Introduction
The blood-retinal barrier (BRB) regulates water,
solutes and ion fluxes in the retina. The inner BRB is mainly the
result of the isolation of the retina by the tight junctions of
retinal endothelial cells (1,2).
Müller cell extensions surrounding retinal blood vessels contribute
to the tightness of the inner BRB. The outer BRB relies on retinal
pigment epithelial (RPE) cell tight junctions, which impede
paracellular flow, and ionic pumps and channels that create a
transepithelial osmotic gradient. Water follows down this gradient
and flows from the subretinal space to the choroid through RPE
cells (3). The exact mechanisms
through which water penetrates RPE cells remain elusive. Aquaporins
(AQPs), the water-specific membrane channels, may be responsible
for this function (4,5). Hence, it has been reported that
human RPE cells express, among several AQPs (6,7),
aquaporin-4 (AQP4) (6). AQP4 is
abundantly expressed in the brain and is involved in the
pathophysiological mechanisms leading to brain oedema (8,9).
On the other hand, in the inner BRB, AQP4 expression in Müller
cells is associated with water transport (1,10,11).
Fluid accumulation into the retina leads to the
formation of macular oedema, a hallmark of severe retinal diseases
and one of the leading causes of central vision loss in developed
countries. It has been demonstrated that macular oedema results in
the disruption of the BRB, leading to the accumulation of proteins
and solutes close to the RPE layer, thus increasing
hyperosmolarity, leading to the accumulation of water into the
retina (12,13). It is therefore postulated that RPE
cells undergo hyperosmotic stress during macular oedema.
Hyperosmolarity modulates AQP expression in various
cell types. Hence, it increases the expression of several AQPs,
such as AQP1 (14–16), AQP2 (16–18), AQP3 (19,20), AQP4 (21), AQP5 (22–27) and AQP9 (21), and induces AQP4 downregulation
during the formation of oedema in the brain cortex (28). Several mechanisms account for
these changes in AQP expression, including the simultaneous
activation of the three main mitogen-activated protein kinases
(MAPKs), ERK, p38 kinase and JNK, as well as the activation of
transcription factors (27,29). Several AQP proteins are also
degraded through lysosomal or proteasome pathways (30,31).
As RPE cells are likely to undergo hyperosmotic
stress during macular oedema and AQP4 is expressed in human RPE
cells, in this study, we investigated the effects of
hyperosmolarity on AQP4 protein expression in the human retinal
pigment epithelial cell line, ARPE-19.
Materials and methods
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM; 4.5 g/l
glucose), streptomycin/penicillin and fetal bovine serum were
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
MG132, chloroquine, calpain and calpeptin were purchased from Sigma
(St. Louis, MO, USA). Anti-phospho p38 and anti-ubiquitin
antibodies were from Cell Signaling Technology, Inc. (Danvers, MA,
USA) and Pierce Biotechnology, Inc. (Rockford, IL, USA),
respectively. Antibodies against glucose transporter 1 (Glut1) were
from Millipore (Billerica, MA, USA). SB203580, SB202474 and
anisomycin were from EDM-Millipore.
Cell culture
Human ARPE-19 cells were grown in DMEM/HAM-F12
medium containing 10% foetal calf serum, 100 UI/ml
streptomycin-penicillin and 4 mM glutamine, and were passaged twice
a week. The cells were treated for different periods of time (10,
30 and 60 min) with a control medium or a hyperosmotic medium
containing an additional 200 mM NaCl or 400 mM sucrose (osmotic
stress). In some experiments, the ARPE-19 cells were pre-treated
for 1 h in the absence or presence of inhibitors of lysosomes, the
proteasome, p38 kinase, MAPK/ERK kinase or activator of p38
kinase.
RT-PCR
Total RNA from the ARPE-19 cells was extracted using
the Aurum™ total RNA kit (Bio-Rad, Hercules, CA, USA) and verified
for its quality using the Experion automated electrophoresis system
(Bio-Rad). The RNA was then reverse transcribed into cDNA using a
RevertAid™ first-strand cDNA synthesis kit (Fermentas, St.
Leon-Rot, Germany). The primers used for the amplification of human
AQP4 cDNA were 5′-GGAATTTCTGGCCATGCTTA-3′ and 5′-AGACTTGGCG
ATGCTGATCT-3′ and for β-actin cDNA were 5′-TGACGGGG
TCACCCACACTGTGCCCGTC-3′ and 5′-CTAGAAGCA TTAGCGGTGGACGATGGAGG-3′
(amplicons, 226 and 661 bp). The PCR reactions were performed in 20
μl reaction volume containing 1 μl of cDNA, 0.5 U GoTaq DNA
polymerase (Promega, Madison, WI, USA), 0.2 mM dNTP, 0.5 μM of each
primer and 4 μl GoTaq Green buffer using the iCycler Thermocycler
(Bio-Rad). PCR conditions were 94°C for 3 min followed by 35 cycles
of 30 sec at 95°C, 30 sec at 57°C and 1 min at 72°C. PCR products
were subjected to electrophoresis in a 1.5% agarose gel. Direct
sequencing of the AQP4 PCR was performed.
Isolation of polyubiquitinated
proteins
Polyubiquitinated proteins were isolated from total
protein from ARPE-19 cells (see below) using an ubiquitin
enrichment kit (Pierce) following the manufacturer’s instructions.
Briefly, 150 μg of total protein from the ARPE-19 cells were
subjected to a high-binding affinity resin allowing the binding of
polyubiquitinated proteins. The polyubiquitinated proteins were
then eluted and subjected to western blot analysis using either
anti-ubiquitin or anti-AQP4 antibodies.
