Introduction
Snake venom contains a variety of enzymes and
peptides that help the snake to overpower, kill, and/or digest its
prey (1–3). As a result, envenomation following a
snake bite evokes serious consequences, including systemic
bleeding, myolysis, coagulopathy, hypovolemia, hemodynamic shock
and acute renal failure, which are accompanied by a series of
systemic deleterious effects in animals (1,2,4,5).
Among the snake venom components, a variety of proteolytic enzymes
are directly or indirectly involved in evoking the symptoms as
preliminary factors (1,3,4).
Proteases are basically classified into snake venom serine
proteases (SVSPs) and snake venom metalloproteases (SVMPs),
depending on their sensitivity to inhibitors (3,5).
Recent proteomic analyses of snake venom have
demonstrated that SVMPs are the major components in venom and
constitute 11% to >65% of the total venom protein contents
(6). In fact, SVMPs are directly
involved in the induction of local and systemic hemorrhaging
(1,7). The proteases degrade the components
of basement membranes underlying capillary endothelial cells
(8). Thus, they trigger the
disruption of the blood vessel wall and eventually evoke the
release of the blood contents into the stroma (1,7).
Studies have indicated that some SVMPs have fibrin(ogen)olytic
activity (6,9), while others serve as prothrombin
activators (10) or inactivators
against blood serpins (11),
demonstrating that the enzymes play pleiotropic roles in the
disturbance of the hemostatic system and the induction of the
leakage of blood components (6).
Macrovipera mauritanica (M.
mauritanica; common name, Moorish viper) is a venomous viper
found in northwestern Africa and one of four species comprising the
genus Macrovipera, together with M. deserti, M.
lebetina and M. schweizeri (12). To date, however, a little is known
about the proteases in their venom. As regards the M.
deserti and M. lebetina, to the best of our knowledge,
there are only two studies availabe which have examined their
venom; one study examined the hemorrhagic, necrotizing and
inflammatory-edematogenic activity (13), and the other surveyed
para-specific neutralization against the venom using a polyvalent
serum (14). M. lebetina
venom, however, has been relatively well documented (15–17). The venom contains several SVSPs,
such as β-F-genase and factor V (FV) activator, and also has SVMPs,
including lebetases and factor X (FX) activator (5,18).
The SVSPs and SVMPs all affect blood coagulation and hemostasis
(4,15). In the case of M.
mauritanica, to the best of our knowledge, there is no study
available to date demonstrating any fibrin(ogen)ase(s) that are
possibly contained in its venom.
SVMPs are classified as α- and β-fibrin(ogen)ases,
according to their proteolytic preference toward the Aα- or
Bβ-chain of fibrin(ogen) (19).
Although they can cleave fibrinogen actively, they cannot induce
the release of fibrinopeptides or fibrin clot formation as thrombin
does (20). In contrast to SVMPs,
fibrin(ogen)olytic serine proteases favorably cleave the Bβ-chain
with lower activity to the Aα-chain and generally do not cleave the
γ-chain (15,18).
In this study, a novel fibrin(ogen)olytic
metalloprotease termed SVMP-M. mauritanica (SVMP-MM),
was purified and characterized from the M. mauritanica snake
venom. We describe the purification and characterization of the
enzyme, with focus on its biochemical properties in terms of enzyme
kinetics and substrate specificity toward a fluorogenic peptide
(newly designed and synthesized in this study) and various blood
coagulation-associated proteins, including plasminogen, fibrinogen
and cross-linked (XL) fibrin. We also describe the effects of
SVMP-MM on the induction of vascular permeability in
vivo.
Materials and methods
Materials
Lyophilized M. mauritanica snake venom was
purchased from Latoxan (Valence, France). All chromatographic
columns, including Superdex 75 10/300 GL, Source 15Q 4.6/100 PE and
Mono Q HR 5/5 were obtained from Amersham Biosciences Biotech Co.
(Uppsala, Sweden). The PD-10 column was from Amersham Pharmacia
Biotech Inc. (Uppsala, Sweden). Protein molecular weight markers
were obtained from Fermentas (St. Leon-Rot, Germany). Human
fibrinogen, α-thrombin, factor XIIIa (FXIIIa),
glycol-bis-(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
(EGTA), ethylenediaminetetraacetic acid (EDTA), 1,10-phenanthroline
(1,10-PT,) N,N′-methylene-bis-acrylamide,
phenylmethanesulfonyl fluoride (PMSF), tetramethylethylenediamine
(TEMED), Trizma base, and other chemicals were purchased from Sigma
(St. Louis, MO, USA). Polyvinylidene fluoride (PVDF) membranes were
obtained from Bio-Rad (Hercules, CA, USA). Synthetic chromogenic
substrates, such as Boc-LGR-para-nitroaniline (pNA)
(typical substrate for FXa) and Boc-VPR-pNA (for thrombin)
were from Seikagaku (Tokyo, Japan). Other chromogenic substrates,
including H-D-VLK-pNA (S-2251 for plasmin),
H-D-IPR-pNA (S-2288 for tPA), pyro-EGR-pNA (S-2444
for urokinase), MeO-Suc-RPY-pNA (S-2586 for chymotrypsin)
and N-α-Z-D-RGR-pNA (S-2765 for FXa) were from Chromogenix
(Milan, Italy). A fluorogenic peptide substrate
[o-aminobenzoic acid (Abz)-HTEKLVTS-2,4-dinitrophenyl
(Dnp)-NH2 for SVMP-MM] was synthesized by GenScript
(Piscataway, NJ, USA). KC1 coagulometer was purchased from
Sigma.
Purification of protease from snake
venom
The lyophilized snake venom powder (121.8 mg) was
dissolved in 1.5 ml of a standard buffer (20 mM sodium acetate, pH
5.5, 100 mM NaCl). To remove the insoluble materials, the crude
venom solution was centrifuged at 9,000 × g for 5 min and the
resulting precipitate was discarded. The proteins (total 40.6 mg)
contained in the supernatant were applied onto a Superdex 75 10/300
GL column equilibrated with standard buffer and then eluted with
the same buffer at a flow rate of 0.5 ml/min. The active fractions
were pooled and applied to a Source 15Q 4.6/100 PE column
equilibrated with the same buffer. The bound proteins were eluted
with a NaCl linear gradient ranging from 0 to 400 mM at a flow rate
of 1.0 ml/min. The active fractions were pooled and desalted on a
PD-10 column equilibrated with standard buffer. The desalted
proteins were then loaded onto a Mono Q HR 5/5 column equilibrated
with the same buffer. The bound proteins were eluted with a NaCl
linear gradient ranging from 0 to 200 mM at a flow rate of 0.5
ml/min. The active fractions were pooled, desalted, concentrated
and stored at −70°C as purified enzyme. In each purification step,
protein concentrations were determined using Bradford reagent
(Sigma). In addition, the fibrinogenolytic activity contained in
each faction was determined by fibrinogen clotting time (FCT)
assay, slightly modified from the original thrombin clotting time
(TCT) method (21). For the
assay, 50 μl of thrombin (2.5 U/ml) pre-incubated at 37°C for 5 min
were mixed with 100 μl of 5% fibrinogen, followed by the addition
of various concentrations of protein samples to be tested with or
without 1 mM EDTA. The clotting time was monitored using a KC1
coagulometer. Protease activity was also examined using azocasein
assay as previously described (22).
Proteolytic activity assay with a
fluorogenic peptide substrate
The fluorogenic peptide substrate (termed
Abz-HTEKLVTS-Dnp-NH2) dissolved in 30% dimethylformamide
(DMF) was resuspended in standard reaction buffer (20 mM sodium
acetate, pH 5.5, 100 mM NaCl) at a final concentration of 80 μM and
incubated at 37°C during which the increase in fluorescence was
monitored at λex =320 nm and λem =420 nm for
15 min using a spectrofluorometer (Molecular Devices Corp.,
Sunnyvale, CA, USA).
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to the method of
Laemmli (23). Protein samples to
be analyzed were mixed with an equal volume of 2X SDS-PAGE sample
buffer, boiled at 100°C for 3 min and then loaded onto 8, 10 or 12%
gels. Following electrophoresis, the protein bands were visualized
by staining the gel with Coomassie brilliant blue. Protein
molecular weight markers used for SDS-PAGE were as follows:
β-galactosidase (116 kDa), bovine serum albumin (66 kDa), ovalbumin
(45 kDa), lactate dehydrogenase (35 kDa), restriction enzyme
Bsp981 (25 kDa), β-lactoglobulin (18.4 kDa) and lysozyme
(14.4 kDa).
Fibrinogen cleavage assay
To examine the fibrinogenolytic activity of the
purified enzyme, human fibrinogen (3.47 mg/ml) was dissolved in
standard reaction buffer and incubated with SVMP-MM enzyme (5.5
mg/ml) at 37°C at a final volume of 140 μl, in which the molar
ratio of enzyme vs. fibrinogen was 1:50. From the reaction mixture,
15 μl each of aliquots was withdrawn at various time periods and
the reaction was terminated by the addition of 3 μl of 6X SDS-PAGE
sample buffer. The samples were then boiled for 3 min and the
resulting products were analyzed by SDS-PAGE as previously
described (22,24).
