Introduction
Osteoarthritis (OA), one of the most chronic
diseases affecting the joint cartilage of middle-aged and elderly
individuals, is characterized by the degradation of articular
cartilage (1). Chondrocytes, the
only type of cell found in cartilage, controls the balance of
catabolism and anabolism to maintain the structural and functional
integrity of the extracellular matrix (ECM) (2–4).
As cartilage has a poor repair and regeneration capacity, enhancing
chondrocyte proliferation may contribute to the inhibition of the
development and progression of OA (5).
The Wnt/β-catenin signaling pathway plays a crucial
role in the processes of cell proliferation (6). Following the activation of the
Wnt/β-catenin signaling pathway, β-catenin accumulates in the
cytosol, and then translocates to the nucleus, subsequently binding
to the transcription factors, T-cell factor (TCF) and lymphoid
enhancer factor (LEF) (7). These
factors accelerate cell cycle progression by regulating the
expression of cyclin D1, an important factor in the cell cycle.
Radix Achyranthis Bidentatae (AB), has been
widely used in traditional Chinese medicinal formulations for the
clinical treatment of OA (8,9).
In a previous study of ours, we demonstrated that Achyranthes
bidentata polysaccharides (ABPS) extracted from AB induce
chondrocyte proliferation by promoting the G1/S transition
(10). However, the specific
mechanisms involved remain to be fully elucidated. In the present
study, we aimed to determine whether ABPS promote chondrocyte
proliferation by activating the Wnt/β-catenin signaling
pathway.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats at 6 weeks of age were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). The care and use of the animals in the present study were
strictly according to the Guidance Suggestions for the Care and Use
of Laboratory Animals administered by the Ministry of Science and
Technology of China and the Fujian University of Traditional
Chinese Medicine, Fuzhou, China.
Preparation of ABPS
The method used to extract ABPS was the one used in
our previous study (10). The
extracted ABPS were dissolved in Dulbecco’s modified Eagle’s medium
(DMEM; HyClone, Logan, UT, USA) containing 10% fetal bovine serum
(FBS; HyClone), then filtered through a 0.22-μm filter and stored
at 4°C.
Isolation, culture and identification of
chondrocytes
Rat articular chondrocytes were isolated and
cultured as previously described (11). Passage (P) 2 chondrocytes were
identified by type II collagen immunohistochemistry. The
chondrocytes were first treated with 50, 100 and 200 μg/ml ABPS for
48 h, as previously described (10). In order to further verify the
mechanisms involved, the chondrocytes were treated with 0.2 μg/ml
Dickkopf-1 (DKK-1; R&D Systems, Minneapolis, MN, USA) and
treated with ABPS (100 μg/ml) in the presence or absence of DKK-1
for 48 h, as previously described (10,12).
Western blot analysis
Following treatment, the proteins were collected
immediately in lysis buffer and quantified using the BCA method.
Proteins (20 μg) were separated on a 12% SDS-PAGE gel and
transferred onto PVDF membranes. After transferring, the membranes
were blocked and incubated overnight at 4°C with the following
primary antibodies (1:1,000): Wnt-4, Frizzled-2 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), β-catenin, glycogen
synthase kinase 3β(GSK-3β) (Cell Signaling Technology, Inc.,
Beverly, MA, USA), cyclin D1, type II collagen (Bio-Word
Technology, Natong, China) or β-actin (Santa Cruz Biotechnology).
Horseradish peroxidase (HRP)-conjugated secondary antibody (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
was then added to the membranes at room temperature. The
immunocomplexes were visualized using the ECL method. β-actin was
used as an internal control.
