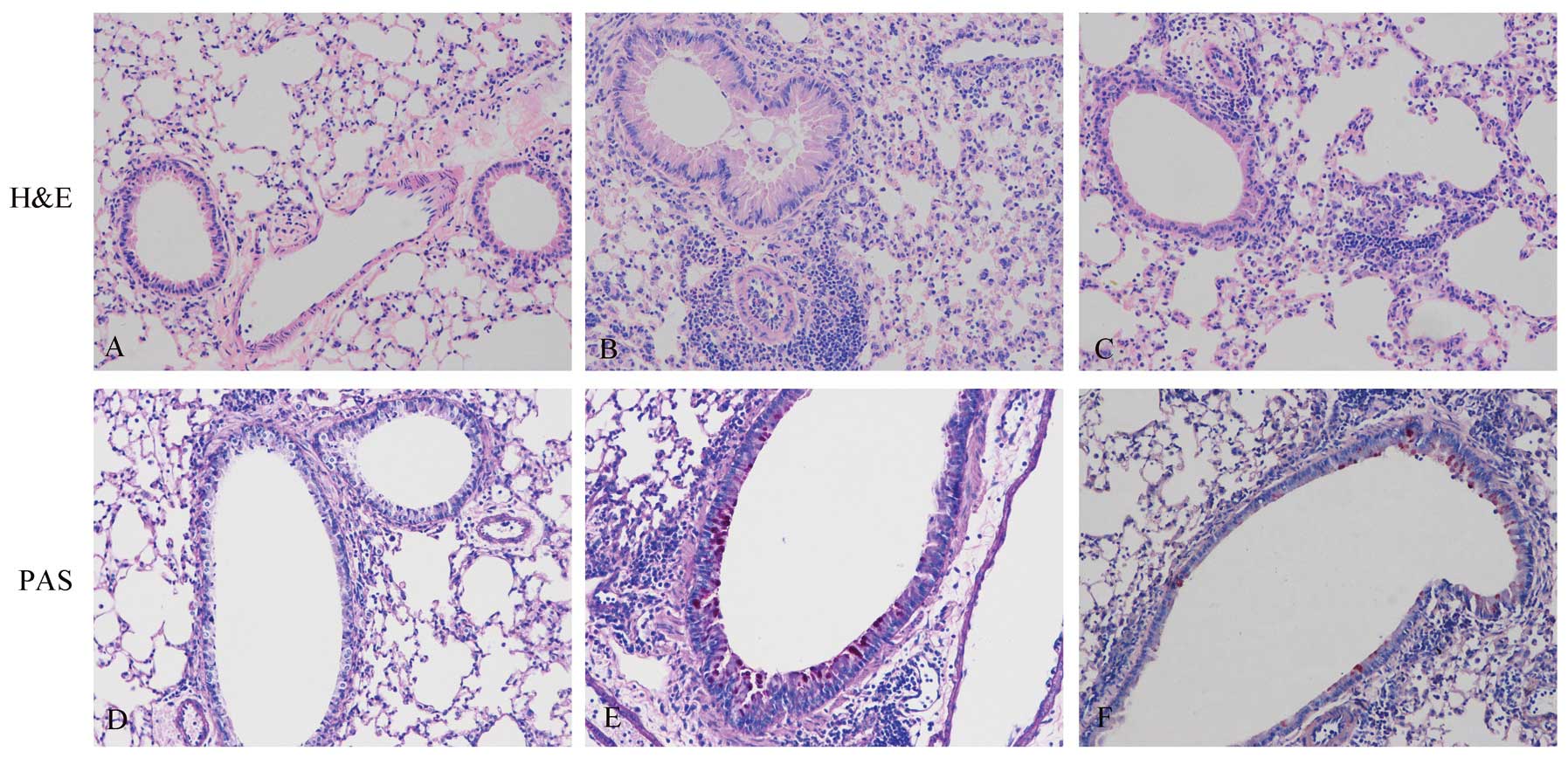
its effects on airway inflammation. Lung tissues were collected 48
h after the final OVA challenge. In histological analysis, the
OVA-exposed mice showed cardinal pathological features of
asthma-like inflammation. In contrast to the PBS-sensitized and
challenged controls (Fig. 2A),
OVA-sensitized and challenged mice displayed numerous inflammatory
cells in the peribronchiolar and perivascular zones (Fig. 2B). Compared to treatment with PBS,
treatment with apyrase markedly decreased the number of
eosinophil-rich inflammatory cells infiltrating the peribronchiolar
and perivascular regions (Fig.
