Introduction
The gastrointestinal tract contains several types of
endocrine cells that control and regulate a number of important
functions of the gastrointestinal tract, enabling it to perform its
main task, the digestion and absorption of ingested nutrients
(1–4). These cells are dispersed among the
epithelial cells of the mucosa and have specialised microvilli that
project into the lumen and function as sensors, responding to
luminal stimuli, particularly nutrients, by releasing hormones that
target other parts of the digestive system (5–14).
There are at least 15 different populations of these cells in the
gastrointestinal tract that interact in an integrated manner with
each other, with the enteric nervous system, and with afferent and
efferent nerve fibres of the central nervous system, in particular
the autonomic nervous system (2,4).
Irritable bowel syndrome (IBS) is a common chronic
functional gastrointestinal disorder that considerably reduces the
quality of life and is an economic burden to both individual
patients and society as a whole due to the direct costs of
diagnostic tests and treatments, and the indirect costs of the low
productivity of patients with IBS (4). Abnormalities of endocrine cells have
been reported in the duodenum, ileum, colon and rectum of patients
with IBS (15–28). However, to the best of our
knowledge, the antral endocrine cells of the stomach have not been
previously investigated. The antrum of the stomach contains 3 types
of endocrine cells: serotonin, gastrin and somatostatin cells.
Therefore, the aim of the present study was to determine whether
there are any endocrine cell or serotonin transporter (SERT)
abnormalities in the antrum of the stomach of patients with
IBS.
Materials and methods
Patients and controls
Seventy-six patients with IBS, classified according
to the Rome III criteria for IBS as previously described (29,30) were included in this study. Forty
patients fulfilled the Rome III criteria for functional dyspepsia
as well. These patients comprised 62 females and 14 males with a
mean age of 32 years (range, 18–55 years). Diarrhoea was the
predominant symptom in 26 of these patients (IBS-D), while in 21
patients, the predominant symptoms were both diarrhoea and
constipation (IBS-M) and in 29 patients, the predominant symptom
was constipation (IBS-C). All the patients (designated as
IBS-total) had experienced their symptoms for many years and were
unable to associate the onset of their IBS symptoms with any
particular event, particularly gastrointestinal infections. They
underwent a complete physical examination and were investigated by
way of blood tests to exclude inflammatory, liver, endocrine or any
other systemic diseases. Moreover, they underwent a colonoscopy
with segmental biopsies, which revealed the presence of a normal
terminal ileum, colon and rectum in all cases. None of these
patients used proton pump inhibitors in the last 3 months. However,
they all tried proton pump inhibitors for short periods of time
without relief of symptoms.
Healthy volunteers without any gastrointestinal
complaints were recruited as the controls through local
announcements at Stord Hospital, Haukeland University Hospital and
the University of Bergen, Norway, as well as in the local
newspapers. Forty-three healthy subjects were included, of whom 15
were residents of Stord and 28 were students or hospital employees.
They comprised 32 females and 11 males with a mean age of 40 years
(range, 20–58 years).
The study was performed in accordance with the
Declaration of Helsinki and was approved by the Regional Committee
for Medical and Health Research Ethics West, Bergen, Norway. All
subjects provided both oral and written consent prior to
participation.
Symptoms and quality of life
assessments
The patients were asked to complete the following
questionnaires: Birmingham IBS symptom questionnaire, the
Short-Form (SF) Nepean Dyspepsia Index (SF-NDI) questionnaire and
the Irritable Bowel Syndrome Quality Of Life (IBS-QOL)
questionnaire. The control subjects were asked to complete the
SF-NDI questionnaire. The Birmingham IBS symptom score
questionnaire is a disease-specific score used to measure the
symptoms of patients with IBS. It has been developed to be suitable
for self-completion and has been found to be acceptable by
patients. Its dimensions have good reliability, external validity
and sensitivity (31). The
questionnaire comprises 11 questions based on the frequency of
IBS-related symptoms. Each question has a standard response scale
with the symptoms all being measured on a 5-point Likert scale
ranging from 0 (‘none of the time’) to 5 (‘all of the time’). There
are 3 underlying dimensions: pain (3 items), diarrhoea (5 items)
and constipation (3 items) (31).
SF-NDI was primarily constructed and validated in patients with
functional dyspepsia (32).
Later, a Norwegian translation of the form was validated and proved
to be acceptable by patients with IBS according to the Rome II
criteria for IBS (33). The form
is a 10-item questionnaire examining the influence of dyspepsia on
the health domains in patients, namely tension/anxiety,
interference with daily activities, disruption to regular
eating/drinking, knowledge towards/control over disease symptoms
and interference with work/study, with each subscale containing 2
items. Each item is measured by a 5-point Likert scale ranging from
1 (not at all or not applicable), 2 (a little), 3 (moderately), 4
(quite a lot) to 5 (extremely). Individual items in each subscale
are aggregated to obtain a score range from 10 [lowest
health-related quality of life (HRQoL) score] to 50 (highest HRQoL
score) as per the original calculation formula of the developer.
High scores indicate worse functioning or symptoms. The IBS-QOL
questionnaire is a 34-item IBS-specific quality of life document
concerning physical and psychosocial functioning as a result of IBS
(34). This questionnaire
includes a 5-point Likert response scale: not at all, slightly,
moderately, quite a lot and extremely. IBS-QOL consists of 8
domains: dysphoria, interference with activity, body image, health
worry, food avoidance, social reaction, sexual function and impact
on relations. The IBS-QOL questionnaire has been validated in
patients with IBS (35).
Gastroscopy, histopathology and
immunohistochemistry
Both the patients and controls underwent a standard
gastroscopy, during which 5 biopsy samples were taken from the
antrum from the area around the pyloric sphincter. Two biopsy
samples were used in a rapid urease test for Helicobacter
pylori (HelicotecUT Plus; Strong Biotech, Taipei, Taiwan). The
remaining biopsy samples were fixed overnight in 4% buffered
paraformaldehyde, embedded in paraffin and cut into 5-μm-thick
sections. The sections were stained with haematoxylin-eosin, and
immunostained using the avidin-biotin complex (ABC) method with a
Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). The
primary antibodies used were monoclonal mouse anti-human serotonin
(clone 5HT-H209, code M0758; Dako, Glostrup, Denmark), polyclonal
rabbit anti-human gastrin-17 (code IR519; Dako), polyclonal rabbit
anti-synthetic cyclic (1–14) somatostatin (code A0566; Dako) and
monoclonal mouse anti-synthetic peptide from human SERT (code
ab136607; Abcam, Cambridge, UK). The sections were incubated at
room temperature for 2 h with all primary antibodies diluted to
1:100, apart from the antibody against gastrin, which was supplied
in a ready-to-use form. The sections were then washed in PBS buffer
(pH 7.4) and incubated with biotinylated swine anti-mouse IgG (in
the case of monoclonal antibodies) or goat anti-rabbit IgG (in the
case of polyclonal antibodies), both diluted to 1:200, for 30 min
at room temperature. After washing the slides in PBS buffer, the
sections were incubated for 30 min with peroxidase-labelled ABC
diluted to 1:100, and then immersed in 3,3′-diaminobenzidine
peroxidase substrate (Vector Laboratories), followed by
counterstaining with haematoxylin.