Western blot analysis
Crude plasma membrane protein or total protein from
the ARPE-19 cells was analyzed by SDS-PAGE in the presence of 5%
β-mercaptoethanol using 12% polyacrylamide gels. For crude plasma
membrane protein preparation, 2 ml of 1 mM NaHCO3
containing a protease inhibitor cocktail (Complete EDTA free; Roche
Diagnostics GmbH, Mannheim, Germany) was added to a Ø10 cm plate of
ARPE-19 cells prior to harvest and immediate freezing in liquid
nitrogen. The disrupted cells were subjected to a 10-min
centrifugation at 1,250 × g at 4°C; the supernatant was subjected
to a further 20-min centrifugation at 25,000 × g at 4°C, and the
pellet was resuspended in 50 mM Tris-HCl, pH 7.4, containing
protease inhibitors (Complete EDTA free; Roche Diagnostics GmbH).
For total protein preparation, the cells were washed with calcium-
and magnesium-free PBS and lysed in 50 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 0.5% NP-40, 50 mM NaF, 1 mM sodium orthovanadate, and
dithiothreitol and protease inhibitors (complete EDTA free, Roche
Diagnostics GmbH). The proteins were transferred onto PVDF
membranes and immunolabeled using specific antibodies against AQP4
(see above, dilution, 1:1,000), Glut1 (1:100,000; used as a loading
control), anti-phospho p38 (dilution, 1:1,000), anti-ubiquitin
(dilution, 1:7,500). Bound antibodies were detected using the ECL
chemiluminescence method (GE Healthcare, Little Chalfont, UK). The
AQP4 antibody, obtained from rabbit immunization using a synthetic
peptide corresponding to amino acids 301–318 of mouse AQP4 (89%
identity to the human AQP4 sequence), was affinity purified, and
its specificity was verified.
Immunofluorescence
The ARPE-19 cells were grown on gelatinized glass
covers, fixed in 4% paraformaldehyde (PAF), permeabilized with 100%
methanol for 10 min at 4°C, then successively incubated with 10%
horse serum for 60 min, primary antibodies [anti-AQP4 and
anti-pan-cytokeratin (Sigma-Aldrich)] overnight at 4°C,
biotinylated anti-rabbit IgG (1/200; GE Healthcare) and
streptavidin-cyanine 2 (1/300; Jackson ImmunoResearch, West Grove,
PA, USA) and/or anti-mouse IgG coupled to cyanine 3 for 60 min. The
cells were mounted using ProLong Gold Antifade reagent with DAPI
(Invitrogen Life Technologies). For the negative controls, the
primary antibody was omitted. Images were acquired using an AxioCam
MRB fluorescent microscope using a ×40 objective (Carl Zeiss, Jena,
Germany).
Results
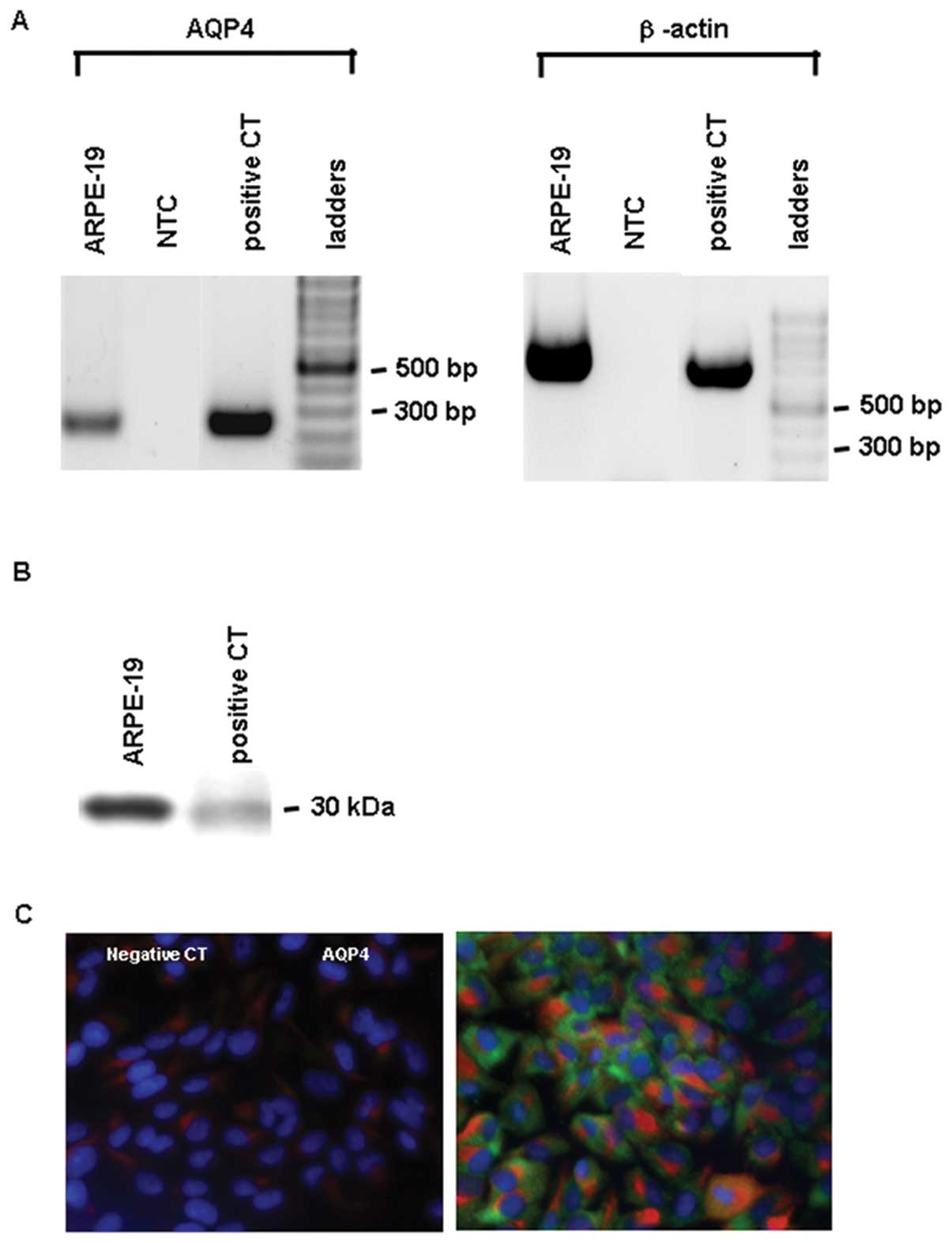
Expression of AQP4 in ARPE-19 cells
AQP4 was expressed in the ARPE-19 cells. RT-PCR of
AQP4 and β-actin using human kidney cDNA as a positive control and
ARPE-19 cell cDNA revealed a unique amplicon of expected size,
i.e., 225 and 660 bp, respectively, while no amplicons were
detected in the negative non-target control (Fig. 1A). Western blot analysis of the
crude plasma membrane proteins of human kidney, used as a positive
control, and of the ARPE-19 cells revealed the presence of AQP4
protein (Fig. 1B). AQP4 was
detected by immunofluorescence in pancytokeratin-positive ARPE-19
cells (Fig. 1C).
