Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer worldwide with an approximate
5-year survival rate of 50% (1).
The prognosis for patients with HNSCC is determined by the stage of
the tumor at presentation, as well as the presence of lymph-node
metastases and distant metastases. Approximately one third of
patients present with early-stage disease, whereas two thirds
present with advanced cancer with lymph node metastases (2). Early-stage tumors are treated with
surgery or radiotherapy and have a favorable prognosis. Thirty-five
to 55% of patients with advanced-stage HNSCC remain disease-free 3
years after standard treatment (3). However, locoregional recurrence
(LRR) develops in 30–40% of patients and distant metastases occur
in 20–30% of HNSCC cases (4).
The standard of care for advanced tumors is surgery
combined with adjuvant radiation therapy and/or chemotherapy.
Survival outcomes are poor (40–50% five-year survival rates), and
the treatment leads to morbidity (5). LRRs often requires a combination of
surgery, radiation therapy, and/or chemotherapy, and metastatic
disease is treated with chemotherapy. However, despite these
therapeutic approaches, the control of LRR has been minimal.
Therefore, addressing the underlying factors associated to
locoregional disease will improve clinical management and decrease
the burden of HNSCC.
Thermal and non-thermal plasma are ionized media
that contain numerous active components, including electrons and
ions, free radicals, reactive molecules and photons (6). Thermal plasma has been widely used
to modify material surfaces; this modification is generally
conducted in a vacuum (7,8). Cold atmospheric plasma (CAP), is a
non-thermal plasma that has been shown to be highly effective in
germicidal irradiation and sterilization, wound healing, blood
coagulation, material surface modifications and crosslinking, as
well as in the treatment of various diseases, including cancer
(9–11).
In contrast to thermal plasma, CAP can reach high
electron temperatures but very low gas temperatures associated with
weak ionization rates (7).
Thermodynamic equilibrium of electron self-collision in CAP occurs
much faster than the equilibrium between electrons and larger
particles, such as ions. Thus, the overall plasma temperature is
much lower than the electron temperature, which is close to room
temperature. Cold plasma has been used in biomedical research as it
can reach ion temperatures closer to those at room temperature
(12).
A number of studies have proposed the use of
different cold plasma modalities for cancer treatment (10,13,14). Our laboratory recently examined
the therapeutic potential of a manually-held CAP jet device in
cancer cell lines and tumors, showing selective tumor eradication
capabilities and apoptotic signaling pathway deregulation in
melanoma cell lines and SCaBER-bearing mouse models (15). We demonstrated that CAP can
potentially offer a minimally invasive surgical approach, allowing
for specific cancer cell or tumor tissue removal without affecting
the surrounding healthy cells and tissues, thus rendering it a
promising technology for cancer therapy.
Despite the wide range of potential biomedical
applications (16,17), the cell-specific effects of cold
plasma treatment are not well understood at the molecular level
(18). The generation of
intracellular reactive oxygen species (ROS) leading to apoptosis
has been proposed by different groups (19,20). Cellular necrosis (11) and senescence (21) have also been proposed to explain
the mechanism of cold plasma treatment on cancer cells. Two
possible underlying mechanisms for the high selectivity of CAP
towards cancer cells can be attributed to the complex composition
of CAP and the diverse characteristics of cancer and normal cells.
While the specific mechanisms of action have not been identified,
it is becoming apparent that cold plasma treatment may be more
beneficial for some tumor sites than others.
The selective tumor eradication capabilities of the
CAP jet device render it a potentially attractive adjuvant
treatment for HPV-negative oropharyngeal squamous cell carcinoma
patients who exhibit a higher rate of residual disease due to LRR
when compared to HPV-positive patients (22). The aim of the present study was to
examine whether CAP treatment for different exposure times shows
selective tumor eradication capabilities in 4 HNSCC and 2 normal
oral epithelial cell lines.
