Introduction
Osteosarcoma is an aggressive bone tumor
characterized by osteoblastic differentiation and malignant osteoid
production (1). It is the most
common type of primary malignant bone tumor affecting children and
adolescents, and the overall 5-year survival rate of osteosarcoma
patients is 68% (2). The
underlying mechanisms in osteosarcoma carcinogenesis have been
investigated in the past few decades, however, the progress has
been slow and the survival rate of patients has reached a plateau
(3,4). The current treatment of osteosarcoma
requires multidisciplinary therapy, incorporating surgery and
systemic chemotherapy (5).
However, the therapies all have different side-effect profiles and
a poor prognosis. Thus, there is a requirement for an improved
understanding of the pathogenesis of osteosarcoma, and the
development of more effective therapeutic targets for this
disease.
The tumor necrosis factor (TNF) receptor-associated
factor (TRAF) family were originally discovered as signaling
adaptors that couple the cytoplasmic regions of receptors of the
TNF-R super-family (6). There are
seven known members of the TRAF family (TRAF1 to 7) in mammals, and
these play an important role in regulating cell survival,
proliferation and stress responses (7). The distinctive feature of all the
TRAF proteins is a C-terminal TRAF domain, which is composed of a
C-terminal β-sandwich (TRAF-C) and an N-terminal coiled-coil region
(TRAF-N) (8). Different members
of the TRAF family mediate different signals. TRAF4 was the first
member of the TRAF protein family found to be upregulated in human
carcinomas. Unlike other canonical TRAFs, TRAF4 only interacts with
limited TNFR-family members, including the p75 neurotrophin
receptor, lichenoid tissue reaction and glucocorticoid-induced TNFR
(GITR) (9), and was originally
identified as a protein localized in the nucleus of breast
carcinoma cells (10). TRAF4 has
also been found in 43% of 623 human tumor samples from the
prostate, ovary, lung and colon, among others (11). Therefore, TRAF4 protein
overexpression is a common characteristic of numerous human
cancers. In the latter study, TRAF4 mRNA was overexpressed
in small cell lung carcinoma, lung adenocarcinoma, colon, ovary and
prostate carcinomas (11). The
amplification and overexpression of TRAF4 indicated that it
is not only a marker of human carcinomas, but also a candidate
oncogene. However, the role and mechanism of TRAF4 in osteosarcoma
remains unclear.
In the present study, the aim was to examine
TRAF4 expression in osteosarcoma tissue and osteosarcoma
cells by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis and to observe the
biological function of Saos-2 cells following TRAF4
knockdown by the RNA interference technique. These data may provide
information for prognosis prediction and targeted therapy for
osteosarcoma.
Materials and methods
Specimens
Primary osteosarcoma tissues were obtained from
biopsies in 38 patients prior to the administration of neo-adjuvant
chemotherapy according to the Chinese National Ethical guidelines
(‘Code for Proper Secondary Use of Human Tissue’, Chinese
Federation of Medical Scientific Societies). In addition, adjacent
normal bone tissue specimens were randomly obtained from 15 of
these 38 osteosarcoma patients following surgical resection. The
patients with osteosarcoma included 23 (60.53%) males and 15
(39.47%) females, aged 11–58 years, with a median age of 23 years.
Without any preoperative treatment, all the 38 cases were
pathologically diagnosed with osteosarcoma postoperatively.
Informed consent was obtained from all the patients prior to
entering the present study, and all the study protocols were
approved by the Ethics Committee for Clinical Research of the Henan
Cancer Hospital, (Henan, China).
Reagents
Mouse anti-TRAF4 antibody, mouse anti-B-cell
lymphoma 2 (Bcl-2), mouse anti-Bax, mouse anti-nuclear factor κB
(NF-κB) were purchased from purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The antibodies against
β-actin were from Good HERE Biotech Inc. (Hangzhou, China). The
horseradish peroxidase-conjugated goat anti-mouse secondary
antibodies were obtained from Abgent Biotechnology Co., Ltd.
(Suzhou, China).
Saos-2 cells culture
The human osteosarcoma cell line, sarcoma osteogenic
(Saos-2), was purchased from the Shanghai Academy of Life Sciences
(Shanghai, China). The cells were cultured in Dulbecco’s modified
Eagle’s medium (Pierce, Rockford, IL, USA) and supplemented with
10% fetal bovine serum, 1% penicillin/streptomycin and 1%
L-glutamine at 37°C under a humidified atmosphere of 5%
CO2.