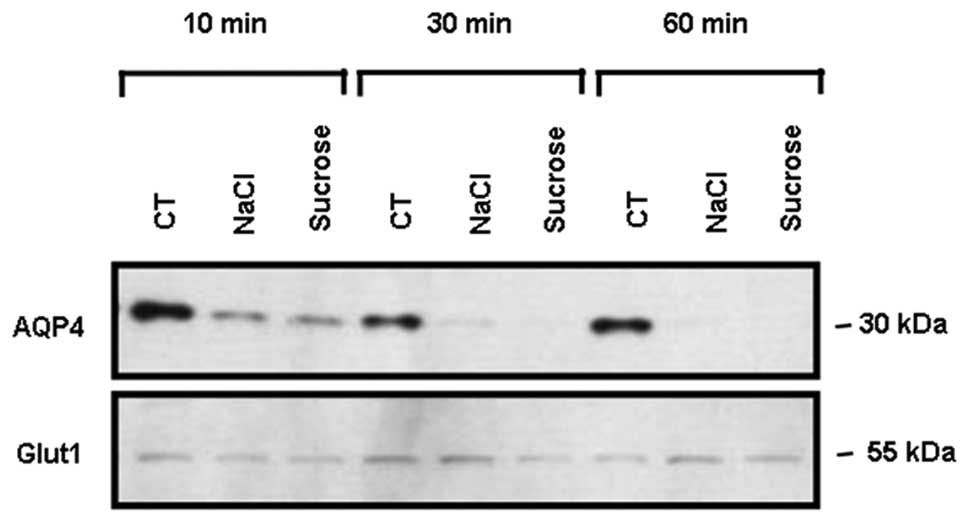
Decreased AQP4 expression observed in
response to osmotic stress
As early as 10 min following hyperosmotic
stimulation with 200 mM NaCl or 400 mM sucrose, membrane AQP4
protein expression was markedly decreased and seemed to reach its
minimum 30–60 min post-stimulation (Fig. 2). The expression of Glut1, used a
loading control, was not modified by the hyperosmotic stimulations
(Fig. 2).
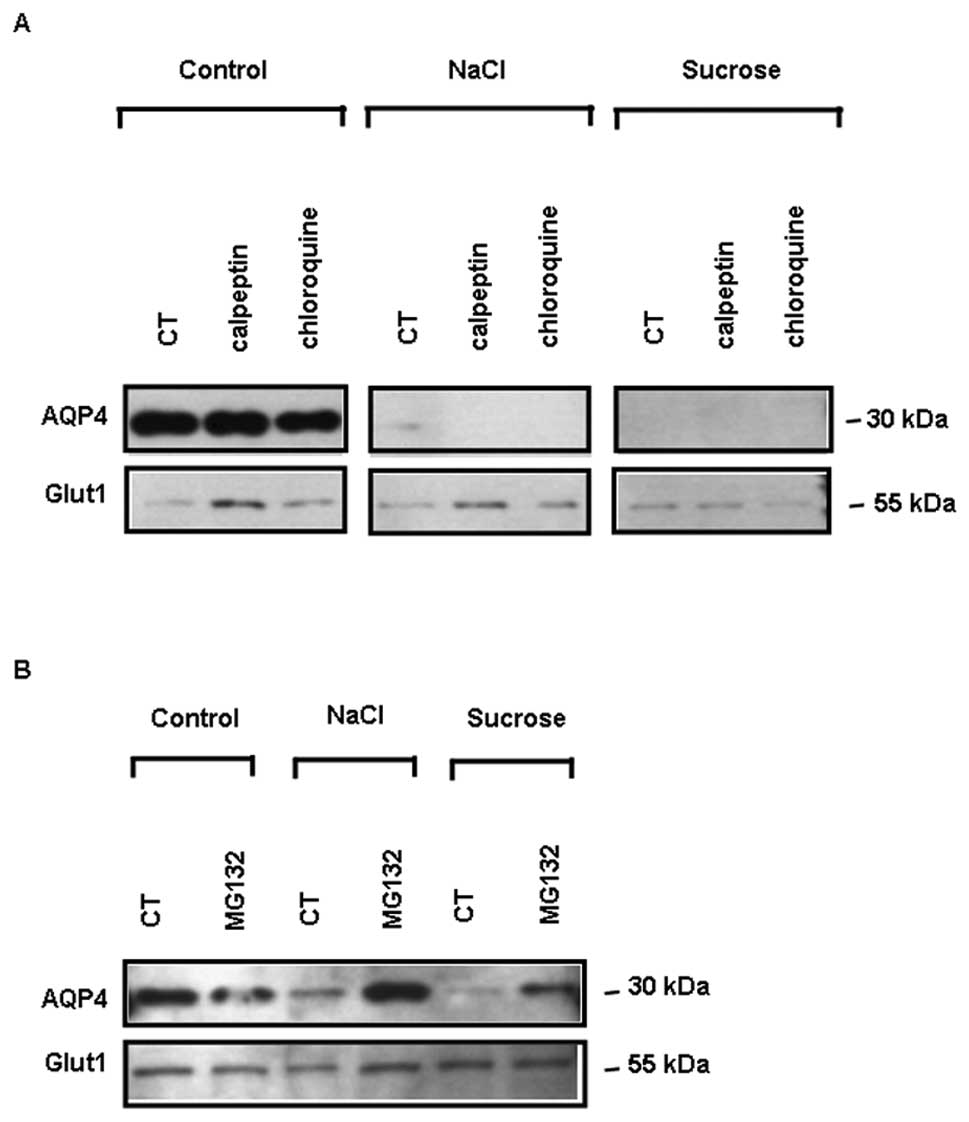
Degradative pathways are involved in the
decrease in AQP4 expression following exposure to osmotic
stress
Pre-incubation of the cells with the lysosomal
inhibitor, chloroquine (100 μM), or the calpain (a non-lysosomal
cysteine protease) inhibitor, calpeptin (50 μM), had no effect on
the decrease in total AQP4 protein expression induced by 4 h of
exposure to osmotic stress (Fig.
3A). By contrast, pre-treatment of the cells with the
proteasome inhibitor, MG132 (10 μM), prior to 4 h of exposure to
osmotic stress, markedly restored AQP4 protein expression under
both NaCl and sucrose stimulation (Fig. 3B). The expression of Glut1, used
