Introduction
Preeclampsia (PE) is one of the leading causes of
maternal and neonatal morbidity and mortality worldwide and is
characterized by a new onset of hypertension and proteinuria after
20 weeks gestation (1). Kidney
damage plays an important role in the pathogenesis of PE. The rates
of complications due to worsening renal function and hypertension
are increased among pregnant women with moderate or severe renal
insufficiency (2). Great advances
have been made in recent years in understanding the pathogenesis of
PE (3). However, further studies
are required in order to fully elucidate the underlying mechanisms
of PE and the development of acute kidney injury and chronic kidney
disease in mothers.
Studies have demonstrated that proteinuria in
pregnant women with PE is due to direct damage to podocytes
(4). Adverse agents that act on
podocytes can induce the proliferation, apoptosis and necrosis of
podocytes, podocyte detachment from the glomerular basement
membrane, as well as a loss of podocyte differentiation markers
(5).
The renin-angiotensin-aldosterone system (RAAS),
particularly renal RAAS, plays an important role in the development
of chronic kidney disease. In recent years, RAS has also been shown
to be involved in the pathogenesis of PE in a series of studies and
has been shown to induce various characteristics of this disease
(6,7). In normal pregnancy (NP), women are
normotensive despite the upregulation of RAS components, suggesting
that there is a counter-regulatory mechanism to reverse the
vasoconstriction and sodium retention induced by angiotensin (Ang)
II (8). In this regard, Valdés
et al (9) found that
plasma levels of angiotensin-(1-7) [Ang-(1-7)] are elevated in
normotensive pregnant women, and that concentrations of urinary
Ang-(1-7) are also markedly increased throughout pregnancy,
indicating that Ang-(1-7) may play a vasodilator role. The
heptapeptide, Ang-(1-7), is an important component of RAS and acts
as a counter-regulatory peptide in RAS, often balancing the
physiological actions of Ang II. It has been shown that Ang-(1-7)
binds to the Mas G protein-coupled receptor to exert many of its
biological effects (10). Merrill
et al (11) found that
Ang-(1-7) was significantly decreased in pregnant women with PE
compared with women NP. Although the levels of renin, Ang I and Ang
II are decreased in pregnant women with PE (12), pregnant women with PE have an
increased sensitivity to Ang II (13), indicating an exaggerated pressor
response. Thus, we hypothesized that the development of proteinuria
in PE may be attributed to the imbalance between the
angiotensin-converting enzyme (ACE)-Ang II-AT1R axis and the
ACE2-Ang-(1-7)-Mas axis and the decreased Ang-(1-7) expression in
the circulation and renal system.
Studies have indicated that Ang-(1-7) plays a
protective role possibly through the downregulation of
mitogen-activated protein kinase (MAPK) phosphorylation in the
kidneys. As previously demonstrated, in cultured rat proximal
tubular cells, Ang-(1-7) potently inhibits the Ang II-stimulated
phosphorylation of ERK1/2, p38 MAPK and JNK (c-Jun N-terminal
kinase), an effect that is reversed by pre-treatment with A779, a
potent and selective Ang-(1-7) antagonist (14). In renal epithelial LLC-PK cells,
high glucose-stimulated protein synthesis and the phosphorylation
of p38 MAPK are also inhibited by Ang-(1-7) (15). Although it appears that podocytes
express ACE2 and are able to generate Ang-(1-7) in culture
(16), to the best of our
knowledge, there is currently no information on the effects of
Ang-(1-7) on podocyte signalling (17).
In the present study, we hypothesized that serum
from pregnant women with PE induces podocyte injury and apoptosis
and that this represents an important mechanism to maintain and
aggravate proteinuria. In addition, we corrected Ang-(1-7)
deficiency in serum from pregnant women with PE in vitro to
explore the protective effects of Ang-(1-7) on podocytes under
these conditions and to elucidate the underlying mechanisms.
Materials and methods
Study participants
From March 2011 to January 2012, pregnant women with
PE (n=20) and gestational age-matched normotensive pregnant women
(n=20) were recruited from the Department of Gynecology and
Obstetrics, The Fifth People’s Hospital of Shanghai, Fudan
University, Shanghai, China. PE was diagnosed by increased blood
pressure (≥140/90 mmHg) and proteinuria (300 mg in one 24-h urine
collection or >1+ by dipstick in a random urine analysis) after
20 weeks of gestation in a previously normotensive and
non-proteinuric woman. Patients with chronic hypertension,
pre-existing proteinuria or renal disease were excluded from this
study. The patients enrolled in the present study were all newly
admitted inpatients and all samples were collected prior to any
medication being administered. The biological study was approved by
the Ethics Committee of the Fifth People’s Hospital of Shanghai.
After obtaining ethical approval and written informed consent from
all participants, blood samples were collected from the pregnant
women with PE and women with NP. Maternal age, parity, gestational
age at delivery, birth weight, blood pressure values, urinary
protein excretion, serum creatinine and serum uric acid, serum urea
levels and estimated glomerular filtration rate (eGFR) were
recorded for each study participant (Table I).
 | Table IClinical characteristics of study
participants. |
Table I
Clinical characteristics of study
participants.
| PE (n=20) | NP (n=20) |
|---|
| Maternal age,
years | 30.3±5.4a | 27.2±2.6 |
| Body mass index
(BMI), Kg/m2 | 27.7±4.3 | 26.7±2.3 |
| Blood pressure,
mmHg |
| Systolic | 156.8±18.1b | 119.3±8.4 |
| Diastolic | 97.4±11.8a | 73.7±7.7 |
| Gestational age
delivery, weeks | 36.7±2.5b | 40.0±1.0 |
| Birth weight of
child, g | 2713±793.5b | 3526±360.4 |
| Primigravida | 14 | 12 |
| Urinary
protein | 1+–3+b | Negative |
| Serum creatinine,
μmol/l | 56.9±9.8b | 45.2±9.6 |
| Serum uric acid,
μmol/l | 361.5±92.0a | 289.0±82.4 |
| Serum urea,
mmol/l | 4.5±2.0a | 2.9±1.2 |
| eGFR (MDRD),
ml/min/1.73 m2 | 162.3±42.8 | 120.0±27.4 |
Cell culture
The conditionally immortalized human podocytes that
were used for the experiments were kindly provided by academician
Zhihong Liu (Research Institute of Nephrology of the Jinling
Hospital of Nanjing University School of Medicine, Nanjing, China),
and the podocyte cell line of Zhihong Liu was provided by Professor
Peter Mundel (Department of Medicine and Department of Anatomy and
Structural Biology, Albert Einstein College of Medicine, New York,
NY, USA). The human podocytes were cultured as previously described
(18). The podocytes were
propagated and seeded at 33°C in RPMI-1640 medium containing 10%
fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1X insulin,
transferrin and selenium solution (ITS) (Invitrogen, Carlsbad, CA,
USA). Type I collagen (Invitrogen) is always used to coat the
culture dishes to promote podocyte proliferation. Under these
permissive conditions, podocytes are small in size, exhibit a
polygonal or ‘cobblestone’ appearance and have a relatively small
cytoplasmic volume. Subsequently, the cells were incubated at
37.0°C for 10–14 days where they grew to 80% confluence. Under
these growth restrictive conditions, the podocytes undergo growth
arrest, increase in size, stop replicating, display a more complex
arborized pattern of foot process extensions, and express markers
of mature podocytic differentiation comparable with filtration
slits in vivo.