Turbidity assay for the spontaneous
polymerization of fibrin monomers
The spontaneous polymerization of fibrin monomers
generated from fibrinogen by thrombin or SVMP-MM cleavage was
examined by measuring the increase in turbidity as previously
described (22). Typically, 90 μg
of fibrinogen dissolved in standard reaction buffer were mixed with
0.02 units of thrombin, 0.04 units of plasmin or SVMP-MM enzyme (3
or 6 μg) at 37°C and the increase in the absorbance at 350 nm was
then recorded using a 96-well plate reader (Molecular Devices
Corp.). The fibrinolytic activity of plasmin or SVMP-MM was
determined by measuring the decrease in turbidity of XL-fibrin.
Typically 90 μl of 1 mg/ml fibrinogen in standard reaction buffer
were added to 10 μl of thrombin (17.7 U/ml) and pre-incubated for 1
h at 25°C to allow the formation of fibrin polymer. Thereafter, 10
μl of SVMP-MM enzyme (3 or 6 μg) or plasmin (0.04 or 0.08 units)
were added and further incubated for 2 h at 37°C. The increase or
decrease in absorbance at 350 nm was then recorded with a 96-well
plate reader.
Analysis of the N-terminal sequence of
the purified enzyme
The purified enzyme was subjected to electrophoresis
on 12% SDS-polyacrylamide gel and transferred onto a PVDF membrane
in 10 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) buffer
(pH 11) containing 10% methanol. The blot was stained with
Coomassie brilliant blue, followed by destaining as previously
described (24). A target band
was excised from the membrane and subjected to N-terminal
sequencing with a Precise 491 HT protein sequencer (Applied
Biosystems, Foster City, CA, USA). The sequencing was performed by
the Korea Basic Research Institute (Daejun, Korea).
Type IV collagen digestion
Type IV collagen (0.16 mg/ml) was incubated with
SVMP-MM (40 μg/ml) in 200 μl of the standard reaction buffer at
37°C. At various time intervals, 30 μl each of aliquots was
withdrawn and mixed with 6 μl of 6X SDS-PAGE sample buffer. The
samples were boiled for 3 min and the proteins were then
electrophoresed on 8% polyacrylamide gel, followed by staining the
gel with Coomassie brilliant blue.
Effects of various protease inhibitors,
divalent ions, pH and temperature on enzyme activity
To examine the effects of various inhibitors [EDTA,
EGTA, dithiothreitol (DTT), tosyl-lysine chloromethyl ketone
(TLCK), tosyl-phenylalanyl chloromethyl ketone (TPCK), 1,10-PT,
bestatin, aprotinin and PMSF] or divalent ions (Ca2+,
Cu2+, Fe2+, Mg2+, Mn2+,
Ni2+ and Zn2+) on enzyme activity, reaction
mixtures were composed of 3 μg of purified enzyme, 1 mM of
corresponding additive and 80 μM of a fluorogenic peptide
(Abz-HTEKLVTS-Dnp-NH2) as a substrate in standard
reaction buffer and incubated for 15 min at 37°C. The effects of
temperature on enzyme activity were also assayed at different
temperatures under the conditions described above. The pH
dependency of the enzyme was also examined at 37°C under different
buffer systems with the same concentrations of enzyme and
fluorogenic peptide substrate. The buffer systems used for the pH
requirements of the enzyme were as follows: 50 mM sodium acetate
(pH 4.0–5.5); 50 mM potassium phosphate (pH 6.0–7.5); 50 mM
Tris-HCl (pH 8.0–8.5); 50 mM glycine-NaOH (pH 9.0–10.5). In all
cases, the reactions were triggered by the addition of 80 μM of the
fluorogenic peptide and the relative fluorescence units (RFUs) were
monitored at λex =320 nm and λem =420 nm for
15 min using a spectrofluorometer.
Vascular permeability assay
Vascular permeability induced by the enzyme was
examined by a modification of the Miles assay (25). Evans blue dye solution was freshly
prepared in 0.6% phosphate-buffered saline (PBS) at a final
concentration of 5% and filtered through a paper (0.2 μm in pore
size) before use. A guinea pig (300 g in body weight, male) was
lightly anesthetized with diethyl ether, and the dye (65 mg/kg body
weight) was administered intravenously, followed by the intradermal
injection of 10 μg of SVMP-MM (dissolved in PBS) at the back of the
animal. After 10 min, the guinea pigs were euthanized by urethane
overdose and photographed to visualize the dye leakage. For the
quantification of the dye leakage, the back skin around the
injection spot (approximately 1 cm2) was cut out, soaked
in 3 ml of formamide, and incubated for 48 h at 60°C to allow the
release of the dye. The amount of dye exclusion was determined by
measuring the absorbance at 620 nm and expressed as a measure in
micrograms of Evans blue dye efflux, as previously described
(25). All efforts were made to
minimize animal suffering and to reduce the number of animals used.
All experimental procedures were performed in accordance with the
NIH Guide for the Care and Use of Laboratory Animals (NIH
publication no. 80–23, 1996.)
Results
Purification of a fibrin(ogen)olytic
enzyme from M. mauritanica snake venom
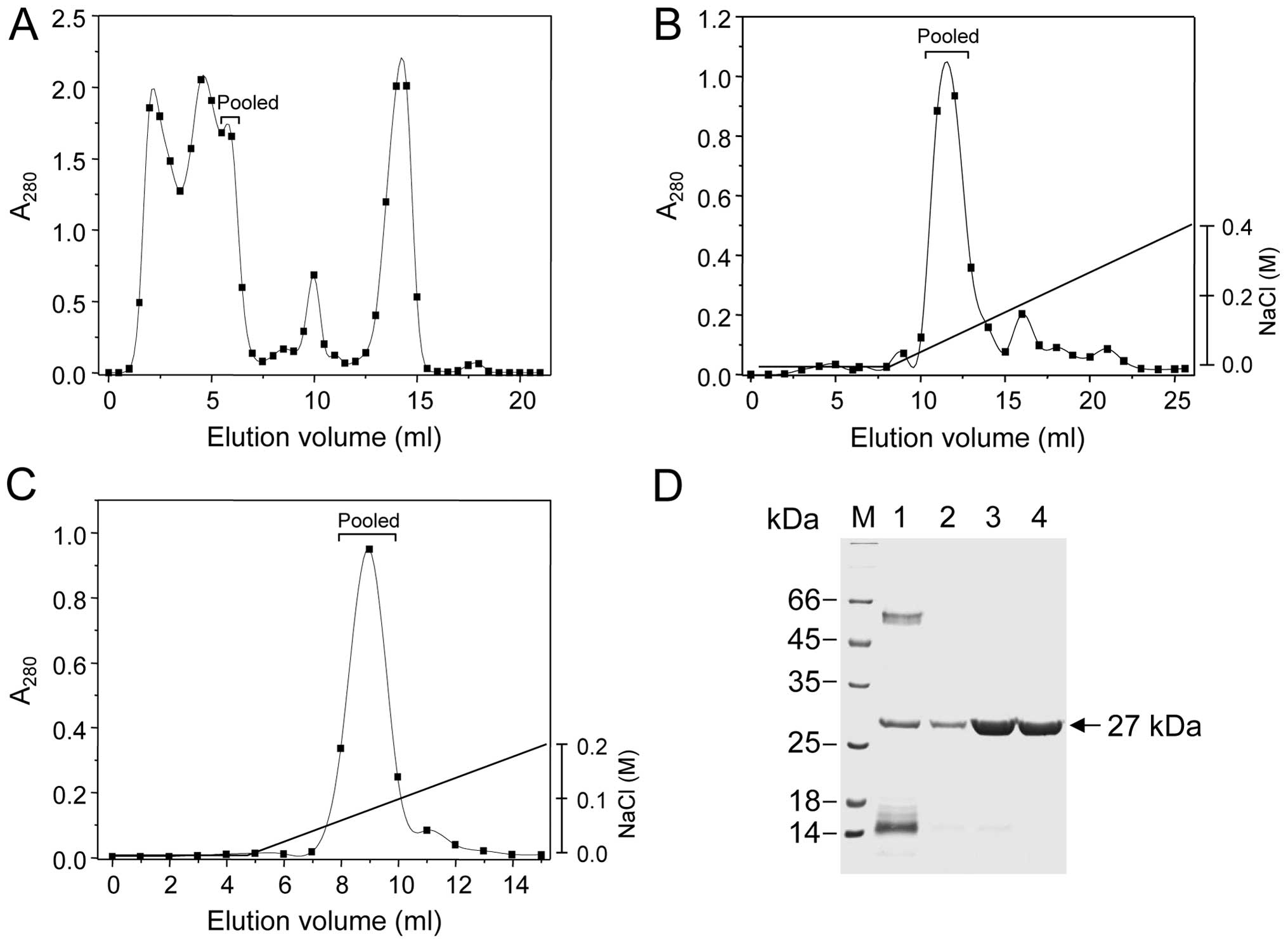
A fibrin(ogen)olytic metalloprotease was purified by
three chromatographic steps, using a size exclusion and two ion
exchanger columns in order. For the purification, the crude venom
powder of M. mauritanica (121.8 mg) was dissolved in
standard buffer and centrifuged for 5 min at 9,000 × g to remove
insoluble materials. The soluble proteins (total 40.6 mg) obtained
were then fractionated by a size exclusion chromatography using a
Superdex 75 10/300 GL column, from which the proteins were eluted
at a flow rate of 0.5 ml/min (Fig.