2C). As shown in Fig. 2D–F,
PAS staining was used to detect goblet cells. In contrast to the
controls (Fig. 2D), the
OVA-exposed mice (Fig. 2E) showed
goblet cell hyperplasia in the airways, which was significantly
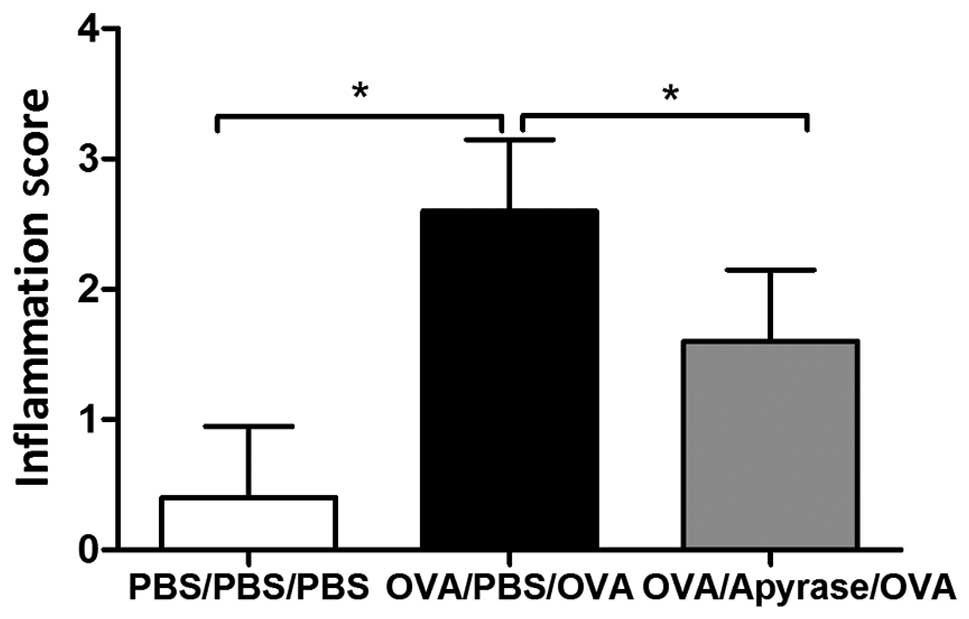
reduced by treatment with apyrase (Fig. 2F). OVA exposure markedly increased
the inflammation scores of the peribronchial and perivascular
regions (Fig. 3), compared to PBS
sensitization and challenge. The increased lung inflammation
following exposure to OVA was reduced by approximately 40%
following treatment with apyrase. Taken together, these results
reveal that apyrase significantly reduces OVA-induced inflammatory
cell infiltration into the lungs, goblet cell hyperplasia in the
airways and airway inflammation.
Treatment with apyrase reduces the number
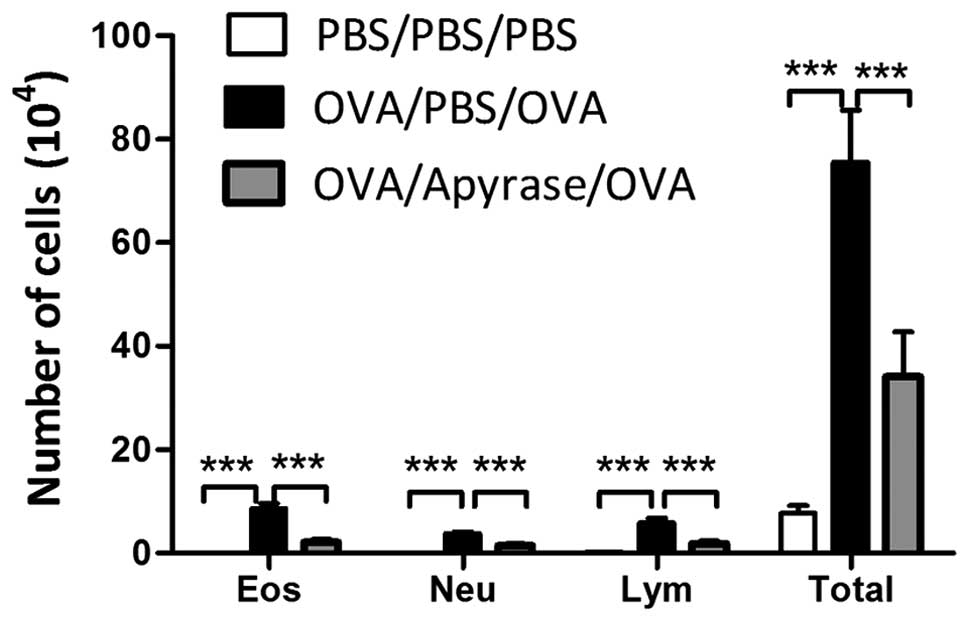
of inflammatory cells in BALF following exposure to OVA
BALF was collected 48 h after the final OVA
challenge and the number of the total cells and the different
inflammatory cells were counted. Exposure to OVA markedly increased
the number of eosinophils, lymphocytes and neutrophils, compared to
PBS sensitization and challenge (Fig.