Materials and methods
Cell culture
The HNSCC cell lines (JHU-022, JHU-028, JHU-029,
SCC25) were cultured in RPMI-1640 cell culture medium (Sigma, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma)
and Pen/Strep (100 units/ml penicillin and 100 μg/ml streptomycin)
(both from Life Technologies, Grand Island, NY, USA). The 2 normal
oral cavity epithelial cell lines (OKF6 and NOKsi) were grown in
Keratinocyte-SFM (1X) supplemented with Keratinocytes Supplements
(both from Gibco/Life Technologies). All cells were obtained from
the Johns Hopkins University Head and Neck Cancer Division cell
bank and incubated at 37°C in an atmosphere of 5%
CO2.
Cold plasma treatment
The CAP device, created in the School of Engineering
and Applied Science of The George Washington University, contains 4
blocks. Block 1 is a DC power supply. Block 2 is a centrally
powered electrode with a ground outer electrode wrapped around a
quartz tube, which is part of the cold plasma production. Block 3
consists of a capacitor, a transistor and a timer; and block 4 is
the helium gas supply, as previously described (6). Cold plasma treatments were carried
out at 8 kV, using a helium flow of 10 l/min−1, with a
distance of 3 cm from the plasma source to the cells, and treatment
durations of 10, 30 and 45 sec.
We seeded the cells in 96-well plates and exposed
them to cold plasma treatment for 10, 30 and 45 sec and a helium
flow of 20 l/min−1 for 10 sec as a positive treatment
control (Fig. 1). Following
treatment, we transferred the cells to 2 sets of 6-well plates per
cell line for MTT and clonogenic assays.
MTT and clonogenic assays
MTT assay (Sigma) was performed on the plated cells
48 h after cold plasma application, according to the manufacturer’s
instructions and the absorbance at 570 nm was measured. Clonogenic
or colony formation assays were performed 7 days after treatment
with cold plasma; colonies were visualized by staining with crystal
violet (Sigma).
Immunoblotting
Western blot analysis of PARP cleavage was performed
48 h after cold plasma treatment as follows: cell lysates were
separated by SDS-PAGE on Tris-glycine gels and transferred to PVDF
membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA) The
membranes were blocked with TBS-T + 5% non-fat dry milk and