Cell viability assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8) (Dojindo Laboratories, Tokyo, Japan). The podocytes
were seeded at a concentration of 105/ml in 96-well
plates. After adherence, the cells were incubated with serum from
pregnant women with PE, or serum from women with NP (1:10 dilution)
for different periods of time (6, 12 and 24 h). When the effects of
preeclamptic serum and serum from women with NP on podocyte cell
viability were investigated, various concentrations of Ang-(1-7)
(10−5, 10−6 and 10−7 mol/l)
(Sigma-Aldrich, St. Louis, MO, USA) and A779 (10−5,
10−6 and 10−7 mol/l) (Bachem, Bubendorf,
Switzerland) were added to the cells for 12 h. At the end of the
treatment period, 10 μl of CCK-8 were added to each well, and the
cells were further incubated at 37°C for 2 h. The absorbance of
CCK-8 was detected at 450 nm using a microplate reader (680
Enzyme-linked Immunosorbent Monitor; Bio-Rad, Tokyo, Japan).
Western blot analysis
The cells were incubated for 12 h with serum derived
from pregnant women with PE or serum from women with NP (1:10
dilution). To verify the concentration of Ang-(1-7) required to
exert protective effects on podocytes incubated with serum pregnant
women with PE, the cells were stimulated with Ang-(1-7) at
concentrations of 10−5, 10−6 and
10−7 mol/l. In some experiments, the cells were
pre-exposed to A779 (10−5 mol/l, 30 min). Serum from
women with NP with A779 [a potent and selective Ang-(1-7)
antagonist (10−5 mol/l)] was used as a control. The
cells were washed twice with ice-cold PBS and harvested in a lysis
buffer containing a protease inhibitor cocktail. Equal amounts of
proteins were run on 10% SDS-PAGE and transferred onto PVDF
membranes. The membranes were blocked at room temperature with 5%
milk in Tris-buffered saline Tween-20 (TBS-T) for 1 h and then
probed at 4°C overnight with the following primary antibodies:
anti-podocin and anti-Wilms’ Tumor-1 (WT-1) were from Abcam
(Burlingame, CA, USA); anti-nephrin antibody was from
Sigma-Aldrich; anti-MAS1 antibody was from Novus Biologicals
(Oakville, ON, Canada); anti-GAPDH and anti-p-JNK were from Cell
Signaling Technology (Beverly, MA, USA); and anti-p-p38 and
anti-p-ERK1/2 were purchased from Epitomics (Burlingame, CA, USA).
The membranes were washed with TBS-T and then incubated with a
peroxidase-conjugated goat anti-rabbit secondary antibody (Cell
Signaling Technology) for 1 h at room temperature. Immunoreactivity
was detected using an enhanced chemiluminescence detection system.
Exposures were recorded on X-ray film.
Immunofluorescence
The cells were incubated with FBS or serum derived
from pregnant women with PE, serum from women with NP (1:10
dilution), or serum derived from pregnant women with PE in the
presence of Ang-(1-7) (10−6 mol/l), and the podocytes
pre-incubated with A779 (10−5 mol/l, 30 min) were
stimulated with serum derived from pregnant women with PE in the
presence of Ang-(1-7) (10−6 mol/l). The cells were grown
on coverslips, washed twice with PBS, fixed in 4% paraformaldehyde
for 30 min, permeabilized using 0.1% Triton X-100 for 15 min and
incubated in a blocking buffer (5% BSA in PBS, pH 7.4). The cells
were then probed with anti-F-actin antibody (1:400)(Abcam) at room
temperature for 2 h. The cells were washed with PBS followed by the
addition of secondary antibody (Cell Signaling Technology) for 2 h
at room temperature. Coverslips were then mounted onto glass
microscope slides with DAPI. The cells were observed and
photographed using a laser scanning confocal fluorescence
microscope (Leica DM5000; Leica Microsystems, Wetzlar,
Germany).
Analysis of apoptosis by flow
cytometry
Treatment of the podocytes was carried out as
described above. The FITC Annexin V Apoptosis Detection kit was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). The cells
were washed twice with cold PBS and then suspended in 1X binding
buffer at a concentration of 1×106 cells/ml.
Subsquently, 100 μl of the solution were transferred to a 5-ml
culture tube, and 5 μl of Annexin V and 5 μl propidium iodide (PI)
were added; the mixture was gently vortex and incubated for 15 min
at room temperture (25°C) in the dark. Subsquently, 400 μl of 1X
binding buffer were added to each tube. Flow cytometric analysis
was performed within 1 h.
Statistical analyses
Values are shown as the the means ± SD. SPSS
(version 16.0) software was used to analyze the data using the
Student’s t-test or one-way ANOVA or the rank sum test. P-values
<0.05 were considered to indicate a statistically significant
difference.
