Introduction
In recent years, radiation has gained tremendous
value in the diagnosis and treatment of a number of malignancies
and radiation in one form or another is now indispensble in
virtually every branch of medicine. It has been used for more than
a century in the treatment of cancer, since rapidly proliferating
cancer cells are more sensitive than other tissue to DNA damage
induced by radiation (1).
However, radiotherapy uses high doses of ionizing radiation to kill
cancer cells, which can cause damage to normal cells. The use of
combination treatments, such as concomitant radiotherapy and
chemotherapy also exacerbates the acute damage to normal tissue
(2). In particular, surrounding
normal tissues, such as lympho-haematopoietic tissue or the
reproductive system are as susceptible to these debilitating
reactions as targeted tumor cells due to their active replication
(3). Thus, the development of
radioprotective agents is crucial in clinical radiotherapy in order
to obtain optimal tumor control and to protect normal tissues from
potential radiation damage.
Exposure to a higher dose of ionizing radiation for
cancer treatment leads to mortality in a mammalian system by
multiple mechanisms, including direct DNA damage and indirect
oxidative stress (4). Direct
effects are the irreparable damage to critical targets within a
cell, such as DNA. Indirect effects result from radiation
interacting with other molecules in cells that are not critical
targets, but are close enough to pass on damage, typically in the
form of free radicals. As mammals are composed of roughly 80%
water, indirect effects include the production of hydroxyl free
radicals, which are potent oxidants capable of breaking chemical
bonds and initiating lipid peroxidation in nano- to microsecond
timeframes (5). These free
radicals interact with critical macromolecules leading to DNA
damage, which may be the most important factor in cell death
(4–7). Although cells and tissues are
equipped with endogenous enzymes [e.g., superoxide dismutase (SOD)]
capable of detoxifying and removing water radiolysis products, when
the production of these reactive oxygen species (ROS) increases
following exposure to irradiation, the system is incapable of
protecting the cells from the hazardous effects of free radicals
(6,7).
A number of compounds have been tested to develop
more effective or less toxic drugs to address the great clinical
need for effective radioprotectant agents. Over the past decade,
attention has been paid to natural compounds, which have lower
toxicity than synthetic radioprotectors (8) as many synthetic compounds have many
drawbacks, including high cost, side-effects and toxicity (9). Furthermore, it is well known that
natural compounds have strong radical scavenging and antioxidant
activity (8). Several plant
extracts, herbal preparations and phytochemicals have been reported
to exert radioprotective effects in in vitro and in
vivo studies, and their radiation-protecting abilities have
been attributed to their antioxidant and free-radical scavenging
properties (10–13). However, few clinical trials for
their efficacy in clinical use have been reported to date.
Cordyceps militaris (C. militaris), the rare
Chinese caterpillar fungus, has been used in traditional medicine
to maintain health and to treat numerous diseases associated with
the circulatory, respiratory, glandular and metabolic systems
(14). It contains many types of
phytochemicals, such as cordycepin, polysaccharides, ergosterol and
mannitol, and due to its various physiological activities, it is
now used for multiple medicinal purposes (15,16). The important bioactive compound,
cordycepin (3′-deoxyadenosine), a nucleoside analogue, is
considered as a nucleic acid antibiotic that may inhibit the
canceration of cells, contributing to the normalization of cancer
cells as one of the constituents of gene DNA (17,18). A number of studies have
demonstrated that the extracts of C. militaris have various
pharmacological actions, such as anti-angiogenetic (19), anti-inflammatory (20), antioxidant (21,22), antitumor (23–25) and immunomodulatory activities
(26). Although C.
militaris extract and cordycepin have been extensively tested
for their pharmacological and biological effects, the
radioprotective effects of C. militaris extract remain
unclear.
In the present study, we investigated the protective
effects of the extract of C. militaris against
radiation-induced DNA damage in Chinese hamster ovary (CHO)-K1
cells. Our data suggest that C. militaris has potential
radioprotective activity.
Materials and methods
Chemicals and reagents
Cordycepin, 1,1diphenyl-2-picryl hydrazyl (DPPH),
nitroblue tetrazolium (NBT), phosphate-buffered saline (PBS),
3-(4,5-dimethyl-2-yl)-2,5-diphenyl-2II tetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO) and other chemical reagents were
purchased from Sigma (St. Louis, MO, USA).
Sample preparation
C. militaris used in the present study was
supplied by Chungwon-Industrial Farm (Busan, Korea) which had
constructed the C. militaris JLM 0636 strain by single spore
fusion of various strains of C. militaris. For the
preparation of the extract from C. militaris JLM 0636
(CM-AE), the powder of dried fruiting bodies was extracted with
distilled deionized water for 3 h at 121°C, the insoluble materials
were removed by centrifugation at 10,000 × g for 30 min and
filtration was carried out using a 0.45-μm membrane filter. The
CM-AE was then lyophilized using a VirTis freeze dryer (VirTis Co.,
Gardiner, NY, USA) for use in later experiments. The cordycepin
content in CM-AE was analyzed by high performance liquid
chromatagraphy (Perkin-Elmer 200 series system; Perkin-Elmer,
Waltham, MA, USA) in our previous study (26). Cordycepin was used as the control
compound.
Free radical scavenging activity of
CM-AE
The following parameters were assayed to determine
the free radical scavenging activity of CM-AE. DPPH radical
scavenging was determined by the method of von Gadow and Hansmann
(27) with some modifications.
Briefly, 10 μl of various concentrations of CM-AE and cordycepin
(diluted to final concentrations of 31.3, 62.5, 125, 250 and 500
μg/ml) were mixed with 190 μl of DPPH in ethanol (final
concentration 0.1 mM) in wells of a 96-well plate. The plate was
kept in the dark for 10 min, and the absorbance of the solution was
measured at 517 nm using a microplate reader (VersaMax; Molecular
Devices, Sunnyvale, CA, USA). Superoxide radical scavenging
activity was assessed by the NBT reduction method of McCord and
Fridovich (28) with some
modifications. The reaction mixture contained 134 μl of buffer (50
mM KH2PO4, pH 7.4), 2 μl of 100 mM
Na2EDTA, 20 μl of 3 mM hypoxanthine, 2 μl of 10 mM NBT,
and 10 μl of various concentrations of CM-AE and cordycepin. The
absorbance of the samples was measured immediately following the
addition of 32 μl of xanthine oxidase (1 unit/10 ml buffer) at 540
nm using a microplate reader. The plate was kept in the dark for 10
min, and absorbance was measured again at 540 nm. Hydroxyl radical
scavenging activity was measured using the OxiSelect™ HORAC
Activity Assay kit (Cell Biolabs, San Diego, CA, USA). This assay
is based on the oxidation-mediated quenching of a fluorescent probe
by hydroxyl radicals produced by a hydroxyl radical initiator and
Fenton’s reagent.