Computerised image analysis
A microscope (type BX 43; Olympus, Tokyo, Japan)
equipped with built-in Koehler illumination for transmitted light,
a light-intensity manager switch, a high-colour-reproductivity LED
light source, a 6 V/30 W halogen bulb and a DP26 Olympus camera was
used for morphometric analysis. This microscope was linked to a
computer with Olympus cellSens imaging software (version 1.7). The
number of immunoreactive cells, the area of epithelial cells and
the immunoreactivity intensity were measured. The number of
immunoreactive cells in each field was counted manually by pointing
and clicking the computer mouse, and the areas of the epithelial
cells were drawn manually using the computer mouse. The areas
considered for quantification were those near to the muscularis
mucosa. The immunoreactivity intensity in each field was measured
using an automatic threshold setting. A ×40 objective was used, for
which each frame (field) on the monitor represented a tissue area
of 0.035 mm2. Measurements were made in 10 randomly
selected fields for each individual. The immunostained sections
from the IBS patients and the controls were coded and mixed, and
measurements were made by the same person (M.E.-S.), who was blind
to the identity of the individual to whom the tissue sections
belonged. The data from the fields were tabulated, and the cell
density of the epithelium (in cells/mm2) and the
immunoreactivity intensity were computed.
Statistical analysis
The gender difference and the occurrence of
Helicobacter pylori infection between the patients and the
controls was examined using Fisher’s exact test. The differences in
age and quality of life measured by SF-NDI were calculated using
the Mann-Whitney non-parametric test. Differences between the
control, IBS-total, IBS-D, IBS-M and IBS-C groups were calculated
using the Kruskal-Wallis non-parametric test with the Dunn’s
post-test. The data are presented as means ± SEM values, and
differences with P<0.05 were considered statistically
significant.
Results
Patients and controls
The gender and age distributions did not differ
significantly between the patients and the controls (P=0.196 and
P=0.360, respectively). A total of 3 patients and 2 control
subjects were positive for Helicobacter pylori infection, as
revealed by both the urease test and histopathological
examinations. The prevalence of Helicobacter pylori
infection did not differ significantly between the patients and the
controls (P=1.0). The total score of the Birmingham IBS symptom
questionnaire was 21.5±0.7. The pain, diarrhoea and constipation
dimensions were 7.2±0.4, 6.6±0.4 and 7.2±0.4, respectively. The
total score of the SF-NDI questionnaire in the controls was
10.5±0.5 and in the IBS patients it was 26±1.2. There was a
significant difference in the reduction of the quality of life
according to the SF-NDI questionnaire between the controls and
patients with IBS (P<0.0001). The total score of the IBS-QOL
questionnaire in the patients with IBS was 72.7±1.5.
Gastroscopy, histopathology and
immunohistochemistry
The oesophagus, stomach and duodenum were
macroscopically and microscopically normal in both the patients and
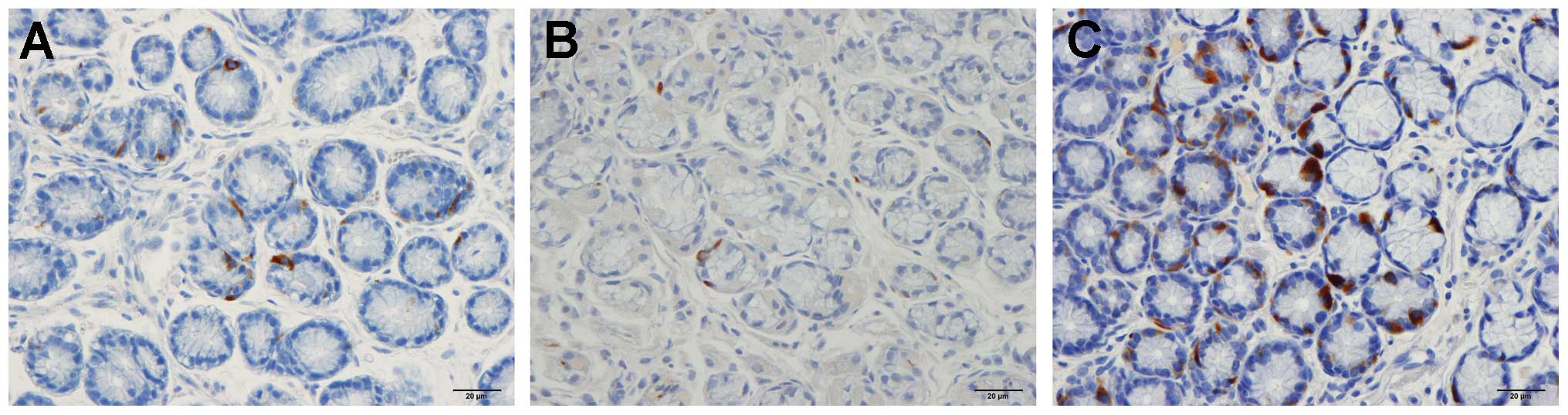
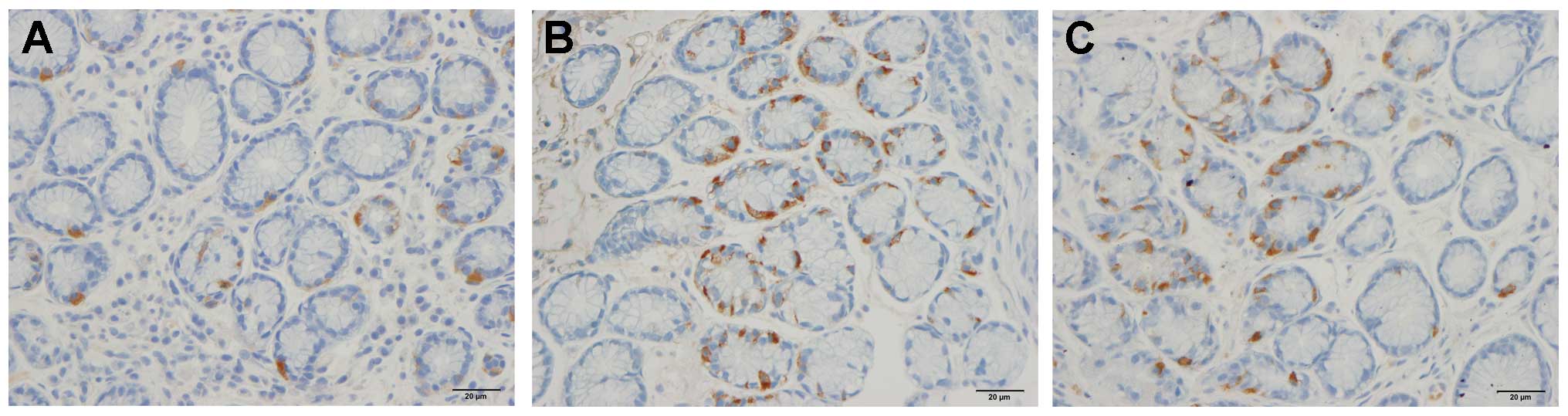
controls. Immunoreactive cells were found in the stomach antrum of
both the patients and the controls, and were either basket- or
flask-shaped, sometimes with a long basal cytoplasmic process.