Results
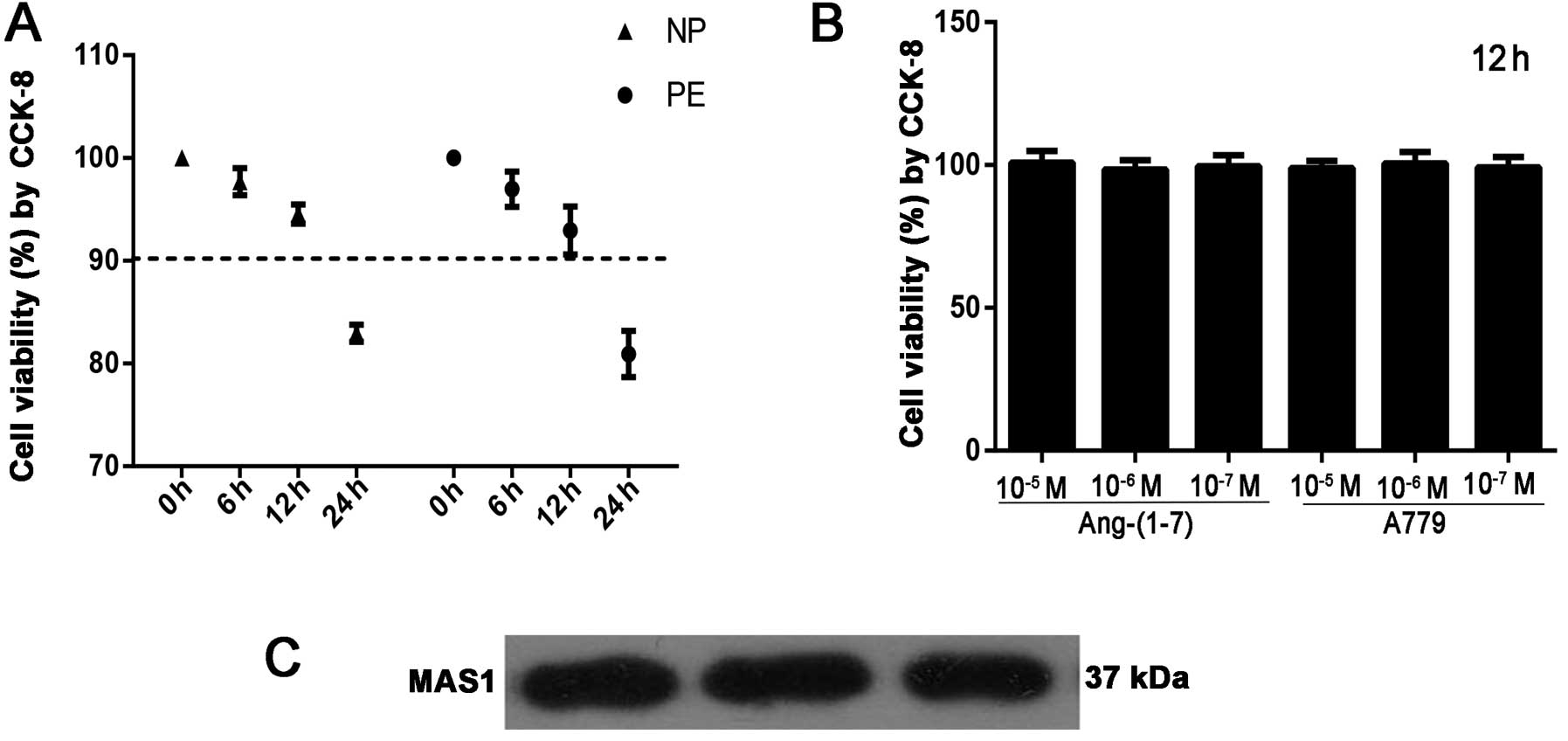
Analysis of podocyte viability
To determine the effects of serum from pregnant
women with PE and serum from women with NP on podocytes, cell
viability was examined by CCK-8 assay. The results revealed that
exposure to serum from pregnant women with PE and from women with
NP induced a decrease in cell viability in a time-dependent manner
(Fig. 1A). At the same time, we
did not observe any significant toxic effects of Ang-(1-7)
(10−5, 10−6 and 10−7 mol/l) and
A779 (10−5, 10−6 and 10−7 mol/l)
on podocytes at 12 h (Fig. 1B).
Thus, we opted to use serum from pregnant women with PE and from
women with NP with 12 h of incubation in the subsequent experiments
to determine the underlying mechanisms.
Expression of Mas G protein-coupled
receptor in podocytes
As is already known, there is no evidence of the
expression of Mas in human podocytes. Thus, western blot analysis
was performed to determine whether human podocytes express Mas, the
putative receptor for Ang-(1-7). In 3 separate experiments, the
results revealed a single band corresponding to the expected 37-kD
product for Mas in human podocytes (Fig. 1C).
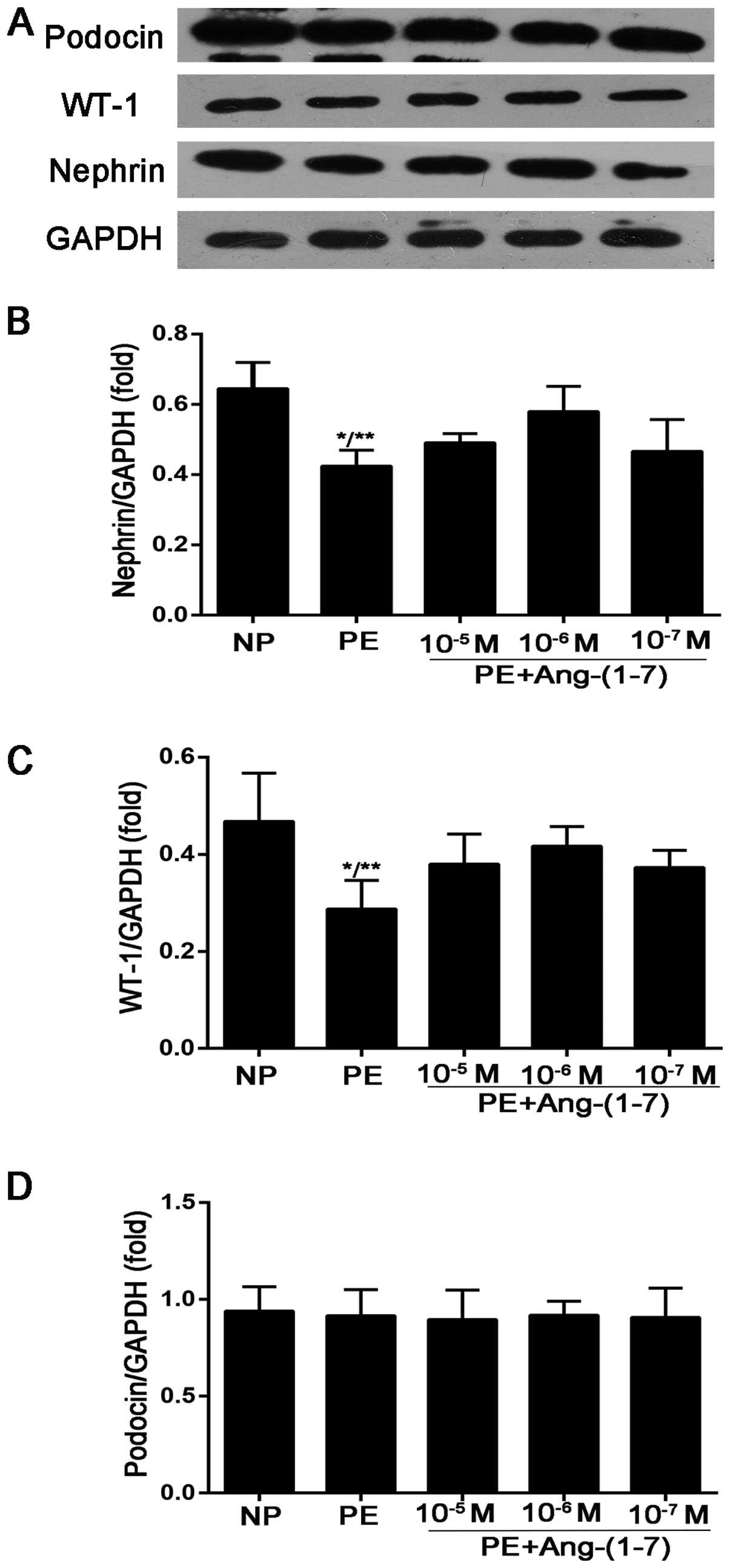
Effects of Ang-(1-7) on the expression of
nephrin, podocin and WT-1 in podocytes treated with preeclamptic
serum
The downregulation of podocyte-specific proteins may
be involved in the appearance and development of proteinuria in
women with PE. Thus, in this study, the expression of nephrin,
podocin and WT-1 was assessed in human cultured podocytes treated
with serum from pregnant women with PE and from women with NP. As
shown in Fig. 2, the expression
of nephrin and WT-1 decreased in the podocytes stimulated with
preeclamptic serum compared with the cells incubated with serum
from women with NP (nephrin, 0.42±0.04-fold vs. 0.64±0.07-fold of
