Introduction
Brain death refers to the irreversible loss of all
functions of the brain, including the brainstem. Legal recognition
of donor sources, as well as the expansion of origin of the donor
source are crucial factors that remain to be addressed (1,2).
Since the establishment of the first brain-death donor model in the
late 1960s and early 1970s, solid organ transplantation from
brain-dead donors has become common in western countries (3). In China, the rapid progress in organ
transplant legislation and establishment of voluntary donation,
donation after brain death (DBD) or cardiac death (DCD) has
gradually replaced deceased donors and is due to become the main
source of donors (4–6). However, the quality of brain-dead
donors was comparatively worse than that of deceased donors.
Subsequently, recent or long-term transplant efficacy was not
successful (7–9).
Numerous studies have reported that the changes of
hemodynamics and metabolic parameters (10,11), hormonal changes and the endocrines
(12–14), the consumption of coagulation
factors (15,16), the release of inflammatory
cytokines and the change of immunological states (10,17–20) may be responsible for the injury of
brain death-donated organs. However, the detailed mechanism
involved remains to be clarified. Additionally, accurate methods
for identifying the quality of brain death donor organs remain to
be identified.
Protein is considered the ultimate performer of
various biological functions and proteomics is the classical method
used for the screening of specific biological markers (21,22). Using two-dimensional gel
electrophoresis and matrix-assisted laser desorption ionization
time of flight mass spectrometry, the purpose of this study was to
detect alterations in the liver protein expression profile between
the brain death and control groups at different time-points after
brain death, and to predict sensitivity factors to explain the
detailed mechanism involved. Additionally, we aimed to establish a
sensitivity method to evaluate the quality of brain death donors
for transplantation.
Materials and methods
Animals
Twelve-week-old male rabbits (Wuhan Wanqianjiahe
Experimental Animal Breeding Center) were randomly divided in the
brain death and sham (control) groups. Each group was subdivided
into four subgroups according to different time-points (2, 4, 6 and
8 h) after brain death (n=5). All the rabbits were kept in the room
with water and food ad libitum in a room with controlled
temperature (22±1°C), humidity (50–70%) and 12-h light/dark cycle
in the Experimental Animal Center of Wuhan University. Animal
experiments were conducted under Institutional guidelines and
approved by the Ethics Committee for Animal Care and Use of the
Wuhan University according to the animal protocol.
Establishment of the model of rabbit
brain death
A brain death model was established using an
intracranial progressive pressurized method, similar to that of
Pratschke et al (23).
After being anesthetized with pentobarbital sodium at a dose of 40
mg/kg, the rabbits were placed on the operating table in a supine
position. Femoral artery and vein cannulation, xiphoid separation
and tracheal intubation were performed, as well as burr hole and
catheter placement. Vital signs of the rabbits including
electrocardiogram, blood pressure, respiratory and
electroencephalogram were monitored using a biological functional
system, rodent ventilator and intelligent temperature control
instrument (Thai Union Technology, Co., Ltd., Chengdu, China).
Intracranial pressure was increased as required until the
occurrence of brain death.
Liver function measuring
Blood samples were collected from each rabbit at 2,
4, 6 and 8 h after brain death. Serum glutamic pyruvic transaminase
(ALT) and glutamic oxaloacetic transaminase (AST) levels reflecting
the liver functions were measured by automatic biochemical analyzer
(Hitachi, Tokyo, Japan).
Histomorphometrical evaluation
At 2, 4, 6 and 8 h after brain death, the liver
tissues were isolated and fixed in 10% buffered formaldehyde for
>24 h and then embedded in paraffin. Serial sections (4 μm) were
stained with hematoxylin and eosin for cell morphometry. Three
sections per animal and five fields per section were scanned and
computerized with a digital image analyzer [Medical Image Analysis
System (MIAS)] (Beijing University of Aeronautics and
Astronautics).
Protein extraction and 2-DE proteomics
profiling
Following the manufacturer’s instructions, the
ReadyPrep Sequential Extraction kit (Bio-Rad, Hercules, CA, USA)
was used to extract proteins from the liver tissues. The tissues
were then washed with the PlusOne 2-D Clean-Up kit (GE Healthcare,
Piscataway, NJ, USA) and dissolved with sample buffer. Proteins
(150 μg) from the control group and the 6-h after brain death group
were mixed with rehydration buffer. Using an Ettan IPGphor
Electrophoresis System (GE Healthcare), the mixture was
isoelectrically focused at 500 V for 1 h; 1,000 V for 1 h; 3,000 V
for 1 h and 8,000 V for 9.5 h subsequent to rehydration for 12 h at
30 V on Immobiline IPG DryStrips (GE Healthcare). IPG strips were
applied for 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) using a PROTEAN® II xi Cell
system (Bio-Rad) following equilibration for 2×15 min in an
equilibration buffer. Each sample was measured in triplicate.