RNA extraction and RT-PCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen, Grand Island, NY, USA), and then quantified followed
by being reverse transcribed into cDNA. We performed PCR to
determine the mRNA levels of Wnt-4, β-catenin, Frizzled-2, GSK-3β,
cyclin D1 and type II collagen. The primers used for PCR were as
follows: Wnt-4 forward, 5′-TCA GCC CAC AGG GTT TCC A-3′ and
reverse, 5′-CGC TCG CCA GCA TGT CTT T-3′; β-catenin forward, 5′-AAG
GAA GCT TCC AGA CAT GC-3′ and reverse, 5′-AGC TTG CTC TCT TGA TTG
CC-3′; Frizzled-2 forward, 5′-TCG AGG CCA ATT CGC AGT A-3′ and
reverse, 5′-CAG GAA GGA TGT GCC GAT G-3′; GSK-3β forward, 5′-AAA
GTG CAT CGC TGG CTT A-3′ and reverse, 5′-GTC GAC GGT TTG TTT CCA
AT-3′; cyclin D1 forward, 5′-AAT GCC AGA GGC GGA TGA GA-3′ and
reverse, 5′-GCT TGT GCG GTA GCA GGA GA-3′; type II collagen
forward, 5′-CCA GAG TGG AAG AGC GGA GAC-3′ and reverse, 5′-CAG TGG
ACA GTA GAC GGA GGA AAG-3′; and β-actin forward, 5′-CAC CCG CGA GTA
CAA CCT TC-3′ and reverse, 5′-CCC ATA CCC ACC ATC ACA CC-3′. The
DNA bands were examined using a Gel Documentation system (Model Gel
Doc 2000; Bio-Rad Laboratories, Hercules, CA, USA). β-actin was
used as an internal control.
Immunofluorescence staining
Following treatment with ABPS, the cells were fixed
in ice-cold methanol and permeabilized with 1% Triton X-100 for 10
min. The cells were blocked with 5% bovine serum albumin, then
incubated with rabbit anti-β-catenin antibody overnight at 4°C
followed by incubation with TRITC-conjugated secondary antibody
(Zymed Laboratories, San Francisco, CA, USA) and DAPI staining. The
signal was visualized and images were acquired using a fluorescence
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the means ± standard
deviation. The data were processed using SPSS software version 18.0
with the Student’s-test or ANOVA. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology and identification of
chondrocytes
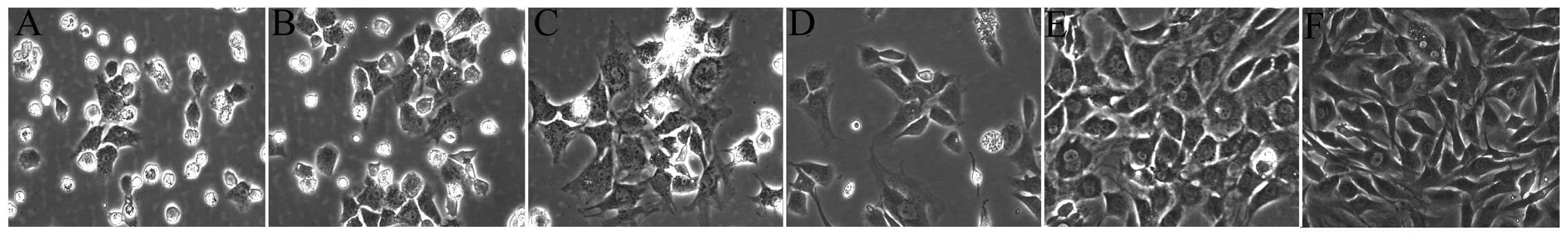
The morphology of the chondrocytes has been
described in previous studies (10,11); the cultured chondrocytes in this
study had the typical characteristics of chondrocytes (Fig. 1). The primary chondrocytes were
small, round cells when first suspended in DMEM. After being
cultured for 2 days, the volumes of cells nestled against the
culture flask became larger and a small number of cells began to
elongate and form protuberances (Fig.
1A). Three days later, the cells showed a fusiform or oval
shape with a clear outline (Fig.
1B). Subsequent to 5 days of proliferation, the cells grew to
be tufted (Fig. 1C). The P1 and
P2 chondrocytes spread across the flask much more rapidly (Fig. 1D, E and F). The morphology of the
P2 chondrocytes showed more typical characteristics of chondrocytes
and the chondrocytes contained abundant levels of type II collagen,
a major secretory molecule of the cartilage extracellular matrix
(ECM). Compared with the negative control, which was not treated
with type II collagen antibody, the cytoplasm was stained brown,
which represented the positive expression of type II collagen in
chondrocytes (Fig. 4G and H).
ABPS increase the expression of Wnt-4,
Frizzled-2, β-catenin and cyclin D1 and decrease the expression of
GSK-3β
To determine the effects of ABPS on the
Wnt/β-catenin signaling pathway in chondrocytes, we used RT-PCR and
western blot analysis to examine the mRNA and protein expression of
Wnt-4, Frizzled-2, β-catenin, GSK-3β and cyclin D1. Compared with
the control group (untreated cells), the protein levels of Wnt-4,
Frizzled-2, β-catenin and cyclin D1 in the ABPS-treated
chondrocytes were significantly upregulated (P<0.01 and
P<0.05), while the protein level of GSK-3β was significantly
downregulated (P<0.01 and P<0.05) (Fig. 2A–F). The mRNA expression of Wnt-4,
Frizzled-2, β-catenin, GSK-3β and cyclin D1 was similar to their
respective protein levels (Fig.