GAPDH, P<0.005; WT-1, 0.28±0.05-fold vs. 0.46±0.09-fold of
GAPDH, P<0.05). However, the expression of podocin showed no
significant difference among the groups. After the podocytes were
treated with preeclamptic serum in the presence of Ang-(1-7)
(10−5, 10−6 and 10−7 mol/l) for 12
h, the results revealed that Ang-(1-7) exerted the maximum
protective effect at a concentration of 10−6 mol/l, and
the expression of nephrin and WT-1 was significantly increased
(nephrin, 0.57±0.07-fold vs. 0.42±0.04-fold of GAPDH, P<0.05;
WT-1, 0.41±0.04-fold vs. 0.28±0.05-fold of GAPDH, P<0.05)
compared with the podocytes incubated only with preeclamptic serum
(Fig. 2).
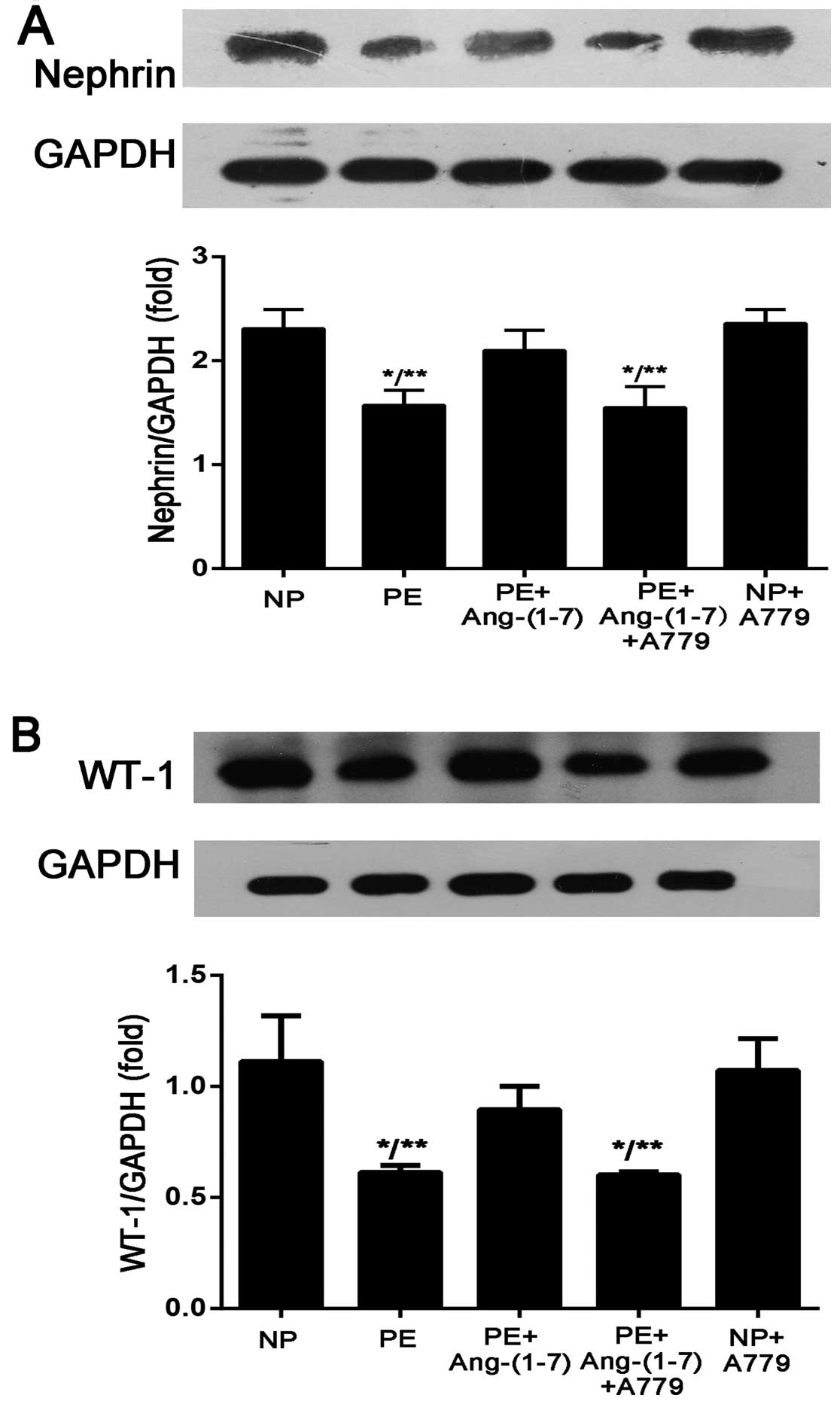
A779 reverses the protective effects of
Ang-(1-7) on podocyte injury induced by preeclamptic serum
The selective Mas receptor antagonist, A779
[D-Ala7-Ang-(1-7)] (10−5 mol/l), was used to verify the
protective role of Ang-(1-7) in another aspect. The results
demonstrated that the addition of A779 reversed the protective
effects of Ang-(1-7) on podocyte injury induced by preeclamptic
serum. The podocytes were pre-treated with A779 for 30 min and then
incubated with preeclamptic serum in the presence of Ang-(1-7)
(10−6 mol/l) for 12 h. As shown in Fig. 3, after the addition of A779, the
expression of nephrin and WT-1 decreased compared with the addition
of Ang-(1-7) only (nephrin, 1.55±0.20-fold vs. 2.1±0.19-fold of
GAPDH, P<0.01; WT-1, 0.60±0.01-fold vs. 0.89±0.10-fold of GAPDH,
P>0.05). Podocytes incubated with serum from NP in the presence
of A779 (10−5 mol/l) served as a control and there was
no significant difference compared with the podocytes incubated
with serum from NP only; this finding suggests that Ang-(1-7) is
required to maintain the increased expression of nephrin and WT-1
in PE.