TRAF4 knockdown by small interfering RNA
(siRNA)
Saos-2 cells were divided into three groups: Control
(treated with Lipofectamine® 2000 only), vector (treated
with Lipofectamine 2000 and control siRNA), and TRAF4-siRNA
group (treated with Lipofectamine 2000 and TRAF4 siRNA). The
Saos-2 cells were seeded into 6-well plates and incubated
overnight, and were subsequently transfected using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions, with slight modifications. The
TRAF4 sense sequence was: 5′-CACCAGCACATTCGAAAGCGA-3′
(GeneChem Co., Ltd., Shanghai, China) (12). For every 1×105 cells,
0.5 μg TRAF4 siRNA or control siRNA was diluted and mixed
with 3 μl transfection reagent. After mixing and incubating 30 min,
the transfection mixture was added to the cells. After 6 h, the
medium was changed to growth medium (13).
Cell proliferation
The MTS assay was used to determine cell
proliferation, as previously described with a few modifications
(14). Approximately
1×104 Saos-2 cells were seeded in each well of a
96-microwell plate. After incubation for 48 h, Cell Titer
96® AQueous One Solution Reagent (Hitachi, Tokyo,
Japan), which is composed of the novel tetrazolium compound MTS and
an electron-coupling reagent, phenazine methosulfate (PES, a redox
intermediary), was added to each well according to the
manufacturer’s instructions. After 3 h in culture, the cell
viability was determined by measuring the absorbance at 490 nm
using an ELISA microplate reader (Invitrogen).
In vivo tumor growth
Athymic nude mice (Vital River Laboratory Animal
Technology Co., Ltd., Beijing, China) were divided into two groups
(n=3) and injected in the right flank with control or
TRAF4-siRNA Saos-2 cells (3×106). The (a) tumor
diameter and the (b) shortest track were measured using a vernier
caliper every five days. The tumor volume (in cubic millimeter) was
calculated according to the formula V=ab2/2 (15). On day 20, the tumors were removed
and weighed. All the studies were performed in compliance with the
Guide for the Care and Use of Laboratory Animals of Henan Province,
China.
Determination of cell cycle by flow
cytometry
Cell cycle analyses were performed as previously
described, with a few modifications (16). The Saos-2 cells were cultured in
serum-free medium for 24 h to complete synchronization;
subsequently, cells were cultured in complete medium for 24 h. The
Saos-2 cells were digested by trypsin, washed in PBS, and fixed by
70% cold ethanol at −20°C. The next day, Saos-2 cells were washed
with citrate phosphate buffer, followed by PBS, before the Saos-2
cells were incubated with RNAse solution (100 μg/ml) for 30 min at
37°C. Subsequently, the Saos-2 cells were incubated in propidium
iodide (PI) solution (100 μg/ml in PBS) at room temperature for 30
min. The cell cycle was detected by flow cytometry (Invitrogen).
The experiment was repeated three times.
Determination of cell apoptosis by flow
cytometry
Cell apoptosis analyses were performed as previously
described, with a few modifications (17). Saos-2 cells were detached by
trypsinization and washed twice in PBS, centrifuged at 1000 × g for
5 min and resuspended in 195 μl Annexin V-fluorescein
isothiocyanate (FITC)-binding buffer. A volume of 5 μl Annexin
V-FITC was added and the solution was mixed. Subsequently, Saos-2
cells were stained in the dark for 10 min at room temperature.
Following this, Saos-2 cells were centrifuged at 1000 × g for 5 min
and resuspended in 190 μl Annexin V-FITC-binding buffer. Finally,
10 μl propidium iodide-staining solution was added and mixed.
Saos-2 cells were maintained on ice in the dark and immediately
subjected to flow cytometric analysis. The data were analyzed using
the CellQuest software (BD Biosciences, San Jose, CA, USA). The
experiment was repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The tissue sample was cryopreserved in liquid
nitrogen. Total RNA was extracted from cultured cells and tissue
samples using the TRIzol Reagent (Invitrogen) according to the
manufacturer’s instructions. RT-qPCR was performed with a SuperRT
One-Step RT-PCR kit (Jiangsu Jiangnan Biotechnology Co., Ltd.,
Jiangsu, China) according to the manufacturer’s instructions. The
primer sequences used for RT-qPCR were as follows: TRAF-4
forward, 5′-CTGGCTAA ACCACAGCACGTC-3′; and reverse, 5′-TCGCTTTCGAAT
GTCCTGG-3′ (18). The 25 μl
reaction mixtures contained 12.5 μl 2× One Step RT-qPCR buffer, 0.5
μM reverse primer, 0.5 μM forward primer, 0.9 μl enzymix, 90 ng RNA
template and 0.5 μM probe. PCR conditions for the reverse
transcription used to obtain cDNA were as follows: 45°C for 10 min,
pre-denaturation at 95°C for 10 min and subsequently 45 cycles at
95°C for 15 sec and 60°C for 45 sec. This was performed using the
ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA). Relative quantification of gene expression was performed
using the 2−ΔΔCt method and with β-actin mRNA as an
internal control (19).