4). The administration of apyrase markedly reduced the number
of eosinophils, lymphocytes and neutrophils in BALF, compared to
the PBS-treated asthmatic mice. In addition, a reduction in the
number of total cells was observed in the apyrase-treated mice.
Administration of apyrase markedly
decreases the levels of Th2 cytokines in BALF and the expression of
GATA3 in lung tissue
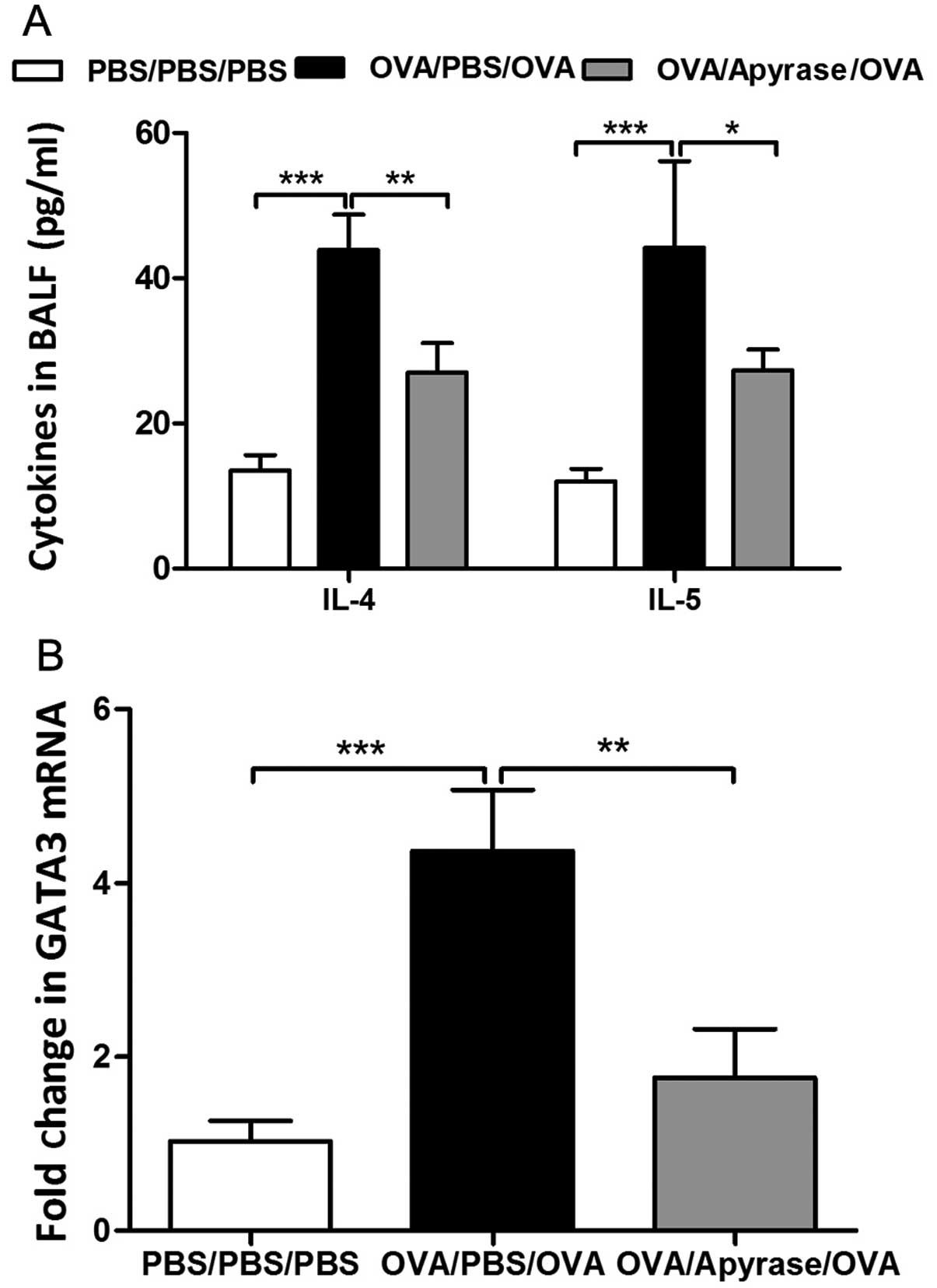
Given the essential role of Th2 cytokines in
triggering the allergic inflammatory response, we detected the
levels of IL-4 and IL-5 in BALF. OVA sensitization and challenge
markedly increased the concentration of cytokines in BALF, as shown
by ELISA (Fig. 5A) compared to
PBS sensitization and challenge. The increased levels of cytokines
in BALF were significantly reduced by apyrase treatment.
GATA3, the key transcription factor, controls the
differentiation of Th2 cells. We further determined whether apyrase
decreases GATA3 mRNA expression in the lungs obtained from
asthmatic mice. In the OVA-exposed mice, the mRNA level of GATA3
was increased by 4-fold in the lung tissues compared with that in
the PBS-sensitized and challenged control mice (Fig. 5B). The administration of apyrase
effectively reduced the expression of GATA3 in the lung tissues
compared with the PBS-treated asthmatic mice (Fig. 5B).
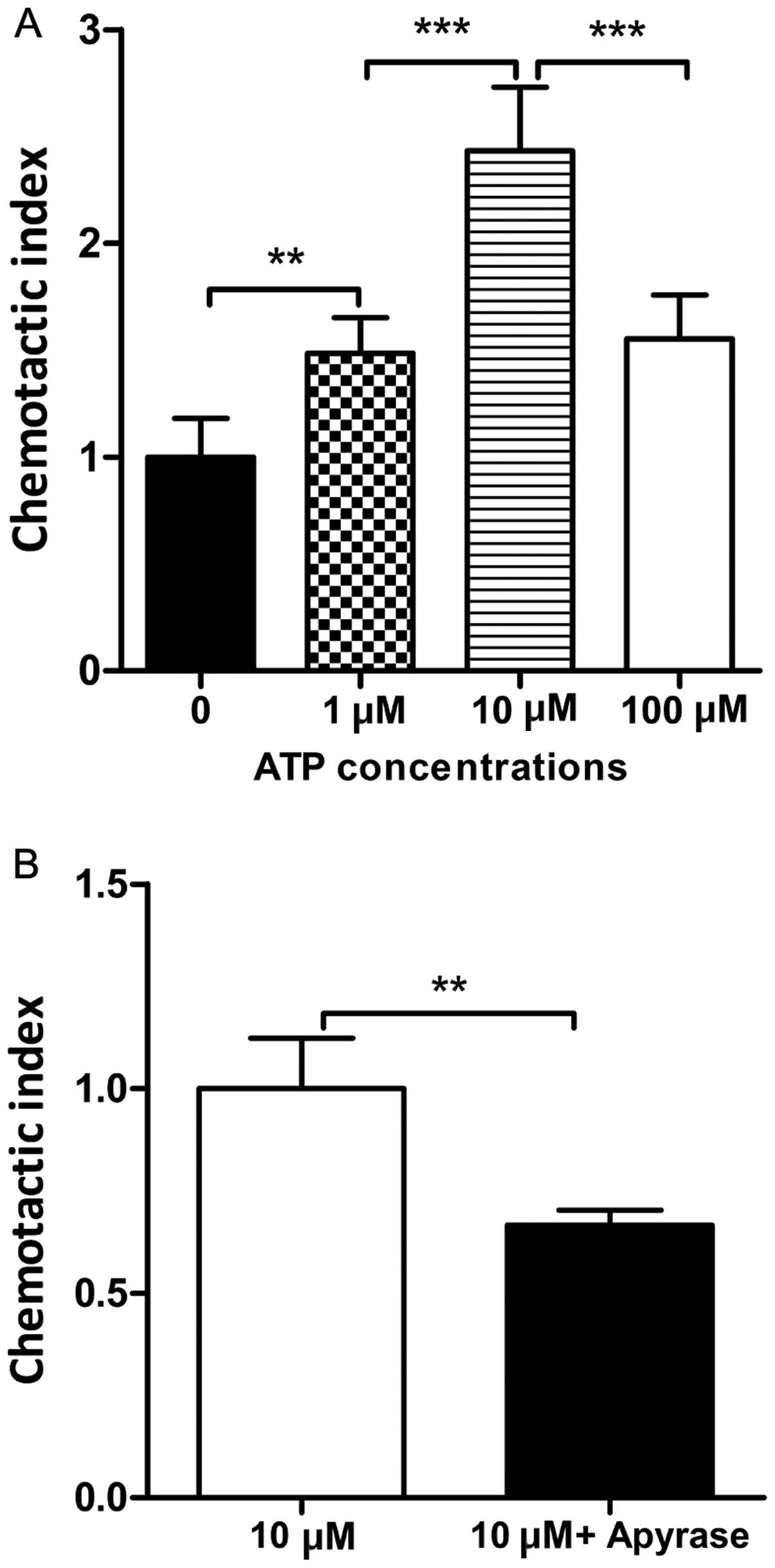
Apyrase decreases the chemotactic