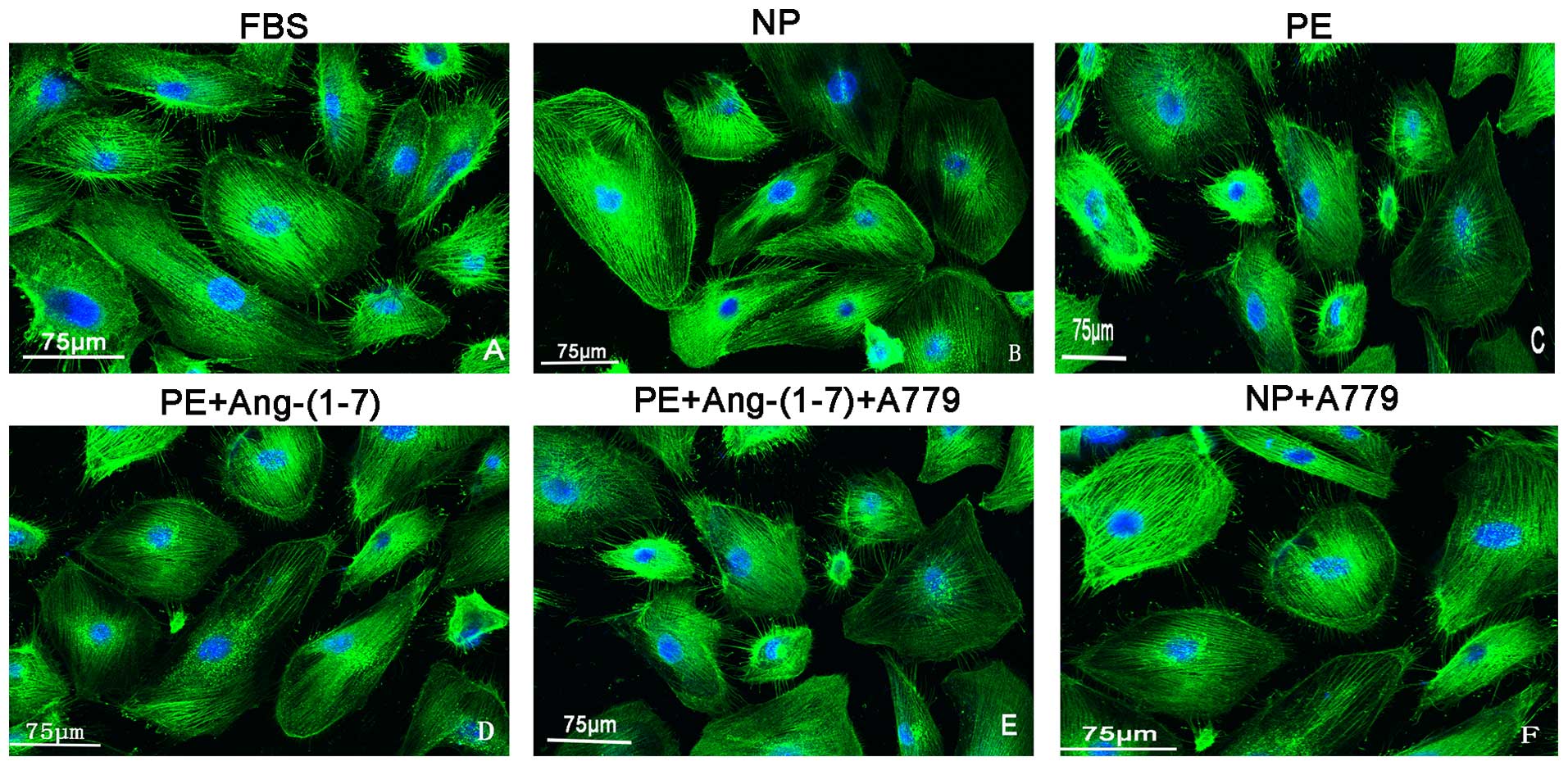
Effects of Ang-(1-7) on the changes in
F-actin in podocytes incubated with preeclamptic serum
Cytoskeletal rearrangement is a crucial early event
in the pathophysiology of proteinuria and has been reported to
result in foot process effacement (19). As shown in Fig. 4, the expression and orientation of
F-actin was changed, including cortical F-actin ring formation and
stress fibre attenuation in the podocytes that were treated with
preeclamptic serum compared with those of the control group. In
addition, this change was attenuated by treatment with Ang-(1-7)
(10−6 mol/l) and this protective role was reversed by
pre-treatment with A779 (Fig
4).