Western blot analysis
Western blot analyses were performed as previously
described (20). Tissue sample or
cells were homogenized in lysis buffer and centrifuged at 4°C for
30 min at 16,000 × g. The supernatant was collected and the same
amount of protein from each sample was separated by sodium SDS-PAGE
on a 12% gel and transferred to a nitrocellulose membrane. The
following anti-TRAF4 or anti-β-actin was used and, subsequently,
horseradish peroxidase-conjugated secondary antibodies were added
(Invitrogen). The proteins were briefly incubated with an enhanced
chemiluminescence reagent (Millipore, Billerica, MA, USA) and
visualized on X-ray film.
Statistical analysis
The SPSS 19.0 software (IBM Corp., Armonk, NY, USA)
was used to analyze the associated data with a t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
TRAF4 mRNA and protein expression in
osteosarcoma tissues and osteosarcoma cells
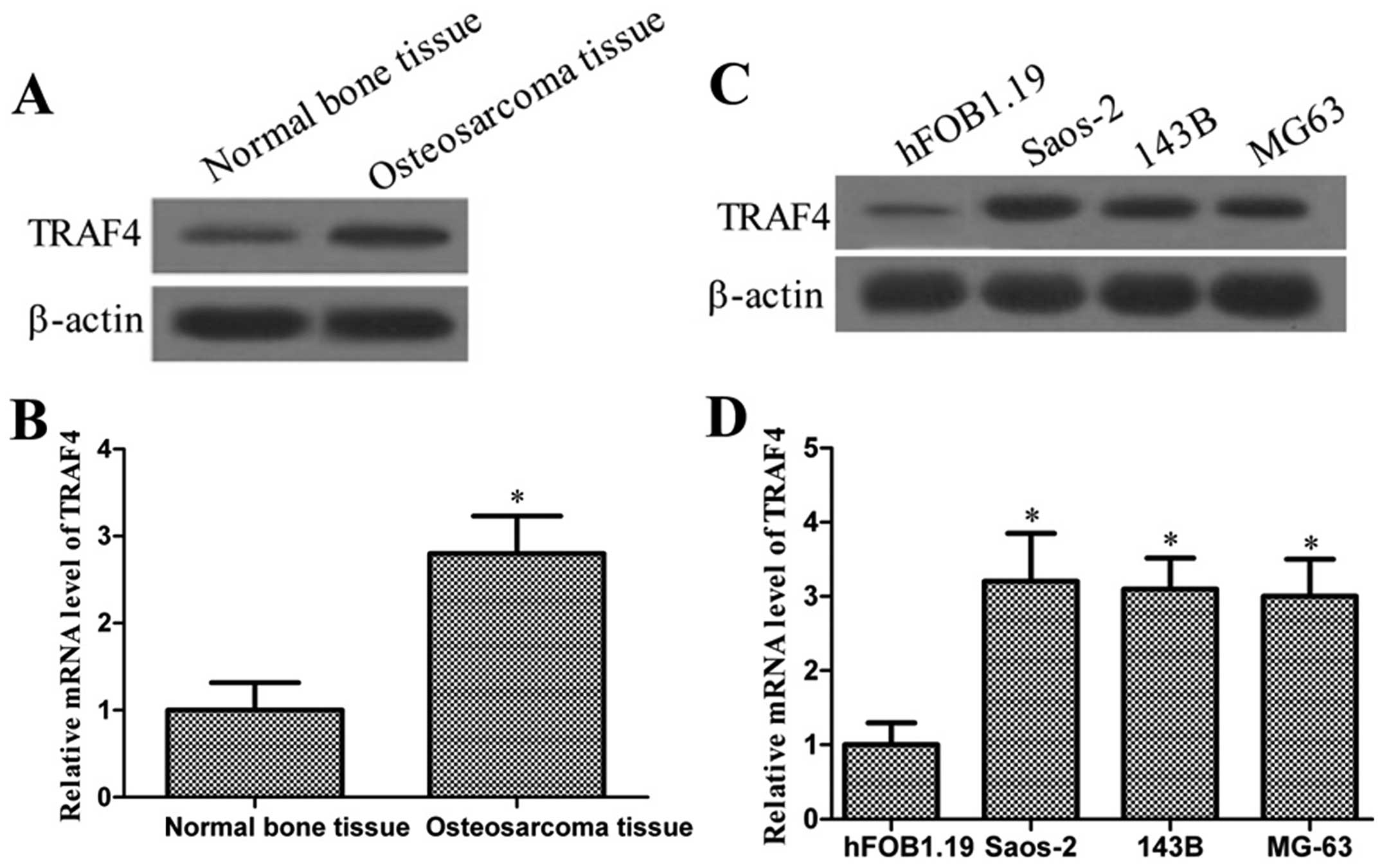
To verify the expression of TRAF4 in osteosarcoma,
the protein and mRNA levels of TRAF4 in osteosarcoma tissue and in
a human osteosarcoma cell line array were determined. As shown in
Fig. 1A and B, the TRAF4 protein
and mRNA expressions in osteosarcoma tissues were significantly
higher compared to normal bone tissues (P<0.05). Consistent with
observations from samples, the protein and mRNA expressions of
TRAF4 were higher in the osteosarcoma cells, Saos-2, 143B and MG63,
compared to the normal human osteoblastic cells, hFOB1.19 (Fig. 1C and D). These results indicated
that TRAF4 may be a critical molecule in osteosarcoma
development.