Gel image acquisition and analysis
The Coomassie Brilliant Blue R-350 (Amersham
Biosciences, Amersham, UK) and PDQuest 2D analysis software
(Bio-Rad) were used to stain 2-DE gel images and detect protein
spots, respectively. Sensitivity parameters were simultaneously
reproduced for each gel image and spot detection and matching were
manually revised in the software. Based on the three independent
pools of biological material, reproducible protein patterns were
measured by the presence of each individual protein in three
replicate gels. The intensity of each protein spot was normalized
to the total density in the gel.
Protein identification
Following excision from the gel, the protein spots
were subjected to destaining, washing and in-gel digestion with
protease trypsin at 37°C overnight. Subsequently, peptides were
extracted from the gel and dried by centrifugal lyophilization. The
peptide mixtures were redissolved in 0.5% TFA and analyzed using a
4700 Proteomics Analyzer (Applied Biosystems, Inc., Foster City,
CA, USA). The Mascot software (Matrix Science, London, UK) was used
for protein identification and the mass spectra were searched in
the Swiss-Prot protein database. Protein scores >56 were
considered as significant. If one spot matched >1 protein
member, the one with the highest score was taken into
consideration.
Re-identification of typical protein
In order to further identify these different
proteins, randomly runt-related transcription factor 1 (RUNX1) was
selected and the difference with immunohistochemistry and western
blot analysis in the brain death and control groups was
re-identified.
The liver tissues obtained from the control and
experimental groups at 2, 4, 6 and 8 h after brain death were
deparaffinized and the endogenous peroxidase was blocked with 3%
H2O2 for 10 min. The tissues were then
incubated with primary polyclonal rabbit antibody against RUNX1
(1:500)and β-actin (1:200; Boster Biological Engineering, Co.,
Ltd., Wuhan, China) for 1 h at 37°C, followed by biotin-labeled
goat anti-rabbit immunoglobulin (Ig) G for 20 min. After sequential
incubation with Streptavidin-Biotin complex (SABC) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and DAB as substrate, the
samples were counterstained with hematoxylin. Five random fields of
each stained section were visualized and analyzed using
morphometric software (MIAS, Beijing University of Aeronautics and
Astronautics) by an investigator who was blinded to the animals’
treatment status.
The proteins extracted from the liver tissues of the
control and brain death groups at 2, 4, 6 and 8 h were prepared by