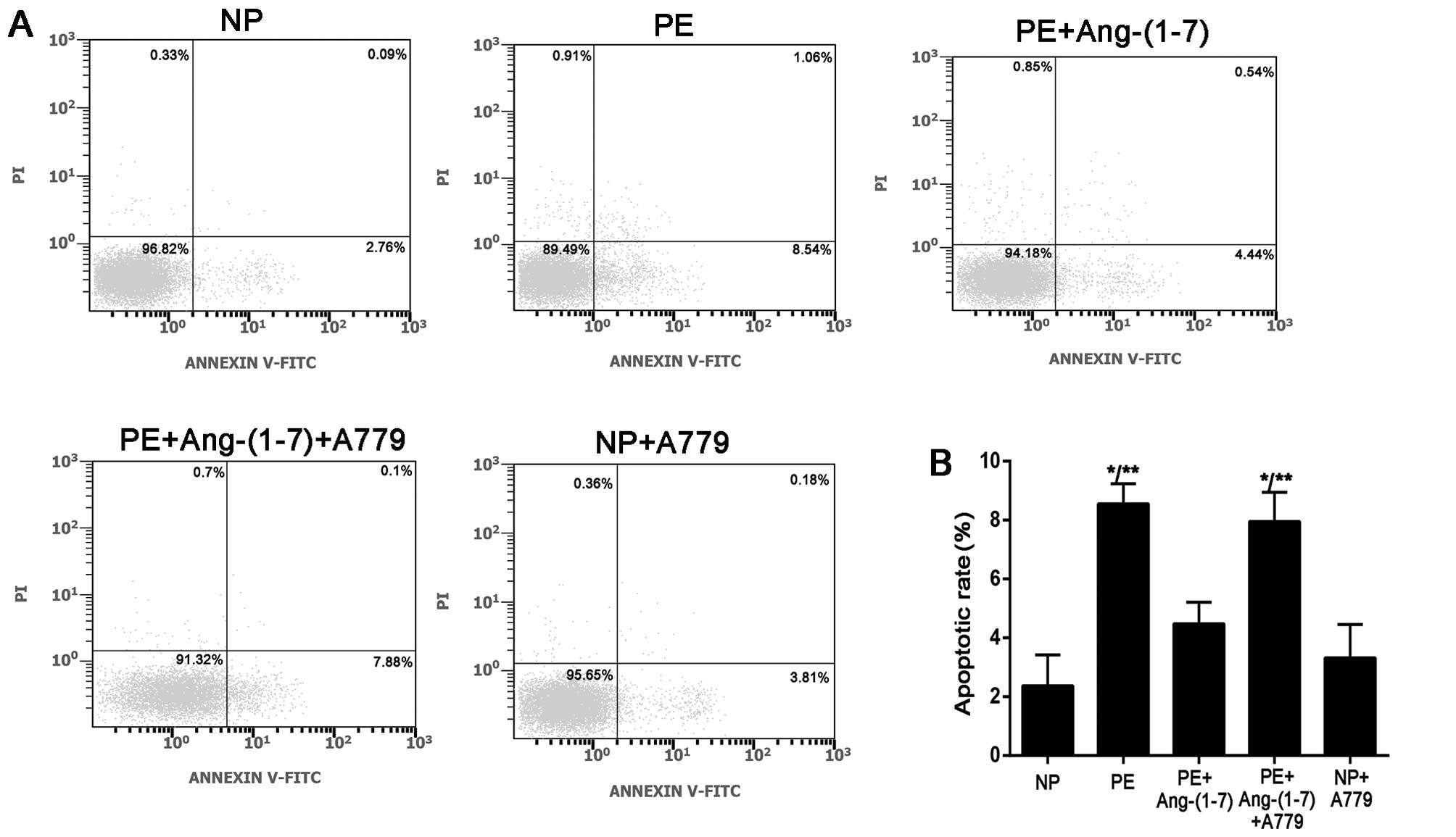
Effects of Ang-(1-7) (10−6
mol/l) on the apoptosis of podocytes induced by preeclamptic
serum
To determine the effects of preeclamptic serum on
the apoptosis of podocytes, we examined podocyte apoptosis by flow
cytometry. The cells were treated with preeclamptic serum in the
presence or absence of Ang-(1-7) (10−6 mol/l) and A779
(10−5 mol/l). Cells incubated with serum from women with
NP served as a control. As shown in Fig. 5, the number of Annexin
V+/PI− (apoptotic) cells was significantly
increased in the PE group; there were 8.55±0.68% apoptotic cells in
the PE group and 2.36±1.05% apoptotic cells in the NP group
(P<0.001). As expected, after the addition of Ang-(1-7)
(10−6 mol/l) in the PE group [PE plus Ang-(1-7)], the
number of apoptotic cells (4.47±0.73%) was significantly decreased
compared with the PE group, and this protective role was reversed
by pre-treatment with A779 (7.95±0.99%, P<0.001). A779 had no
effect on podocyte apoptosis, as shown in the NP plus A779 group
(3.59±0.74%); this finding suggests that Ang-(1-7) is required to
prevent the apoptosis of podocytes in PE and that the increased
apoptosis in the group treated with serum from women with PE plus
Ang-(1-7) and A779 did not result from A779 alone.
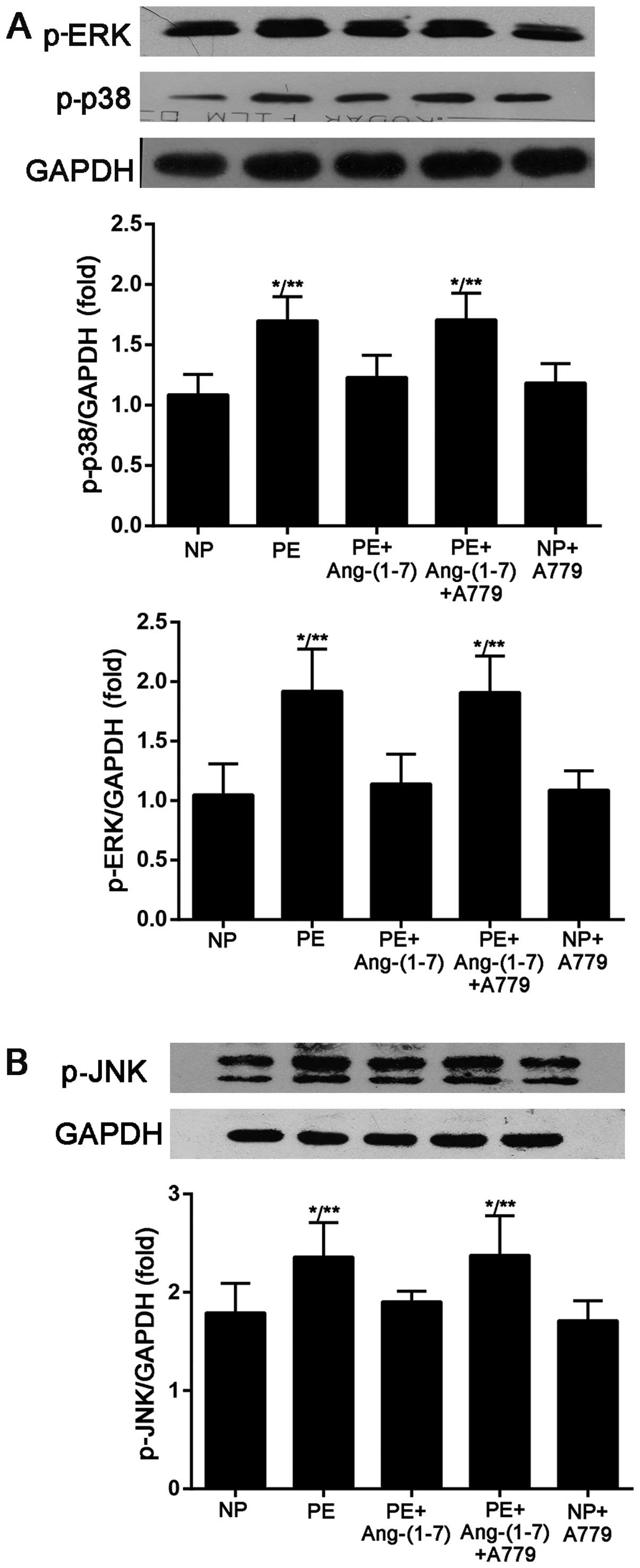
Effect of Ang-(1-7) on MAPK (p38, ERK 1/2
and JNK) phosphorylation
As illstrated in Fig.