Estimation of plasmid pSK DNA damage
A 5.5 kb length of plasmid pSK was transformed in
E. coli and purified using an EndoFree Plasmid Maxi kit
(Qiagen, Valencia, CA, USA). The pSK DNA (0.5 μg) in PBS was
exposed to 5 Gy-radiation in the presence and absence of CM-AE and
cordycepin at various concentrations. Following irradiation, the
DNA was electrophoresed on a 1% agarose gel in 0.08 M Tris
borate/0.2 mM EDTA buffer (pH 8.3). The bands of supercoiled DNA
(SC) and open circular DNA or broken DNA (OC) were visualized with
SYBR Safe DNA gel staining (Invitrogen, Carlsbad, CA, USA) under UV
light, and quantified by scanning and densitometric measurements
using BIO-1D analysis software (Vilber Lourmat, Marne-la-Vallée,
France). DNA lesions were expressed as a density ratio of the OC
form.
γ-irradiation
γ-irradiation by 137Cs was carried out
using a Biobeam 8000 (Gamma-Service Medical GmbH, Leipzig, Germany)
irradiator with a dose rate of 1.88 Gy/min.
Cell culture
The Chinese hamster ovary cell line, CHO-K1, was
obtained from the American Type Tissue Collection (ATCC, Manassas,
VA, USA). The CHO-K1 cells were cultured in F-12 nutrient mixtures
(Ham’s F-12; Welgene, Daegu, Korea) supplemented with 10% fetal
bovine serum (FBS; HyClone, Logan, UT, USA). The cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
Cell viability assay
The number of viable cells was determined by the
ability of mitochondria to convert MTT to formazan dye. The CHO-K1
cells were cultured overnight in 96-well plates, at a density of
2×104 cells/200 μl in each well. The following day, the
cells were co-incubated with various concentrations of CM-AE and
cordycepin for 24 h. Following incubation, the medium was removed,
and the cells were supplemented with 10 μl of 10 mg/ml MTT in each
well. Following a further 4 h of incubation at 37°C in a humidified
5% CO2 atmosphere, the MTT was removed, and the cells
were lysed with 150 μl DMSO. The absorbance was measured at 550 nm
using a microplate reader.
2′,7′-Dichlorofluorescein (DCFH)
assay
The CHO-K1 cells were cultured in 96-well plates, at
a density of 3×104 cells/200 μl in each well and treated
with various concentrations of CM-AE and cordycepin at 37°C in a
humidified atmosphere with 5% CO2 for 1 h. The cells
were supplemented with 25 μM 2′,7′-dichlorfluorescein-diacetate
(DCFH-DA; Sigma-Aldrich) solution and were immediately exposed to 2
Gy of 137Cs γ-radiation. Following irradiation, the
cells were incubated at 37°C for 10 min and the fluorescence
intensity of DCFH-DA was measured using Paradigm™ Detection
Platform and Multimode Analysis Software version 3.1.0.1 (Beckman
Coulter, Fullerton, CA, USA). The excitation and emission
wavelengths were 480 and 530 nm, respectively.
Comet assay
The CHO-K1 cells were cultured overnight in 6-well
plates, at a density of 2×105 cells/3 ml in each well.
The following day, the cells were treated with various
concentrations of CM-AE and cordycepin for 15 min, exposed to 2 Gy
of 137Cs γ-radiation, and incubated at 37°C in a
humidified atmosphere with 5% CO2 for 15 min. The cells
were collected and mixed with low melting point agarose at 37°C.
This mixture was placed on the top of the previous layer of 0.5%
normal melting point agarose on a slide covered with a coverslip,
and returned to 4°C until solid. The coverslip was gently removed
and some NMP agarose was added to the slide. The slide was covered
again with a coverslip and placed at 4°C until the mixture was
solid. The slide was placed in chilled lysis buffer (100 mM EDTA,
2.5 M sodium chloride, 10 mM Trizma base and 1%
N-lauroylsarcosinate, adjusted to pH 10.0, with 1% Triton X-100)
and unwinding buffer (1 mM EDTA and 300 mM sodium hydroxide, pH
>13), respectively, and subjected to electrophoresis.
Thereafter, the slides were gently washed with 0.4 M Tris buffer,
stained with GelGreen DNA dye (Biotium, Inc., Hayward, CA, USA),
and analyzed under a fluorescence microscope (Carl Zeiss,
Oberkochen, Germany). The images were captured, and a minimum of
100 comets per slide, in triplicate for a group, were analyzed
using Metafer 4 software (MetaSystems; Carl Zeiss) which yields the
percentage DNA in the tail, tail length, tail moment (TM) and olive
tail moment (OTM) directly. The parameter TM is the product of the
tail length and percentage DNA in the tail, and the OTM is the
product of the distance between the center of the head and the
center of the tail and percentage DNA in the tail (29).
Immunofluorescence staining of
phoshphorylated H2AX
The CHO-K1 cells were cultured overnight in 6-well
plates, at a density of 3×105 cells/3 ml in each well.
The following day, the cells were treated with various
concentrations of CM-AE and cordycepin for 15 min, exposed to 2 Gy
of 137Cs γ-radiation and incubated at 37°C in a
humidified atmosphere with 5% CO2 for 45 min. The cells
were cytocentrifuged on slides, fixed with 4% formaldehyde
(Biosesang, Seoul, Korea), permeabilized for 10 min on ice in 0.2%
Triton X-100 in PBS, and washed thoroughly with PBS. The slides
were then incubated with anti-phosphorylated histone H2AX (serine
139) antibody (Abcam, Cambridge, MA, USA) in PBS at room
temperature for 1 h. The primary antibodies were washed with PBS,
and Texas Red Goat anti-mouse IgG secondary antibody (Vector
Laboratories, Inc., Burlingame, CA, USA) was added. The slides were
incubated at room temperature for 1 h, washed with PBS, and
incubated at room temperature with 4 μg/ml Hoechst 33342
(4′,6-diamidino-2-phenylindole; Invitrogen) for 15 min. All sldies
were mounted with 0.05 ml PBS containing 10% glycerol (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) and were examined using a
Zeiss fluorescence microscope. The red intensity of the
phospho-H2AX signal on the digitized images was analyzed using
AxioVision Rel. 4.8 software (Carl Zeiss).