homogenizing in RIPA buffer containing protease inhibitors (Boston
BioProducts, Inc., MA, USA) followed by centrifugation at 10,000 ×
g for 10 min at 4°C. Samples were stored at −80°C until use.
Protein concentrations of the lysates were determined by the BCA
Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Proteins
were then separated using 12.5% SDS polyacrylamide gel
electrophoresis and transferred to a PVDF membrane (0.2 μm;
Millipore, Bedford, MA, USA). The membrane was then blocked with 5%
non-fat dry milk overnight and probed with primary rabbit
polyclonal antibody against RUNX1 (1:500, Boster Biological
Engineering, Co., Ltd.). The proteins were then detected on the
blot using infrared-labeled secondary antibodies visualized in 800
nm fluorescence channels. The blot was developed and quantified
using the Odyssey Infrared Imaging System (LI-COR Biosciences,
Lincoln, NE, USA) following the manufacturer’s instructions.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Calculations were performed using SPSS 18.0 software. One-way
ANOVA was used to compare the differences between two groups. The
intergroup differences were compared with repeated measurement
design. P<0.05 was considered statistically significant.
Results
Alteration of liver function
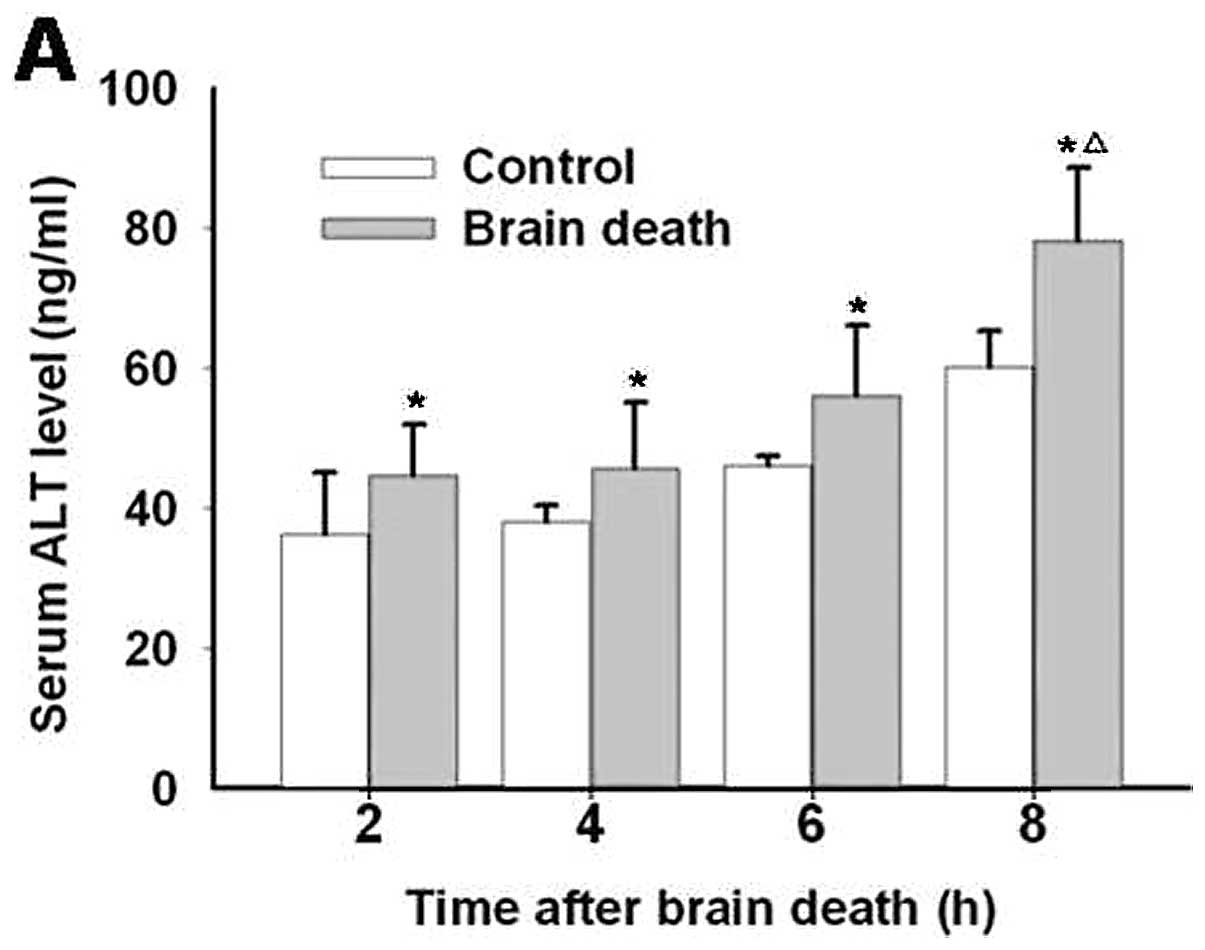
Compared with the previous group, there were obvious
differences for serum ALT and AST level in each brain death group
from initiation to 8 h after rabbits’ brain death. Compared with
the control groups, no marked changes in the ALT and AST levels
were observed for the 2, 4 and 6 h brain death groups. However, a
significant difference between the two groups was observed at 8 h
(Fig. 1).
The morphological alteration of
liver
No obvious morphological alteration occurred for the
liver cells for the control groups at 2, 4 and 6 h. However, some
inflammatory cell infiltration occurred for the 8-h groups. For the
brain death groups, the liver cells were almost normal in the 2-h
group, while mild edema, osteoporosis and compression of the
hepatic sinus part was evident in the 4-h group. Liver injuries
gradually became exacerbated in a time-dependent manner. In
particular, the ballooning degeneration, sinusoidal pressure, no
significant hepatic cord structure, abundant periportal lymphocytic
infiltration and part of focal necrosis were found in all 8-h brain
death group livers (Fig. 2).
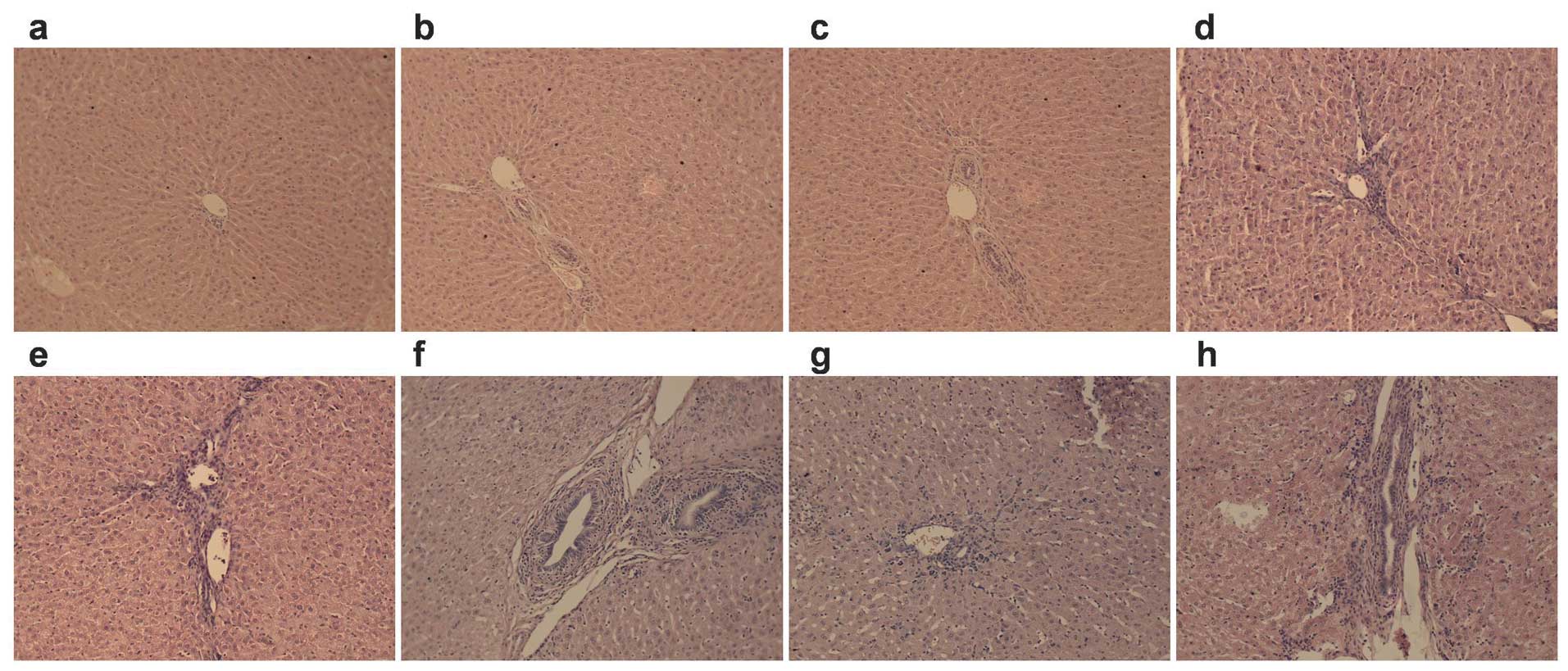 | Figure 2Effects of brain death at different
time-points on the morphological alteration of liver (n=5).