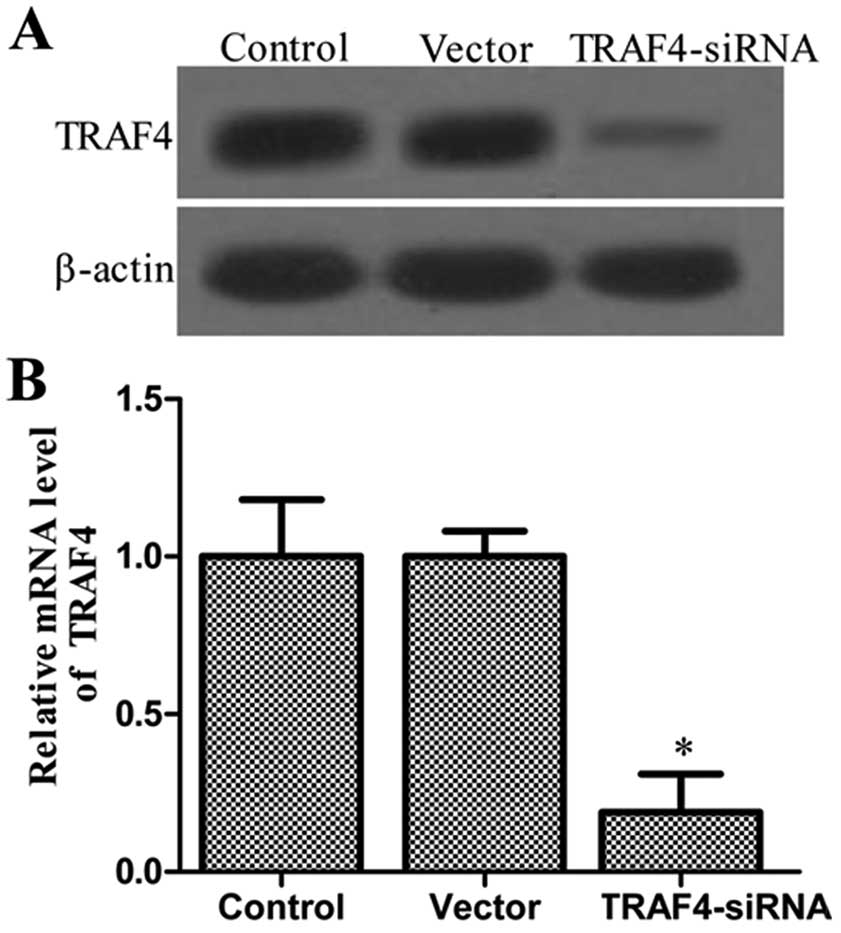
Determination of transfection
effects
On the basis of the observations, it was
hypothesized that TRAF4 may affect the tumorigenic properties in
osteosarcoma. Thus, the stable knockdown TRAF4 Saos-2 line
was generated. To test the efficiency of TRAF4 transfection,
western blotting and RT-qPCR were employed to determine the
expression level of the protein and mRNA. As shown in Fig. 2, expression levels of the TRAF4
protein and mRNA were significantly decreased in the TRAF4
siRNA-transfected group. There was no significant difference in the
expression level of TRAF4 protein and mRNA between the control and
vector groups, which also demonstrated that Lipofectamine and
control siRNA did not affect the expression of TRAF4 protein and
mRNA. Collectively, these data also demonstrated that the TRAF4
protein and mRNA were inhibited in Saos-2 cells.