Statistical analysis
All data are expressed as the means ± standard
deviation. Statistical significance was tested using the
Statistical Package for the Social Sciences statistical software
for Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA). Data were
tested for normality using the Kolmogorov-Smirnov test and for
homogeneity of variance using Levene’s test, prior to any
statistical analysis. The data were normally distributed and the
variances were homogeneous. Therefore, significant differences
between 2 groups were evaluated using the Student’s t-test and
significant differences between more than 2 groups were evaluated
by one-way analysis of variance with Dunnett’s post hoc test for
multiple comparisons. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
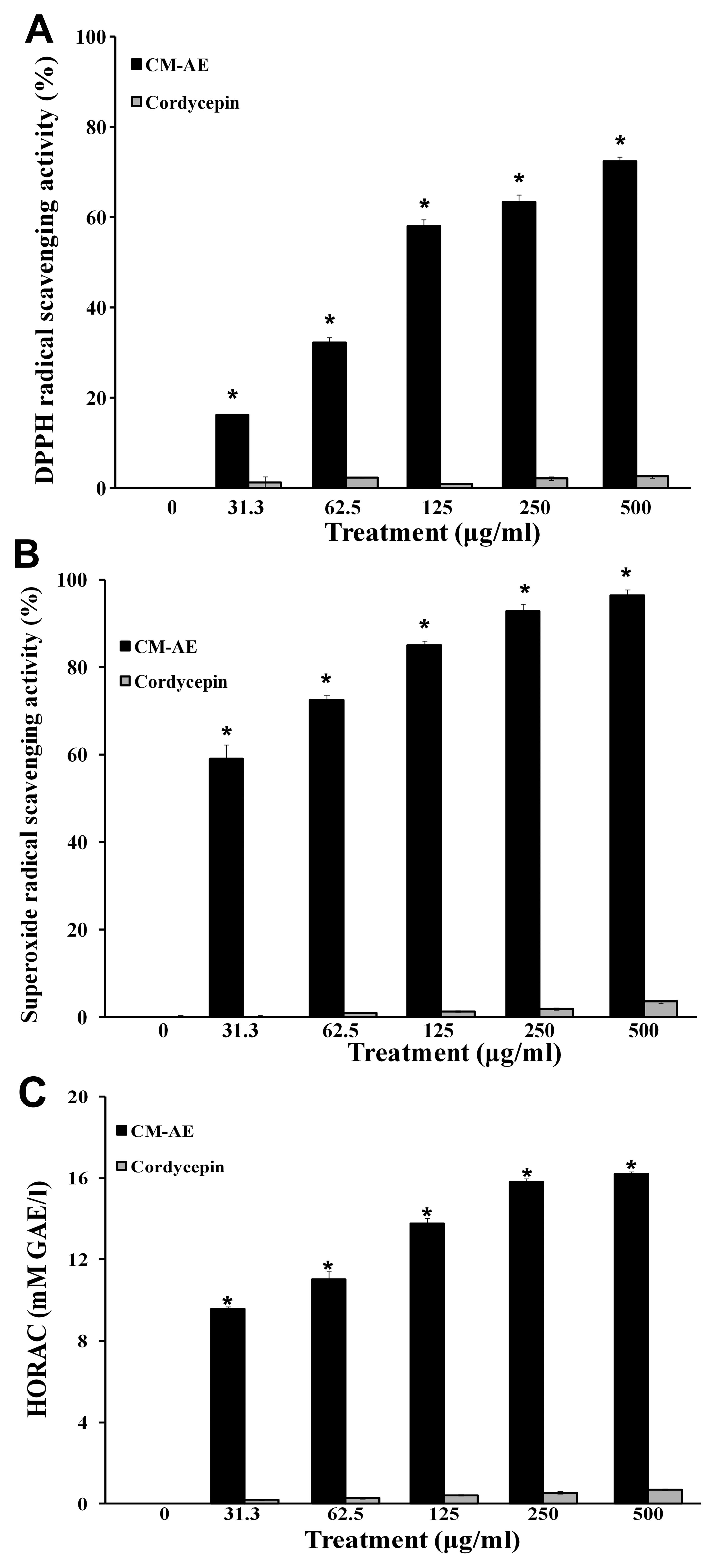
Effect of CM-AE on free radical
scavenging activity
The concentration of cordycepin contained in the JLM
0636 strain of C. militaris was 7.42 mg/g (dry weight) as
shown in our previous study (26), which was calculated from the peak
area shown in the standard curve of commercial cordycepin. To
investigate the free radical scavenging activity of
cordycepin-enriched CM-AE, we performed DPPH assay, NBT/XO assay,
and the oxidation of a fluorescent probe by hydroxyl radicals, and
compared the results with those of cordycepin as the control
compound. The stable free radical scavenging activity of DPPH with
characteristic absorption at 517 nm was significantly increased by
CM-AE (P<0.05) (Fig. 1A).
CM-AE also inhibited the generation of superoxide radicals and
hydroxyl radical production in a concentration-dependent manner, as
shown by the increased superoxide radical scavenging activity
(Fig. 1B and C). However,
cordycepin showed little free radical scavenging activity as
regards DPPH radicals, superoxide radicals and hydroxyl radicals at
the tested concentrations (Fig.
1).