6, the results from western blot analysis showed that the p38,
ERK and JNK phosphorylation levels in the cultured podocytes that
were stimulated with preeclamptic serum increased compared with
those stimulated with serum from NP (p-p38, 1.69±0.20-fold vs.
1.08±0.16-fold of GAPDH, P<0.005; p-ERK, 1.92±0.35-fold vs.
1.04±0.26-fold of GAPDH, P<0.005; p-JNK, 2.35±0.35-fold vs.
1.79±0.30-fold of GAPDH, P<0.05). The addition of Ang-(1-7) to
the cells prevented the preeclamptic serum-induced increase in
p-p38, ERK and JNK phosphorylation (p-p38, 1.23±0.18-fold vs.
1.69±0.20-fold of GAPDH, P<0.05; p-ERK, 1.14±0.25-fold vs.
0.87±0.18-fold of GAPDH, P<0.01; p-JNK, 1.90±0.10-fold vs.
2.35±0.35-fold of GAPDH, P<0.05) and the effects of Ang-(1-7)
were blocked by the Mas receptor inhibitor, A-779.
Discussion
In the present study, we used a conditionally
immortalized differentiated human podocyte cell line to explore the
damage sustained by glomerular epithelial cells from preeclamptic
serum and found that cultured podocytes underwent functional and
structural morphologic changes that resulted from the
downregulation of podocyte-specific proteins (nephrin and WT-1),
cytoskeletal rearrangement (specialized arrangement of F-actin) and
apoptosis. In addition, we determined the role of Ang-(1-7)
deficiency under these conditions (PE), and the results revealed
that Ang-(1-7) attenuated podocyte injury induced by preeclamptic
serum through the downregulation of MAPK phosphorylation.
Podocyte injury is usually characterized by the
disappearance or effacement of foot processes leading to
proteinuria. The foot processes of neighbouring podocytes form an
interdigitating pattern with slits that are bridged by a protein
complex known as the slit diaphragm (SD) (20). The downregulation of specialized
proteins associated with slit-pores (e.g., nephrin and podocin) and
podocyte-specific transcriptional factors (e.g., WT-1) may be
involved in the initiation and development of proteinuria in
pregnant women with PE. Our data indicated that the incubation of
podocytes with preeclamptic serum resulted in the decreased
expression of nephrin and WT-1 in podocytes and had no effect on
podocin expression; these results are consistent with those of
other studies (4). The unique
shape of podocytes and the maintenance of the processes
characterize the well-developed cytoskeleton (20). Cytoskeletal rearrangement has been
suggested to underlie foot process effacement, which is a crucial
early event in the pathophysiology of proteinuria. The F-actin
cytoskeleton in podocyte foot processes is believed to be
dynamically maintained at a steady-state with a low turnover
(21). In the present study, the
expression and orientation of F-actin was altered in the podocytes
that were treated with preeclamptic serum compared with the control
group (treated with serum from women with NP), which may contribute
to podocyte injury in PE. Podocyte depletion has long been shown to
be a typical characteristic of glomerulosclerosis and is now
considered a key factor in the progression of renal diseases
(22). In this study, we found
that the apoptosis of podocytes in the PE group was increased
compared to the NP group. Our previous study indicated that the
number of urinary podocytes in pregnant women with PE was
significantly higher than that in women with gestational
hypertension without proteinuria and women with NP (23). These results indicate that the
progressive depletion of podocytes in PE is associated with an
increase in podocyte apoptosis and the detachment from the
glomerular basement membrane.
Treatment with Ang-(1-7) has been shown to reduce
proteinuria in stroke-prone spontaneously hypertensive rats (SHRSP)
(24), and Ang-(1-7) has been
shown to have therapeutic potential for reversing
glomerulosclerosis by counteracting the effects of Ang II in a rat
model of experimental glomerulonephritis (25). The data from the present study
indicate that correcting the Ang-(1-7) deficiency in PE can partly
reverse the downregulation of podocyte-specific proteins,
cytoskeletal rearrangement and the apoptosis of podocytes.
However, the effects of Ang-(1-7) on the kidneys
remain controversial. Shao et al (26) found that exogenous Ang-(1-7)
injection did not ameliorate diabetic rat renal injury induced by
streptozotocin (STZ), and it accelerated progressive diabetic
nephropathy. Many factors result in these inconsistent results,
such as different experimental designs and different doses of
Ang-(1-7). In this study, the doses of Ang-(1-7) used in the
experiments ranged from 10−7 to 10−5 mol/l,
and we found that Ang-(1-7) exerted a maximum protective effect on
podocyte injury in PE at a concentration of 10−6
mol/l.
In the present study, we elucidated the underlying
mechanisms of the protective role of Ang-(1-7) in PE. The results
revealed that Ang-(1-7) partly inhibited the phosphorylation of
p38, ERK1/2 and JNK in cultured podocytes treated with preeclamptic
serum, which was reversed by pre-treatment with A779, suggesting an
effect mediated by the binding of Ang-(1-7) to the receptor, Mas.
In primary cultures of mouse mesangial cells, Moon et al
(27) showed that Ang-(1-7)
attenuated AngII-induced MAPK phosphorylation, as well as the
expression of transforming growth factor (TGF)-β1, fibronectin and
collagen IV. However, Zimpelmann and Burns (28) demonstrated that treatment of a
human mesangial cell line with Ang-(1-7) caused a rapid and
significant increase in arachidonic acid release, as well as the
phosphorylation of MAPKs. Overall, Ang-(1-7)/Mas signaling in the
kidneys is complex and more detailed studies at the cellular level
are required.