migration of DCs towards ATP
Our results revealed that apyrase attenuated airway
inflammation. ATP is a chemoattractant for DCs in vitro
(8,19). Therefore, we explored whether
apyrase decreases the chemotactic migration of DCs towards ATP.
Various concentrations of ATP were added to the medium in the lower
chamber. As shown in Fig. 6A, the
DCs showed the strongest chemotactic activity with 10 μM ATP.
Apyrase was then added to the medium containing 10 μM ATP. We found
that upon the administration of apyrase, the chemotactic migration
of DCs towards ATP was markedly decreased compared to the cells
treated with ATP alone (Fig.
6B).
Discussion
CD39 is an integral membrane protein that hydrolyzes
ATP and is constitutively expressed in the spleen, thymus, lung and
placenta (20,21). CD39 is structurally characterized
by 2 transmembrane regions, a small cytoplasmic region and a large
extracellular region that is essential for the catabolic activity
of the enzyme (22). Increasing
evidence demonstrates the regulation of immunity by CD39 in
infectious diseases (23–26), autoimmune diseases (27–29) and ischemia-reperfusion injury
(30,31). However, little is known of the
role of CD39 in allergic asthma.
In the present study, we found that CD39 was located
at the surface of bronchial epithelial cells and the CD39
expression was reduced in the lungs obtained from allergic
asthmatic mice, which was associated with the pathogenesis of