as a loading control, was not modified under these conditions
(Fig. 3A and B). Therefore, the
proteasome is likely to be involved in AQP4 degradation.
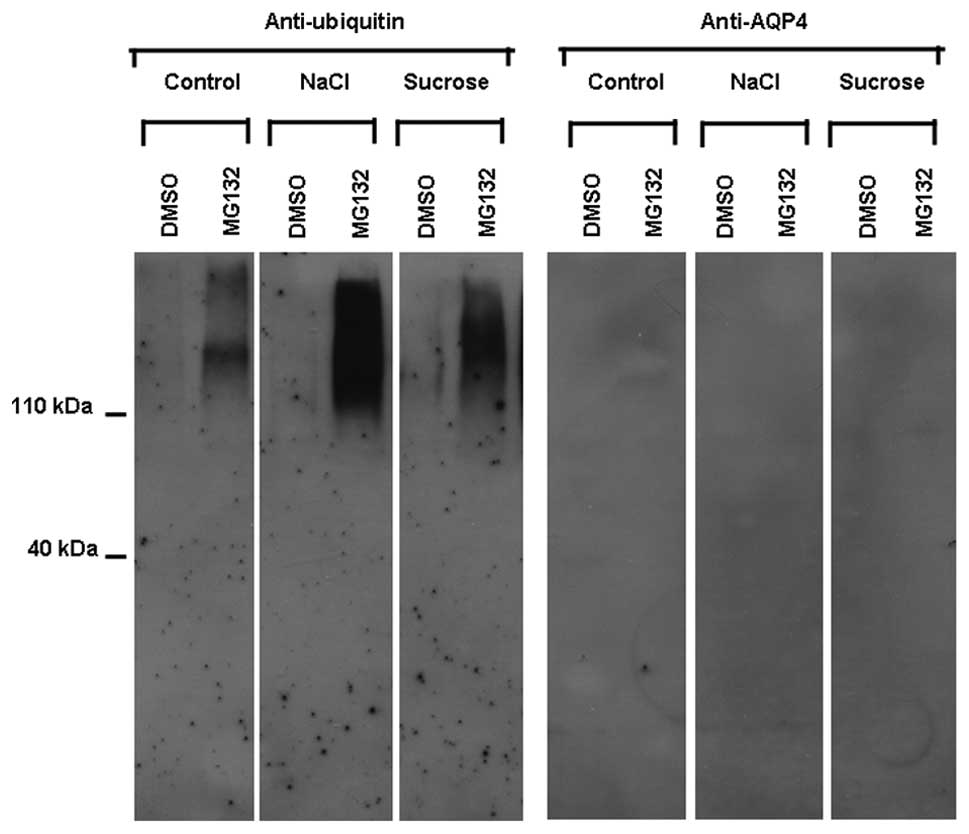
Ubiquitinylation is not likely to be
involved in AQP4 degradation by the proteasome
Following the exposure of the ARPE-19 cells to
osmotic stress, polyubiquitinated proteins, purified from total
ARPE-19 cell proteins, were subjected to western blot analysis
using anti-ubiquitin and anti-AQP4 antibodies. The use of
anti-ubiquitin antibodies revealed a diffuse pattern of bands that
increased in the presence of MG132 as compared to its absence in
both the unstimulated (control) and stimulated (NaCl and sucrose)
ARPE-19 cells. The accumulation of ubiquitinylated proteins
observed in the presence of MG132 indicated that it effectively
inhibited the proteasome. The use of anti-AQP4 antibodies did not
reveal any diffuse pattern of bands in the absence or presence of
MG132 in both the unstimulated (control) and stimulated (NaCl and
sucrose) ARPE-19 cells (Fig.