Alterations of morphometry of liver cells stained by hematoxylin
and eosin (×100) are shown in Fig. 2. (a–d) No obvious differences
were evident in (a–c) at 2, 4, 6 and 8 h for the control group,
whereas some inflammatory cell infiltration is evident in (d).
(e–h) Morphological alterations in liver at 2, 4, 6 and 8 h for the
brain death group. Liver cells were almost normal in the (e) brain
death group, while mild edema, osteoporosis and compression of the
hepatic sinus part were observed in the liver of the (f) group.
Obvious ballooning degeneration, sinusoidal pressure, no
significant hepatic cord structure, abundant periportal lymphocytic
infiltration and part of focal necrosis were found in (g) and (h)
of the livers of the brain death group. |
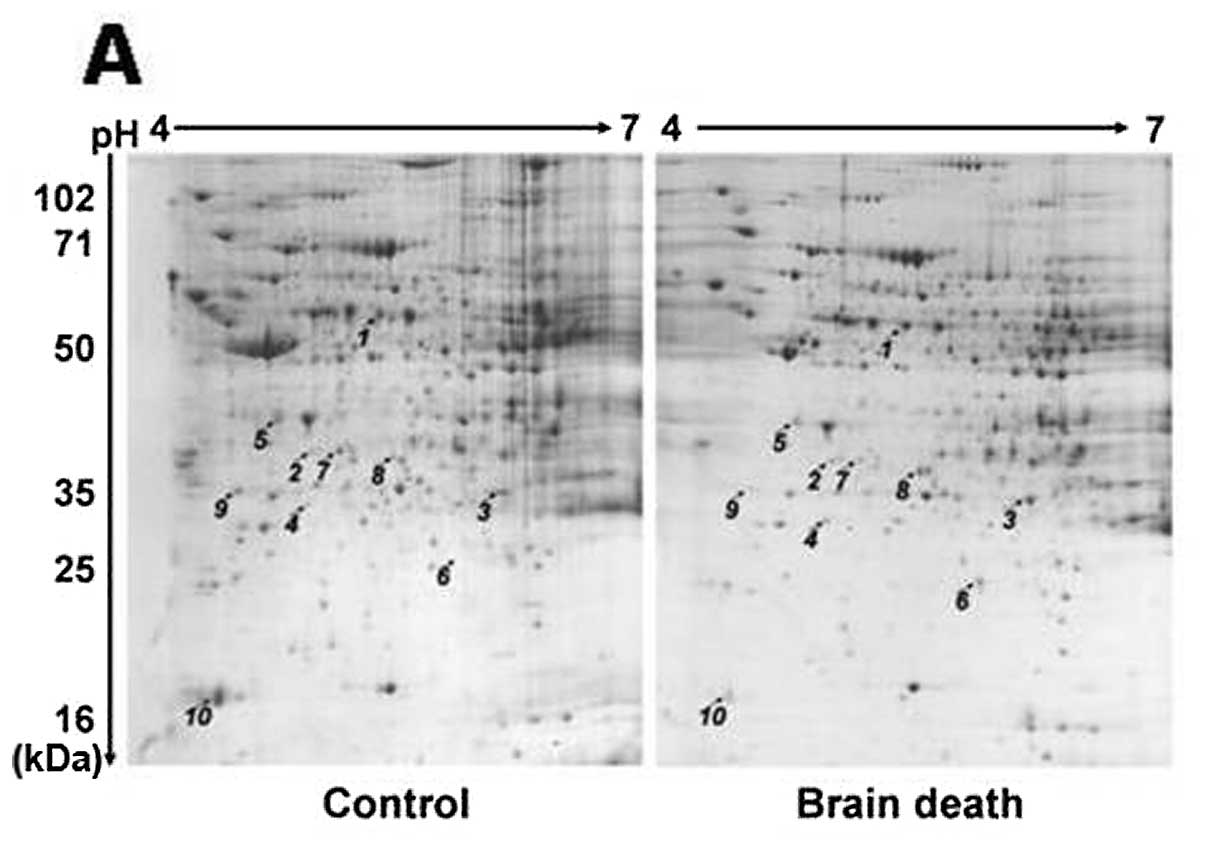
2-DE proteomics profiling of different
proteins
Different proteins were obtained by analyzing and
comparing the 2-DE-based proteomic profiling of the control and
brain death group at 6 h after brain death. PDQuest 2D analysis
software was employed to detect 973±34 protein spots in the control
group and 987±38 protein spots in the brain death group. Results of
the statistical analysis of 2-DE-based proteomic profiling revealed
that there were 52 obvious different protein spots between the
control and brain death group. Ten of the 52 protein spots
differentially expressed in a >2-fold increase or decrease were
identified by MS/MS analysis. The positions on the 2-DE-based
profiling were annotated in Fig.