incubated overnight at 4°C with an antibody specific for PARP
(Santa Cruz Biotechnology, Inc. Dallas, TX, USA). The membranes
were washed and incubated with horseradish-peroxidase conjugated
secondary antibodies. Protein detection was performed by enhanced
chemiluminescence.
Results
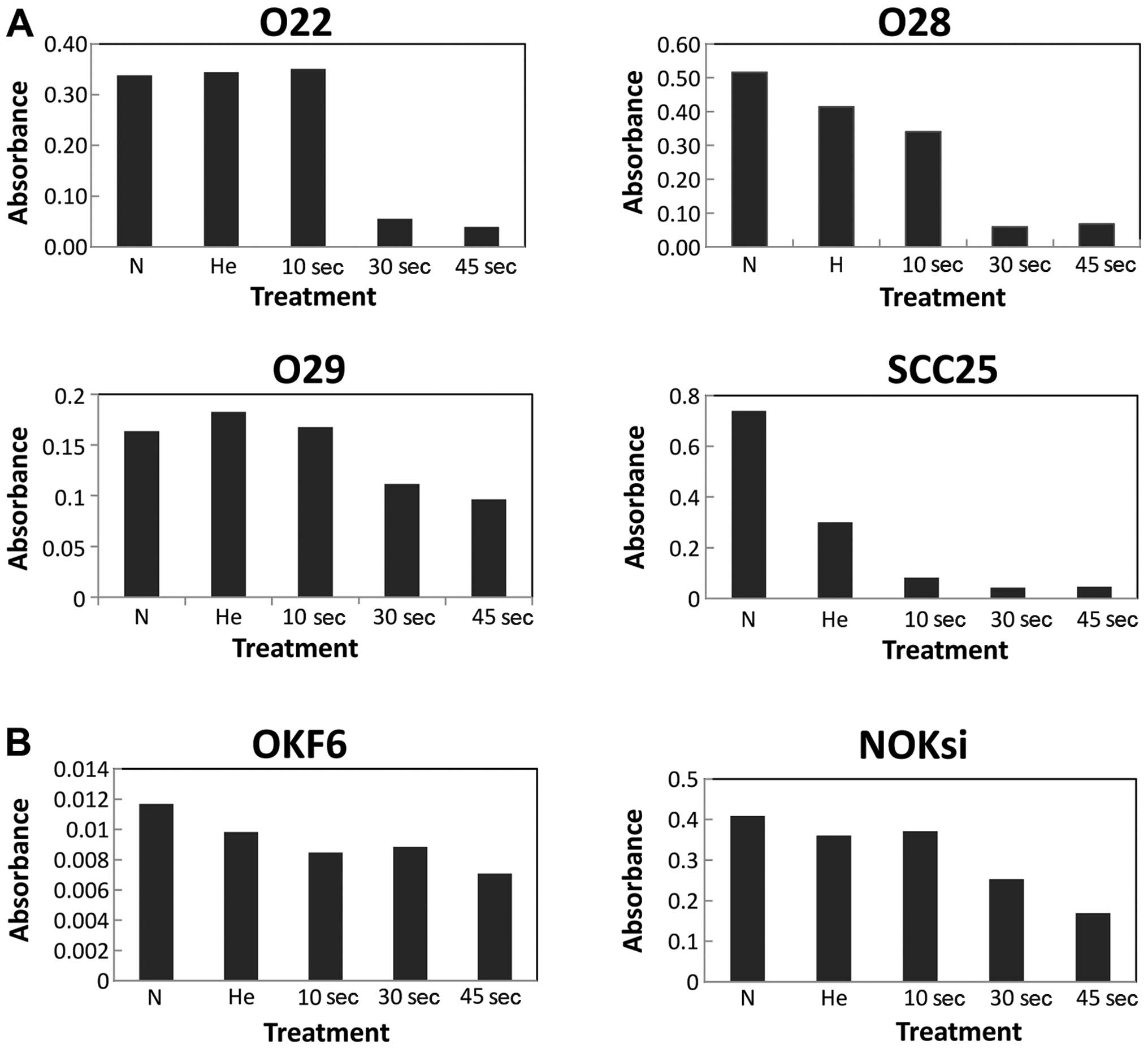
The results from MTT assay revealed that cold plasma
selectively diminished the viability of the SCC25 and JHU-O28 HNSCC
cells in a dose- response manner (Fig. 2A). The JHU-O22 and JHU-O29 cells
showed a diminished cell viability only after 30 and 45 sec of
treatment. The viability of the OKF6 cells was not affected by the
cold plasma, while the viability of the NOKsi cell lines was
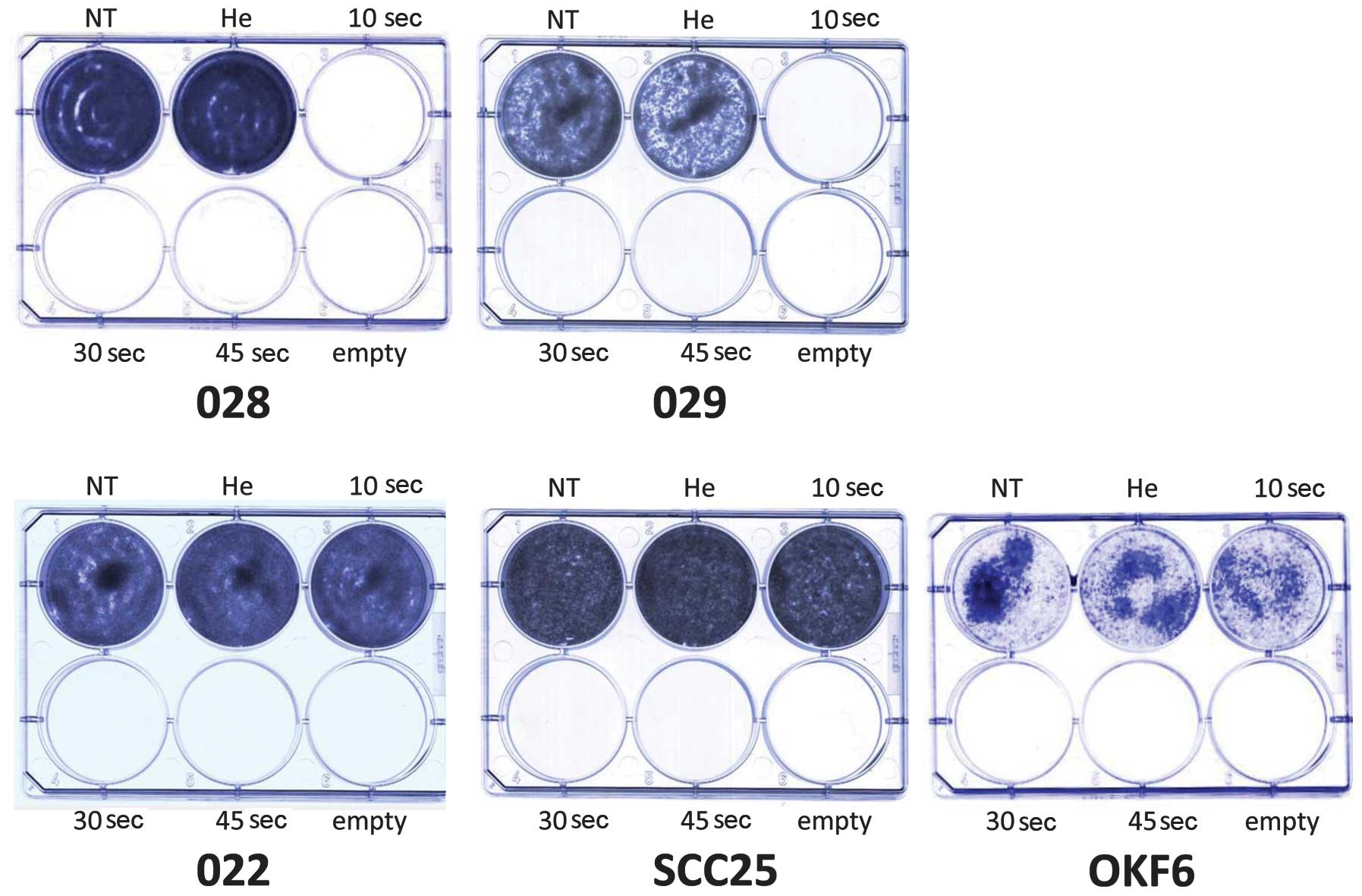
slightly diminished after 30 and 45 sec of treatment (Fig. 2B). The results of the colony
formation assay also revealed a cell-specific response to cold
plasma application. Exposure to helium flow for 10 sec did not
impede colony formation. The JHU-O28 and JHU-O29 cells did not form
any colonies following treatment with cold plasma for the 3
different time periods (Fig. 3).