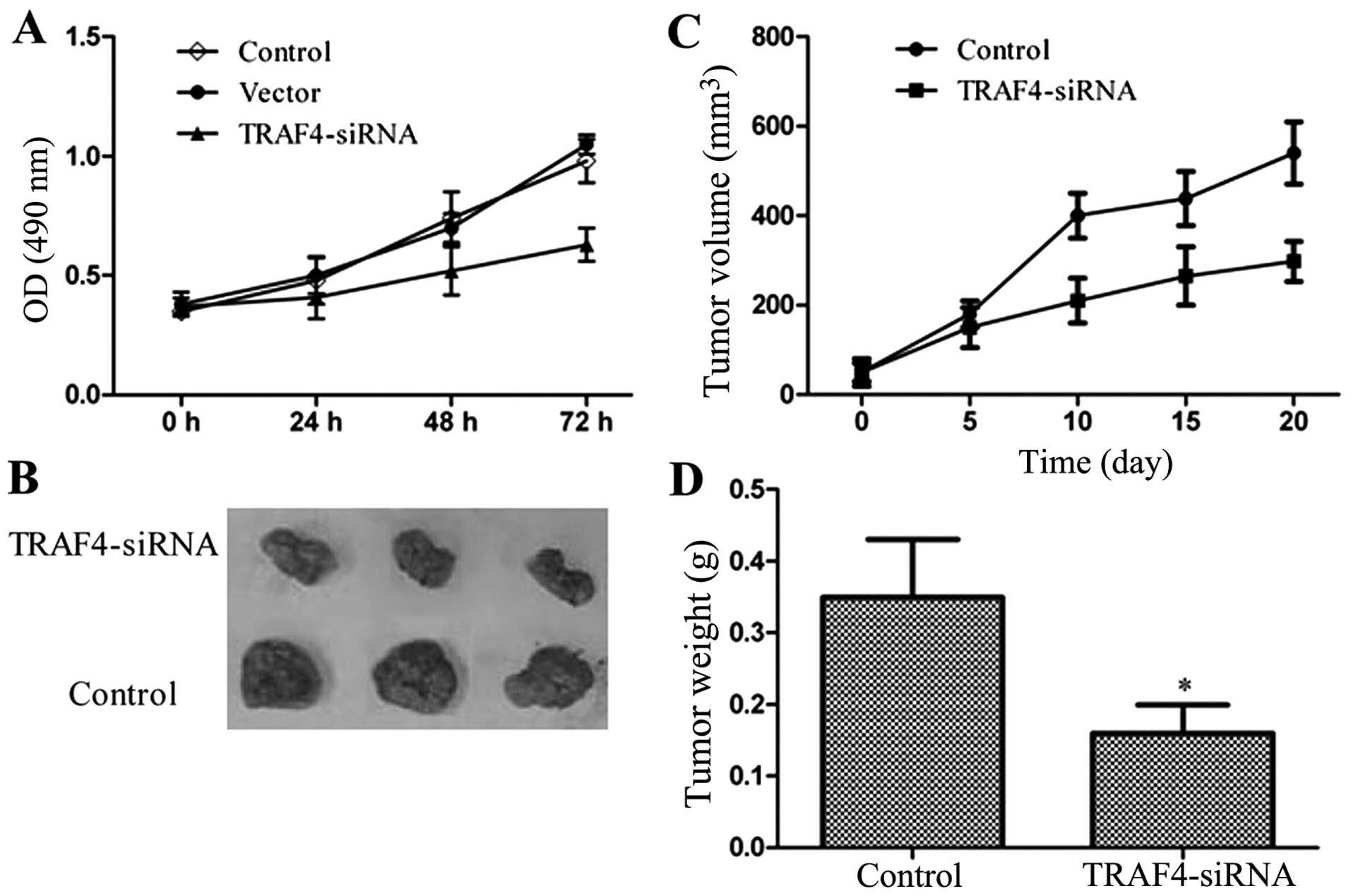
Effects of TRAF4 on Saos-2 cells in vitro
and in vivo
The impact of TRAF4 on Saos-2 cell proliferation was
determined via an MTS assay every 24 h after transfection, for up
to 72 h. The results revealed that the viability of the cell was,
to a certain extent, inhibited by TRAF4 in a time-dependent manner.
As shown in Fig. 3, the
TRAF4-transfected group grew more slowly compared to the
control and vector groups. Furthermore, xenograft growth of Saos-2
cells in athymic nude mice was also attenuated following the
knockdown of TRAF4 (Fig.
3B). These results demonstrated that the downregulation of
TRAF4 inhibited the tumorigenic properties of Saos-2
cells.