3A–F).
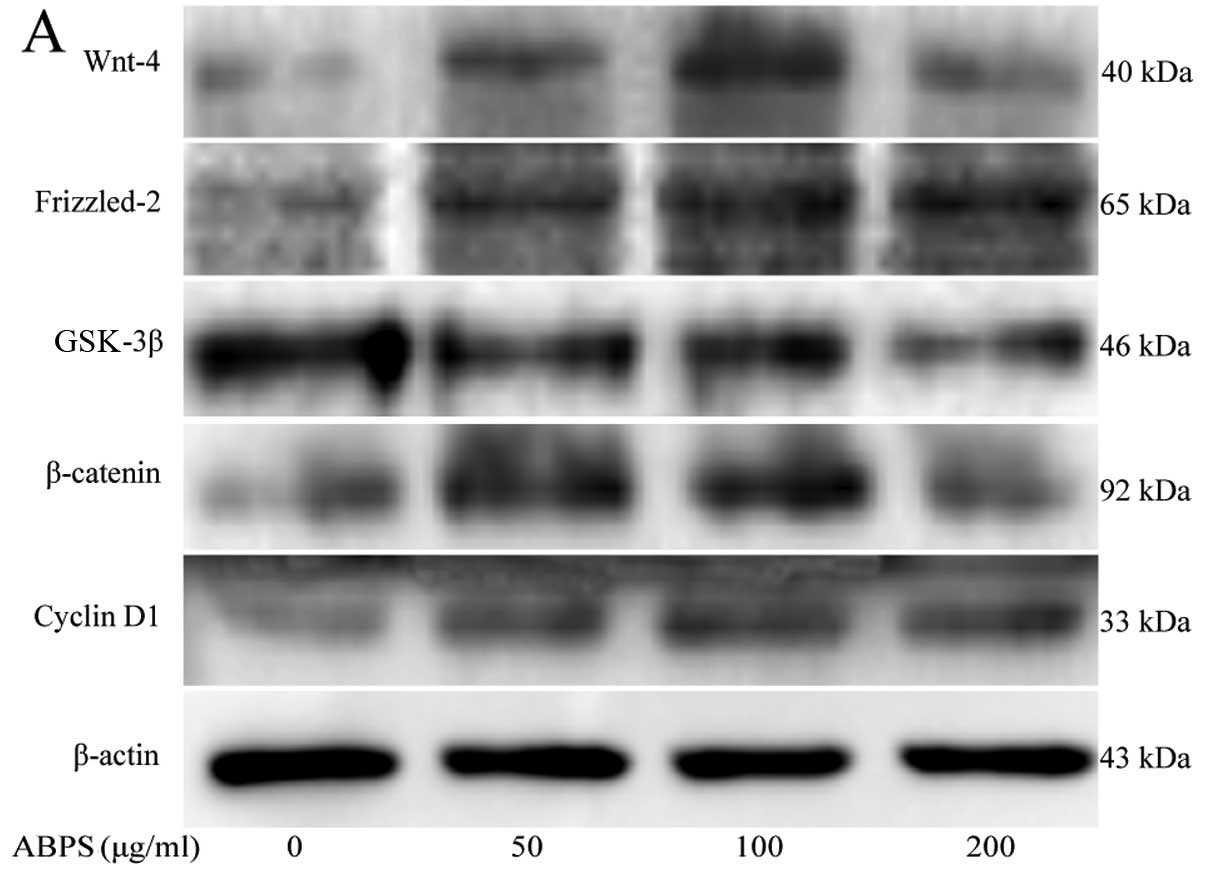 | Figure 2Protein levels of Wnt-4, Frizzled-2,
glycogen synthase kinase (GSK)-3β, β-catenin, cyclin D1 and type II
collagen in chondrocytes following treatment with Achyranthes
bidentata polysaccharides (ABPS). (A) Western blots showing the
protein levels of Wnt-4, Frizzled-2, GSK-3β, β-catenin, cyclin D1
and collagen II. β-actin was used as the internal control. (B–G)
Quantification of the protein levels of Wnt-4, Frizzled-2, GSK-3β,
β-catenin, cyclin D1 and type II collagen by western blot analysis.