Computerised image analysis
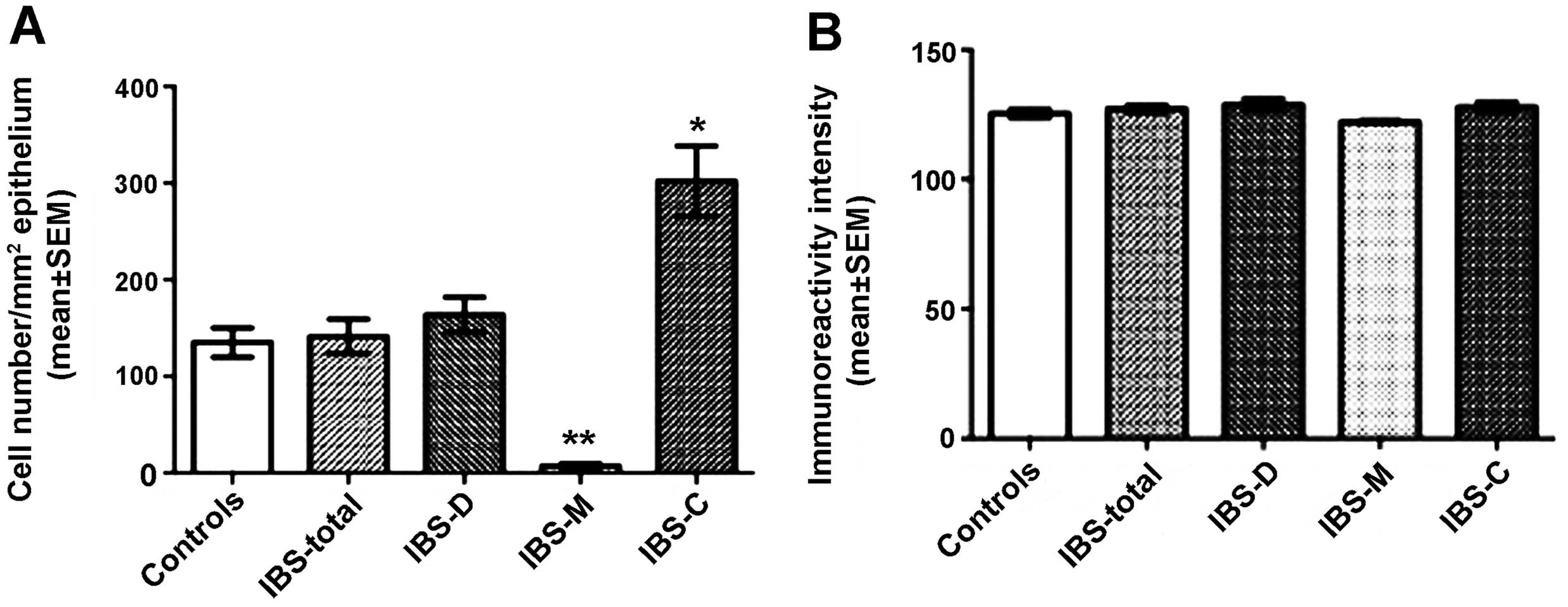
Serotonin
The densities of the serotonin-immunoreactive cells
were 135.0±15.0, 141.3±17.9, 163.4±18.2, 7.2±2.3 and 302.3±36.3
cells/mm2 in the control, IBS-total, IBS-D, IBS-M and
IBS-C groups, respectively. The density of the
serotonin-immunoreactive cells was significantly lower in the IBS-M
group and higher in the IBS-C group compared with the controls
(IBS-C, P<0.05; IBS-M, P<0.01 compared to controls; Figs. 1 and 2). The immunoreactivity intensities of
serotonin were 125.3±1.5, 127.1±1.3, 128.8±2.1, 122.2±0.4 and
127.8±1.8 in the control, IBS-total, IBS-D, IBS-M and IBS-C groups,
respectively, with no statistically significant differences between
any of these groups (P=0.2; Figs.
1 and 2).