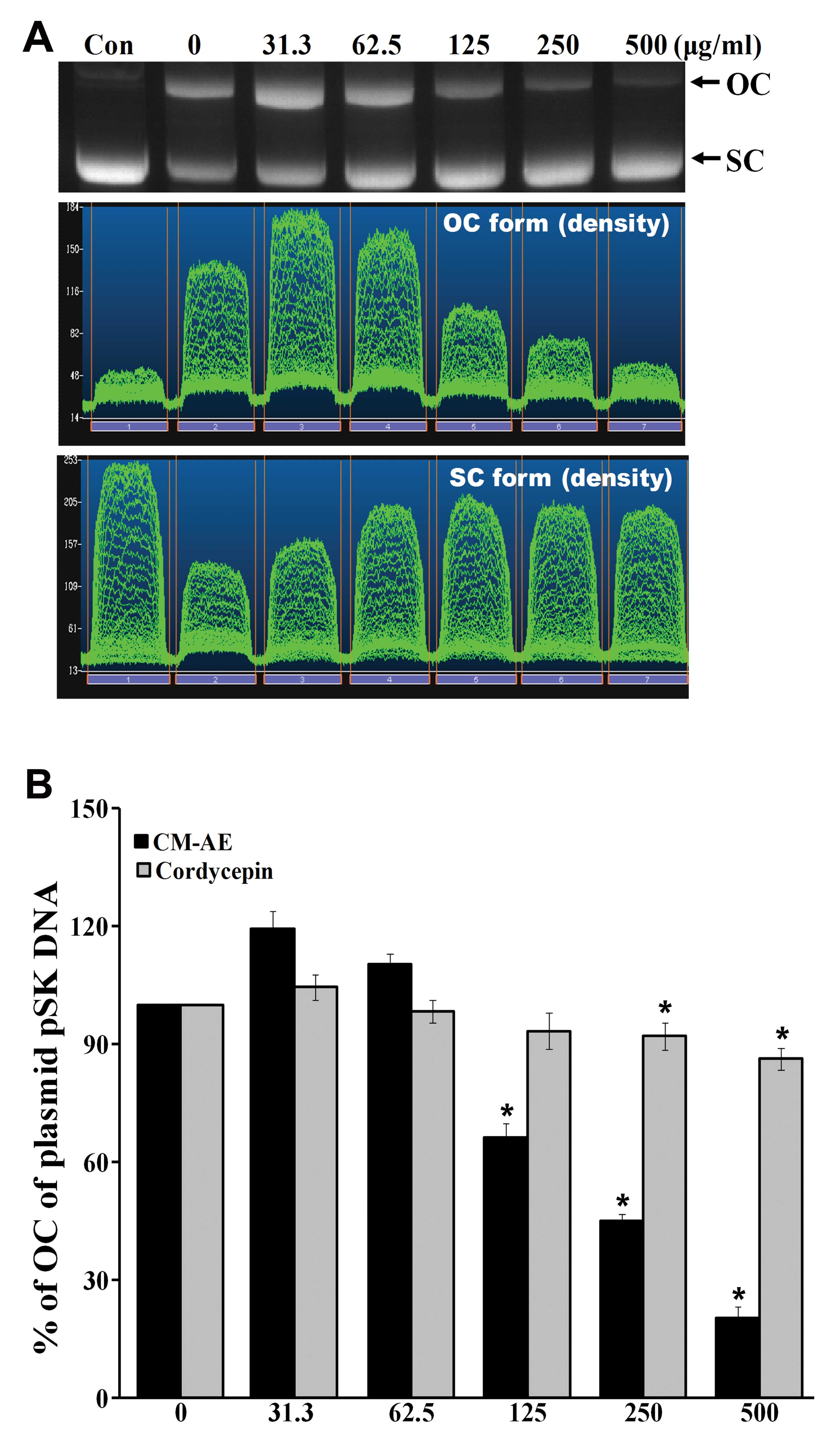
Effect of CM-AE on γ-radiation-induced
DNA damage in plasmid pSK
To determine the DNA protecting activity of CM-AE
in vitro, we measured plasmid pSK DNA damage with 5 Gy of
γ-irradiation in the absence or prescence CM-AE and compared the
results with those of cordycepin. The exposure of plasmid pSK DNA
to 137Cs γ-radiation resulted in the production of
strand breaks in which the supercoiled covalently closed circular
(SC) form of DNA was converted to the open circular or linear forms
(OC) in a radiation dose-dependent manner, as demonstrated in a
previous study of ours (30).
Hence, this plasmid DNA relaxation assay with pSK DNA was thought
to be a useful tool for the study of the radioprotective efficacy
of CM-AE against direct DNA damage. The data on the effects of
various concentrations of CM-AE on radiation-induced disappearance
of the OC form of plasmid pSK DNA are presented in Fig. 2. There was a
concentration-dependent inhibition of the disappearance of the OC
form of plasmid DNA following exposure to 5 Gy of γ-radiation at
125, 250 and 500 μg/ml. There was significant reduction of the OC
form of plasmid DNA following treatment with cordycepin at 250 and
500 μg/ml; however, the inhibitory effect was low when it was
compared to the effect produced by CM-AE (P<0.05; Fig. 2B).
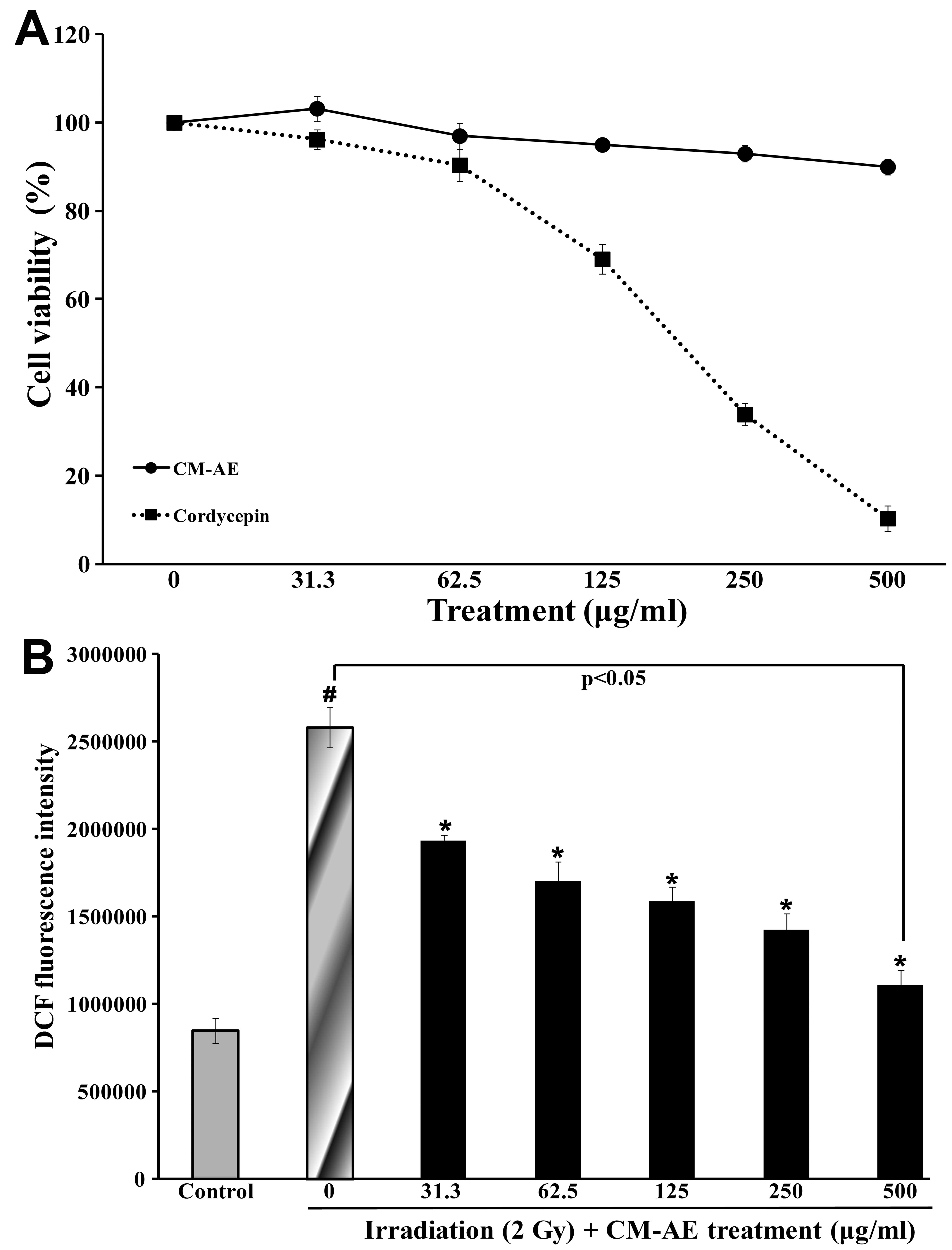
Effect of CM-AE on cellular ROS
production in irradiated CHO-K1 cells
To evaluate the effect of CM-AE on cellular ROS
production, radiation-exposed CHO-K1 cells were used. The
experimental doses of CM-AE and cordycepin were determined as
follows: the viability of the CHO-K1 cells was measured by MTT
assay in the presence of CM-AE or cordycepin. CM-AE retained the
viability of the CHO-K1 cells at 90% at a dose of up to 500 μg/ml.