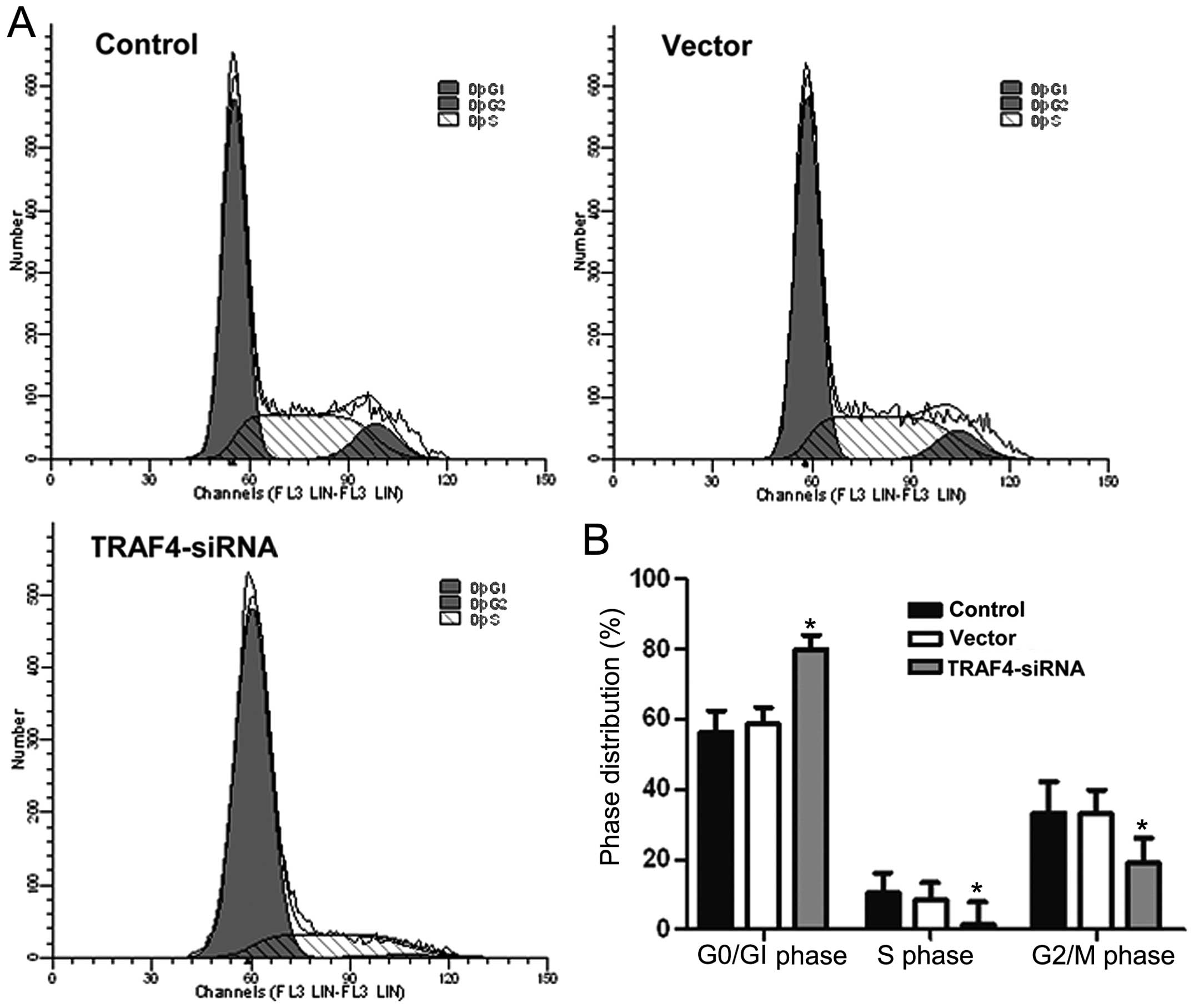
Effects of TRAF4 on cell cycle
progression in Saos-2 cells
Flow cytometry cell cycle analysis showed that the
Saos-2 cells in the TRAF4-siRNA group had significantly more
cells in the G1 phase and significantly fewer cells in
the S and G2 phases compared to the control and vector
groups (P<0.05) (Fig. 4).
These results indicated that the downregulation of TRAF4 may
affect the cell cycle distribution of Saos-2 cells.