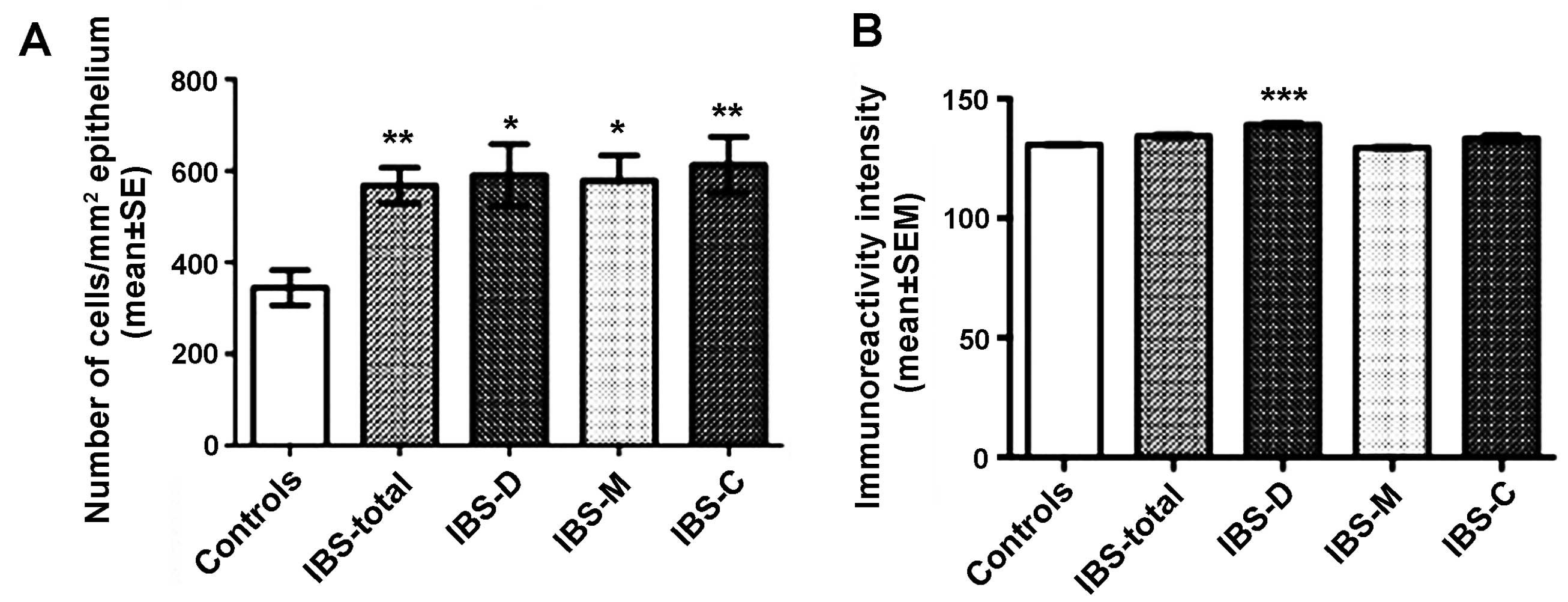
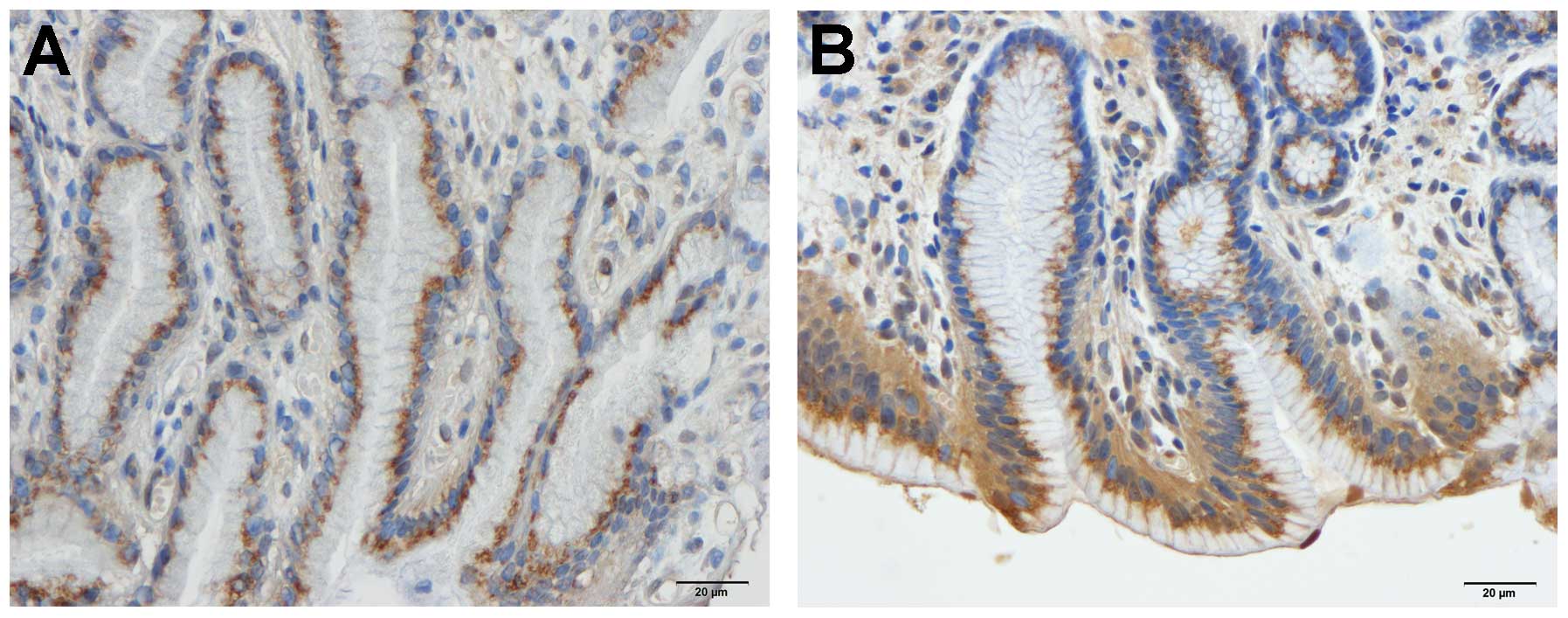
Gastrin
The densities of the gastrin-immunoreactive cells
were 344.8±38.2, 567.9±38.9, 591.0±67.6, 579.1±54.7 and 612.9±61.1
cells/mm2 in the control, IBS-total, IBS-D, IBS-M and
IBS-C groups, respectively. The densities of gastrin-immunoreactive
cells differed significantly between the controls and the IBS-total
and the IBS subgroups (P<0.0001). The density of the
gastrin-immunoreactive cells differed significantly between the
controls and the IBS-total, IBS-D, IBS-M and IBS-C patients
(P<0.01, P<0.05, P<0.05 and P<0.01, respectively;
Figs. 3 and 4). The immunoreactivity intensities of
gastrin were 130.8±0.8, 134.6±1.0, 139.3±1.3, 129.7±0.9 and
133.7±1.7 in the control, IBS-total, IBS-D, IBS-M and IBS-C groups,
respectively, with statistically significant differences between
all groups (P=0.0001). The gastrin immunoreactivity intensity was
significantly higher in the IBS-D patients than in the controls
(P=0.0001; Figs. 3 and 4).