3.
Mass spectrum identification and the
function classification of different proteins
Following MALDI-TOF/TOF tandem mass spectrometry
analysis and the Swiss-Prot protein database search, we found that
5/10 identified different proteins were upregulated while the
remaining five were downregulated. The biological associations
between the alterations of identified proteins and the progression
of brain death-induced liver injury were searched on the www.uniprot.org according to the individual biological
and molecular functions. The major biological functions of these 10
proteins were divided into six classifications, including material
metabolism (3/10) and redox regulation (2/10), energy metabolism
(1/10), cell proliferation and differentiation (3/10), lipid
metabolism (1/10) and detoxification (2/10) and neurodevelopment
(1/10). Basic information of these proteins and their
classifications are listed in detail in Table I.
 | Table IProteins identified by mass
spectrometry. |
Table I
Proteins identified by mass
spectrometry.
| Spot no. | Protein namea | Gene name | Accession no.b | pIc | Sequence coverage
(%) | Mascot score | MWc (Da) | Subcellular
localization | Biological
function | Protein
expression |
|---|
| 1 |
Dihydropyrimidinase-related protein 4 | DPYL4 | Q62951 | 6.30 | 22 | 53 | 61617 | Cytoplasm | Neurodevelopment | Up |
| 2 | Aldehyde
dehydrogenase, mitochondrial | ALDH2 | P05091 | 6.63 | 17 | 82 | 56859 | Mitochondrial
matrix | Material metabolism,
redox regulation | Down |
| 3 | Peroxiredoxin-6 | PRDX6 | O35244 | 5.64 | 40 | 100 | 24860 | Mitochondrion | Antioxidant,
catabolism | Up |
| 4 |
3-Phosphoinositide-dependent protein
kinase-1, isoform CRA_b | PDK1 | O55173 | 7.88 | 53 | 72 | 19833 | Cytoplasm | Cell proliferation