4).
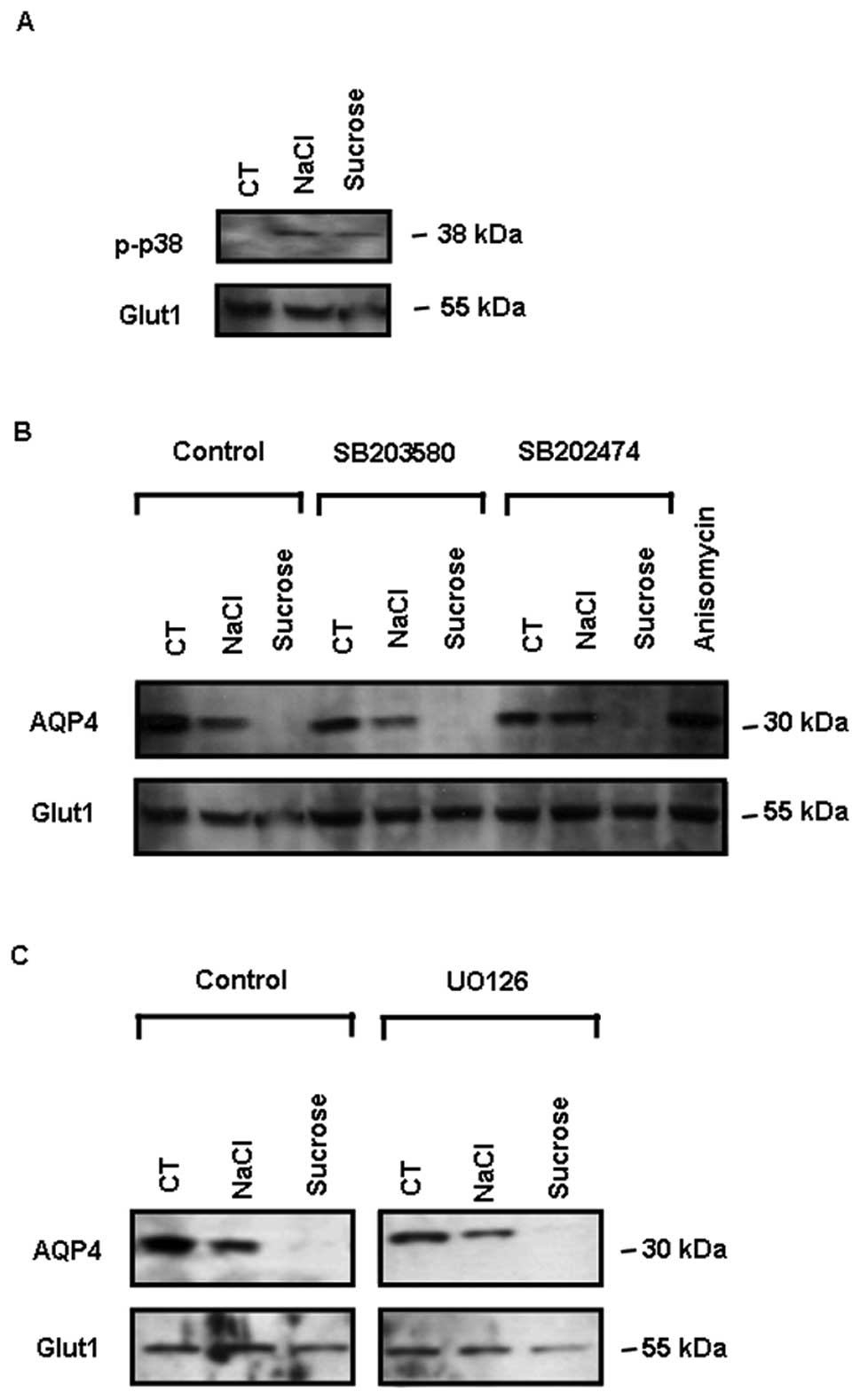
p38 activation following exposure to
osmotic stress
In response to 4 h of exposure to osmotic stress
(200 mM NaCl and 400 mM sucrose), the MAPK pathway was activated in
the ARPE-19 cells, as revealed by the detection of p38 activation
using an anti-phospho p38 antibody specifically recognizing the
active double-phosphorylated protein (Fig. 5A). However, 1 μM SB203580, a p38
kinase inhibitor and 1 μM SB202474 (an inactive analog of
SB203580), as well as 10 μM UO126, an inhibitor of MAPK/ERK kinase,
had no effect on the decrease in AQP4 expression following exposure
to osmotic stress (Fig. 5B and
C). Furthermore, the presence of a p38 activator (10 μg/ml
anisomycin) for 4 h had no effect on AQP4 expression in ARPE-19
cells incubated under the control conditions (Fig. 5B).
Discussion
Tightly regulated water movements through RPE cells
are required for normal retinal function. Under several
pathophysiological situations, the osmotic gradient is modified and
water consequently accumulates in the subretinal space and the
sensory retina, leading to the formation of macular oedema.