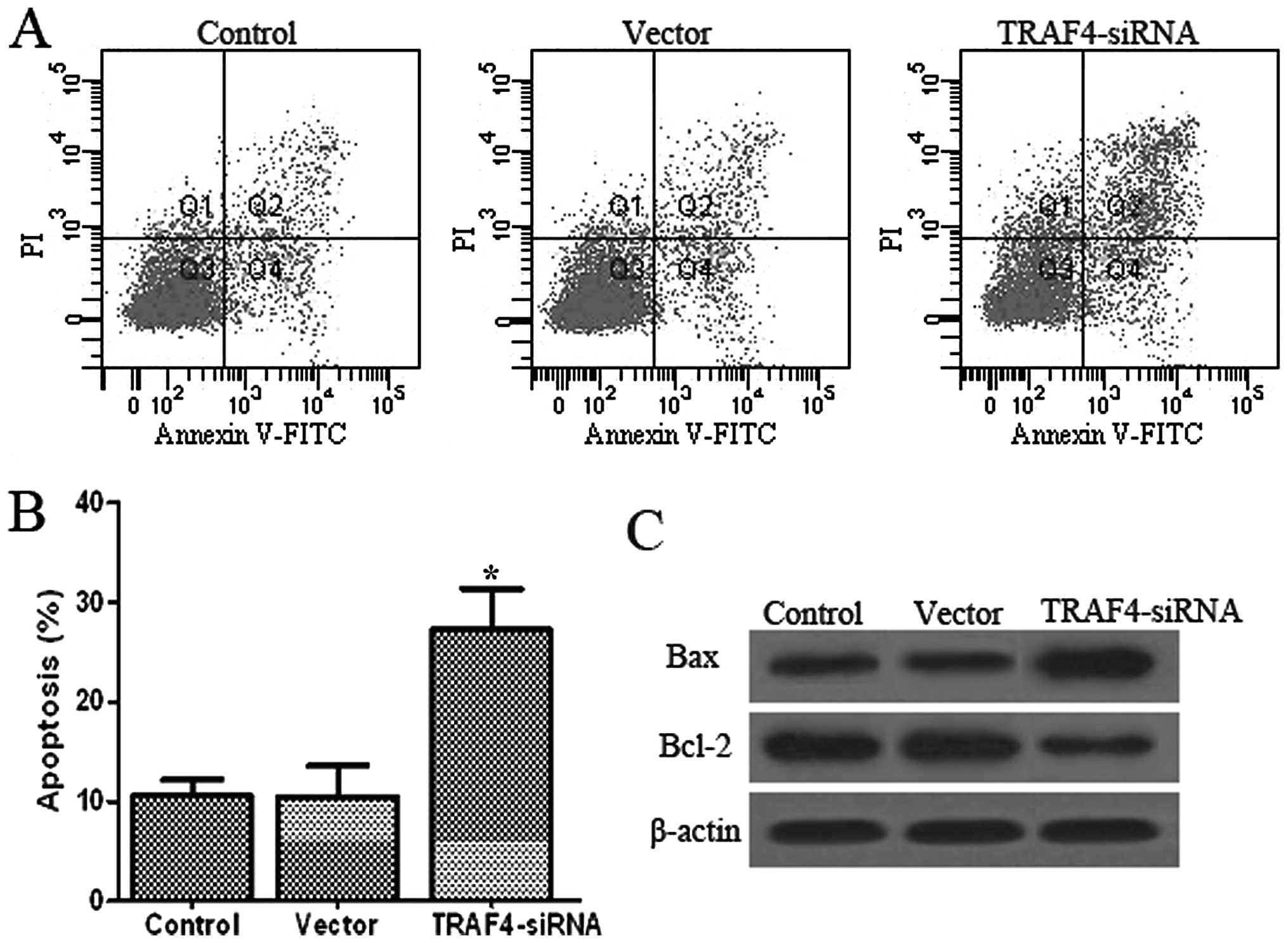
Effects of TRAF4 on apoptosis in Saos-2
cells
Apoptosis of Saos-2 cells was detected via PI
staining and the Annexin V method after 48 h of TRAF4
transfection, followed by flow cytometry. As shown in Fig. 5A, there was an extremely low level
(10.6 and 10.5%) of Saos-2 cell apoptosis in the control and vector
groups, but the percentage of apoptosis significantly increased to
27.3% (P<0.05) in the TRAF4-siRNA group. Furthermore, the
percentage of apoptotic cells did not differ significantly between
the control and vector groups (P<0.05).
To investigate whether TRAF4 induces
apoptosis in Saos-2 cells, the possible molecular mechanisms of
TRAF4 associated with apoptosis were investigated. Thus, the
expression of Bcl-2 and Bax proteins were measured in Saos-2 cells
(Fig. 5C). The results showed
that the expression of Bcl-2 decreased and the expression of Bax
was simultaneously upregulated in the TRAF4-siRNA group
compared to the control and vector groups (P<0.05). The
apoptosis rate was significantly higher due to the upregulation of
pro-apoptotic genes. These data revealed that TRAF4 plays a
critical role in promoting apoptosis of Saos-2 cells.