However, cordycepin showed cytotoxicity in dose-dependent manner in
the CHO-K1 cells (Fig. 3A). When
irradiated with 2 Gy of 137Cs γ-radiation, ROS
production determined as by DCFH-DA assay was approximately 3-fold
greater than that in the non-irradiated CHO-K1 cells (Fig. 3B). CM-AE significantly reduced ROS
production in the irradiated CHO-K1 cells in a dose-dependent
manner (Fig. 3B); however, little
difference was observed in the production of ROS following
treatment with cordycepin compared to the irradiated cells at the
tested concentration (data not shown).
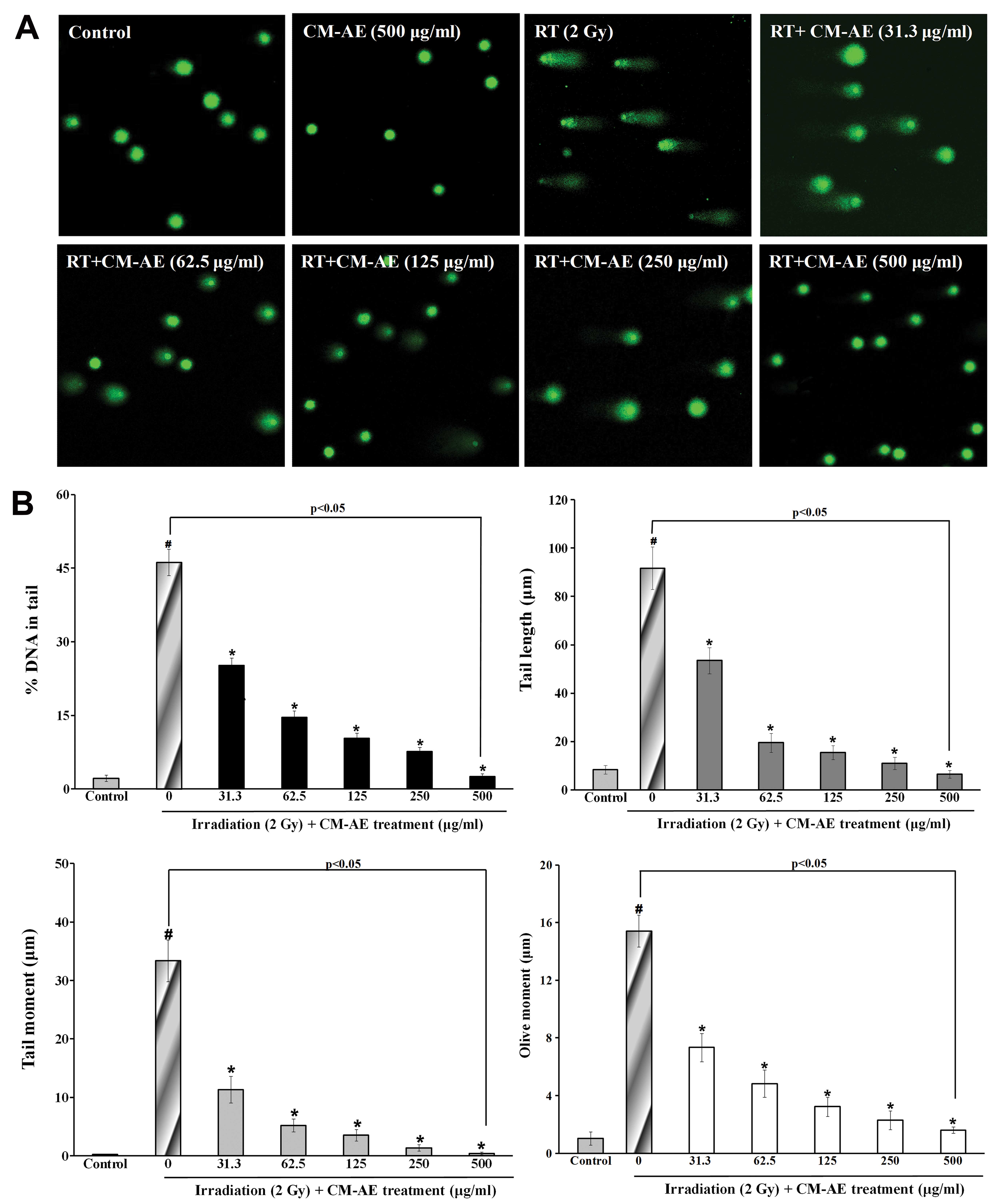
Effect of CM-AE on cellular DNA damage in
irradiated CHO-K1 cells
To demonstrate the protective effects of CM-AE on
cellular DNA damage, radiation-exposed CHO-K1 cells were used. We
performed alkaline single-cell gel electrophoresis (comet assay)
and immunefluorescence staining of phoshphorylated H2AX. As shown
Fig. 4, there was a significant
increase in comet parameters on DNA damage, such as percentage DNA
in the tail, the tail length, tail moment, and olive tail moment in
the irradiated CHO-K1 cells compared to the non-irradiated cells.