1A). As shown in the elution profile of Fig. 1A, the proteins were separated into
at least 5 peaks according to their sizes, among which the third
one (12 to 14 in the elution volumes) showed fibrinogenolytic
activity, as judged by FCT assay. From this column chromatography,
a total of 16.8 mg proteins were obtained by pooling the 3 active
fractions (Table I). The proteins
acquired were further separated by an anion exchanger column
chromatography on a Source 15Q 4.6/100 PE column (Fig. 1B). In this chromatography, the
proteins bound were eluted with a NaCl linear gradient of 0 to 400
mM at a flow rate of 1.0 ml/min, from which 3 active fractions (10
to 12 in the elution volumes) were pooled to acquire a total of 4.2
mg proteins (Fig. 1B and Table I). Thereafter, the proteins
obtained were lastly applied and fractionated by another anion
exchanger Mono Q HR 5/5 column chromatography. The proteins bound
were eluted with a NaCl linear gradient ranging from 0 to 200 mM at
flow rate of 0.5 ml/min, and 4 active fractions (7 to 10 in the
elution volumes) were pooled (Fig.
1C). From this last chromatography, a total 2.8 mg of proteins
could be obtained as a purified enzyme (Table I). The purified enzyme was
designated to SVMP-MM. Table I
summarizes the purification results. The specific activity of
purified enzyme was 1,000 U/mg proteins and approximately 2.8 mg of
enzymes were obtained in homogeneity, with 2.3% in yield (Table I).
 | Table ISummary of the purification process
for the SVMP-MM protease from M. mauritanica snake
venom. |
Table I
Summary of the purification process
for the SVMP-MM protease from M. mauritanica snake
venom.
| Purification
step | Total protein
(mg) | Total activity
(U)a | Specific activity
(U/mg) | Yield (%)b |
|---|
| Crude venom | 121.8 | 19,500 | 160 | 100 |
| Superdex 75 | 16.8 | 12,800 | 762 | 13.8 |
| Source Q | 4.2 | 3,360 | 800 | 3.5 |
| Mono Q | 2.8 | 2,800 | 1,000 | 2.3 |
Estimation of molecular weight and
N-terminal amino acid sequence of SVMP-MM
The purified SVMP-MM enzyme appeared as single bands
on an SDS-polyacrylamide gel stained with Coomassie brilliant blue
and the molecular mass was estimated to be 27 kDa (Fig. 1D), a size similar to the
metalloproteases, BlaH1 (28 kDa) from Bothrops lanceolatus
(B. lanceolatus) (26) and
VIF (26 kDa) from M. lebetina venom (27). In addition, SVMP-MM formed just
one peak when it was eluted through gel filtration on a Superdex 75
10/300 GL column (data not shown). These results suggest that
SVMP-MM is a monomeric protease with a small size. The N-terminus
of SVMP-MM was composed of NH2-QRFAPRYIEL-COOH, as
analyzed by amino acid sequencing. The comparison of amino acid
sequences showed that the N-terminal sequence of SVMP-MM was highly
conserved in several metalloproteases derived from snake venoms, in
which BAP1 from Bothrops asper (28), BmooMPα-I from Bothrops
moojeni (29), neuwiedase
from B. neuwiedi (30),
and 3 proteases (VIF, lebetases-II and −4) from M. lebetina
(16,27,31) were included and there was an
average of 80.5% identity between them (Table II).
 | Table IIComparison of the N-terminal amino
acid sequence of SVMP-MM with those of several snake venom
proteases. |
Table II
Comparison of the N-terminal amino
acid sequence of SVMP-MM with those of several snake venom
proteases.
| Snake species | Enzyme | N-terminal
sequencea | Identity (%) | Position of
sequence | Accession no. |
|---|
| M.
mauntanica | SVMP-MM | --QRFAPRYIEL | 100 | 1–10 | This study |
| B.
asper | BAP1 |
--ERFSP-YIEL | 77.8 | 192–202 | P83512 |
| M.
lebetina | Lebetase-II |
--QRFEPRYIEL | 90.0 | 195–205 | Q98995 |
| M.
lebetina | Lebetase-4 |
-QQRFDPRYIEL | 90.0 | 15–25 | Q3ZD74 |
| M.
lebitina | VIF |
--ERFAPRYIEL | 90.0 | 1–10 | P83255 |
| B.
moojeni | BmooMPα-I |
---RFSP-HIEL | 75.0 | 1–8 | 3GBO_A |
| B.
neuwiedi | Neuwiedase |
QQRFFPQRYIEL | 60.0 | 1–12 | Q9I9R4 |
Fibrinogenolytic and fibrinolytic
activities of SVMP-MM
Various plasma proteins, including fibrinogen,
plasminogen and prothrombin, which are involved in blood
coagulation and hemostasis, were digested by the SVMP-MM enzyme
(data not shown). However, there were no detectable plasmin and
thrombin activities when the plasminogen and prothrombin were
incubated with SVMP-MM in the presence of S-2251 (typical substrate
for plasmin) and Boc-VPR-pNA (for thrombin) (data not
shown). These results suggest that SVMP-MM can neither activate
plasminogen nor prothrombin, as other SVMPs can (6,32).
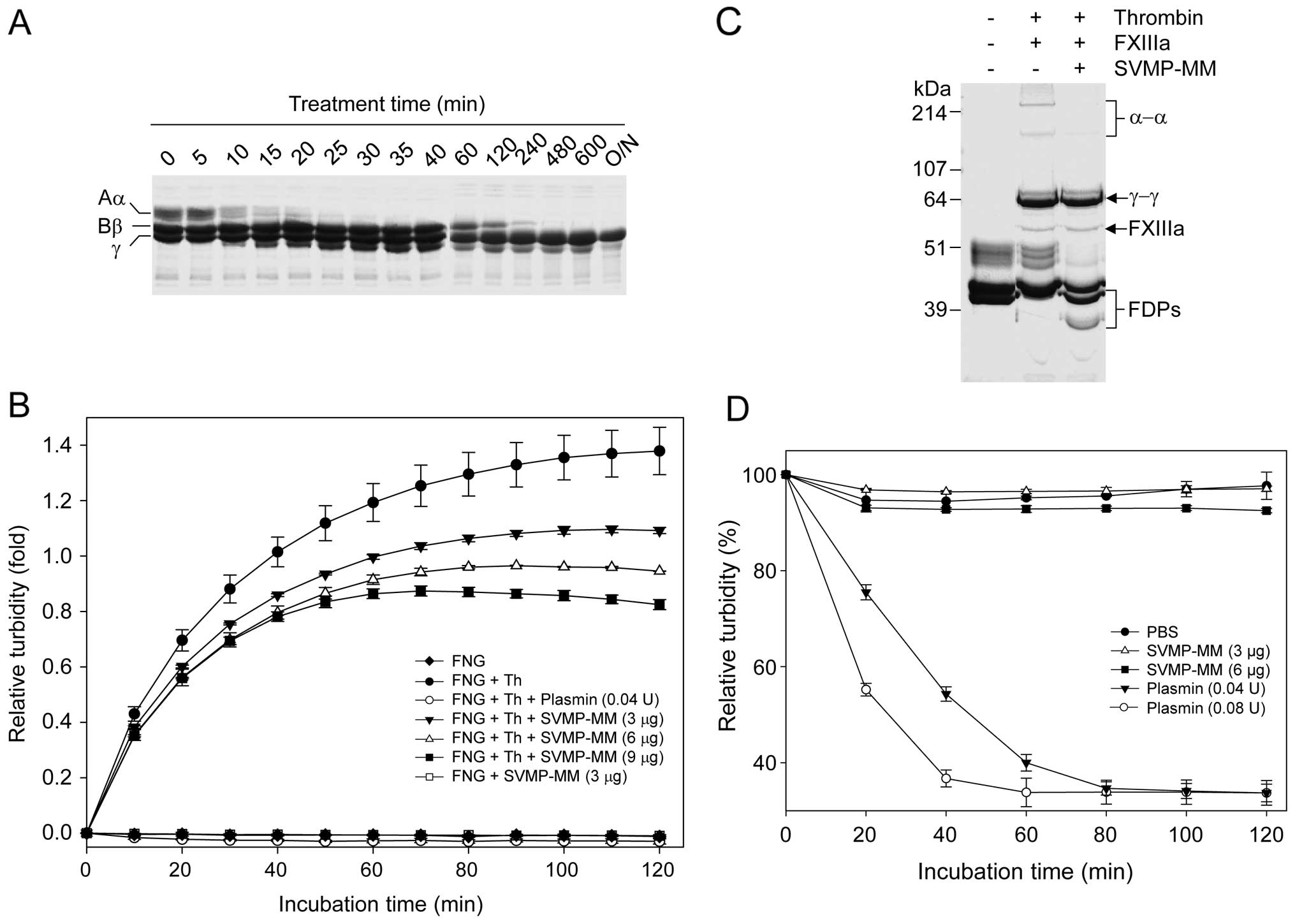
Fibrinogen was also one of efficient protein substrates for SVMP-MM
(Fig. 2). The enzyme actively
digested the Aα- and the Bβ-chains of fibrinogen to a different
extent (Fig. 2A). The Aα- and the
Bβ-chains of fibrinogen were completely degraded by the enzyme
within 20 and 480 min, respectively. However, the γ-chain was much
more resistant to digestion by the enzyme, even with overnight
incubation (Fig. 2A). It is well
known that fibrin monomers generated from thrombin-cleaved
fibrinogen can be polymerized spontaneously in the absence of
FXIIIa in vitro, accompanied with an increase in turbidity
as the polymerization proceeds (24). In this study, the spontaneous
polymerization of fibrin monomers generated by thrombin or possibly
SVMP-MM fibrinogen cleavage was examined by measuring the increase
in turbidity. As expected, thrombin increased the turbidity
effectively, but SVMP-MM did not. The thrombin-induced increase in
turbidity was decreased by plasmin since the fibrin polymers were
cleaved by the enzyme (Fig. 2B).