allergic asthma. In addition, CD39 expression is decreased in
patients with Crohn’s disease (32). Furthermore, the deletion of CD39
has been demonstrated to increase the disease severity and
pre-treatment of apyrase has been shown to improve the outcome in a
murine model of DSS-induced colitis (32). These findings demonstrate a
protective role of CD39 in inflammatory diseases. In our study, the
administration of apyrase, a soluble factor with enzymatic activity
essentially identical to CD39, to OVA-sensitized mice attenuated
airway inflammation, which was consistent with the results of a
previous study (8). This mainly
included a significant decrease in airway eosinophilic
inflammation, goblet cell hyperplasia and the levels of Th2
cytokines in BALF.
Th2 cells play a crucial role in allergic asthma by
triggering the recruitment of eosinophils and mast cells and
inducing goblet cell hyperplasia (33). However, the polarization of Th2
cells is regulated by the transcription factor, GATA3 (34). In our study, the expression of
GATA3 was markedly increased in the lungs obtained from
OVA-sensitized and challenged mice compared to those from
PBS-sensitized and challenged mice. In a previous study of ours,
GATA3 mRNA expression was decreased in allergic asthma patients
(33). Treatment with apyrase
reduced its expression in the lungs, which resulted in the reduced
levels of Th2-cytokines.