**P<0.01, *P<0.05, compared with the
control group (untreated cells). |
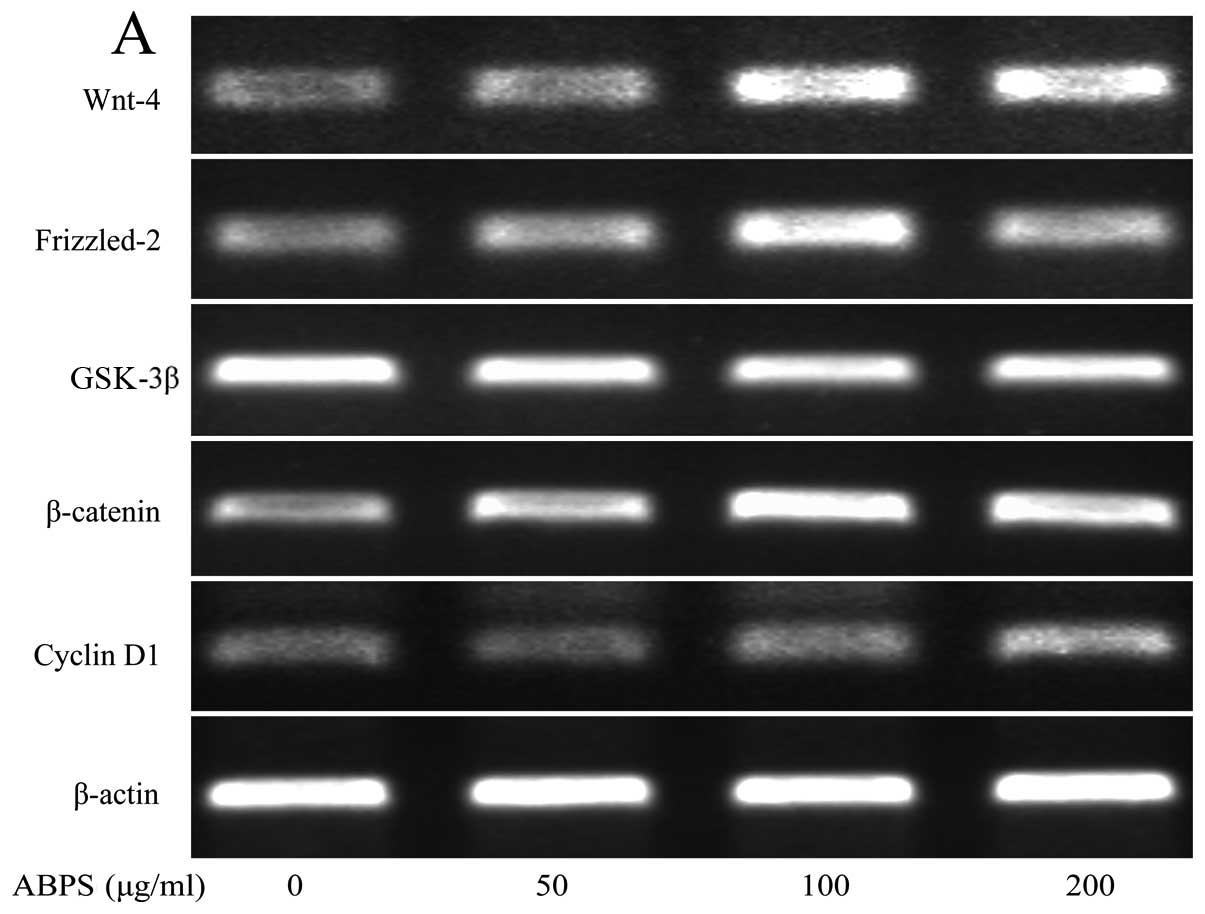 | Figure 3Achyranthes bidentata
polysaccharides (ABPS) increase the mRNA expression of Wnt-4,
Frizzled-2, β-catenin, cyclin D1 and type II collagen, and decrease
the expression of glycogen synthase kinase (GSK-3β). (A) mRNA
expression of Wnt-4, Frizzled-2, GSK-3β, β-catenin, cyclin D1 and
collagen II analyzed by RT-PCR. (B–G) Quantification of mRNA
expression of Wnt-4, Frizzled-2, GSK-3β, β-catenin, cyclin D1 and
type II collagen. **P<0.01, *P<0.05,
compared with the control group (untreated cells). |
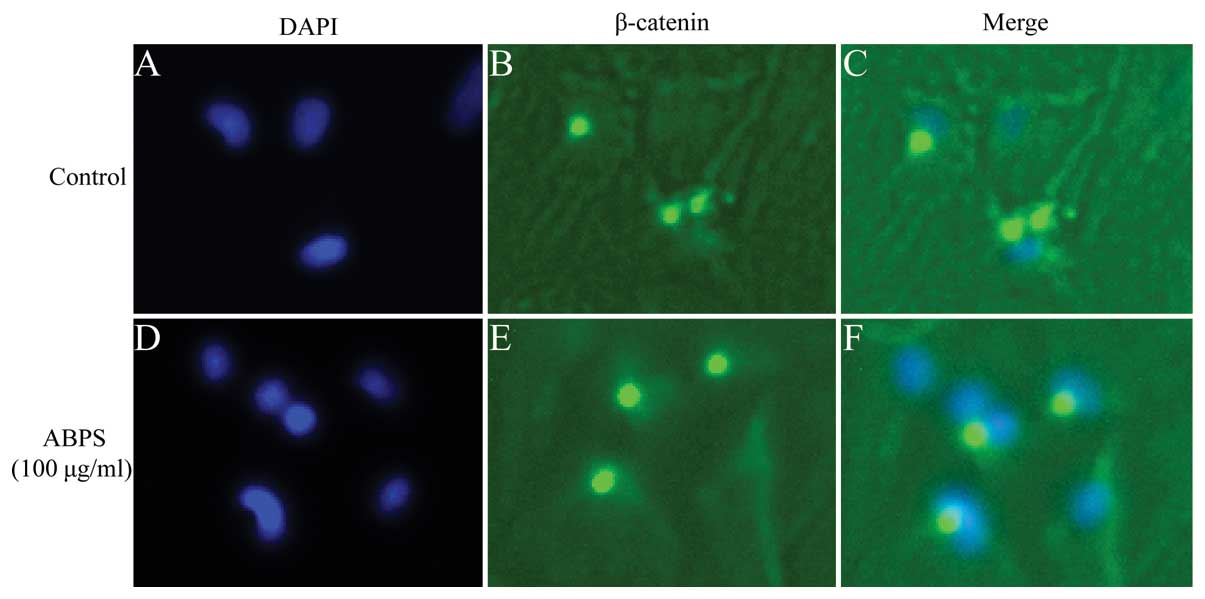
ABPS promote β-catenin nuclear
translocation
The effects of ABPS on the nuclear translocation of
β-catenin were further confirmed by immunouorescence staining.
Immunouorescence staining revealed that ABPS markedly promoted the
translocation β-catenin into the nucleus (Fig. 4A–F). β-catenin mainly existed in