As with plasmin, SVMP-MM decreased the thrombin-induced increase in
turbidity in a dose-dependent manner. These results suggest that
SVMP-MM cannot allow the fibrin monomers to be polymerized
spontaneously when it cleaves fibrinogen. SVMP-MM was also able to
cleave the XL-fibrin formed by thrombin in the presence of FXIIIa
and fibrinogen (Fig. 2C). The
enzyme showed α-α polymer-cleaving activity, accompanied with the
generation of fibrin degrading products (FDPs); however, it could
not digest γ-γ polymers effectively (Fig. 2C), as in the case of its
fibrinogen cleavage (Fig. 2A).
This XL-fibrin-cleaving activity of SVMP-MM was also examined by
turbidity assay (Fig. 2D).
Plasmin clearly decreased the turbidity of XL-fibrin in a
dose-dependent manner, but SVMP-MM did not at the doses used. These
results seem to be related to the inability of SVMP-MM in cleaving
both the γ-chain of fibrinogen and the γ-γ polymers of XL-fibrin
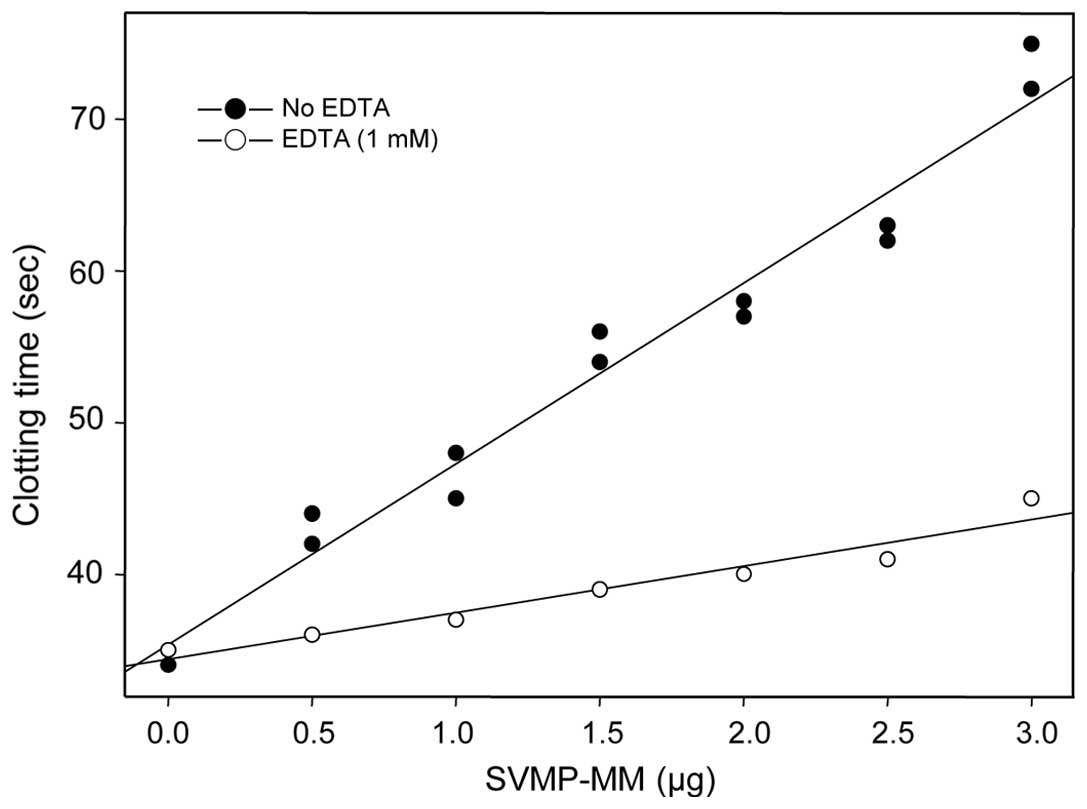
(Fig. 2D). SVMP-MM inhibited the
thrombin-induced clotting time in a dose-dependent manner, as
judged by FCT assay (Fig. 3). The
clotting time was prolonged 2.2-fold, compared to that of the
untreated control; however, the delaying effect of the enzyme was
diminished in the presence of 1 mM of EDTA (Fig. 3), suggesting that SVMP-MM can
delay thrombin-induced clotting time through a defibrinogenation
and this is related directly to the enzyme activity. The results
presented in Figs. 2 and 3 suggest that SVMP-MM belongs to the
fibrin(ogen)ase family which has no clot formation activity, as it
can just cleave predominantly on the Aα-chain and less on both the
Bβ- and the γ-chains of fibrinogen subunits (33,34).
Design and synthesis of a
fluorescence-quenching peptide substrate for SVMP-MM
In this study, a fluorescence-quenching peptide
substrate was designed and synthesized on the basis of the cleavage
site of SVMP-MM on its protein substrate. To locate the cleavage
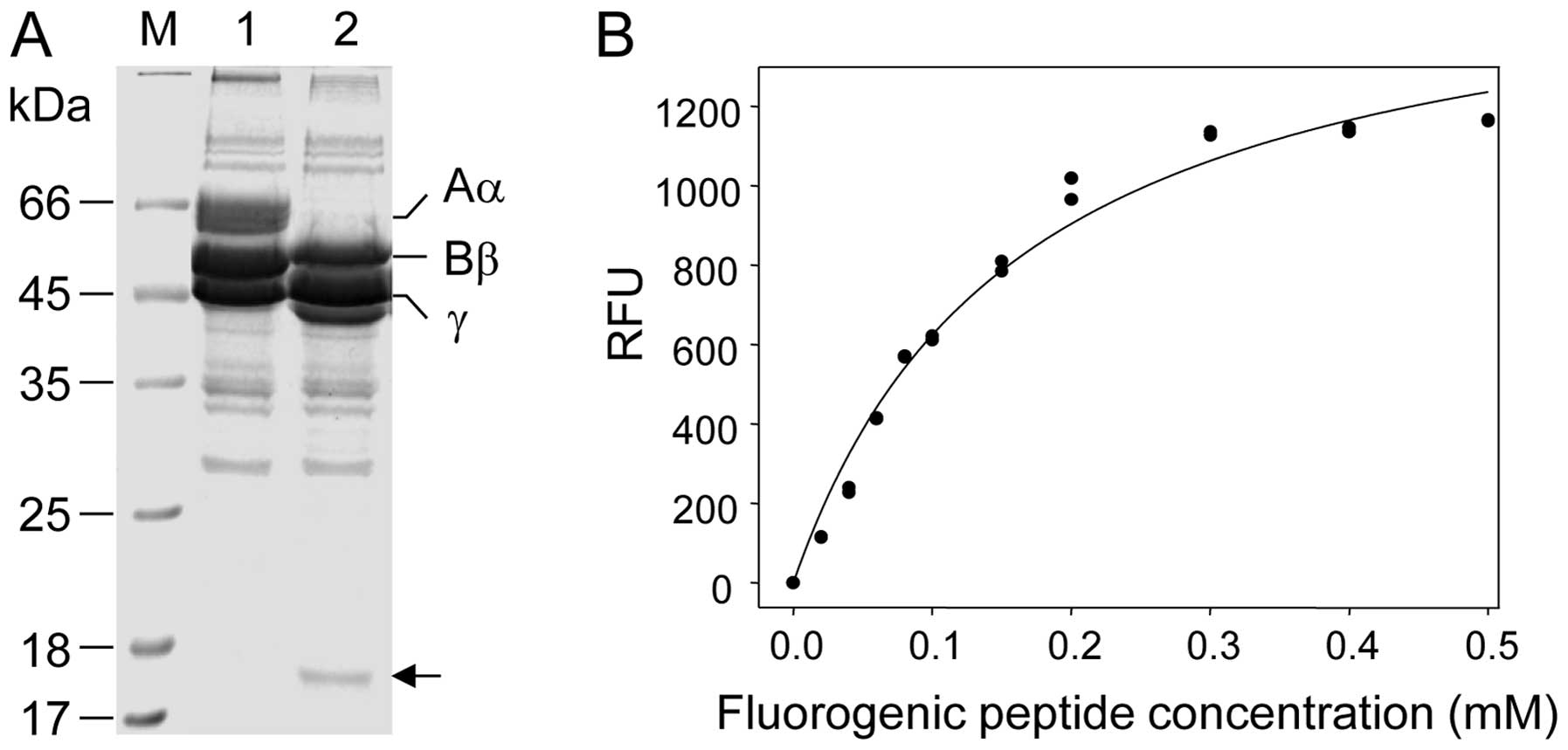
site of the enzyme on the fibrinogen substrate first, 140 μg of
fibrinogen were digested with 1 μg of enzyme for 20 min at 37°C and
the peptide products generated were separated on a 12%
SDS-polyacrylamide gel (Fig. 4A).