Although it is accepted that water passes through
RPE cells, the exact molecular mechanisms responsible for this flux
remain elusive. As AQPs are recognized water channels, it seems
likely that the expression of AQPs in RPE cells may play an
important role in this phenomenon. Human RPE cells appear to
express several AQPs (6,7), including AQP4 (6). These data prompted us to investigate
the expression of AQP4 and its regulation following hyperosmotic
stress stimulation in the human ARPE-19 cell line. We found that
the ARPE-19 cells indeed expressed AQP4 at the mRNA and protein
level.
Based on the fact that osmotic pressure is the main
force involved in water movement, we hypothesized that osmotic
stress may affect AQP4 expression in ARPE-19 cells. Indeed, upon
hyperosmotic stress stimulation, we observed a rapid decrease in
AQP4 expression that was found to be more pronounced following
stimulation with sucrose than with NaCl. Since an inhibitor of the
proteasome, MG132, blocked AQP4 degradation upon hyperosmotic
stress, it is likely that this degradation occurs through the
proteasome.
Despite their possible exposure to extreme
hyperosmotic stress, cells can survive and function owing to
protective adaptation, including the accumulation of large amounts
of organic osmolytes that normalize cell volume and intracellular
ionic strength. In RPE cells, it has been shown that
hyperosmolarity regulates aldose reductase activity, and taurine
transporter expression and function (32,33).
In other tissues and cells, hyperosmolarity has
generally been reported to increase the expression of several AQPs
(14–20,22–29,23,34). By contrast, in ARPE-19 cells, a
decreased AQP4 protein expression was observed in response to the
hyperosmotic stress induced by 200 mM NaCl and 400 mM sucrose using
both membrane protein (Fig. 2)
and total protein (Fig. 3).
Therefore, the reduced membrane AQP4 expression is unlikely due to
the decreased insertion of AQP4 into the membrane. Our results are
in agreement with those of a previous study, which demonstrated a
decrease in AQP4 expression observed in response to hyperosmotic
stress during oedema formation in the contusional brain cortex
(28).
The activation of p38 (21) or ERK (24) has been considered to be essential
for the induction of adaptative responses to osmotic stress
(35) and the increased
expression of several AQPs in response to hyperosmotic stress.
However, despite the simultaneous activation of the three MAPKs
(ERK, p38 kinase and JNK) (14,18), these mechanisms do not seem to
account for the decreased AQP4 protein expression in response to
hyperosmotic stress in ARPE-19 cells. Indeed, our data demonstrated
that the pre-incubation of ARPE-19 cells with inhibitors of these
kinases did not affect the decrease in AQP4 protein expression in
response to the subsequent hyperosmotic stress.
Some AQP proteins have also been shown to be the
target for proteolysis through lysosomal degradation (16,30,31,36–38). However, in our study, the
lysosomal pathway did not appear to participate in the protein
degradation of AQP4 in ARPE-19 cells subjected to hyperosmotic
stress. Indeed, the lysosomal protease inhibitors, calpeptin and
chloroquine, had no effect on the decrease in AQP4 protein levels
observed in response to hyperosmotic stress.
The ubiquitinylation (39–42) and proteasomal degradation
(16,31,36) of several AQPs has been previously
described. The proteasomal pathway may participate in the protein
degradation of AQP4 in ARPE-19 cells subjected to hyperosmotic
stress, as MG132, an inhibitor of the proteasome, prevented the
decrease in the AQP4 protein level in response to hyperosmotic
stress. Our data are in agreement with those of a previous study
which demonstrated the presence of an active ubiquitin-proteasome
pathway in ARPE-19 cells as anti-ubiquitin antibodies detected a
diffuse pattern of bands that increased in the presence of MG132
(43). However, no apparent AQP4
ubiquitinylation could be detected in the absence or presence of
hyperosmotic stress under our experimental conditions, as anti-AQP4
antibodies did not reveal any bands following polyubiquitinated
protein enrichment. Therefore, AQP4 is likely to be degraded in a
ubiquitin-independent manner by the proteasome as described for
other proteins, such as ornitine decarboxylase, p53,
p21Cip, steroid receptor coactivator, p300 or nuclear
factor of activated T cells 5 (NFAT5) (44–49). However, we cannot rule out the
possibility that if ubiquitinylation occurs in one or several of
the three lysine residues present in the AQP4 C-terminal peptide
sequence used to produce the antibodies, this would impair the
peptide epitope recognition by the antibodies. Further proteomic
studies are required to undoubtedly confirm the absence or presence
of ubiquitinylated AQP4 in response to hyperosmotic stress.
The decreased AQP4 protein expression observed in
the ARPE-19 cells under osmotic stress may reflect one of the
underlying pathophysiological mechanisms occurring during the
formation of macular oedema and/or a protective/adaptative
mechanism in response to the hyperosmotic cellular stress occurring
during the formation of macular oedema.
In conclusion, hyperosmotic stress in the ARPE-19
cells induced a marked decrease in AQP4 expression due to AQP4
protein degradation by the proteasome that is possibly independent
of ubiquitinylation.
Acknowledgements
This study was supported by the Fund for Medical
Scientific Research (FRSM, Belgium; grant no. 3.4502.09) and the
Funds for Research in Ophthalmology (FRO, Belgium). S.J. is a
recipient of a Vésale Foundation Award; T.A. is a recipient of a
FNRS fellowship (FNRS, Belgium).
References
|
1
|
Bringmann A, Reichenbach A and Wiedemann
P: Pathomechanisms of cystoid macular edema. Ophthalmic Res.
36:241–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bringmann A, Uckermann O, Pannicke T,
Iandiev I, Reichenbach A and Wiedemann P: Neuronal versus glial
cell swelling in the ischaemic retina. Acta Ophthalmol Scand.