and differentiation | Up |
| 5 | Runt-related
transcription factor 1, isoform CRA_b | RUNX1 | Q63046 | 9.08 | 29 | 67 | 41392 | Nucleus | Cell proliferation
and differentiation | Down |
| 6 | 3-Mercaptopyruvate
sulfurtransferase | MPST | P97532 | 6.13 | 27 | 85 | 33443 | Cytoplasm | Detoxification | Up |
| 7 | Inorganic
pyrophosphatase | PPA1 | Q15181 | 5.26 | 21 | 71 | 33206 | Cytoplasm | Metabolism, redox
regulation | Down |
| 8 | Alcohol
dehydrogenase [NADP+] | ALDR1 | P14550 | 6.84 | 41 | 111 | 36711 | Cytoplasm | Energy
metabolism | Up |
| 9 | Glutamate-cysteine
ligase regulatory subunit | GLCLR | P48508 | 5.36 | 28 | 70 | 30871 | CytoplasmCell
proliferation | Down and
differentiation | |
| 10 | Microsomal
cytochrome B5 | CYB5 | P00169 | 5.14 | 71 | 110 | 10788 | Endoplasmic
reticulum microsomes | Lipid metabolism,
detoxification | Down |
Identification and re-identifications of
RUNX1 proteins
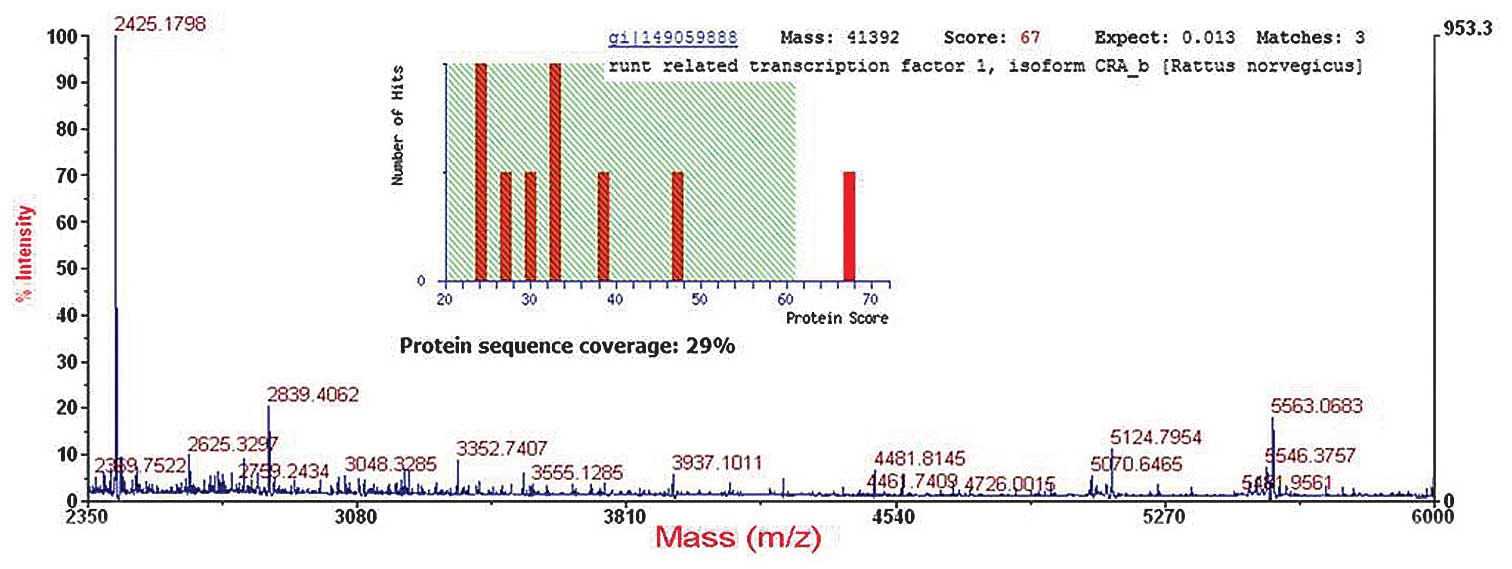
The predicted molecular mass/isoelectric point (pI)
value for RUNX1 was 49 kDa/9.08 which was suitable to the position
of the corresponding spot (spot 5) on the 2-DE gel. MS/MS analysis
showed that RUNX1 was identified with a Mascot score of 67 and 29%
sequence coverage (Fig. 4).
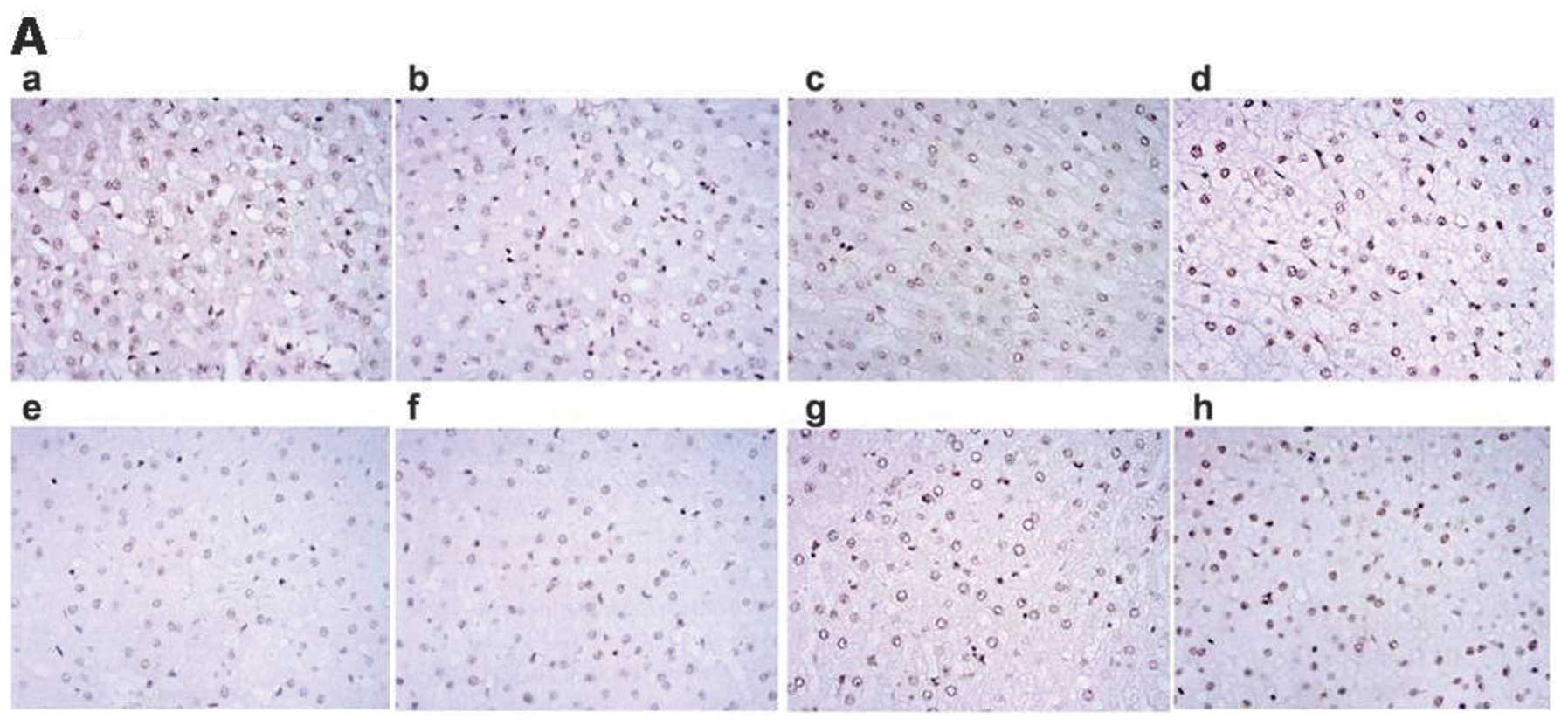
Results of the immunohistochemical analysis of RUNX1
revealed that the expression of RUNX1 was decreased in a
time-dependent manner in the brain death group. Additionally, the
level of RUNX1 for each time-point to some extent decreased
compared with the control groups (Fig. 5A). Similarly, results of the
western blot analysis revealed a gradual decrease in the brain
death group at each time-point compared with control group
(Fig. 5B).