Among the peptide fragments produced, a distinct 17.5 kDa protein
band was excised from the gel and its N-terminus was sequenced. The
sequencing results showed that SVMP-MM specifically cleaved the
peptide bond of Lys413 and Leu414, which is
located in the α-chain of fibrinogen (35). On the basis of this result, a
peptide flanked by the Abz and Dnp groups, namely,
Abz-HTEKLVTS-Dnp-NH2 containing SVMP-MM cleavage site
(K-L) was designed and synthesized in order to form a fluorescent
donor (Abz group)-acceptor (Dnp group) pair. Therefore, Abz
fluorescence would be quenched by the Dnp group (36) until the enzyme cleaves the peptide
bond located between K and L residues in the synthetic peptide. The
RFU clearly increased in a dose-dependent manner, when SVMP-MM (3
μg) was incubated with various concentrations of the fluorogenic
substrate and then the fluorescence produced was monitored for 15
min at λex =320 nm and λem =420 nm (Fig. 4B). This result suggests that the
fluorescence-quenching peptide synthesized may be used as a
suitable substrate for elucidating the enzymatic properties of
SVMP-MM.
Enzymatic properties and kinetic
parameters of SVMP-MM
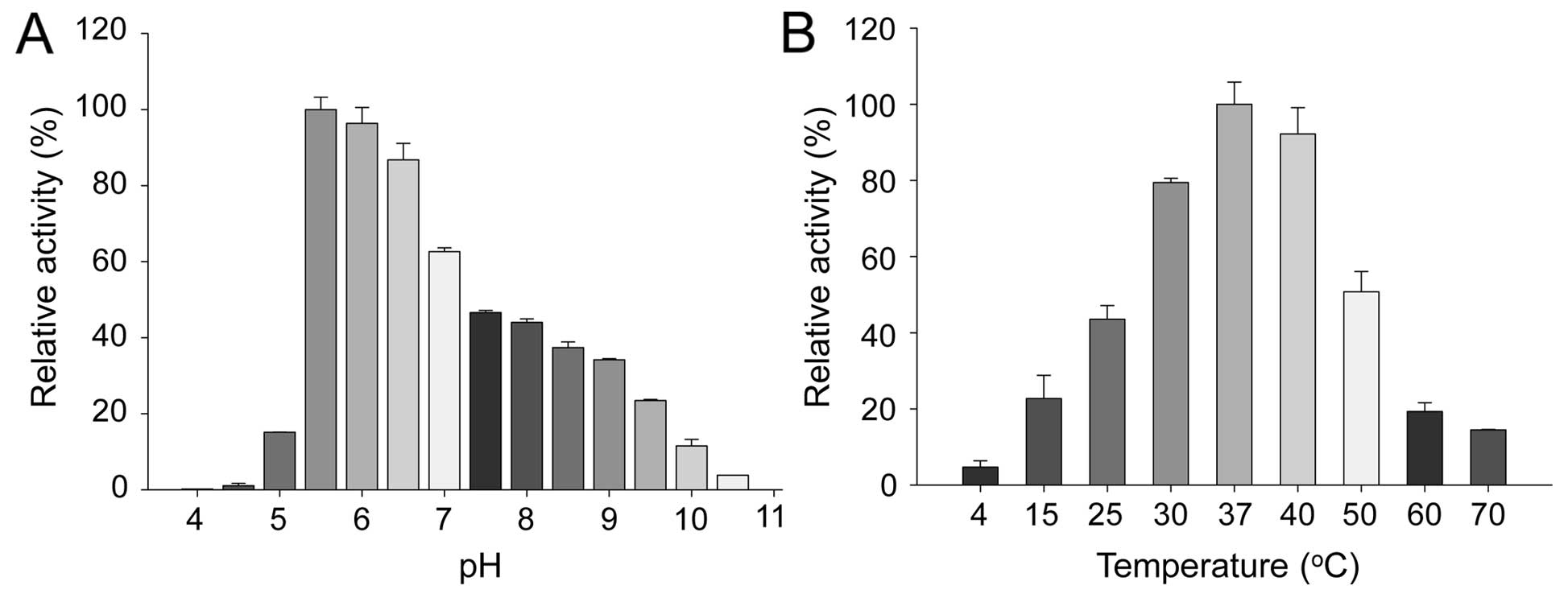
The optimal pH and temperature for SVMP-MM activity
were examined with the fluorescence-quenching peptide,
Abz-HTEKLVTS-Dnp-NH2, as a substrate (Fig. 5). The enzyme exhibited its optimum
pH (Fig. 5A) and temperature
(Fig. 5B) at 5.5 and 37°C,
respectively, with a total loss of activity under pH 10.5 and at
4°C. Considering the pH, temperature and ionic strength (data not
shown) for the maximal enzyme activity, a standard reaction buffer
consisting of 20 mM sodium acetate (pH 5.5) and 100 mM NaCl was
prepared and used for further experiments. The effects of various
divalent cations and protease inhibitors on SVMP-MM activity were
examined (Table III). Divalent
cations, such as Ca2+, Mg2+ and
Mn2+ showed no significant stimulatory or inhibitory
effects on SVMP-MM activity; however, Fe2+ and
Zn2+ (1 mM) exhibited a slight inhibitory effect on the
enzyme activity at different levels. However, a low dose of
Zn2+ (<0.1 mM) did not show such a significant
inhibitory effect on the activity (Table III). In addition,
Ni2+ and Cu2+ showed relatively strong
inhibitory effects on enzyme activity, with an average of 69.6%
inhibition, compared to those of the untreated control. More
importantly, the enzyme activity was clearly inhibited by treatment
with 1,10-PT, EDTA and EGTA, which are all known metalloprotease
inhibitors (37). Among the
metalloprotease inhibitors used, bestatin did not act as an
effective inhibitor against the enzyme activity. However, TLCK,
TPCK, PMSF and aprotinin, which are typical serine protease
inhibitors (5), showed no
significant inhibitory effects on the enzyme activity (Table III). These results suggest that
SVMP-MM is a typical metalloprotease requiring a certain metal ion
as a co-factor for the enzymatic activity and not a serine
protease. In addition, the enzyme activity was completely abolished
by treatment with a reducing agent, such as DTT (Table III). This result suggests that a
disulfide bond(s) located in the enzyme plays an important role in
maintaining the enzyme activity. On the other hand, the enzyme
kinetic parameters for SVMP-MM were also estimated using the
fluorescence-quenching peptide as a substrate (Table IV). The KM and
kcat values for the enzyme were estimated to be
0.015 mM and 0.031 sec−1, respectively, when
Abz-HTEKLVTS-Dnp-NH2 was used as a substrate (Table IV). The
kcat/KM value of SVMP-MM was
found to be 20.67 mM−1sec−1, as well with the
same fluorogenic substrate (Table
IV).
 | Table IIIEffects of various divalent cations,
protease inhibitors and chemical reagents on SVMP-MM activity. |
Table III
Effects of various divalent cations,
protease inhibitors and chemical reagents on SVMP-MM activity.
| Additive | Concentration
(mM) | Relative activity
(%)a |
|---|
| Control | 0 | 100 |
|
Ca2+ | 1 | 95.1 |
|
Cu2+ | 1 | 17.6 |
|
Fe2+ | 1 | 88.6 |
|
Mg2+ | 1 | 98.2 |
|
Mn2+ | 1 | 104.1 |
|
Ni2+ | 1 | 43.2 |
|
Zn2+ | 0.1 | 95.8 |
|
Zn2+ | 1 | 78.5 |
| TLCK | 1 | 103.5 |
| TPCK | 1 | 99.7 |
| EDTA | 1 | 0 |
| 1,10-PT | 1 | 1.2 |
| Bestatin | 0.01 | 81.6 |
| PMSF | 1 | 92.6 |
| Aprotinin | 1 | 97.9 |
| EGTA | 1 | 3.1 |
| DTT | 1 | 0 |
 | Table IVKinetic parameters for the cleavage
of a fluorogenic peptide substrate by SVMP-MM. |
Table IV
Kinetic parameters for the cleavage
of a fluorogenic peptide substrate by SVMP-MM.