The presence of CM-AE during irradiation reduced these parameters
in a dose-dependent manner in the CHO-K1 cells (Fig. 4). To verify the protective effect
of CM-AE against cellular DNA double-strand breaks following
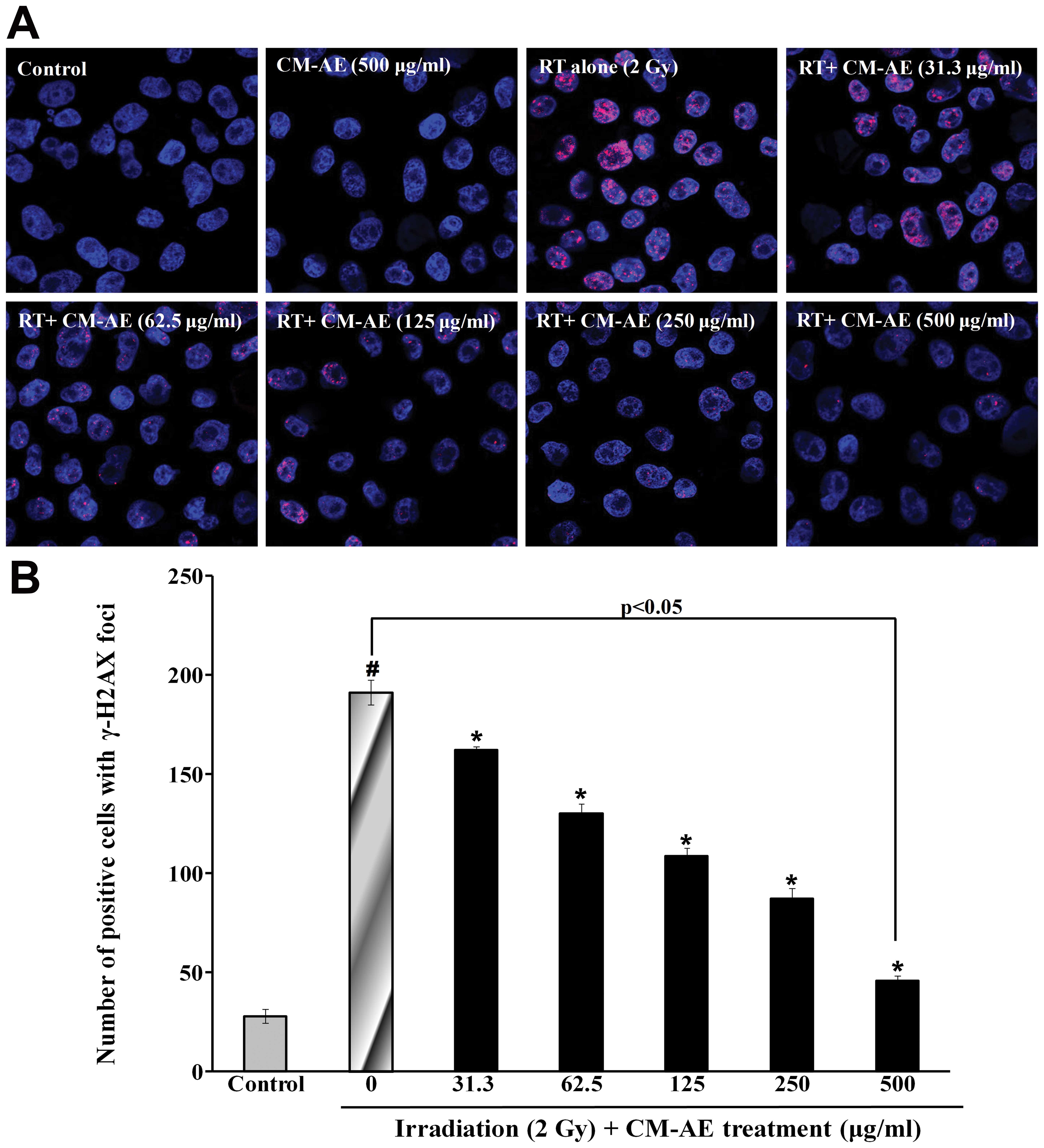
irradiation, the number of γ-H2AX foci was also measured in the
CHO-K1 cells. As shown in Fig. 5,
a greater number of red phosphorylated H2AX foci were clearly
observed in the nucleus following irradiation compared to the
non-irradiated cells. Similarly, CM-AE reduced the number of
positive cells with γ-H2AX foci in the CHO-K1 cells in a
dose-dependent manner (Fig. 5).
However, there was no reduction in cellular DNA damage follwing
treatment with cordycepin, as shown by comet assay and
immunefluorescence staining (γ-H2AX foci) compared to the
irradiated cells at tested concentration (data not shown).
Discussion
It is well known that most of the damage induced by
radiation in living cells is due to the generation of aqueous free
radicals. In particular, water, the most abundant intracellular
material, decomposes following exposure to ionizing radiation and
generates primary hydroxyl radicals (•OH) and secondary superoxide
radicals, which leads to serious cell damage from DNA strand breaks
(31). Hence, compounds that
protect DNA breaks from ionizing radiation-induced free radicals
have considerable potential as radioprotectors. Recent studies on
the development of radioprotectors have focused on searching for
effective and non-toxic compounds with herbal preparation. Herbal
products have various pharmacological properties and have long been
used for the treatment of various diseases. Therefore, the
screening of herbal drugs offers a major focus for new drug
discovery. In this regard, attention over the past 15 years has
shifted towards the evaluation of herbal products as
radioprotectors, due to their efficacy and low toxicity. The
suggested radioprotective efficacy of herbal extracts is a result
of the fact that they contain a large number of active constituents
which have antioxidant activity (32).
The pharmacological effects of several medicinal
mushrooms are related to their free radical scavenging properties,
and C. militaris is one of the most important medicinal
mushrooms (33). C.
militaris extracts have been reported to have antioxidant
properties (34,35). It has been reported that the
water-soluble crude extract of C. militaris exhibits
scavenging activity towards hydroxyl radicals (35). In addition, the protective effects
of C. militaris against oxidative damage have been compared,
and the free radical scavenging ability of C. militaris has
been shown to reduce the oxidative damage of biomolecules (36). The present study demonstrated that
CM-AE obtained from the cordycepin-enriched JLM 0636 strain of
C. militaris effectively scavenged in vitro DPPH
radicals, superoxide radicals and hydroxyl radicals. Furthermore,
the protective effects of CM-AE against plasmid DNA damage
following irradiation, such as DNA strand breaks in vitro
were demonstrated by quantifying the amount of DNA in both nicked
circular and supercoiled forms. However, there was little increase
in the free radical scavenging activity or the reduction of plasmid
DNA damage following treatment with cordycepin. These results
provide evidence that the radioprotective effects of CM-AE against
harmful chemicals and radiation are much more potent than those of
cordycepin.