Discussion
Previously, the detailed mechanism involving the
effect of brain death on the quality of the transplant donor was
unclear. In their study, Van Der Hoeven et al (24) used a rat brain death model and
found that brain death-induced injury is associated with apoptosis.
Avlonitis et al (10) also
demonstrated that brain death in rats induced an inflammatory
response represented by the elevated levels of IL-6, TNF-α,
neutrophil CD11b/CD18, cytokine-induced neutrophil chemoattractant
(CINC)-1 and CINC-3. Similar to the establishment of the pig brain
death model (25), in the present
study, a new rabbit brain death model using an intracranial
progressive pressurized method was established using the biological
functional system, rodent ventilator and intelligent temperature
control instrument. Maintaining of continuous breathing and the
monitoring of electroencephalography ensured the model was similar
to the state of brain death utilized in the clinic.
Weiss et al (26) reported that significantly
upregulated levels of MIP-1α, IL-4, IFN-γ, HO-1, CD3 and CD25 in
brain death donor transplantation may be due to the phase of
ischemic reperfusion prior to transplantation. In the present
study, we have demonstrated that from initiation to 6 h after brain
death, there were no obvious functional or morphological
alterations (Figs. 1 and 2). Result of the rabbit brain death
model also show that efficient information for the quality
evaluation of donor livers is lacking. Thus, it appears that in
early brain death, the ‘traditional criteria’ may not be
sufficiently efficient to identify an appropriate transplant
donor.
The proteomics analysis identified 52 different
protein spots indicating that complex pathological changes occurred
in the state of brain death. Ten significantly different proteins
identified were classified into material metabolism and redox
regulation, energy metabolism, cell proliferation and
differentiation, lipid metabolism and detoxification and
neurodevelopment. The appropriate systemic physiologic changes,
which are considered to be principally a manifestation of brain
death affecting all organs suitable for transplantation, were
presumably the result of the ischemia/reperfusion injury (8,10,11,26), followed by oxidative stress
(12,27), apoptosis (28,29) and inflammatory response (2,10,20). Evidence suggests that brain death
results in the development of a systemic inflammatory response in
the donor, which can damage all organs with deleterious impact on
their function following transplantation.
RUNX1 is a nucleus gene whose major functions
include cell proliferation and differentiation with the activation
of PKC-θ and reactive oxygen species (30). Subsequent to the identification of
the different RUNX1 protein by immunohistochemistry after
proteomics and western blot analysis in the present study, we came
to a primary conclusion that in addition to the development of
brain death, a gradual decrease of RUNX1 expression was also
induced. In other words, the expression of RUNX1 may be an
indicator of the degree of brain death-induced liver injury,
However, more investigations on the role of RUNX1 in brain death
liver injury is required.
Acknowledgements
This investigation was supported by Research Fund
for the Doctoral Program of Higher Education (20100141110016);
Natural Science Fund of Hubei Province (2012FFA044; 2013CFB258);
Science and technology projects of Wuhan city (2013060705010326;
2013060602010247).
References
|
1
|
Zhang SJ and Wang T: The influence of
brain death on donor liver and the potential mechanisms of
protective intervention. Front Med. 5:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watts RP, Thom O and Fraser JF:
Inflammatory signalling associated with brain dead organ donation:
from brain injury to brain stem death and posttransplant ischaemia
reperfusion injury. J Transplant. 2013:5213692013. View Article : Google Scholar
|
|
3
|
Machado C: The first organ transplant from
a brain-dead donor. Neurology. 64:1938–1942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang J, Mao Y and Millis JM: Government
policy and organ transplantation in China. Lancet. 372:1937–1938.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Mao Y, Wang Y, Zhang ZJ, Zhao MG
and Liu Y: Modernization of the organ transplantation program in
China. Transplantation. 86:1649–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Millis JM, Mao Y, Millis MA, Sang
X and Zhong S: A pilot programme of organ donation after cardiac
death in China. Lancet. 379:862–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terasaki PI, Cecka JM, Gjertson DW and
Takemoto S: High survival rates of kidney transplants from spousal
and living unrelated donors. New Engl J Med. 333:333–336. 1995.