In this study, we determined the role of Ang-(1-7)
in the pathogenesis of PE and found that Ang-(1-7) reversed
podocyte injury in PE through the activation of the podocyte Mas
receptor and inhibition of the MAPK pathway. However, a role for
correcting Ang-(1-7) deficiency in this condition as a therapeutic
strategy requires further investigation.
Acknowledgements
The present study was supported by grants from the
Major State Basic Research Development Program of China (973
Program) (no. 2012CB517700), Phase III of the Nephropathy
Discipline Construction of 211 Project of State Education
Commission, the Shanghai Biopharmaceutics Major Project
(08dz1900603), the Fudan University ‘985’ Hospital Key Discipline
Construction Project (2012FDYSXK02), the Shanghai Municipal Science
and Technology Project (124119a7802), the Shanghai City Health
Bureau Project (20114311) and Minhang District Key Disciplines
Construction. We thank academician Zhihong Liu for the donation of
the podocyte cells. We thank Professor Zhaoxia Wang for providing
technical advice and Peng Fang for technical support.
References
|
1
|
Walker JJ: Pre-eclampsia. Lancet.
356:1260–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones DC and Hayslett JP: Outcome of
pregnancy in women with moderate or severe renal insufficiency. N
Engl J Med. 335:226–232. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levine RJ, Maynard SE, Qian C, et al:
Circulating angiogenic factors and the risk of preeclampsia. N Engl
J Med. 350:672–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garovic VD, Wagner SJ, Petrovic LM, et al:
Glomerular expression of nephrin and synaptopodin, but not podocin,
is decreased in kidney sections from women with preeclampsia.
Nephrol Dial Transplant. 22:1136–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herman-Edelstein M, Weinstein T and Gafter
U: TGFβ1-dependent podocyte dysfunction. Curr Opin Nephrol
Hypertens. 22:93–99. 2013.
|
|
6
|
Shah DM: Role of the renin-angiotensin
system in the pathogenesis of preeclampsia. Am J Physiol Renal
Physiol. 288:F614–F625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Irani RA and Xia Y: Renin angiotensin
signaling in normal pregnancy and preeclampsia. Semin Nephrol.
31:47–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anton L and Brosnihan KB: Systemic and
uteroplacental renin--angiotensin system in normal and
pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis. 2:349–362.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valdes G, Germain AM, Corthorn J, et al:
Urinary vasodilator and vasoconstrictor angiotensins during
menstrual cycle, pregnancy, and lactation. Endocrine. 16:117–122.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santos RA, Ferreira AJ, Pinheiro SV,
Sampaio WO, Touyz R and Campagnole-Santos MJ: Angiotensin-(1-7) and
its receptor as a potential targets for new cardiovascular drugs.
Expert Opin Investig Drugs. 14:1019–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merrill DC, Karoly M, Chen K, Ferrario CM
and Brosnihan KB: Angiotensin-(1-7) in normal and preeclamptic
pregnancy. Endocrine. 18:239–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herse F, Staff AC, Hering L, Muller DN,
Luft FC and Dechend R: AT1-receptor autoantibodies and
uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med
(Berl). 86:697–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdul-Karim R and Assalin S: Pressor
response to angiotonin in pregnant and nonpregnant women. Am J
Obstet Gynecol. 82:246–251. 1961.PubMed/NCBI
|
|
14
|
Su Z, Zimpelmann J and Burns KD:
Angiotensin-(1-7) inhibits angiotensin II-stimulated
phosphorylation of MAP kinases in proximal tubular cells. Kidney
Int. 69:2212–2218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gava E, Samad-Zadeh A, Zimpelmann J, et
al: Angiotensin-(1-7) activates a tyrosine phosphatase and inhibits
glucose-induced signalling in proximal tubular cells. Nephrol Dial
Transplant. 24:1766–1773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Velez JC, Bland AM, Arthur JM, Raymond JR
and Janech MG: Characterization of renin-angiotensin system enzyme
activities in cultured mouse podocytes. Am J Physiol Renal Physiol.
293:F398–F407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zimmerman D and Burns KD:
Angiotensin-(1-7) in kidney disease: a review of the controversies.
Clin Sci (Lond). 123:333–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saleem MA, O’Hare MJ, Reiser J, et al: A
conditionally immortalized human podocyte cell line demonstrating
nephrin and podocin expression. J Am Soc Nephrol. 13:630–638.
2002.PubMed/NCBI
|
|
19
|
Moller CC, Wei C, Altintas MM, et al:
Induction of TRPC6 channel in acquired forms of proteinuric kidney
disease. J Am Soc Nephrol. 18:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pavenstadt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307.
2003.
|
|
21
|
Tryggvason K, Pikkarainen T and Patrakka
J: Nck links nephrin to actin in kidney podocytes. Cell.
125:221–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kriz W, Gretz N and Lemley KV: Progression
of glomerular diseases: is the podocyte the culprit? Kidney Int.
54:687–697. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen G, Zhang L, Jin X, et al: Effects of
angiogenic factors, antagonists, and podocyte injury on development
of proteinuria in preeclampsia. Reprod Sci. 20:579–588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giani JF, Munoz MC, Pons RA, et al:
Angiotensin-(1-7) reduces proteinuria and diminishes structural
damage in renal tissue of stroke-prone spontaneously hypertensive
rats. Am J Physiol Renal Physiol. 300:F272–F282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benter IF, Yousif MH, Anim JT, Cojocel C
and Diz DI: Angiotensin-(1-7) prevents development of severe
hypertension and end-organ damage in spontaneously hypertensive
rats treated with L-NAME. Am J Physiol Heart Circ Physiol.
290:H684–H691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao Y, He M, Zhou L, Yao T, Huang Y and
Lu LM: Chronic angiotensin (1-7) injection accelerates STZ-induced
diabetic renal injury. Acta Pharmacol Sin. 29:829–837. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon JY, Tanimoto M, Gohda T, et al:
Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated
NAD(P)H oxidase activation in type 2 diabetic nephropathy of
KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 300:F1271–F1282. 2011.
View Article : Google Scholar
|
|
28
|
Zimpelmann J and Burns KD:
Angiotensin-(1-7) activates growth-stimulatory pathways in human
mesangial cells. Am J Physiol Renal Physiol. 296:F337–F346. 2009.
View Article : Google Scholar : PubMed/NCBI
|