The exposure of cells to ionizing radiation can lead
to the increased generation of ROS, including hydroxyl radicals
(OH), superoxide anions (O2−), singlet oxygen
(1O2), and hydrogen peroxide
(H2O2), which are major determinants of
cellular damage. Excessive ROS production leads to impaired
intracellular ionic homeostasis by damaging cellular
macromolecules, including DNA, proteins and lipids. Damaged DNA may
lead to cell apoptosis or cancerization (37). Therefore, ROS-scavenging activity
is also crucial for the development of radioprotectors (38). In a previous study, C.
militaris was shown to reduce the intracellular ROS generation
of human umbilical vein endothelial cells (HUVECs) exposed to high
amounts of glucose (39) and
cordycepin has also been reported to have the ability to scavenge
ROS, and inhibit platelet-derived growth factor (PDGF)-induced ROS
generation (40). The present
study revealed that CM-AE is a strong inhibitor of cellular ROS
production in CHO-K1 cells irradiated with 2 Gy of 137Cs
γ-radiation, while pre-treatment with cordycepin did not attenuate
the ROS levels in the irradiated cells at the tested
concentrations. Our results suggest that CM-AE effectively
attenuated cellular ROS levels induced by radiation and that these
effects were more potent to those induced by cordycepin.
DNA damage is the main event in radiation-induced
cell death. ROS is a DNA damage agent producing a series of DNA
lesions, including base damage, single- or double-strand breaks,
DNA-DNA or DNA-protein crosslinks and others. Double-strand breaks
are the most important for cell killing (41). Since the amount of DNA damage
caused by ionizing radiation correlates with the intensity of
oxidative stress, there are several possible means to diminish
macromolecular damage due to ionizing radiation. Several strategies
have been shown to ameliorate radiation-induced damage, one of
which is to reduce the amount of double-strand breaks, a critical
source of radiation-induced damage, through antioxidant activity
(32). In the present study, we
investigated the comet parameters of γ-radiation-exposed CHO-K1
cells, in which most of the strand breaks measured by the alkaline
comet assay were single-strand breaks. The increased parameters
induced by radiation were effectively prevented by a short-term
incubation with CM-AE prior to irradiation. We further evaluated
double-strand breaks in γ-radiation-exposed CHO-K1 cells through
the frequency of γ-H2AX foci. DNA DSBs are potentially damaging
events in cells that are highly mutagenic when misrepaired and
lethal if left unrepaired. Following DSB induction, phosphorylation
mediated either by ataxia-telangiectasia-mutated,
ataxia-telangiectasia-related or DNA-dependent protein kinase,
occurs on serine 139 at the C-terminus of H2AX molecules flanking
the DSBs in chromatin. The phosphorylated form of H2AX is termed
γ-H2AX (42). The appearance of
γ-H2AX in chromatin in the form of discrete nuclear foci, each of
which represents a single double-strand break, can be detected
immunocytochemically shortly after the induction of double-strand
breaks (43). The increased
number of γ-H2AX foci induced by the radiation of CHO-K1 cells was
also effectively prevented by short-term incubation with CM-AE
prior to irradiation. However, pre-treatment with cordycepin did
not protect against cellular DNA damage, such as single- and
double-strand breaks.
Nowadays, the major concern related to the
development of radioprotectors in radiotherapy is an enhancement of
the antitumor efficacy of radiation without causing unacceptable
toxicity. Hence, the normal tissues should be protected against
radiation injury to obtain optimal tumor control with a higher dose
(44). Previous studies have
reported that isolated compounds from C. militaris, such as
polysaccharides or cordycepin show antitumor activity (21,22), and it has also demonstrated that
extracts obtained from C. militaris induce immunomodulation
and tumor growth delay in mouse-derived breast cancer (23). Therefore, further studies are
required to investigate potential candidate material from CM-AE of
C. militaris as adjuvant materials for radiotherapy.
Acknowledgements
The present study was supported by the 2014 National
R&D Program through the Dong Nam Institute of Radiological and
Medical Sciences (DIRAMS) funded by the Ministry of Education,
Science and Technology (50493-2014).
References
|
1
|
Formenti SC and Demaria S: Systemic
effects of local radiotherapy. Lancet Oncol. 10:718–726. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaanders JH and Ang KK: Early reactions as
dose-limiting factors in radiotherapy. Semin Radiat Oncol. 4:55–67.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welsh JS, Limmer JP, Howard SP, Diamond D,
Harari PM and Tome W: Precautions in the use of intensity-modulated
radiation therapy. Technol Cancer Res Treat. 4:203–210. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karbownik M and Reiter RJ: Antioxidative
effects of melatonin in protection against cellular damage caused
by ionizing radiation. Proc Soc Exp Biol Med. 225:9–22. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Sonntag C: The chemical basis of
radiation biology. Taylor & Francis; London: pp. 9–30. 1987
|
|
6
|
Löbrich M and Jeggo PA: The impact of a
negligent G2/M checkpoint on genomic instability and cancer
induction. Nat Rev Cancer. 7:861–869. 2007.PubMed/NCBI
|
|
7
|
van Gent DC, Hoeijmakers JH and Kanaar R:
Chromosomal stability and the DNA double-stranded break connection.
Nat Rev Genet. 2:196–206. 2001.
|
|
8
|
Weiss JF and Landauer MR: Protection
against ionizing radiation by antioxidant nutrients and
phytochemicals. Toxicology. 189:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maurya DK, Devasagayam TP and Nair CK:
Some novel approaches for radioprotection and the beneficial effect
of natural products. Indian J Exp Biol. 44:93–114. 2006.PubMed/NCBI
|
|
10
|
Hsu H, Yang JJ, Ho YH and Lin CC:
Difference in the effects of radioprotection between aerial and
root parts of Lycium chinense. J Ethnopharmacol. 64:101–108.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Devi PU and Ganasoundari A: Modulation of
antioxidant enzymes by Ocimum sanctum and its role in
protection against radiation injury. Indian J Exp Biol. 37:262–268.
1999.
|
|
12
|
Kamat JP, Boloor KK, Devasagayam TPA and
Venkatachalam SR: Antioxidant properties of Asparagus
racemosus against damage induced by gamma-radiation in rat
liver mitochondria. J Ethnopharmacol. 71:425–435. 2000.
|
|
13
|
Zhang C, Zheng S, Zhang Y, Luo C and Guo
C: The protective effective polysaccharide and C-phycocyanin from
Spirulina platensis on acute radiation injury in mice. Acta
Nutrimenta Sinica. 18:327–331. 1997.
|
|
14
|
Paterson RR: Cordyceps: a traditional
Chinese medicine and another fungal therapeutic biofactory?
Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizuno T: Medicinal effects and
utilization of Cordyceps (Fr.) Link (Ascomycetes) and
Isaria Fr (Mitosporic Fungi) Chinese caterpillar fungi,
‘Tochukaso’ (Review). Int J Med Mushroom. 1:251–261. 1999.
|
|
16
|
Nag TB and Wang HX: Pharmacological
actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol.
57:1509–1519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cunningham KG, Hutchinson SA, Manson W and
Spring FS: Cordycepin, a metabolic product from cultures of
Cordyceps militaris (Linn.) Link Part I Isolation and
characterisation. J Chem Soc. 23:2299–3200. 1951. View Article : Google Scholar
|
|
18
|
Ahn YJ, Park SJ, Lee SG, Shin SC and Choi
DH: Cordycepin: selective growth inhibitor derived from liquid
culture of Cordyceps militaris against Clostridium
spp. J Agric Food Chem. 48:2744–2748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoo HS, Shin JW, Cho JH, et al: Effects of
Cordyceps militaris extract on angiogenesis and tumor
growth. Acta Pharmacol Sin. 25:657–665. 2004.
|
|
20
|
Won SY and Park EH: Anti-inflammatory and
related pharmacological activities of cultured mycelia and fruiting
bodies of Cordyceps militaris. J Ethnopharmacol. 96:555–561.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu HM, Wang BS, Huang SC and Duh PD:
Comparison of protective effects between cultured Cordyceps
militaris and natural Cordyceps sinesis against
oxidative damage. J Agric Food Chem. 54:3132–3138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C, Luo SS, Li Y, Sun YJ and Zhang CK:
Study on antioxidant activity of three Cordyceps sp. by
chemiluminescence Shanghai. J Trad Chinese Med. 38:53–55. 2004.
|
|
23
|
Liu J, Yang S, Yang X, Chen Z and Li J:
Anticarcinogenic effect and hormonal effect of Cordycceps
militaris. Zhongguo Zhong Yao Za Zhi. 22:111–113. 1997.(In
Chinese).
|
|
24
|
Wu WC, Hsiao JR, Lian YY, Lin CY and Huang
BM: The apoptotic effect of cordycepin on human OEC-M1 oral cancer
cell line. Cancer Chemother Pharmacol. 60:103–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin YW and Chiang BH: Anti-tumor activity
of the fermentation broth of Cordyceps militaris cultured in
the medium of Radix astragali. Proc Biochim. 43:244–250.
2008. View Article : Google Scholar
|
|
26
|
Jeong MH, Lee CM, Lee SW, et al:
Cordycepin-enriched Cordyceps militaris induces
immunomodulation and tumor growth delay in mouse-derived breast
cancer. Oncol Rep. 30:1996–2002. 2013.
|
|
27
|
Von Gadow A and Hansmann CF: Comparison of
the antioxidant activity of aspalathin with that of other plant
phenols of rooibos tea (Aspalathus linearis),
alpha-tocopherol, BHT, and BHA. J Agric Food Chem. 45:632–638.
1997.
|
|
28
|
McCord JM and Fridovich I: Superoxide
dismutase. An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 244:6049–6055. 1969.PubMed/NCBI
|
|
29
|
Końca K, Lankoff A, Banasik A, et al: A
cross-platform public domain PC image-analysis program for the
comet assay. Mutat Res. 534:15–20. 2003.PubMed/NCBI
|
|
30
|
Jeong MH, Yang KM, Jeong DH, et al:
Protective activity of novel resveratrol analogue, HS-1793 against
DNA damage in 137Cs irradiated CHO-K1 cells. J Radiat
Res. 55:464–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Londhe JS, Devasagayam TP, Foo LY and
Ghaskadbi SS: Antioxidant activity of some polyphenol constituents
of the medicinal plant Phyllanthus amarus Linn. Redox Rep.
13:199–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hosseinimehr SJ: Trends in the development
of radioprotective agents. Drug Discov Today. 12:794–805. 2007.
View Article : Google Scholar
|
|
33
|
Das SK, Masuda M, Sakurai A and Sakakibara
M: Medicinal uses of the mushroom Cordyceps militaris:
current state and prospects. Fitoterapia. 81:961–968.
2010.PubMed/NCBI
|
|
34
|
Yu RM, Yang W, Song LY, Yan CY, Zhang Z
and Zhao Y: Structural characterization and antioxidant activity of
a polysaccharide from the fruiting bodies of cultured Cordyceps
militaris. Carbohydr Polym. 70:430–436. 2007. View Article : Google Scholar
|
|
35
|
Wu F, Yan H, Ma X, et al: Structural
characterization and antioxidant activity of purified
polysaccharide from cultured Cordyceps militaris. Afr J
Microbiol Res. 5:2743–2751. 2011.
|
|
36
|
Hamburger M: Comment on comparison of
protective effects between cultured Cordyceps militaris and
natural Cordyceps sinensis against oxidative damage. J Agric
Food Chem. 55:7213–7214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogawa Y, Kobayashi T, Nishioka A, et al:
Radiation-induced reactive oxygen species (ROS) formation prior to
oxidative DNA damage in human peripheral T cells. Int J Mol Med.
11:149–152. 2003.PubMed/NCBI
|
|
38
|
Gudkov SV, Shtarkman IN, Smirnova VS,
Chernikov AV and Bruskov VI: Guanosine and inosine display
antioxidant activity, protect DNA in vitro from oxidative damage
induced by reactive oxygen species, and serve as radioprotectors in
mice. Radiat Res. 65:538–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chu HL, Chien JC and Duh PD: Protective
effect of Cordyceps militaris against high glucose-induced
oxidative stress in human umbilical vein endothelial cells. Food
Chem. 129:871–876. 2011.
|
|
40
|
Won KJ, Lee SC, Lee CK, et al: Cordycepin
attenuates neointimal formation by inhibiting reactive oxygen
species-mediated responses in vascular smooth muscle cells in rats.
J Pharmacol Sci. 109:403–412. 2009. View Article : Google Scholar
|
|
41
|
Dizdaroglu M, Jaruga P, Birincioglu M and
Rodriguez H: Free radical induced damage to DNA: mechanisms and
measurement. Free Radic Biol Med. 32:1102–1115. 2002.PubMed/NCBI
|
|
42
|
Rogakou EP, Pilch DR, Orr AH, et al: DNA
double-stranded breaks induce histone H2AX phosphorylation on
serine 139. J Biol Chem. 273:5858–5868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sedelnikova OA, Rogakou EP, Panyutin IG,
et al: Quantitative detection of 125IdU-induced DNA
double-strand breaks with γ-H2AX antibody. Radiat Res. 158:486–492.
2002.
|
|
44
|
Nair CK, Parida DK and Nomura T:
Radioprotectors in radiotherapy. J Radiat Res. 42:21–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|