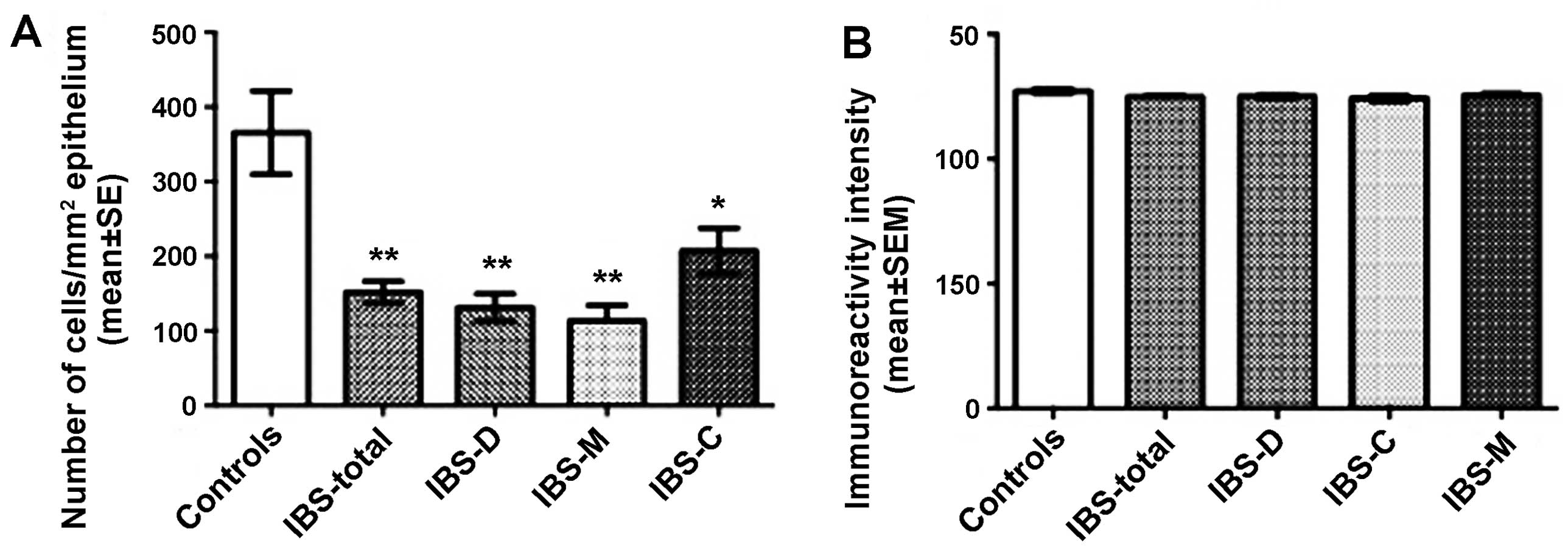
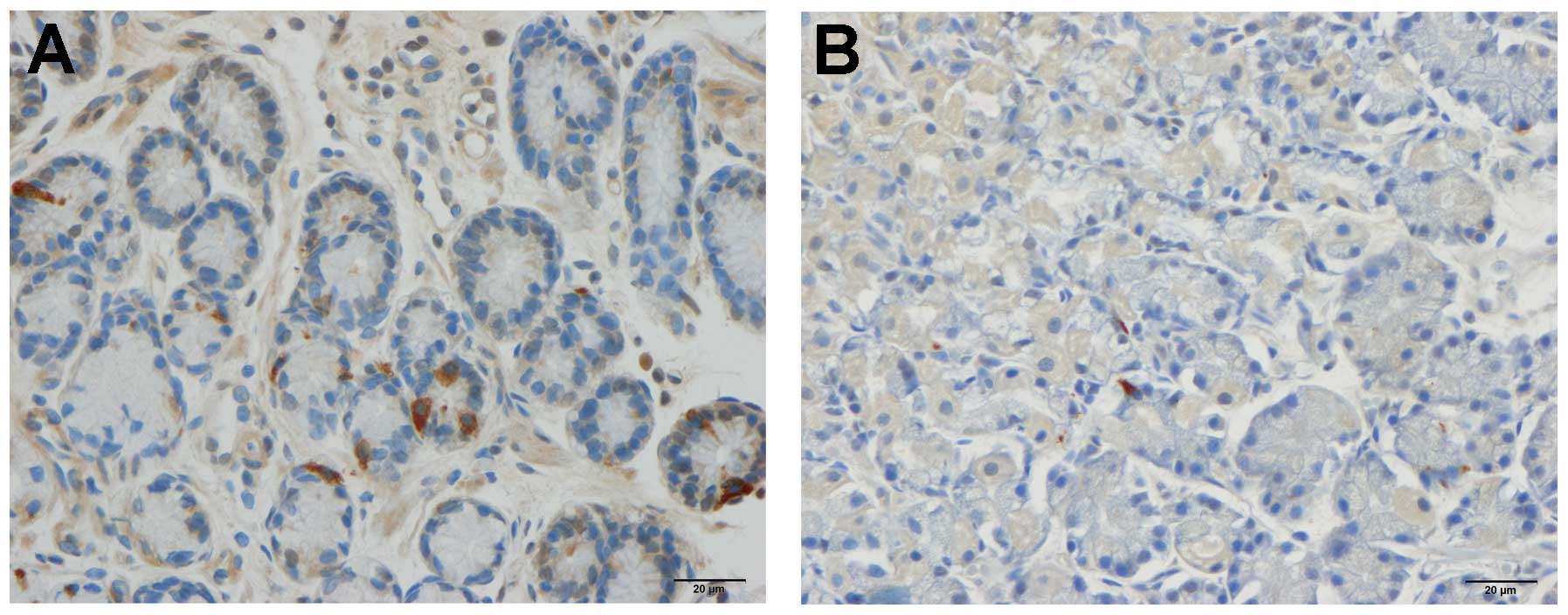
Somatostatin
The densities of somatostatin-immunoreactive cells
were 365.6±55.9, 152.0±14.6, 131.5±18.3, 113.4±21.2 and 207.3±30.5
cells/mm2 in the control, IBS-total, IBS-D, IBS-M and
IBS-C groups, respectively. There was a statistical difference
between the controls and the IBS subgroups (P=002). The density of
the somatostatin-immunoreactive cells was significantly lower in
the IBS-total, IBS-D, IBS-M and IBS-C patients than in the controls
(P<0.01, P<0.01, P<0.01 and P<0.05, respectively).
There was no significant difference in somatostatin
immunoreactivity intensity between the controls (127.1±0.9) and the
IBS-total (124.8±0.5), IBS-D (125.0±0.7), IBS-M (125.6±0.6) and
IBS-C (124.3±1.0) patients (P=0.369; Figs. 5 and 6).