the cytoplasm in the untreated cells; however, following treatment
with ABPS, the staining was more intense and localized in the
nucleus. These results demonstrated that ABPS activated the
Wnt/β-catenin signaling pathway by promoting the nuclear
localization of β-catenin.
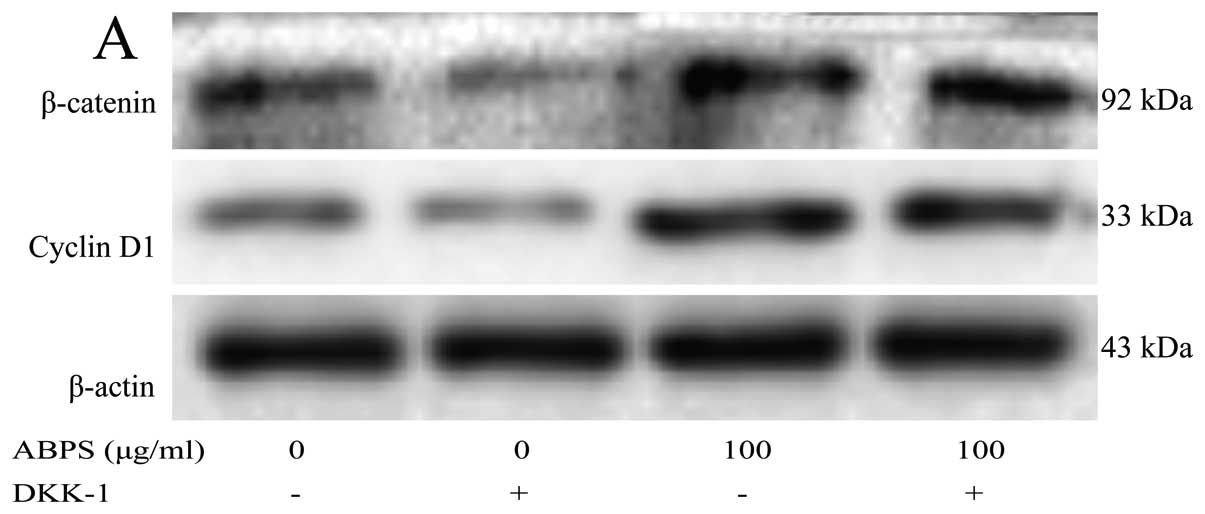
The expression of cyclin D1 and β-catenin
is partly decreased following the inhibition of the Wnt/β-catenin
singaling pathway
To further confirm that ABPS promote chondrocyte
proliferation through the Wnt/β-catenin singaling pathway, DKK-1,
an inhibitor of the Wnt/β-catenin receptor, was added to block the
activation of the Wnt/β-catenin signaling pathway. The results
revealed that the expression of cyclin D1 and β-catenin was partly
inhibited (P<0.01 and P<0.05), indicating that ABPS
participate in the regulation of chondrocyte proliferation through
the Wnt/β-catenin singaling pathway, and perhaps also through other
channels (Fig. 5).