The JHU-O22, SCC25 and OKF6 cells only formed colonies following
cold plasma treatment for 10 sec (Fig. 3). The NOKsi cells formed colonies
following treatment with cold plasma at all 3 time periods (data
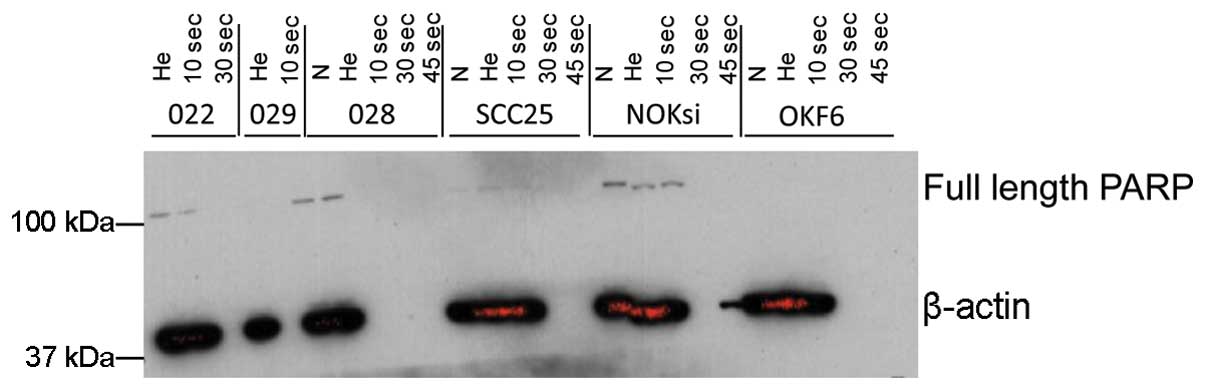
not shown). Western blot analysis did not provide evidence that the
cleavage of PARP occurred following cold plasma treatment (Fig. 4), suggesting that cold plasma
application leads to selective cell death possibly through
non-apoptotic pathways in HNSCC.
Discussion
The main purpose of this study was to assess the
selectivity of cold plasma in HNSCC cell lines and the mechanisms
underlying this selectivity. Our results suggest that cold plasma
application selectively impairs some HNSCC cell lines through
non-apoptotic mechanisms, as the cleavage of PARP was not
significantly altered in the treated cells, while having a minimal
effect on normal oral cavity epithelial cell lines.
A number of studies have suggested possible
molecular mechanisms for the effects of cold plasma on cancer
cells. Several in vitro mechanisms have been suggested to be
associated with a decrease in the expression of cell-surface
proteins, such as integrins and FAK: cell detachment, the induction
of apoptosis, the induction of senescence and the generation of ROS
(17,20,21,23–25). The selective response of tumor
cells to CAP may also be due to the phase of the cell cycle. It is
known that the percentage of cancer cells is higher in the S phase
of the cell cycle and this may render the cancer cells more
susceptible to the effects of CAP, as previously shown in the 308
and PAM 212 cancer cell lines (26).
Mouse xenograft models of melanoma, bladder cancer,
neuroblastoma and glioma treated with cold plasma have been found
to have a decreased tumor volume and an increased survival rate
(15,17,27). In addition, although some tumors
recurred, their growth rate was reduced as compared to the tumors
in the untreated mice.
In the present in vitro study, we observed
that cold plasma application selectively targeted the HNSCC cell
lines, JHU-O28 and SCC25, while it had a moderate effect on the
JHU-022 and JHU-029 cells, and a minimal effect on normal oral
cavity epithelial cell lines. The mechanisms appear to involve
non-apoptotic pathways, as the cleavage of PARP was not detected
following cold plasma treatment. One reason for the moderate effect
on HNSCC JHU-022 and JHU-029 cells may be due to cold
plasma-induced TP53 inactivation. In this regard, Skinner et
al showed that disruptive TP53 mutations render head and
neck cancer cells more resistant to treatment with radiation
(28). Since the mechanisms of
action of cold plasma are not yet clearly known, it is tempting to
speculate that cold plasma induced-TP53 mutations may also
cause resistance to treatment with cold plasma. However, our data
suggest a mechanism of action independent of p53, as cold plasma
had different effects on HNSCC regardless of the p53-status of
these cells; the 3 JHU cell lines express wild-type p53 (29,30), while SCC25 cells express mutant
p53 (31).