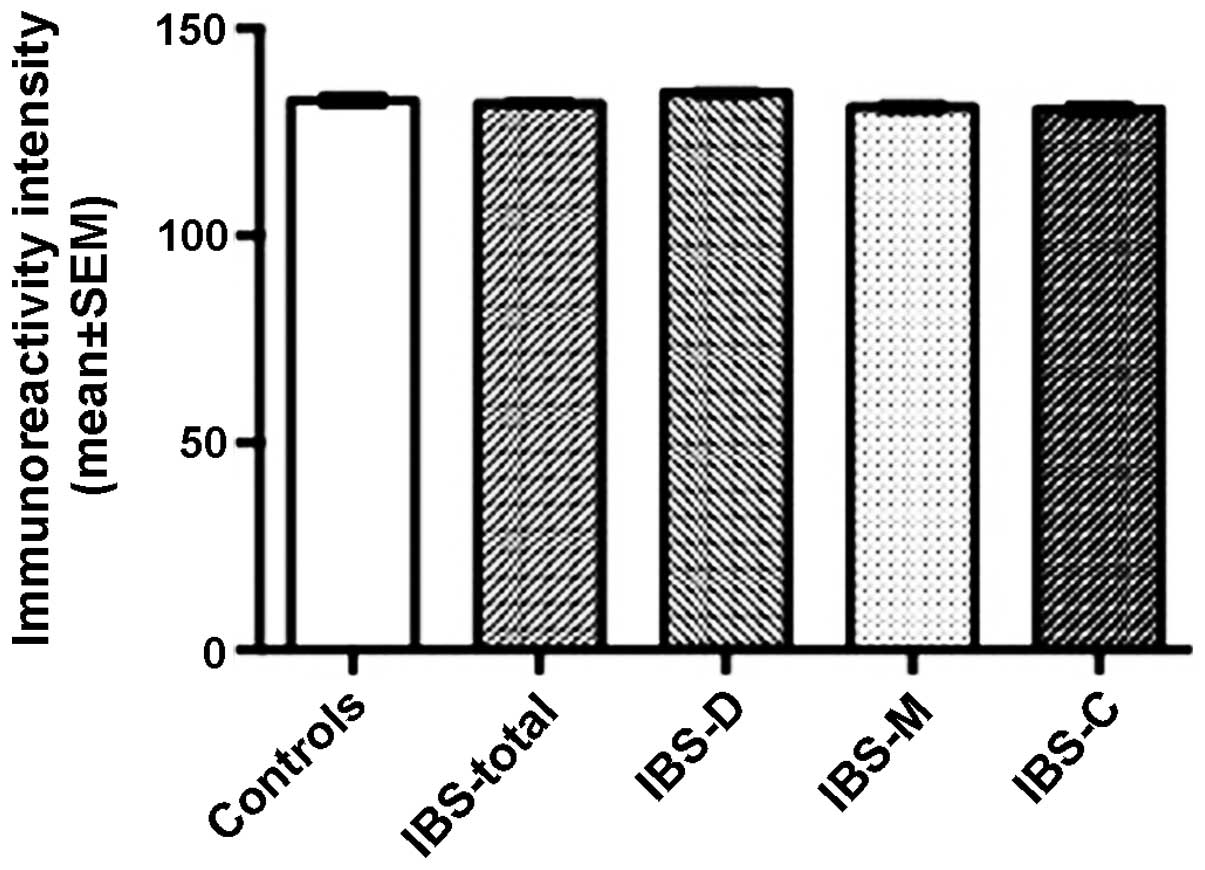
SERT
The immunoreactivity intensities for SERT were
132.7±1.4, 132.0±0.7, 134.5±0.8, 131.1±1.2 and 130.5±1.4 in the
control, IBS-total, IBS-D, IBS-M and IBS-C groups, respectively,
with no significant differences between any of these groups
(P=0.142; Figs. 7 and 8).
Discussion
The patients with IBS examined in this study had
moderate symptoms. These symptoms appeared, however, to
considerably reduce their quality of life. Approximately 53% of
these patients suffered from functional dyspepsia in addition to
IBS.
The present study measured the density of endocrine
cells, which are the anatomical units responsible for the
production of hormones. Furthermore, the immunoreactivity
intensity, which reflects the cellular hormone (secretory granules)
content, and hence the summation of the cellular synthesis and
release of that particular hormone was detected. The
immunoreactivity intensity is a semi-quantative method measured in
arbitrary units and is useful for comparing groups immunostained
under the same conditions. Modern advances in the illumination used
to visualise specimens on microscopes and in computer software have
now made it possible to obtain reliable measurements of this
parameter. The findings of the present study revealed abnormalities
in all the endocrine cell types in the stomach antrum, namely the
serotonin-, gastrin- and somatostatin-secreting endocrine cells. In
patients with IBS, regardless of the subtype, the density of
gastrin-secreting cells increased, while that of the
somatostatin-secreting cells decreased.
The density of serotonin-secreting cells differed
between the IBS subtypes. Whereas the density of
serotonin-secreting cells was unaltered in the IBS-D patients
(relative to the controls), it was decreased in IBS-M patients and
increased in the IBS-C patients. Serotonin activates the submucosal
sensory branch of the enteric nervous system, which conveys
sensation from the gut to the central nervous system and stimulates
the intrinsic primary afferent neurons that initiate peristaltic
reflexes (36–39). The increased density of
serotonin-secreting cells in the IBS-C patients may reflect an
attempt to increase motility and initiate peristaltic reflexes in
the presence of constipation. The increased serotonin levels in
IBS-C patients may explain the nausea that these patients
experience. However, it is difficult to ascertain why the density
of serotonin-secreting cells decreased in the IBS-M patients, but
not in the IBS-D patients based on a secondary effect on motility.
Genetic abnormalities in SERT have been observed in patients with
IBS and it has been reported that SERT levels are decreased in the
large intestine and increased in the ileum of patients with IBS
(20,40–46). There were no abnormalities in SERT
immunoreactivity in the antrum of the patients with IBS included in
the present study.
The density of gastrin-immunoreactive cells was
higher in the patients with IBS (regardless of subtype) than in the
controls. The increased gastrin immunoreactivity intensity in the
IBS-D patients may be caused by either increased synthesis or
decreased release of the hormone. On the other hand, the density of
somatostatin-immunoreactive cells was lower in all the subtypes of
IBS than in the healthy controls. Gastrin is the main hormonal
stimulant of acid secretion in the stomach (47–51); it stimulates parietal cells both
directly and indirectly by releasing histamine from
enterochromaffin-like cells (47,48,51,52). Somatostatin inhibits acid
secretion directly by acting on parietal cells and indirectly by
inhibiting histamine and gastrin secretion (47–51). The present findings of increased
gastrin and decreased somatostatin in all IBS subtypes may cause a
high level of gastric acid secretion, which may account for the
high incidence of dyspepsia and gastro-oesophageal reflux observed
in patients with IBS (53–61).
In conclusion, the present study found abnormalities
in all 3 endocrine cell types in the stomach antrum of patients
with IBS. The nature of the abnormalities in the
serotonin-secreting cells differed between the IBS subtypes, while
all IBS subtypes had a high density of gastrin-immunoreactive cells
and a low density of somatostatin-immunoreactive cells. Since
gastrin is the main stimulator and somatostatin is the principal
inhibitor of gastric acid secretion, a high gastric secretion is to
be expected in patients with IBS. These findings may explain the
overlap of IBS with dyspepsia and gastro-oesophageal reflux.