83:528–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:45–881. 2005. View Article : Google Scholar
|
|
4
|
Agre P: Aquaporin water channels (Nobel
Lecture). Angew Chem Int Ed Engl. 43:4278–4290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verkman AS: Role of water channels in eye
function. Exp Eye Res. 76:137–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollborn M, Rehak M, Iandiev I, Pannicke
T, Ulbricht E, Reichenbach A, Wiedemann P, Bringmann A and Kohen L:
Transcriptional regulation of aquaporins in the ischemic rat
retina: upregulation of aquaporin 9. Curr Eye Res. 37:514–531.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stamer WD, Bok D, Hu J, Jaffe GJ and McKay
BS: Aquaporin-1 channels in human retinal pigment epithelium: role
in transepithelial water movement. Invest Ophthalmol Vis Sci.
44:2803–2808. 2003. View Article : Google Scholar
|
|
8
|
Badaut J, Ashwal S and Obenaus A:
Aquaporins in cerebrovascular disease: a target for treatment of
brain edema? Cerebrovasc Dis. 31:521–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papadopoulos MC and Verkman AS:
Aquaporin-4 and brain edema. Pediatr Nephrol. 22:778–784. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Da T and Verkman AS: Aquaporin-4 gene
disruption in mice protects against impaired retinal function and
cell death after ischemia. Invest Ophthalmol Vis Sci. 45:4477–4483.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagelhus EA, Veruki ML, Torp R, Haug FM,
Laake JH, Nielsen S, Agre P and Ottersen OP: Aquaporin-4 water
channel protein in the rat retina and optic nerve: polarized
expression in Muller cells and fibrous astrocytes. J Neurosci.
18:2506–2519. 1998.PubMed/NCBI
|
|
12
|
Marmor MF and Tan F: Central serous
chorioretinopathy: bilateral multifocal electroretinographic
abnormalities. Arch Ophthalmol. 117:184–188. 1999. View Article : Google Scholar
|
|
13
|
Soliman W, Sander B and Jorgensen TM:
Enhanced optical coherence patterns of diabetic macular oedema and
their correlation with pathophysiology. Acta Ophthalmol.
85:613–617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Umenishi F and Schrier RW:
Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by
the activation of MAPK pathways and hypertonicity-responsive
element in the AQP1 gene. J Biol Chem. 278:15765–15770. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umenishi F, Narikiyo T and Schrier RW:
Hypertonic induction of aquaporin-1 water channel independent of
transcellular osmotic gradient. Biochem Biophys Res Commun.
325:595–599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umenishi F, Narikiyo T and Schrier RW:
Effect on stability, degradation, expression, and targeting of
aquaporin-2 water channel by hyperosmolality in renal epithelial
cells. Biochem Biophys Res Commun. 338:1593–1599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasler U, Nunes P, Bouley R, Lu HAJ,
Matsuzaki T and Brown D: Acute hypertonicity alters aquaporin-2
trafficking and induces a MAPK-dependent accumulation at the plasma
membrane of renal epithelial cells. J Biol Chem. 283:26643–26661.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hasler U: Controlled aquaporin-2
expression in the hypertonic environment. Am J Physiol.
296:C641–C653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki T, Suzukin T and Takata K:
Hypertonicity-induced expression of aquaporin 3 in MDCK cells. Am J
Physiol. 281:C55–C63. 2001.PubMed/NCBI
|
|
20
|
Sugiyama Y, Ota Y, Hara M and Inoue S:
Osmotic stress up-regulation of aquaporin-3 expression in cultured
human keratinocytes. Biochim Biophys Acta. 1522:82–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arima H, Yamamoto N, Sobue K, Umenishi F,
Tada T, Katsuya H and Asai K: Hyperosmolar mannitol simulates
expression of aquaporins 4 and 9 through a p38 mitogen-activated
protein kinase-dependent pathway in rat astrocytes. J Biol Chem.
278:44525–44534. 2003. View Article : Google Scholar
|
|
22
|
Hansen AK and Galtung HK: Aquaporin
expression and cell volume regulation in the SV40 immortalized rat
submandibular acinar cell line. Pflugers Arch. 453:787–796.
2007.PubMed/NCBI
|
|
23
|
Herrlich A, Leitch V and King LS: Role of
proneuregulin 1 cleavage and human epidermal growth factor receptor
activation in hypertonic aquaporin induction. Proc Natl Acad Sci
USA. 101:15799–15804. 2004. View Article : Google Scholar
|
|
24
|
Hoffert JD, Leitch V, Agre P and King LS:
Hypertonic induction of aquaporin-5 expression through an
ERK-dependent pathway. J Biol Chem. 275:9070–9077. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang SM, Lee RH, Song JM, Yoon S, Kim YS,
Lee SJ, Kang SK and Jung JS: Expression of aquaporin-5 and its
regulation in skeletal muscle cells. Exp Mol Med. 34:69–74. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pedersen PS, Braunstein TH, Jorgensen A,
Larsen PL, Holstein-Rathlou NH and Frederiksen O: Stimulation of
aquaporin-5 and transepithelial water permeability in human airway
epithelium by hyperosmotic stress. Pflugers Arch. 453:777–785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou B, Ann DK, Li X, Kim KJ, Lin H, Minoo
P, Crandall ED and Borok Z: Hypertonic induction of aquaporin-5:
novel role of hypoxia-inducible factor-1alpha. Am J Physiol.