| Substrate used |
KM (mM)a |
Kcat
(sec−1)a |
Kcat/KM
(mM−1sec−1) |
|---|
|
Abz-HTEKLVTS(Dnp)-NH2 | 0.015±0.009 | 0.31±0.042 | 20.67 |
Induction of vascular permeability and
digestion of type IV collagen by SVMP-MM
In general, vascular permeability is a leakage of
fluids and molecules from the blood stream to the extravascular
space when the vascular basement membrane comprised of various
protein components, such as laminin and type IV collagen is
destroyed under a circumstance (38). This leakage can also be enhanced
by effectors, such as inflammatory mediators and vascular
endothelial growth factor (VEGF) (39). In this study, the activity of
SVMP-MM related to the induction of vascular permeability was also
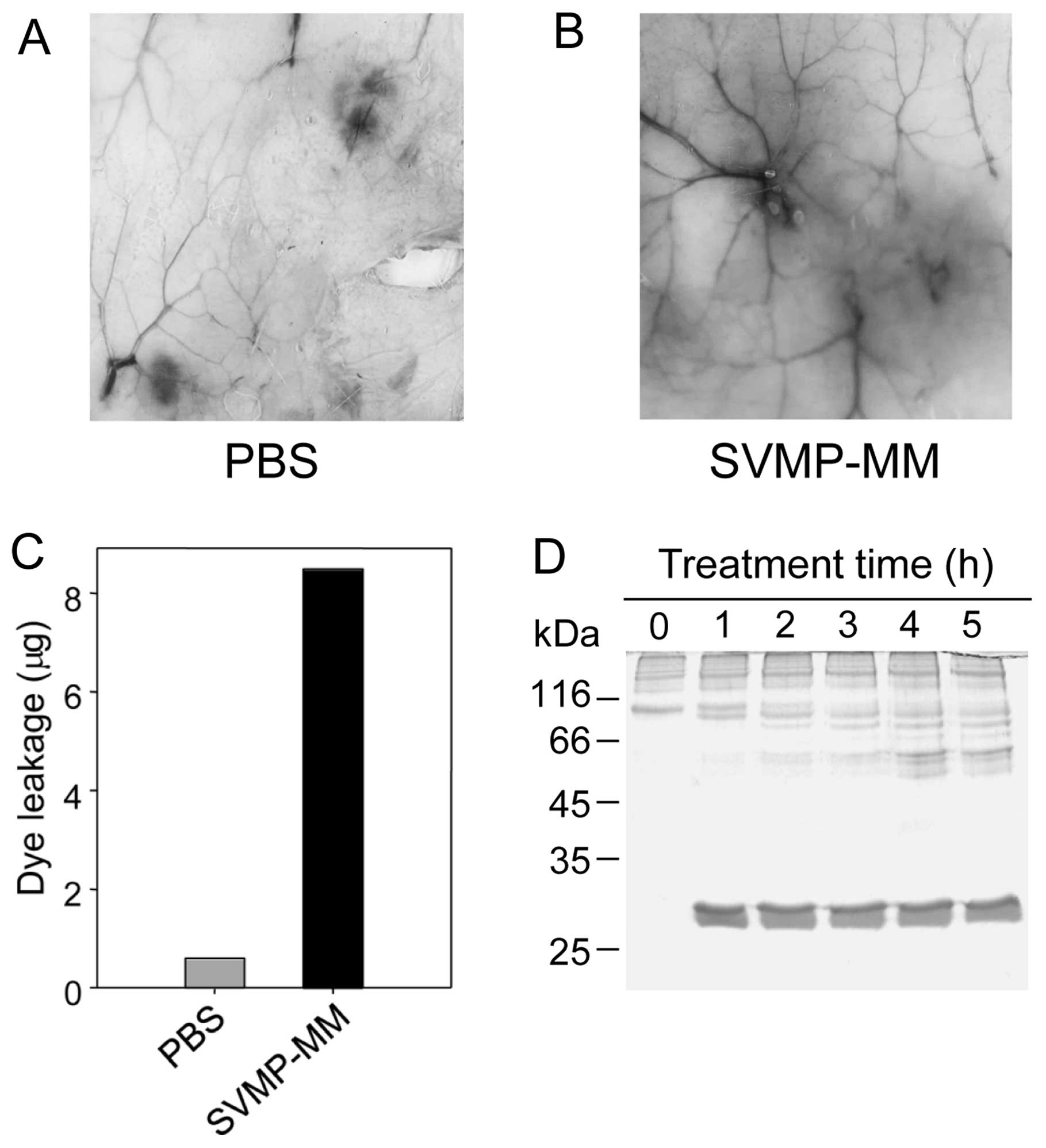
observed in vivo (Fig. 6)
using Miles assay as described in the Materials and methods, and as
previously described (8,25). As shown in Fig. 6A–C, PBS only did not evoke the
leakage of Evans blue dye (Fig.
6A); however, SVMP-MM induced vascular permeability (Fig. 6B), during which approximately 8 μg
of dye leakage occurred following the injection of 10 μg of SVMP-MM
(Fig. C). In addition, the enzyme
digested type IV collagen, which is a major component of the
vascular basement membrane (Fig.
6D). These results suggest that the induction of vascular
permeability may be related to the SVMP-MM activity capable of
digesting some basal membranes and extracellular matrix proteins,
such as type IV collagen (32,39).
Discussion
In this study, a novel fibrin(ogen)olytic
metalloprotease termed SVMP-MM from the M. mauritanica snake
venom was purified and biochemically characterized in terms of
enzyme kinetics and substrate specificity. The enzyme was purified
homogeneously by three chromatographic steps, including one size
exclusion and two sorts of anion exchangers in order (Fig. 1). The purified SVMP-MM is a
monomeric protease and 27 kDa in size, as judged by SDS-PAGE
(Fig. 1D) and a gel filtration
(data not shown). The size of SVMP-MM seems to be similar to that
of several snake venom metalloproteases, such as BlaH1 (28 kDa)
(26) and VIF (26 kDa) (27). In addition, the SVMP-MM has an
amino acid stretch (that is N-QRFAPRYIEL-C) at its N-terminus,
which shows high homology with those of metalloproteases, including
BAP1 (28), lebetase-II,
lebetase-4 and VIF (16,17,27,31,40) (Table II).
SVMP-MM actively digested various plasma proteins,
including fibrinogen, plasminogen, and prothrombin, which are
participating in blood coagulation and hemostasis. However, the
enzyme did not show any amidolytic activity towards typical
chromogenic substrates for plasmin (S-2251) and for thrombin
(Boc-VPR-pNA). In addition, there was no occurrence of
spontaneous polymerization of fibrin monomers, possibly produced
from the fibrinogen cleavage by SVMP-MM, as judged by turbidity
assay (Fig. 2B). These results
demonstrate that the enzyme does not act as a plasminogen or
prothrombin activator, as do other SVMPs from snake venom (6,32,41).
It should be noted that the Aα-chain of fibrinogen
was completely digested by the SVMP-MM protease for 20 min, while
the Bβ- and the γ-chains remained intact for the same incubation
time (Fig. 2A). As regards the
Bβ-chain of fibrinogen, it was completely digested after 480 min of
incubation. However, the γ-chain and the γ-γ polymer of
fibrin(ogen) were not completed digested by the enzyme (Fig. 2A and D). A number of SVMPs,
including fibrolase from Agkistrodon contortrix contortrix
(A. contortrix contortrix) (42), atroxase from Crotalus atrox
(C. atrox) (9) and
lebetase from M. lebetina (43) preferentially cleave the Aα-chain
and the Bβ-chain of fibrinogen slowly, together with their
digesting activities which favor towards the α-α polymer of fibrin.
However, they cannot cleave the γ-chain and the γ-γ polymer of
fibrin(ogen), apart from atrolysin F from C. atrox (9). Therefore, SVMP-MM seems to be a
fibrin(ogen)ase belonging to snake venom metalloprotease family,
which can digest both the Aα- and the Bβ-chains of fibrinogen.
Azocasein assay is routinely used for examining the
proteolytic activity of most proteases (22,24). Unfortunately, SVMP-MM did not
effectively cleave azocasein as a substrate. Therefore, the assay
seemed not to be suitable for examining SVMP-MM activity. The
enzyme also showed only a background level of amidolytic activity
against various chromogenic substrates listed in the Materials and
methods. This inability of the amidolytic activity of the enzyme
towards the chromogenic substrates may be due to its ability to
cleave the peptide bond only at the amino side (termed
forward-amino acid cleaving activity), rather than at the carboxyl
side of the peptide substrate. Since the chromogenic substrates
used in this study are all coupled to pNA groups at their
carboxyl sides, an amino acid-tethered pNA would be released
by the enzyme action, which makes no measurable yellowish color
change. For example, one protease of SVMPs from B. moojeni
venom shows the forward-serine cleaving activity, accordingly the
enzyme activity can be estimated only with a fluorescence-quenching
peptide substrate MCA-GXXPSXQED-Dnp (29). Thus, it was absolutely necessary
to obtain a sensitive peptide substrate for SVMP-MM, which could be
used for investigating the pH- and temperature-dependencies, the
responsiveness to various protease inhibitors and salts, and the
determination of kinetic parameters. One way to prepare a peptide
substrate for SVMP-MM was to synthesize it on the basis of the
cleavage site of enzyme. Since SVMP-MM specifially cleaved the K-L
bond located in the α-chain of fibrinogen substrate (Fig. 2A), an octameric
fluorescence-quenching peptide containing the K-L cleavage site was
synthesized, to which Abz (as a fluorescent donor) and Dnp (as a
quencher) groups were attached at the N- and the C-termini,
respectively (36,44). The SVMP-MM activity was
sensitively assayed with this fluorescence-quenching peptide as a
substrate by monitoring the increase in RFU at λex =320
nm and λem =420 nm (Fig.