IBS is considered to be a large intestinal disorder
and consequently the colonic and rectal endocrine cells have been
the subjects of several studies to elucidate a possible role of
these cells in the pathophysiology of IBS. Thus, colonic serotonin
and PYY cell densities have been found to be low in both IBS-D and
IBS-C patients (18). In the
rectum of patients with sporadic (non-specific) IBS, the densities
of PYY and enteroglucagon cells have been shown to be significantly
lower and those of somatostatin-secreting cells to be significantly
higher in both IBS-D and IBS-C patients compared to the controls,
whereas the density of serotonin-secreting cells in these patients
did not differ from that in the healthy controls (19,20). Rectal serotonin and PYY cell
densities in post-infectious IBS have been reported to be elevated
(22,24,26,62,63). Recently, however, abnormalities in
the endocrine cells in the stomach and duodenum have been reported
in patients with IB (15–28). The density of ghrelin cells in the
oxyntic mucosa of the stomach has been shown to be lower in IBS-C
and higher in IBS-D patients than in healthy controls (15). In the duodenum, the densities of
GIP- and somatostatin-secreting cells have been shown to be
decreased in both IBS-D and IBS-C patients (17). The densities of duodenal secretin
and cholecystokinin (CCK) cells are decreased in IBS-D patients but
unaltered in IBS-C patients (17). The duodenal serotonin cells are
not affected in both IBS-D and IBS-C patients (17). Post-infectious IBS has been found
to be associated with increased numbers of duodenal CCK cells but
decreased numbers of serotonin cells (16). The present observation of
abnormalities in the antral endocrine supports the suggestion that
the endocrine cells are abnormal in all the segments of the
gastrointestinal tract and IBS is a disorder that is not restricted
to the large intestine. The present findings emphasise the role of
gut endocrine cells in the pathophysiology of IBS. Moreover, they
lend support to the assumption that abnormalities in
gastrointestinal cells can explain the dysmotility, abdominal
visceral hypersensitivity and abnormal gut secretion observed in
patients with IBS (64,65).
Acknowledgements
The present study was supported by a grant from
Helse-Fonna.
References
|
1
|
Moran GW, Leslie FC, Levison SE,
Worthington J and McLaughlin JT: Enteroendocrine cells: neglected
players in gastrointestinal disorders? Therap Adv Gastroenterol.
1:51–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: the role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (Review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
4
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: diagnosis, pathogenesis and
treatment options. Nova Science Publishers Inc; New York: 2012
|
|
5
|
Sternini C, Anselmi L and Rozengurt E:
Enteroendocrine cells: a site of ‘taste’ in gastrointestinal
chemosensing. Curr Opin Endocrinol Diabetes Obes. 15:73–78.
2008.
|
|
6
|
Sternini C: Taste receptors in the
gastrointestinal tract. IV. Functional implications of bitter taste
receptors in gastrointestinal chemosensing. Am J Physiol
Gastrointest Liver Physiol. 292:G457–G461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raybould HE: Gut chemosensing:
interactions between gut endocrine cells and visceral afferents.
Auton Neurosci. 153:41–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raybould HE: Nutrient sensing in the
gastrointestinal tract: possible role for nutrient transporters. J
Physiol Biochem. 64:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertrand PP and Bertrand RL: Serotonin
release and uptake in the gastrointestinal tract. Auton Neurosci.
153:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akiba Y and Kaunitz JD: Luminal
chemosensing in the duodenal mucosa. Acta Physiol (Oxf). 201:77–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinert RE and Beglinger C: Nutrient
sensing in the gut: interactions between chemosensory cells,
visceral afferents and the secretion of satiation peptides. Physiol
Behav. 105:62–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura E, Hasumura M, Uneyama H and
Torii K: Luminal amino acid-sensing cells in gastric mucosa.
Digestion. 83(Suppl 1): 13–18. 2011. View Article : Google Scholar
|
|
13
|
Tolhurst G, Reimann F and Gribble FM:
Intestinal sensing of nutrients. Handb Exp Pharmacol. 309–335.
2012. View Article : Google Scholar
|
|
14
|
Mace OJ, Schindler M and Patel S: The
regulation of K- and L-cell activity by GLUT2 and the
calcium-sensing receptor CasR in rat small intestine. J Physiol.
590:2917–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Salhy M, Lillebo E, Reinemo A and
Salmelid L: Ghrelin in patients with irritable bowel syndrome. Int
J Mol Med. 23:703–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dizdar V, Spiller R, Singh G, et al:
Relative importance of abnormalities of CCK and 5-HT (serotonin) in
Giardia-induced post-infectious irritable bowel syndrome and
functional dyspepsia. Aliment Pharmacol Ther. 31:883–891.
2010.PubMed/NCBI
|
|
17
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small-intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dis Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Salhy M, Gundersen D, Ostgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Abnormal rectal endocrine cells in patients with
irritable bowel syndrome. Regul Pept. 188:60–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coates MD, Mahoney CR, Linden DR, et al:
Molecular defects in mucosal serotonin content and decreased
serotonin reuptake transporter in ulcerative colitis and irritable
bowel syndrome. Gastroenterology. 126:1657–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang SH, Dong L, Luo JY, et al: Decreased
expression of serotonin in the jejunum and increased numbers of
mast cells in the terminal ileum in patients with irritable bowel
syndrome. World J Gastroenterol. 13:6041–6047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK
and Cho SW: The alteration of enterochromaffin cell, mast cell, and
lamina propria T lymphocyte numbers in irritable bowel syndrome and
its relationship with psychological factors. J Gastroenterol
Hepatol. 23:1689–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Rhee PL, Kim G, et al:
Enteroendocrine cell counts correlate with visceral
hypersensitivity in patients with diarrhoea-predominant irritable
bowel syndrome. Neurogastroenterol Motil. 18:539–546. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Lim JH, Park H and Lee SI:
Increased immunoendocrine cells in intestinal mucosa of
postinfectious irritable bowel syndrome patients 3 years after
acute Shigella infection: an observation in a small case
control study. Yonsei Med J. 51:45–51. 2010.PubMed/NCBI
|
|
25
|
Dunlop SP, Coleman NS, Blackshaw E, et al:
Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel
syndrome. Clin Gastroenterol Hepatol. 3:349–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spiller RC, Jenkins D, Thornley JP, et al:
Increased rectal mucosal enteroendocrine cells, T lymphocytes, and
increased gut permeability following acute Campylobacter
enteritis and in post-dysenteric irritable bowel syndrome. Gut.
47:804–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Salhy M, Lomholt-Beck B and Hausken T:
Chromogranin A as a possible tool in the diagnosis of irritable
bowel syndrome. Scand J Gastroenterol. 45:1435–1439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225.
2012.PubMed/NCBI
|
|
29
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spiller R, Aziz Q, Creed F, et al:
Guidelines on the irritable bowel syndrome: mechanisms and
practical management. Gut. 56:1770–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roalfe AK, Roberts LM and Wilson S:
Evaluation of the Birmingham IBS symptom questionnaire. BMC
Gastroenterol. 8:302008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talley NJ, Verlinden M and Jones M:
Quality of life in functional dyspepsia: responsiveness of the
Nepean Dyspepsia Index and development of a new 10-item short form.