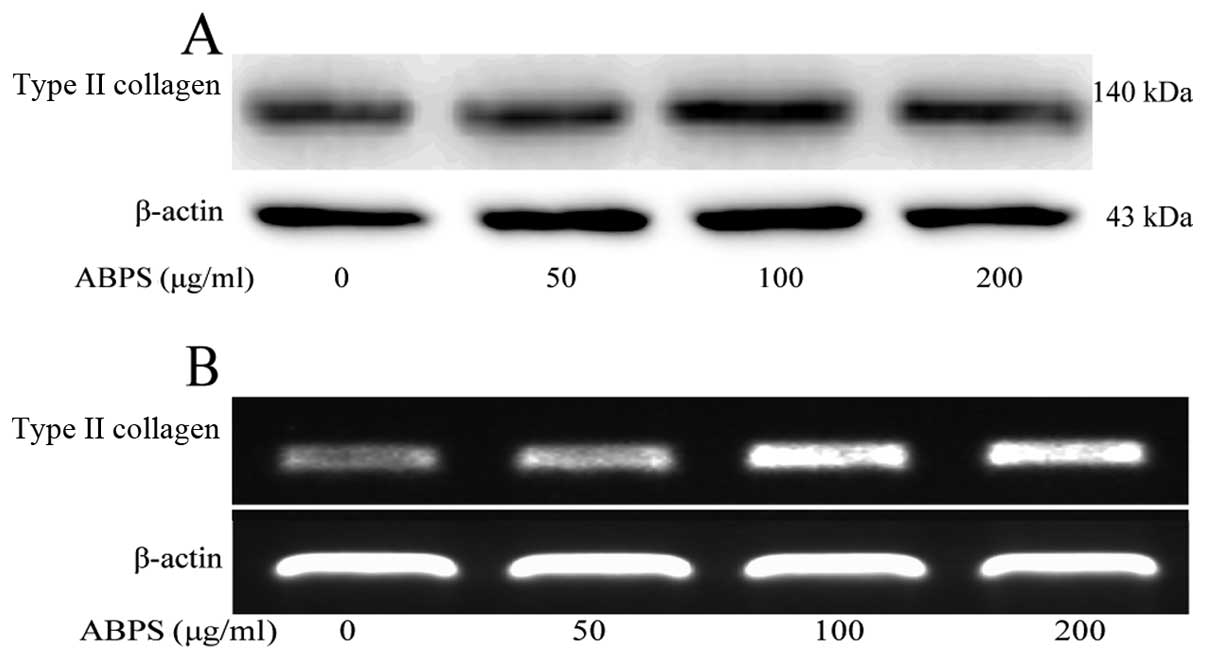
ABPS increase the expression of type II
collagen
The development of articular cartilage degradation
results in the loss of type II collagen, a main constituent of
articular cartilage (13,14); thus, in the present study, we
examined the levels of type II collagen in the chondrocytes. The
results revealed that the expression of type II collagen was
significantly increased compared with the control group (untreated
cells) (P<0.01 and P<0.05) (Figs. 6, 2G and 3G), indicating that ABPS promoted
chondrocyte proliferation by increasing the expression of type II
collagen, a major secretory molecule of the ECM.