The control of LRR in HNSCC is of one of the most
important clinical management goals. Failure to achieve this goal
leads to complex clinical scenarios associated with persistent or
recurrent disease at the primary tumor site or in regional lymph
nodes. Furthermore, patients can develop metastatic disease, either
as a consequence of the spreading from the primary tumor before the
initial diagnosis or from treatment-resistant persistent/recurrent
locoregional disease. Both of these clinical scenarios
(persistent/recurrent locoregional or metastatic disease) represent
extremely difficult management problems (32). Salvage treatment is possible but
frequently unsuccessful, particularly in patients in whom
macroscopic disease is evident at or within 6 months after the end
of initial chemoradiotherapy. Salvage treatment usually entails
both acute and long-term morbidity (33). Systemic metastatic disease may be
palliated by cytotoxic chemotherapy, biological agents or low-dose
radiotherapy, but remains incurable with a median survival of
approximately 6–9 months (34).
Other therapies include simultaneous chemoradiotherapy, and the
combination of radiotherapy and targeted therapies (e.g., EGFR
antibody, cetuximab) (35,36).
However, despite these therapeutic approaches, locoregional control
and survival rates have shown only a modest increase.
Patient mortality with HPV-negative HNSCC is
primarily driven by tumor cell radioresistance leading to LRR.
Overall and disease-specific survival is higher in patients with
HPV-positive HNSCC tumors (37),
which, as a distinct molecular and pathologic subtype, displays an
average of 4 somatic mutations per tumor, while HPV-negative HNSCC
tumors harbor 20. HVP-positive HNSCC patients have a different
molecular profile than HPV-negative patients, which may modulate
their sensitivity to cold plasma. For example, HPV-positive HNSCC
patients usually do not have TP53 mutations in their tumors,
but the cell cycle is still deregulated in these patients, as the
E6 HPV protein silences TP53 (38,39). CDKN2A, a principal
cyclin-dependent kinase inhibitor that decelerates the cell cycle,
is lost in HPV-negative HNSCC (40) and amplified in HPV-positive HNSCC
(41). All the HNSCC cell lines
we used in this study were HPV-negative. Thus, cold plasma may be
successfully used as an adjuvant treatment for HPV-negative HNSCC
patients with or without p53 mutations.
In a recent study by Wang et al (6), it was shown that, due to the complex
composition and parameters of CAP, the high selectivity towards
cancer cells may vary. In fact, the various components that compose
the CAP as a variety of ROS, reactive nitrogen species, charge
particles and UV, and parameters, such as voltage, resistance,
plasma emission and power may alter the response of cells to
treatment, thus promoting specific chemical reactions between
charged particles and living cells, triggering intracellular
biochemical reactions. In that study by Wang et al, the CAP
treatment parameters were optimized to selectively kill human
metastatic breast cancer (BrCa) cells, while minimally affecting
healthy human bone marrow mesenchymal stem cells (MSCs) (6). Similarly, in the present study, we
showed that it is feasible to fine-tune the settings of CAP to
specifically target cancer cells, while leaving the adjacent normal
tissue unharmed.
Cold plasma treatment represents an alternative
means to selectively targeting HNSCC cells, while having a minimal
effect on the normal adjacent tissue and a feasible therapeutic
strategy if coupled with endoscopic technology. It could also be
potentially used in the first-line eradication of small malignant
growths and as an adjuvant irradiation treatment of malignant
tissue prior to surgery and surgical margins after surgery. Cold
plasma represents an alternative adjuvant therapy that may lead to
a reduction in LRR, particularly in HPV-negative patients and, as
such, it warrants further investigation.
Acknowledgements
This study was supported by National Cancer
Institute grants (U01CA84986 and K01CA164092).