Aliment Pharmacol Ther. 15:207–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arslan G, Lind R, Olafsson S, Florvaag E
and Berstad A: Quality of life in patients with subjective food
hypersensitivity: applicability of the 10-item short form of the
Nepean Dyspepsia Index. Dig Dis Sci. 49:680–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patrick DL, Drossman DA, Frederick IO,
DiCesare J and Puder KL: Quality of life in persons with irritable
bowel syndrome: development and validation of a new measure. Dig
Dis Sci. 43:400–411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drossman DA, Patrick DL, Whitehead WE, et
al: Further validation of the IBS-QOL: a disease-specific
quality-of-life questionnaire. Am J Gastroenterol. 95:999–1007.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gershon MD and Tack J: The serotonin
signaling system: from basic understanding to drug development for
functional GI disorders. Gastroenterology. 132:397–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gershon MD: Plasticity in serotonin
control mechanisms in the gut. Curr Opin Pharmacol. 3:600–607.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gershon MD: 5-Hydroxytryptamine
(serotonin) in the gastrointestinal tract. Curr Opin Endocrinol
Diabetes Obes. 20:14–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gershon MD: Serotonin is a sword and a
shield of the bowel: serotonin plays offense and defense. Trans Am
Clin Climatol Assoc. 123:268–280. 2012.PubMed/NCBI
|
|
40
|
Camilleri M, Andrews CN, Bharucha AE, et
al: Alterations in expression of p11 and SERT in mucosal biopsy
specimens of patients with irritable bowel syndrome.
Gastroenterology. 132:17–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Camilleri M, Busciglio I, Carlson P, et
al: Candidate genes and sensory functions in health and irritable
bowel syndrome. Am J Physiol Gastrointest Liver Physiol.
295:G219–G225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumar S, Ranjan P, Mittal B and Ghoshal
UC: Serotonin transporter gene (SLC6A4) polymorphism in patients
with irritable bowel syndrome and healthy controls. J
Gastrointestin Liver Dis. 21:31–38. 2012.PubMed/NCBI
|
|
43
|
Park JM, Choi MG, Park JA, et al:
Serotonin transporter gene polymorphism and irritable bowel
syndrome. Neurogastroenterol Motil. 18:995–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saito YA, Locke GR III, Zimmerman JM, et
al: A genetic association study of 5-HTT LPR and GNbeta3 C825T
polymorphisms with irritable bowel syndrome. Neurogastroenterol
Motil. 19:465–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wendelbo I, Mazzawi T and El-Salhy M:
Increased serotonin transporter immunoreactivity intensity in the
ileum of patients with irritable bowel disease. Mol Med Rep.
9:180–184. 2013.PubMed/NCBI
|
|
46
|
El-Salhy M, Wendelbo I and Gundersen D:
Serotonin and serotonin transporter in the rectum of patients with
irritable bowel disease. Mol Med Rep. 8:451–455. 2013.PubMed/NCBI
|
|
47
|
Schubert ML: Gastric secretion. Curr Opin
Gastroenterol. 27:536–542. 2011. View Article : Google Scholar
|
|
48
|
Schubert ML: Gastric secretion. Curr Opin
Gastroenterol. 26:598–603. 2010. View Article : Google Scholar
|
|
49
|
Schubert ML: Gastric secretion. Curr Opin
Gastroenterol. 23:595–601. 2007. View Article : Google Scholar
|
|
50
|
Schubert ML: Gastric secretion. Curr Opin
Gastroenterol. 18:639–649. 2002. View Article : Google Scholar
|
|
51
|
Chu S and Schubert ML: Gastric secretion.
Curr Opin Gastroenterol. 28:587–593. 2012. View Article : Google Scholar
|
|
52
|
Van Citters GW and Lin HC: Ileal brake:
neuropeptidergic control of intestinal transit. Curr Gastroenterol
Rep. 8:367–373. 2006.PubMed/NCBI
|
|
53
|
Pourhoseingholi A, Vahedi M,
Pourhoseingholi MA, et al: Irritable bowel syndrome,
gastro-oesophageal reflux disease and dyspepsia: overlap analysis
using loglinear models. Arab J Gastroenterol. 13:20–23. 2012.
View Article : Google Scholar
|
|
54
|
Kim HG, Lee KJ, Lim SG, Jung JY and Cho
SW: G-protein beta3 subunit C825T polymorphism in patients with
overlap syndrome of functional dyspepsia and irritable Bowel
syndrome. J Neurogastroenterol Motil. 18:205–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Suzuki H and Hibi T: Overlap syndrome of
functional dyspepsia and irritable bowel syndrome - are both
diseases mutually exclusive? J Neurogastroenterol Motil.
17:360–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nakajima S, Takahashi K, Sato J, et al:
Spectra of functional gastrointestinal disorders diagnosed by Rome
III integrative questionnaire in a Japanese outpatient office and
the impact of overlapping. J Gastroenterol Hepatol. 25(Suppl 1):
S138–S143. 2010. View Article : Google Scholar
|
|
57
|
Olafsdottir LB, Gudjonsson H, Jonsdottir
HH and Thjodleifsson B: Stability of the irritable bowel syndrome
and subgroups as measured by three diagnostic criteria - a 10-year
follow-up study. Aliment Pharmacol Ther. 32:670–680.
2010.PubMed/NCBI
|
|
58
|
Kaji M, Fujiwara Y, Shiba M, et al:
Prevalence of overlaps between GERD, FD and IBS and impact on
health-related quality of life. J Gastroenterol Hepatol.
25:1151–1156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Noh YW, Jung HK, Kim SE and Jung SA:
Overlap of erosive and non-erosive reflux diseases with functional
gastrointestinal disorders according to rome III criteria. J
Neurogastroenterol Motil. 16:148–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hori K, Matsumoto T and Miwa H: Analysis
of the gastrointestinal symptoms of uninvestigated dyspepsia and
irritable bowel syndrome. Gut Liver. 3:192–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ford AC, Marwaha A, Lim A and Moayyedi P:
Systematic review and meta-analysis of the prevalence of irritable
bowel syndrome in individuals with dyspepsia. Clin Gastroenterol
Hepatol. 8:401–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang LH, Fang XC and Pan GZ: Bacillary
dysentery as a causative factor of irritable bowel syndrome and its
pathogenesis. Gut. 53:1096–1101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dunlop SP, Jenkins D, Neal KR and Spiller
RC: Relative importance of enterochromaffin cell hyperplasia,
anxiety, and depression in postinfectious IBS. Gastroenterology.
125:1651–1659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Salhy M, Gundersen D, Gilja OH,
Hatlebakk JG and Hausken T: Is irritable bowel syndrome an organic
disorder? World J Gastroenterol. 2:384–400. 2014. View Article : Google Scholar
|
|
65
|
El-Salhy M, Hatlebakk JG, Gilja OH and
Hausken T: Irritable bowel syndrome: recent developments in
diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol
Hepatol. 8:435–443. 2014. View Article : Google Scholar : PubMed/NCBI
|