Discussion
Polysaccharides, a type of macromolecule with a
broad range of biological activities, have attracted attention as
they play a crucial role in human diseases (15,16). Recently, studies have demonstrated
that polysaccharides contribute to chondrocyte proliferation
(10,11). Thus, polysaccharides may be a
potentially novel therapeutic target for OA by promoting
chondrocyte proliferation. Our results revealed that ABPS
upregulated the expression of Wnt-4, Frizzled-2, β-catenin and
cyclin D1, and downregulated the expression of GSK-3β. ABPS induced
the translocation of β-catenin into the nucleus in the
chondrocytes. Furthermore, the expression of β-catenin and cyclin
D1 was partly decreased following the addition of DKK-1, indicating
that ABPS activated the Wnt/β-catenin signaling pathway to promote
chondrocyte proliferation.
Currently, western pharmacological intervention that
addresses chronic pain in OA is frequently insufficient or poorly
tolerated (17,18). Non-steroidal anti-inflammatory
drugs (NSAIDs) are the most common treatments for OA (19,20). However, NSAIDs have a wide variety
of side-effects. There are growing interests in herbal medicines,
which seem to have an encouraging risk-benefit profile (21). ABPS, extracted from AB, are
one of the most effective natural elements used for the treatment
of OA.
The Wnt/β-catenin signaling pathway is important to
the regulation of proliferation (22). The Wnt/β-catenin pathway is
activated when Wnt proteins bind to Frizzled family receptors and
low density lipoprotein receptor-related protein (LRP) 5/6, which
results in the activation of dishevelled (Dvl) family proteins
(23). The activation of Dvl
leads to the inhibition of GSK-3β that causes non-phosphorylated
β-catenin to accumulate in the cytoplasm and migrate to the nucleus
(24). Subsequently, β-catenin
interacts with transcription factors, thus altering the expression
of Wnt/β-catenin signaling target genes, such as cyclin D1, that is
a positive regulator of the G1/S transition. Our results
demonstrated that ABPS increased the expression of Wnt-4,
Frizzled-2, β-catenin and cyclin D1, whereas it decreased the
expression of GSK-3β, and significantly promoted the translocation
of β-catenin into the nucleus. These results suggest that ABPS
activate the Wnt/β-catenin signaling pathway by inhibiting GSK-3β
to promote the translocation of non-phosphorylated β-catenin into
the nucleus to enhance cyclin D1 expression.
DKK-1 binds to LRP5/6 on target cells, resulting in
the inhibition of Wnt/β-catenin signaling pathway by preventing the
binding of Wnt with LRP5/6 (25,26). In the Wnt/β-catenin signaling
pathway, GSK-3β is thought to phosphorylate without Wnt signaling,
and consequently induces the degradation of β-catenin, thus,
decreasing the expression of cyclin D1. Our results suggested that
the expression of β-catenin and cyclin D1 was partly inhibited by
DKK-1, which further demonstrates that ABPS promote chondrocyte
proliferation through the Wnt/β-catenin singaling pathway.
In conclusion, our results demonstrate that ABPS
activate the Wnt/β-catenin signaling pathway, thus contributing to
chondrocyte proliferation. However, further studies are required
using animals to verify this conclusion. In our study, we also
found that ABPS increased the expression of type II collagen in the
chondrocytes, which suggests that ABPS inhibits cartilage
degradation by increasing the expression of type II collagen, a
major secretory molecule of the ECM.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no.
81373818&81102609), the Special Research Fund for Doctor
Discipline in College (20123519110001), the Key Natural Sciences
Foundation of Fujian Province (2014Y0032), and the Natural Science
Foundation of Fujian Province (2014J01357).
References
|
1
|
Sulzbacher I: Osteoarthritis: histology
and pathogenesis. Wien Med Wochenschr. 163:212–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen C, Tambe DT, Deng L and Yang L:
Biomechanical properties and mechanobiology of the articular
chondrocyte. Am J Physiol Cell Physiol. 305:C1202–C1208. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clouet J, Vinatier C, Merceron C, et al:
From osteoarthritis treatments to future regenerative therapies for
cartilage. Drug Discov Today. 14:913–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schroeppel JP, Crist JD, Anderson HC and
Wang J: Molecular regulation of articular chondrocyte function and
its significance in osteoarthritis. Histol Histopathol. 26:377–394.