References
|
1
|
Agrawal N, Frederick MJ, Pickering CR, et
al: Exome sequencing of head and neck squamous cell carcinoma
reveals inactivating mutations in NOTCH1. Science. 333:1154–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
3
|
Fletcher EV: Epidermal growth factor
receptor inhibitor induces interleukin-6 via NADPH oxidase enzymes
in head and neck cancer cells. Master’s thesis. University of Iowa;
2012
|
|
4
|
Forastiere AA, Goepfert H, Maor M, et al:
Concurrent chemotherapy and radiotherapy for organ preservation in
advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hardisson D: Molecular pathogenesis of
head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
260:502–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang M, Holmes B, Cheng X, et al: Cold
atmospheric plasma for selectively ablating metastatic breast
cancer cells. PLoS One. 8:e737412013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimizu T, Steffes B, Pompl R, et al:
Characterization of microwave plasma torch for decontamination.
Plasma Process Polym. 5:577–582. 2008. View Article : Google Scholar
|
|
8
|
Yonson S, Coulombe S, Léveillé V and Leask
RL: Cell treatment and surface functionalization using a miniature
atmospheric pressure glow discharge plasma torch. J Phys D Appl
Phys. 39:3508–3513. 2008. View Article : Google Scholar
|
|
9
|
Kim JY, Ballato J, Foy P, et al:
Single-cell-level cancer therapy using a hollow optical fiber-based
microplasma. Small. 6:1474–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lupu AR, Georgescu N, Călugăru A, et al:
The effects of cold atmospheric plasma jets on B16 and COLO320
tumoral cells. Roum Arch Microbiol Immunol. 68:136–144.
2009.PubMed/NCBI
|
|
11
|
Lupu AR and Georgescu N: Cold atmospheric
plasma jet effects on V79-4 cells. Roum Arch Microbiol Immunol.
69:67–74. 2010.PubMed/NCBI
|
|
12
|
Moisan M, Barbeau J, Moreau S, et al:
Low-temperature sterilization using gas plasmas: a review of the
experiments and an analysis of the inactivation mechanisms. Int J
Pharm. 226:1–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Ballato J, Foy P, et al: Apoptosis
of lung carcinoma cells induced by a flexible optical fiber-based
cold microplasma. Biosens Bioelectron. 28:333–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panngom K, Baik KY, Nam MK, et al:
Preferential killing of human lung cancer cell lines with
mitochondrial dysfunction by nonthermal dielectric barrier
discharge plasma. Cell Death Dis. 4:e6422013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keidar M, Walk R, Shashurin A, et al: Cold
plasma selectivity and the possibility of a paradigm shift in
cancer therapy. Br J Cancer. 105:1295–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffmann M, Bruch HP, Kujath P and Limmer
S: Cold-plasma coagulation in the treatment of malignant pleural
mesothelioma: results of a combined approach. Interact Cardiovasc
Thorac Surg. 10:502–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walk RM, Snyder JA, Srinivasan P, et al:
Cold atmospheric plasma for the ablative treatment of
neuroblastoma. J Pediatr Surg. 48:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volotskova O, Hawley TS, Stepp MA and
Keidar M: Targeting the cancer cell cycle by cold atmospheric
plasma. Sci Rep. 2:6362012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sensenig R, Kalghatgi S, Cerchar E, et al:
Non-thermal plasma induces apoptosis in melanoma cells via
production of intracellular reactive oxygen species. Ann Biomed
Eng. 39:674–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vandamme M, Robert E, Lerondel S, et al:
ROS implication in a new antitumor strategy based on non-thermal
plasma. Int J Cancer. 130:2185–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arndt S, Wacker E, Li YF, et al: Cold
atmospheric plasma, a new strategy to induce senescence in melanoma
cells. Exp Dermatol. 22:284–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandulache VC, Ow TJ, Daram SP, et al:
Residual nodal disease in patients with advanced-stage
oropharyngeal squamous cell carcinoma treated with definitive
radiation therapy and posttreatment neck dissection: association
with locoregional recurrence, distant metastasis, and decreased
survival. Head Neck. 35:1454–1460. 2013.
|
|
23
|
Kieft IE, Kurdi M and Stoffels E:
Reattachment and apoptosis after plasma-needle treatment of
cultured cells. IEEE T Plasma Sci. 34:1331–1336. 2006. View Article : Google Scholar
|
|
24
|
Lee HJ, Shon CH, Kim YS, et al:
Degradation of adhesion molecules of G361 melanoma cells by a
non-thermal atmospheric pressure microplasma. New J Phys.