292:C1280–C1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ke C, Poon WS, Ng HK, Pang JCS and Chan Y:
Heterogeneous responses of aquaporin-4 in oedema formation in a
replicated severe traumatic brain injury model in rats. Neurosci
Lett. 301:21–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kasono K, Saito T, Saito T, Tamemoto H,
Yanagidate C, Uchida S, Kawakami M, Sasaki S and Ishikawa SE:
Hypertonicity regulates the aquaporin-2 promoter independently of
arginine vasopressin. Nephrol Dial Transplant. 20:509–515. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gan SW, Ran JH, Chen H, Ren ZQ, Sun SQ,
Zhu SJ, Lu WT, Xu J, Zhang B, Huang J, Wang KJ and Chen Z:
Lysosomal degradation of retinal glial AQP4 following its
internalization induced by acute ocular hypertension. Neurosci
Lett. 516:135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hasler U, Mordasini D, Bens M, Bianchi M,
Cluzeaud F, Rousselot M, Vandewalle A, Feraille E and Martin PY:
Long term regulation of aquaporin-2 expression in
vasopressin-responsive renal collecting duct principal cells. J
Biol Chem. 277:10379–10386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El Sherbeny A, Naggar H, Miyauchi S, Ola
MS, Maddox DM, Martin PM, Ganapathy V and Smith SB: Osmoregulation
of taurine transporter function and expression in retinal pigment
epithelial, ganglion, and Muller cells. Invest Ophthalmol Vis Sci.
45:694–701. 2004.PubMed/NCBI
|
|
33
|
Lin LR, Carper D, Yokoyama T and Reddy VN:
The effect of hypertonicity on aldose reductase, alpha
B-crystallin, and organic osmolytes in the retinal pigment
epithelium. Invest Ophthalmol Vis Sci. 34:2352–2359.
1993.PubMed/NCBI
|
|
34
|
Storm R, Klussmann E, Geelhaar A,
Rosenthal W and Maric K: Osmolality and solute composition are
strong regulators of AQP2 expression in renal principal cells. Am J
Physiol. 284:F189–F198. 2003.PubMed/NCBI
|
|
35
|
de Nadal E, Alepuz PM and Posas F: Dealing
with osmostress through MAP kinase activation. EMBO Rep. 3:735–740.
2002.PubMed/NCBI
|
|
36
|
Lehmann GL, Larocca MC, Soria LR and
Marinelli RA: Aquaporins: their role in cholestatic liver disease.
World J Gastroenterol. 14:7059–7067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Madrid R, Le Maout S, Barrault MB, Janvier
K, Benichou S and Merot J: Polarized trafficking and surface
expression of the AQP4 water channel are coordinated by serial and
regulated interactions with different clathrin-adaptor complexes.
EMBO J. 20:7008–7021. 2001. View Article : Google Scholar
|
|
38
|
Sidhaye V, Hoffert JD and King LS: cAMP
has distinct acute and chronic effects on aquaporin-5 in lung
epithelial cells. J Biol Chem. 280:3590–3596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dibas A, Yang MH, He S, Bobich J and Yorio
T: Changes in ocular aquaporin-4 (AQP4) expression following
retinal injury. Mol Vis. 14:1770–1783. 2008.PubMed/NCBI
|
|
40
|
Kamsteeg EJ, Hendriks G, Boone M, Konings
IBM, Oorschot V, van der Sluijs P, Klumperman J and Deen PM:
Short-chain ubiquitination mediates the regulated endocytosis of
the aquaporin-2 water channel. Proc Natl Acad Sci USA.
103:18344–18349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leitch V, Agre P and King LS: Altered
ubiquitination and stability of aquaporin-1 in hypertonic stress.
Proc Natl Acad Sci USA. 98:2894–2898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schweitzer K, Li E, Sidhaye V, Leitch V,
Kuznetsov S and King LS: Accumulation of aquaporin-1 during
hemolysin-induced necrotic cell death. Cell Mol Biol Lett.
13:195–211. 2008. View Article : Google Scholar
|
|
43
|
Fernandes AF, Guo W, Zhang X, Gallagher M,
Ivan M, Taylor A, Pereira P and Shang F: Proteasome-dependent
regulation of signal transduction in retinal pigment epithelial
cells. Exp Eye Res. 83:1472–1481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Asher G, Tsvetkov P, Kahana C and Shaul Y:
A mechanism of ubiquitin-independent proteasomal degradation of the
tumor suppressors p53 and p73. Genes Dev. 19:316–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ito T, Fujio Y, Takahashi K and Azuma J:
Degradation of NFAT5, a transcriptional regulator of osmotic
stress-related genes, is a critical event for doxorubicin-induced
cytotoxicity in cardiac myocytes. J Biol Chem. 282:1152–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Lonard DM, Jung SY, Malovannaya A,
Feng Q, Qin J, Tsai SY, Tsai MJ and O’Malley BW: The SRC-3/AIB1
coactivator is degraded in a ubiquitin- and ATP-independent manner
by the REGgamma proteasome. Cell. 124:381–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murakami Y, Matsufuji S, Kameji T, Hayashi
S, Igarashi K, Tamura T, Tanaka K and Ichihara A: Ornithine
decarboxylase is degraded by the 26S proteasome without
ubiquitination. Nature. 360:597–599. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Poizat C, Sartorelli V, Chung G, Kloner RA
and Kedes L: Proteasome-mediated degradation of the coactivator
p300 impairs cardiac transcription. Mol Cell Biol. 20:8643–8654.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sheaff RJ, Singer JD, Swanger J,
Smitherman M, Roberts JM and Clurman BE: Proteasomal turnover of
p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell.
5:403–410. 2000. View Article : Google Scholar : PubMed/NCBI
|