View Article : Google Scholar
|
|
8
|
Avlonitis VS, Wigfield CH, Golledge HD,
Kirby JA and Dark JH: Early hemodynamic injury during donor brain
death determines the severity of primary graft dysfunction after
lung transplantation. Am J Transplant. 7:83–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stiegler P, Sereinigg M, Puntschart A, et
al: Oxidative stress and apoptosis in a pig model of brain death
(BD) and living donation (LD). J Transl Med. 11:2442013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avlonitis VS, Wigfield CH, Kirby JA and
Dark JH: The hemodynamic mechanisms of lung injury and systemic
inflammatory response following brain death in the transplant
donor. Am J Transplant. 5:684–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rostron AJ, Avlonitis VS, Cork DM, Grenade
DS, Kirby JA and Dark JH: Hemodynamic resuscitation with arginine
vasopressin reduces lung injury after brain death in the transplant
donor. Transplantation. 85:597–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leber B, Stadlbauer V, Stiegler P, et al:
Effect of oxidative stress and endotoxin on human serum albumin in
brain-dead organ donors. Transl Res. 159:487–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vespa PM: Hormonal dysfunction in
neurocritical patients. Curr Opin Crit Care. 19:107–112. 2013.
View Article : Google Scholar
|
|
14
|
Ranasinghe AM and Bonser RS: Endocrine
changes in brain death and transplantation. Best Pract Res Clin
Endocrinol Metab. 25:799–812. 2011. View Article : Google Scholar
|
|
15
|
Hvas CL, Fenger-Eriksen C, Høyer S,
Sørensen B and Tønnesen E: Hypercoagulation following brain death
cannot be reversed by the neutralization of systemic tissue factor.
Thromb Res. 132:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Du Z, Yu J, et al: Activity of
factor VIII in patients with isolated blunt traumatic brain injury:
association with coagulopathy and progressive hemorrhagic injury. J
Trauma Acute Care Surg. 76:114–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuecuek O, Mantouvalou L, Klemz R, et al:
Significant reduction of proinflammatory cytokines by treatment of
the brain-dead donor. Transplant Proc. 37:387–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nijboer WN, Schuurs TA, van der Hoeven JA,
et al: Effects of brain death on stress and inflammatory response
in the human donor kidney. Transplant Proc. 37:367–369. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koudstaal LG, ‘t Hart NA, Ottens PJ, et
al: Brain death induces inflammation in the donor intestine.
Transplantation. 86:148–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Auråen H, Mollnes TE, Bjørtuft Ø, et al:
Multiorgan procurement increases systemic inflammation in brain
dead donors. Clin Transplant. 27:613–618. 2013.PubMed/NCBI
|
|
21
|
Ignatova M, Guével B, Com E, et al:
Two-dimensional fluorescence difference gel electrophoresis
analysis of Listeria monocytogenes submitted to a redox shock. J
Proteomics. 79:13–27. 2013. View Article : Google Scholar
|
|
22
|
Qiao B, Wang J, Xie J, et al: Detection
and identification of peroxiredoxin 3 as a biomarker in
hepatocellular carcinoma by a proteomic approach. Int J Mol Med.
29:832–840. 2012.PubMed/NCBI
|
|
23
|
Pratschke J, Wilhelm MJ, Kusaka M,
Laskowski I and Tilney NL: A model of gradual onset brain death for
transplant-associated studies in rats. Transplantation. 69:427–430.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Der Hoeven JA, Moshage H, Schuurs T,
Nijboer M, Van Schilfgaarde R and Ploeg RJ: Brain death induces
apoptosis in donor liver of the rat. Transplantation. 76:1150–1154.
2003.PubMed/NCBI
|
|
25
|
Sereinigg M, Stiegler P, Puntschart A, et
al: Establishing a brain-death donor model in pigs. Transplant
Proc. 44:2185–2189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiss S, Kotsch K, Francuski M, et al:
Brain death activates donor organs and is associated with a worse
I/R injury after liver transplantation. Am J Transplant.
7:1584–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golling M, Jahnke C, Fonouni H, et al:
Distinct effects of surgical denervation on hepatic perfusion,
bowel ischemia, and oxidative stress in brain dead and living donor
porcine models. Liver Transpl. 13:607–617. 2007. View Article : Google Scholar
|
|
28
|
Lau A, Arundine M, Sun HS, Jones M and
Tymianski M: Inhibition of caspase-mediated apoptosis by
peroxynitrite in traumatic brain injury. J Neurosci.
26:11540–11553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pérez López S, Vázquez Moreno N, Escudero
Augusto D, et al: A molecular approach to apoptosis in the human
heart during brain death. Transplantation. 86:977–982.
2008.PubMed/NCBI
|
|
30
|
Giambra V, Jenkins CR, Wang H, et al:
NOTCH1 promotes T cell leukemia-initiating activity by
RUNX-mediated regulation of PKC-θ and reactive oxygen species. Nat
Med. 18:1693–1698. 2012.PubMed/NCBI
|