11:1150262009. View Article : Google Scholar
|
|
25
|
Shashurin A, Stepp MA, Hawley TS, et al:
Influence of cold plasma atmospheric jet on surface integrin
expression of living cells. Plasma Process Polym. 7:294–300. 2010.
View Article : Google Scholar
|
|
26
|
Keidar M, Shashurin A, Volotskova O, et
al: Cold atmospheric plasma in cancer therapy. Physics of Plasmas.
20:0571012013. View Article : Google Scholar
|
|
27
|
Vandamme M, Robert E, Pesnel S, et al:
Antitumor effect of plasma treatment on U87 glioma xenografts:
preliminary results. Plasma Process Polym. 7:264–273. 2010.
View Article : Google Scholar
|
|
28
|
Skinner HD, Sandulache VC, Ow TJ, et al:
TP53 disruptive mutations lead to head and neck cancer treatment
failure through inhibition of radiation-induced senescence. Clin
Cancer Res. 18:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tokumaru Y, Yamashita K, Osada M, et al:
Inverse correlation between cyclin A1 hypermethylation and p53
mutation in head and neck cancer identified by reversal of
epigenetic silencing. Cancer Res. 64:5982–5987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu X, Song X, Dong Y, et al: Vitamin E
succinate induces ceramide-mediated apoptosis in head and neck
squamous cell carcinoma in vitro and in vivo. Clin Cancer Res.
14:1840–1848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burns JE, Baird MC, Clark LJ, et al: Gene
mutations and increased levels of p53 protein in human squamous
cell carcinomas and their cell lines. Br J Cancer. 67:1274–1284.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho AS, Kraus DH, Ganly I, et al: Decision
making in the management of recurrent head and neck cancer. Head
Neck. 36:144–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strojan P, Corry J, Eisbruch A, et al:
Recurrent and second primary squamous cell carcinoma of the head
and neck: when and how to reirradiate. Head Neck. Nov 7–2013.(Epub
ahead of print).
|
|
34
|
Mehanna HM and Ang KK: Head and Neck
Cancer Recurrence: Evidence-based Multidisciplinary Management. 1st
edition. Thieme Medical Publishers, Inc; New York, NY: pp.
3162012
|
|
35
|
Hoffmann TK: Systemic therapy strategies
for head-neck carcinomas: current status. GMS Curr Top
Otorhinolaryngol Head Neck Surg. 11:Doc032012.PubMed/NCBI
|
|
36
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ang KK, Harris J, Wheeler R, et al: Human
papillomavirus and survival of patients with oropharyngeal cancer.
N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kelloff GJ, Lippman SM, Dannenberg AJ, et
al: Progress in chemoprevention drug development: the promise of
molecular biomarkers for prevention of intraepithelial neoplasia
and cancer-a plan to move forward. Clin Cancer Res. 12:3661–3697.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Myers MF, Chang MH, Jorgensen C, et al:
Genetic testing for susceptibility to breast and ovarian cancer:
evaluating the impact of a direct-to-consumer marketing campaign on
physicians’ knowledge and practices. Genet Med. 8:361–370.
2006.PubMed/NCBI
|
|
40
|
Whitworth A: New research suggests access,
genetic differences play role in high minority cancer death rate. J
Natl Cancer Inst. 98:6692006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Svatek RS, Lee JJ, Roehrborn CG, Lippman
SM and Lotan Y: The cost of prostate cancer chemoprevention: a
decision analysis model. Cancer Epidemiol Biomarkers Prev.
15:1485–1489. 2006. View Article : Google Scholar : PubMed/NCBI
|