2011.PubMed/NCBI
|
|
5
|
Ciorba A and Martini A: Tissue engineering
and cartilage regeneration for articular reconstruction. Int J
Pediatr Otorhinolaryngol. 70:1507–1515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aman A, Nguyen M and Piotrowski T:
Wnt/β-catenin dependent cell proliferation underlies segmented
lateral line morphogenesis. Dev Biol. 349:470–482. 2011.
|
|
7
|
Teo JL and Kahn M: The Wnt signaling
pathway in cellular proliferation and differentiation: a tale of
two coactivators. Adv Drug Deliv Rev. 62:1149–1155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen FP, Chang CM, Hwang SJ, Chen YC and
Chen FJ: Chinese herbal prescriptions for osteoarthritis in Taiwan:
analysis of national health insurance dataset. BMC Complement
Altern Med. 14:912014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Q, Liu Z and He J: Achyranthes
bidentata polysaccharide enhances immune response in weaned
piglets. Immunopharmacol Immunotoxicol. 31:253–260. 2009.
View Article : Google Scholar
|
|
10
|
Yu F, Li X, Cai L, et al: Achyranthes
bidentata polysaccharides induce chondrocyte proliferation via
the promotion of the G1/S cell cycle transition. Mol Med Rep.
7:935–940. 2013.
|
|
11
|
Li H, Li X, Liu G, et al: Bauhinia
championi (Benth.) Benth polysaccharides upregulate
Wnt/β-catenin signaling in chondrocytes. Int J Mol Med.
32:1329–1336. 2013.
|
|
12
|
Lim JC, Kania KD, Wijesuriya H, et al:
Activation of β-catenin signalling by GSK-3 inhibition increases
p-glycoprotein expression in brain endothelial cells. J Neurochem.
106:1855–1865. 2008.
|
|
13
|
Poole AR: Biochemical/immunochemical
biomarkers of osteoarthritis: utility for prediction of incident or
progressive osteoarthritis. Rheum Dis Clin North Am. 29:803–818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elsaid KA and Chichester CO: Review:
collagen markers in early arthritic diseases. Clin Chim Acta.
365:68–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu Z, Che J, Ma X and He JM: Effect of
Aloe vera polysaccharides on immunity and antioxidant
activities in oral ulcer animal models. Carbohyd Polym. 75:307–311.
2009.
|
|
16
|
Chen Y, Shen Z and Chen X: Modulatory
effect of Ganoderma lucidum polysaccharides on serum
antioxidant enzymes activities in ovarian cancer rats. Carbohyd
Polym. 78:258–262. 2009.
|
|
17
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: an update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gallelli L, Galasso O, Falcone D, et al:
The effects of nonsteroidal anti-inflammatory drugs on clinical
outcomes, synovial fluid cytokine concentration and signal
transduction pathways in knee osteoarthritis. A randomized open
label trial. Osteoarthritis Cartilage. 21:1400–1408. 2013.
View Article : Google Scholar
|
|
19
|
Flood J: The role of acetaminophen in the
treatment of osteoarthritis. Am J Manag Care. 16:S48–S54. 2010.
|
|
20
|
Bannuru RR, Vaysbrot EE, Sullivan MC and
McAlindon TE: Relative efficacy of hyaluronic acid in comparison
with NSAIDs for knee osteoarthritis: a systematic review and
meta-analysis. Semin Arthritis Rheum. 43:593–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang M, Jung I, Hur J, et al: The
analgesic and anti-inflammatory effect of WIN-34B, a new herbal
formula for osteoarthritis composed of Lonicera japonica
Thunb and Anemarrhena asphodeloides BUNGE in vivo. J
Ethnopharmacol. 131:485–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tao HY, He B, Liu SQ, et al: Effect of
carboxymethylated chitosan on the biosynthesis of NGF and
activation of the Wnt/β-catenin signaling pathway in the
proliferation of Schwann cells. Eur J Pharmacol. 702:85–92.
2013.PubMed/NCBI
|
|
23
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009.
|
|
24
|
Kim W, Kim M and Jho EH: Wnt/β-catenin
signalling: from plasma membrane to nucleus. Biochem J. 450:9–21.
2013.
|
|
25
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signaling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semenov MV, Tamai K, Brott BK, Kuhl M,
Sokol S and He X: Head inducer Dickkopf-1 is a ligand for Wnt
coreceptor LRP6. Curr Biol. 11:951–961. 2001. View Article : Google Scholar : PubMed/NCBI
|