DCs are pivotal for the initiation and maintenance
of adaptive Th2 cell responses to inhaled allergens in asthma
(4). Our findings demonstrated
that ATP induced the migration of DCs, which was in accordance with
a previous study that demonstrated that ATP promoted the migration
of DCs by activating P2Y2 receptor (9). However, in our study, apyrase
reduced the chemotactic migration of DCs, which was associated with
the hydrolysis of ATP by apyrase. Furthermore, in another study of
ours, we showed that ATP induced the migration of mast cells and
the exogenous administration of soluble CD39 inhibited the
migration of ATP-induced mast cells in an in vitro assay
(unpublished data).
In conclusion, the present study provides evidence
of the role of associated ectonucleotidases in a murine model of
OVA/aluminum hydroxide-induced allergic asthma. As shown by our
resutls, the reduced CD39 expression was associated with the
development of allergic airway inflammation. Apyrase decreased the
expression of GATA3 and reduced the chemotactic migration of DCs
towards ATP, which resulted in the alleviation of airway
inflammation. Thereby, targeting eATP or ectonucleotidases may
provide a novel therapeutic approach for allergic asthma.
Acknowledgements
We would like to thank the National Natural Science
Foundation of China. We would also like to thank the faculties at
the Center for Medical Research, Hubei Key Laboratory of Allergy
and Immune-Related Disease of Wuhan University. This study was
supported by a grant from the National Natural Science Foundation
of China (81170029).
References
|
1
|
Lloyd CM and Hessel EM: Functions of T
cells in asthma: more than just T(H)2 cells. Nat Rev Immun.
10:838–848. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adcock IM, Caramori G and Chung KF: New
targets for drug development in asthma. Lancet. 372:1073–1087.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holgate ST and Polosa R: Treatment
strategies for allergy and asthma. Nat Rev Immunol. 8:218–230.
2008. View
Article : Google Scholar
|
|
4
|
Lambrecht BN and Hammad H: Biology of lung
dendritic cells at the origin of asthma. Immunity. 31:412–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HY, DeKruyff RH and Umetsu DT: The
many paths to asthma: phenotype shaped by innate and adaptive
immunity. Nat Immunol. 11:577–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trautmann A: Extracellular ATP in the
immune system: more than just a ‘danger signal’. Sci signal.
2:pe62009.
|
|
7
|
Robson SC, Wu Y, Sun X, Knosalla C, Dwyer
K and Enjyoji K: Ectonucleotidases of CD39 family modulate vascular
inflammation and thrombosis in transplantation. Semin Thromb
Hemost. 31:217–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Idzko M, Hammad H, van Nimwegen M, et al:
Extracellular ATP triggers and maintains asthmatic airway
inflammation by activating dendritic cells. Nat Med. 13:913–919.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller T, Robaye B, Vieira RP, et al: The
purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic
cells and eosinophils in allergic lung inflammation. Allergy.
65:1545–1553. 2010.PubMed/NCBI
|
|
10
|
Müller T, Vieira RP, Grimm M, et al: A
potential role for P2X7R in allergic airway inflammation in mice
and humans. Am J Respir Cell Mol Biol. 44:456–464. 2011.PubMed/NCBI
|
|
11
|
Manthei DM, Jackson DJ, Evans MD, et al:
Protection from asthma in a high-risk birth cohort by attenuated
P2X(7) function. J Allergy Clin Immunol. 130:496–502. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crikis S, Lu B, Murray-Segal LM, et al:
Transgenic overexpression of CD39 protects against renal
ischemia-reperfusion and transplant vascular injury. Am J
Transplant. 10:2586–2595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Künzli BM, Berberat PO, Dwyer K, et al:
Variable impact of CD39 in experimental murine colitis. Dig Dis
Sci. 56:1393–1403. 2011.PubMed/NCBI
|
|
14
|
Künzli BM, Nuhn P, Enjyoji K, et al:
Disordered pancreatic inflammatory responses and inhibition of
fibrosis in CD39-null mice. Gastroenterology. 134:292–305.
2008.PubMed/NCBI
|
|
15
|
Dwyer KM, Deaglio S, Gao W, Friedman D,
Strom TB and Robson SC: CD39 and control of cellular immune
responses. Purinergic Signal. 3:171–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deaglio S and Robson SC: Ectonucleotidases
as regulators of purinergic signaling in thrombosis, inflammation,
and immunity. Adv Pharmacol. 61:301–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antonioli L, Pacher P, Vizi ES and Haskó
G: CD39 and CD73 in immunity and inflammation. Trends Mol Med.