2B). The results revealed that the fluorescence-quenching
peptide synthesized may be used for effectively testing the
activity of SVMP-MM from crude venom.
SVMP-MM showed its maximum activity at 37°C and
under pH 5.5 (Fig. 5). Although
one type of SVMP, such as CcHaseII from Cerastes cerastes
also shows the maximal activity at the same conditions (45), similar to SVMP-MM, the relative
weak acidic pH requirement for the SVMP-MM activity seems to be a
slightly different characteristic from other SVMPs that exhibit
optimal activities, in general, under neutral or weak alkalic
conditions (16,47,48). The enzyme activity of SVMP-MM was
greatly decreased at low temperatures (approximately 4°C) (Fig. 5B). This cold sensitivity of the
enzyme was not expected as the majority of SVMPs, such as CcHaseII
(45) and BpirMP (46) are still active at this
temperature. The enzyme activity of SVMP-MM was inhibited by
Zn2+ at 1 mM, and did not show significance at a low
dose (Table III). This property
is in accordance with those of some metalloproteases that contain
Zn2+ in their catalytic centers (30). The enzyme activity of SVMP-MM was
completedly inhibited by a reducing agent DTT (Table III), demonstrating that a
disulfide bond(s) located in the enzyme may play an important role
in maintaining the enzyme activity. A number of SVMPs, including
BAP1, lebetases, BmooMPα-I, and neuwiedase are also completely
inhibited by DTT (30).
SVMPs are classified as P-I, P-II and P-III
classes, depending on their size and domain structure differences
(30). P-I proteases are small
SVMPs in size (molecular masses of 20–30 kDa) and contain only a
pro and a catalytic domain. P-II SVMPs are medium-sized enzymes
(30–60 kDa) and are composed of pro, catalytic and disintegrin
domains. P-IIIs are large enzymes (60–100 kDa) and contain pro,
catalytic, disintegrin-like and cysteine-rich domains (29,30). A recent study on the crystal
structures of P-I class SVMPs have demonstrated that SVMPs have a
consensus HEXXHXXGXXHD sequence and a Met-turn structure that
contains a conserved Met residue forming a hydrophobic basement for
the three zinc-binding histidine residues in the consensus sequence
(30). By these criteria, SVMP-MM
can be categorized as a P-I class enzyme, as its molecular mass is
27 kDa composed of a single polypeptide (Fig. 1D) and its activity is completely
inhibited by typical metalloprotease inhibitors, such as 1,10-PT
and EGTA (Table III). However,
further studies are required to reveal the domain structure of
SVMP-MM and whether the enzyme has a catalytic domain containing a
consensus Zn2+ binding motif and Met-turn structure,
even though it has a high homology amino acid stretch at its
N-terminus with those of typical P-I enzymes (Table II).
On the other hand, SVMP-MM has two typical
activities: i) to induce a vascular permeability, as judged by
Miles assay (Fig. 6B and C); and
ii) to cleave type IV collagen (Fig.
6D), one of the basal membrane and extracellular matrix
proteins, together with other proteins, such as fibronectin and
laminin (32). Moreover, it also
hydrolyzes plasma proteins, such as prothrombin, plasminogen,
fibrinogen and XL-fibrin, which are involved in blood coagulation
and hemostasis. Therefore, the ability of SVMP-MM to induce
vascular permeability and degrade the plasma proteins and type IV
collagen may likely be related to the disturbance of blood
hemostasis by the enzyme. However, it cannot be ruled out that
SVMP-MM can induce vascular permeability by activating mast cells
to release mediators, such as histamine, which provoke a rapid
increase in plasma extravasation in animal skin (49). To verify the direct induction
ability of SVMP-MM, further studies related to the activation of
mast cells and the release of cellular mediators, such as histamine
are required.
Taken together, the data from present study
demonstrate that: i) SVMP-MM is a metalloprotease which is small in
size (27 kDa molecular mass) from M. mauritanica snake venom
(Fig. 1D); ii) it can cleave
fibrinogen and XL-fibrin (Fig. 2)
without having prothrombin- and prothrombin-activating abilities
(data not shown); iii) it is very sensitive to divalent cation
chelators, such as EDTA and EGTA and a reducing agent, such as DTT
(Table III); iv) it can be
inhibited by a typical metalloprotease inhibitor, such as 1,10-PT
(Table III); v) it has a high
sequence identity at its N-terminus to those of typical P-I SVMPs
(Table II); and vi) it can
induce vascular permeability by digesting type IV collagen
(Fig. 6). In conclusion, these
results demonstrate that SVMP-MM is a fibrin(ogen)olytic
metalloprotease of the P-I class, which can induce a hemorrhagic
reaction in vivo.
Acknowledgements
This study was supported by a research fund from
Chosun University, 2013.
Abbreviations:
|
1,10-PT
|
1,10-phenanthroline
|
|
Abz
|
o-aminobenzoic acid
|
|
CAPS
|
3-(cyclohexylamino)-1-propanesulfonic
acid
|
|
DMF
|
dimethylformamide
|
|
Dnp
|
2,4-dinitrophenyl
|
|
DTT
|
dithiothreitol
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
FV
|
factor V
|
|
EGTA
|
glycol-bis-(2-aminoethylether)-N,N,N′,N′-tetraacetic
acid
|
|
FX
|
factor X
|
|
FXIIIa
|
factor XIIIa
|
|
PBS
|
phosphate-buffered saline
|
|
PMSF
|
phenylmethanesulfonyl fluoride
|
|
pNA
|
para-nitroaniline
|
|
PVDF
|
polyvinylidene fluoride
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
serpin
|
serine proteinase inhibitors
|
|
TEMED
|
tetramethylethylenediamine
|
|
TLCK
|
tosyl-lysine chloromethyl ketone
|
|
TPCK
|
tosyl-phenylalanyl chloromethyl
ketone
|
|
XL-fibrin
|
cross-linked fibrin.
|
References
|
1
|
Gutiérrez JM, Escalante T and Rucavado A:
Experimental pathophysiology of systemic alterations induced by
Bothrops asper snake venom. Toxicon. 54:976–987. 2009.
|
|
2
|
Rucavado A, Soto M, Escalante T, Loria GD,
Arni R and Gutiérrez JM: Thrombocytopenia and platelet
hypoaggregation induced by Bothrops asper snake venom.
Toxins involved and their contribution to metalloproteinase-induced
pulmonary hemorrhage. Thromb Haemost. 94:123–131. 2005.PubMed/NCBI
|
|
3
|
Takeda S, Takeya H and Iwanaga S: Snake
venom metalloproteinases: Structure, function and relevance to the
mammalian ADAM/ADAMTS family proteins. Biochim Biophys Acta.
1824:164–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White J: Snake venoms and coagulopathy.
Toxicon. 45:951–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsui T, Fujimura Y and Titani K: Snake
venom proteases affecting hemostasis and thrombosis. Biochim
Biophys Acta. 1477:146–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markland FS Jr and Swenson S: Snake venom
metalloproteinases. Toxicon. 62:3–18. 2013. View Article : Google Scholar
|
|
7
|
Anderson SG and Ownby CL: Systemic
hemorrhage induced by proteinase H from Crotalus adamanteus
(eastern diamondback rattlesnake) venom. Toxicon. 35:1301–1313.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagy JA, Benjamin L, Zeng H, Dvorak AM and
Dvorak HF: Vascular permeability, vascular hyperpermeability and
angiogenesis. Angiogenesis. 11:109–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tu AT, Baker B, Wongvibulsin S and Willis
T: Biochemical characterization of atroxase and nucleotide sequence
encoding the fibrinolytic enzyme. Toxicon. 34:1295–1300. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berger M, Pinto AF and Guimarães JA:
Purification and functional characterization of bothrojaractivase,
a prothrombin-activating metalloproteinase isolated from
Bothrops jararaca snake venom. Toxicon. 51:488–501. 2008.
View Article : Google Scholar
|
|
11
|
Kress LF and Catanese J: Enzymatic
inactivation of human antithrombin III. Limited proteolysis of the
inhibitor by snake venom proteinases in the presence of heparin.
Biochim Biophys Acta. 615:178–186. 1980. View Article : Google Scholar
|
|
12
|
Garrigues T, Dauga C, Ferquel E, Choumet V
and Failloux A-B: Molecular phylogeny of Vipera Laurenti,
1768 and the related genera Macrovipera (Reuss, 1927) and
Daboia (Gray, 1842), with comments about neurotoxic
Vipera aspis aspis populations. Mol Phylogenet Evol.
35:35–47. 2005.
|
|
13
|
Lago NR, de Adolfo Roodt R, Archundia I,
et al: Local damage produced by Vipera and
Macrovipera venoms and some immunochemical characteristics.
Toxicon. 60:2272012.
|
|
14
|
Archundia IG, de Roodt AR, Ramos-Cerrillo
B, et al: Neutralization of Vipera and Macrovipera
venoms by two experimental polyvalent antisera: A study of
paraspecificity. Toxicon. 57:1049–1056. 2011.PubMed/NCBI
|
|
15
|
Samel M, Subbi J, Siigur J and Siigur E:
Biochemical characterization of fibrinogenolytic serine proteinases
from Vipera lebetina snake venom. Toxicon. 40:51–54. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siigur J, Samel M, Tõnismägi K, Subbi J,
Siigur E and Tu AT: Biochemical characterization of lebetase, a
direct-acting fibrinolytic enzyme from Vipera lebetina snake
venom. Thromb Res. 90:39–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trummal K, Vija H, Subbi J and Siigur J:
MALDI-TOF mass spectrometry analysis of substrate specificity of
lebetase, a direct-acting fibrinolytic metalloproteinase from
Vipera lebetina snake venom. Biochim Biophys Acta.
1476:331–336. 2000. View Article : Google Scholar
|
|
18
|
Swenson S and Markland FS Jr: Snake venom
fibrin(ogen)olytic enzymes. Toxicon. 45:1021–1039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leonardi A, Fox JW, Trampus-Bakija A and
Krizaj I: Ammodytase, a metalloprotease from Vipera
ammodytes venom, possesses strong fibrinolytic activity.
Toxicon. 49:833–842. 2007.
|
|
20
|
Markland FS Jr: Snake venom
fibrinogenolytic and fibrinolytic enzymes: an updated inventory.
Registry of Exogenous Hemostatic Factors of the Scientific and
Standardization Committee of the International Society on
Thrombosis and Haemostasis. Thromb Haemost. 79:668–674. 1998.
|
|
21
|
Buschek S, Ignjatovic V, Summerhayes R and
Lowe R: The effect of different snake venoms and anti-venoms on
thrombin clotting time in human plasma. Thromb Res. 125:e149–e152.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JW, Park JE, Choi HK, Jung TW, Yoon
SM and Lee JS: Purification and characterization of three
thermostable alkaline fibrinolytic serine proteases from the
polychaete Cirriformia tentaculata. Process Biochem.
48:979–987. 2013. View Article : Google Scholar
|
|
23
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage
T4. Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang AK, Kim HY, Park JE, et al:
Vibrio vulnificus secretes a broad-specificity
metalloprotease capable of interfering with blood homeostasis
through prothrombin activation and fibrinolysis. J Bacteriol.
187:6909–6916. 2005. View Article : Google Scholar
|
|
25
|
Miles AA and Miles EM: Vascular reactions
to histamine, histamine-liberator and leukotaxine in the skin of
guinea-pigs. J Physiol. 118:228–257. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stroka A, Donato JL, Bon C, Hyslop S and
de Araújo AL: Purification and characterization of a hemorrhagic
metalloproteinase from Bothrops lanceolatus (Fer-de-lance)
snake venom. Toxicon. 45:411–420. 2005. View Article : Google Scholar
|
|
27
|
Gasmi A, Srairi N, Karoui H and El Ayeb M:
Amino acid sequence of VlF: identification in the C-terminal domain
of residues common to non-hemorrhagic metalloproteinases from snake
venoms. Biochim Biophys Acta. 1481:209–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe L, Shannon JD, Valente RH, et al:
Amino acid sequence and crystal structure of BaP1, a
metalloproteinase from Bothrops asper snake venom that
exerts multiple tissue-damaging activities. Protein Sci.
12:2273–2281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okamoto DN, Kondo MY, Oliveira LC, et al:
P-I class metalloproteinase from Bothrops moojeni venom is a
post-proline cleaving peptidase with kininogenase activity:
Insights into substrate selectivity and kinetic behavior. Biochim
Biophys Acta. 1844:545–552. 2014.
|
|
30
|
Rodrigues VM, Soares AM, Guerra-Sá R,
Rogrigues V, Fontes MR and Giglio JR: Structural and functional
characterization of neuwiedase, a nonhemorrhagic
fibrin(ogen)olytic. Arch Biochem Biophys. 38:213–224. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siigur E, Aaspõllu A, Tu AT and Siigur J:
cDNA cloning and deduced amino acid sequence of fibrinolytic enzyme
(lebetase) from Vipera lebetina snake venom. Biochem Biophys
Res Commun. 224:229–236. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sajevic T, Leonardi A, Kovačič L, et al:
VaH3, one of the principal hemorrhagins in Vipera ammodytes
ammodytes venom, is a homodimeric P-IIIc metalloproteinase.
Biochimie. 95:1158–1170. 2013.PubMed/NCBI
|
|
33
|
Huang KF, Hung CC, Pan FM, Chow LP,
Tsugita A and Chiou SH: Characterization of multiple
metalloproteinases with fibrinogenolytic activity from the venom of
Taiwan habu (Trimeresurus mucrosquamatus): protein
microsequencing coupled with cDNA sequence analysis. Biochem
Biophys Res Commun. 216:223–233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao R, Li QW, Perrett S and He RQ:
Characterisation of the fibrinogenolytic properties of the buccal
gland secretion from Lampetra japonica. Biochimie.
89:383–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doolittle RF, Watt KWK, Cottrell BA,
Strong DD and Riley M: The amino acid sequence of the alpha-chain
of human fibrinogen. Nature. 280:464–468. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Araujo MC, Melo RL, Cesari MH, Juliano MA,
Juliano L and Carmona AK: Peptidase specificity characterization of
C- and N-terminal catalytic sites of angiotensin I-converting
enzyme. Biochemistry. 39:8519–8525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baker AH, Edwards DR and Murphy G:
Metalloproteinase inhibitors: biological actions and therapeutic
opportunities. J Cell Sci. 115:3719–3727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Norris LA: Blood coagulation. Best Pract
Res Clin Obstet Gynaecol. 17:369–383. 2003. View Article : Google Scholar
|
|
39
|
Yamazaki Y, Nakano Y, Imamura T and Morita
T: Augmentation of vascular permeability of VEGF is enhanced by
KDR-binding proteins. Biochem Biophys Res Commun. 355:693–699.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gasmi A, Srairi N, Guermazi S, Dekhil H,
Karoui H and El Ayeb M: Amino acid structure and characterization
of a heterodimeric disintegrin from Vipera lebetina venom.
Biochim Biophys Acta. 1547:51–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pereira AL, Fritzen M, Faria F, Motta G
and Chudzinski-Tavassi AM: Releasing or expression modulating
mediator involved in hemostasis by Berythractivase and Jararhagin
(SVMPs). Toxicon. 47:788–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Randolph A, Chamberlain SH, Chu HL,
Retzios AD, Markland FS Jr and Masiarz FR: Amino acid sequence of
fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon
contortrix contortrix venom. Protein Sci. 1:590–600. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aaspõllu A, Siigur J and Siigur E: cDNA
cloning of a novel P-I lebetase isoform Le-4. Toxicon. 46:591–594.
2005.PubMed/NCBI
|
|
44
|
Oyama E, Kitagawa Y and Takahashi H:
Primary structure and characterization of a non hemorrhagic
metalloproteinase with fibrinolytic activity, from the snake venom
of Protobothrops tokarensis (Tokara-habu). Toxicon.
70:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wahby AF, Mahdy el SM, El-Mezayen HA,
Salama WH, Abdel-Aty AM and Fahmy AS: Egyptian horned viper
Cerastes cerastes venom hyaluronidase: purification, partial
characterization and evidence for its action as a spreading factor.
Toxicon. 60:1380–1389. 2012.
|
|
46
|
Bernardes CP, Menaldo DL, Camacho E, et
al: Proteomic analysis of Bothrops pirajai snake venom and
characterization of BpirMP, a new P-I metalloproteinase. J
Proteomics. 80:250–267. 2013.
|
|
47
|
Bernardes CP, Santos-Filho NA, Costa TR,
et al: Isolation and structural characterization of a new
fibrin(ogen)olytic metalloproteinase from Bothrops moojeni
snake venom. Toxicon. 51:574–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun MZ, Liu S and Greenaway FT:
Characterization of a fibrinolytic enzyme (ussurenase) from
Agkistrodon blomhoffii ussurensis snake venom: insights into
the effects of Ca2+ on function and structure. Biochim
Biophys Acta. 1764:1340–1348. 2006.PubMed/NCBI
|
|
49
|
Rubinstein I, Nadel JA, Graf PD and
Caughey GH: Mast cell chymase potentiates histamine-induced wheal
formation in the skin of ragweed-allergic dogs. J Clin Invest.
86:555–559. 1990. View Article : Google Scholar : PubMed/NCBI
|