19:355–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwak YG, Song CH, Yi HK, et al:
Involvement of PTEN in airway hyperresponsiveness and inflammation
in bronchial asthma. J Clin Invest. 111:1083–1092. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Idzko M, Dichmann S, Ferrari D, et al:
Nucleotides induce chemotaxis and actin polymerization in immature
but not mature human dendritic cells via activation of pertussis
toxin-sensitive P2y receptors. Blood. 100:925–932. 2002. View Article : Google Scholar
|
|
20
|
Enjyoji K, Sévigny J, Lin Y, et al:
Targeted disruption of cd39/ATP diphosphohydrolase results in
disordered hemostasis and thromboregulation. Nat Med. 5:1010–1017.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapojos JJ, van den Berg A, Borghuis T, et
al: Enhanced ecto-apyrase activity of stimulated endothelial or
mesangial cells is downregulated by glucocorticoids in vitro. Eur J
Pharmacol. 501:191–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heine P, Braun N, Sévigny J, Robson SC,
Servos J and Zimmermann H: The C-terminal cysteine-rich region
dictates specific catalytic properties in chimeras of the
ectonucleotidases NTPDase1 and NTPDase2. Eur J Biochem.
268:364–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paletta-Silva R and Meyer-Fernandes JR:
Adenosine and immune imbalance in visceral leishmaniasis: the
possible role of ectonucleotidases. J Trop Med. 2012:6508742012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Souza Vdo C, Schlemmer KB, Noal CB, et al:
E-NTPDase and E-ADA activities are altered in lymphocytes of
patients with indeterminate form of Chagas’ disease. Parasitol Int.
61:690–696. 2012.PubMed/NCBI
|
|
25
|
Fan J, Zhang Y, Chuang-Smith ON, et al:
Ecto-5′-nucleotidase: a candidate virulence factor in
Streptococcus sanguinis experimental endocarditis. PloS One.
7:e380592012.
|
|
26
|
Théâtre E, Frederix K, Guilmain W, et al:
Overexpression of CD39 in mouse airways promotes bacteria-induced
inflammation. J Immunol. 189:1966–1974. 2012.PubMed/NCBI
|
|
27
|
Fletcher JM, Lonergan R, Costelloe L, et
al: CD39+Foxp3+ regulatory T cells suppress
pathogenic Th17 cells and are impaired in multiple sclerosis. J
Immunol. 183:7602–7610. 2009.PubMed/NCBI
|
|
28
|
Moncrieffe H, Nistala K, Kamhieh Y, et al:
High expression of the ectonucleotidase CD39 on T cells from the
inflamed site identifies two distinct populations, one regulatory
and one memory T cell population. J Immunol. 185:134–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peelen E, Damoiseaux J, Smolders J, et al:
Th17 expansion in MS patients is counterbalanced by an expanded
CD39+ regulatory T cell population during remission but not during
relapse. J Neuroimmunol. 240–241:97–103. 2011.PubMed/NCBI
|
|
30
|
Hart ML, Gorzolla IC, Schittenhelm J,
Robson SC and Eltzschig HK: SP1-dependent induction of CD39
facilitates hepatic ischemic preconditioning. J Immunol.
184:4017–4024. 2010. View Article : Google Scholar
|
|
31
|
Bönner F, Borg N, Burghoff S and Schrader
J: Resident cardiac immune cells and expression of the
ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PloS
One. 7:e347302012.PubMed/NCBI
|
|
32
|
Friedman DJ, Künzli BM, A-Rahim YI, et al:
CD39 deletion exacerbates experimental murine colitis and human
polymorphisms increase susceptibility to inflammatory bowel
disease. Proc Nat Acad Sci USA. 106:16788–16793. 2009. View Article : Google Scholar
|
|
33
|
Chen X, Gao YD and Yang J: Elevated
interferon regulatory factor 4 levels in patients with allergic
asthma. J Asthma. 49:441–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stellato C, Gubin MM, Magee JD, et al:
Coordinate regulation of GATA-3 and Th2 cytokine gene expression by
the RNA-binding protein HuR. J Immunol. 187:441–449. 2001.
View Article : Google Scholar : PubMed/NCBI
|