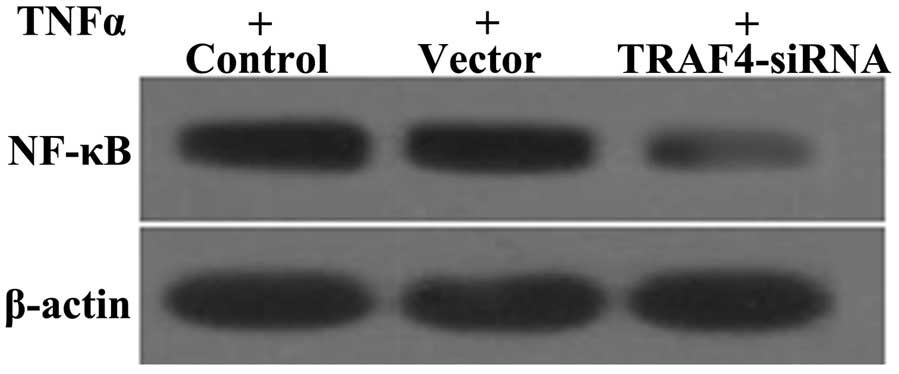
Effect of TRAF4 on NF-κB expression
TRAF4 is an important regulatory factor for the
expression of NF-κB (28). The expression of NF-κB was examined in
the Sao-2 cell line by western blotting. Compared to the control
and vector groups, the TRAF4-siRNA group had significantly
reduced expression levels of NF-κB following TNF-α treatment
(Fig. 6). These results indicate
that TRAF4 may promote the activation of NF-κB induced by
TNF-α in Saos-2 cells.
Discussion
Osteosarcoma is the most common primary malignant
bone lesion and a highly malignant tumor with extensively
destructive potential (21).
Despite great advancements in the diagnosis and treatment of
osteosarcoma thus far, substantial improvements in overall survival
rate have been elusive and overall survival has remained relatively
constant for over 2 decades (22). Thus, establishing the molecular
mechanism of tumorigenesis and the progression of osteosarcoma and
exploring the effective treatments for osteosarcoma is vital.
TRAF4 was initially identified as an
overexpressed gene in human breast carcinoma (23). Overexpression of the TRAF4 protein
is the consequence of its gene amplification in approximately
one-quarter of human carcinomas (11). Several previous studies have
hypothesized that TRAF4 may be involved in apoptosis. For instance,
TRAF4 provides resistance to an apoptotic stimulus in HEK293 cells
(24). By contrast, Sax and
El-Deiry (25) reported that
TRAF4 may play a role in p53-mediated pro-apoptotic signaling in
response to cellular stress. However, depending on the study the
role of TRAF4 in apoptosis is controversial. Currently, TRAF4 has
been found highly expressed in human carcinomas, but its biological
functions in tumorigenesis remain unclear.
The results of the present study showed that
TRAF4 is overexpressed in osteosarcoma tissues and cell
lines. To study the function of TRAF4, a stable TRAF4
knockdown Saos-2 cell line was generated and the effects of the
downregulation of TRAF4 on the proliferation, cell cycle
arrest and apoptosis ability in the Saos-2 cell line, as well as
tumor development in a xenograft mouse model were examined. The
study showed that the knockdown of TRAF4 inhibited the
proliferation of the Saos-2 cell line in vitro and slowed
down tumor growth in a xenograft mouse model. These results
indicated that TRAF4 plays a crucial role in osteosarcoma
carcinogenesis. However, TRAF4 as a p53-regulated
pro-apoptotic gene in a p53 temperature-sensitive cell line VM10
induced apoptosis and inhibited colony formation (25). Simultaneously, TRAF4
knockdown may lead to cell cycle arrest in the G1 phase
and promote Saos-2 cell apoptosis. Knockdown of TRAF4 can
downregulate Bcl-2 expression and upregulate Bax expression.
TRAF4 knockdown possibly induces Saos-2 cell apoptosis by
inhibiting Bcl-2 and activating Bax expression. This apparent
discrepancy indicates that the same gene can perform various
biological functions that are dependent upon the form of the cell
and the type of stimulation.
Previous studies have indicated that TRAF4
positively regulates GITR-induced NF-κB activation (26). NF-κB plays an essential role in
preventing TNF-α-induced cell death (27). Therefore, the expression of NF-κB
was examined. Following TNF-α treatment, NF-κB exhibited
downregulation in Saos-2 cells from the TRAF4-siRNA group
compared to the control and vector groups. These data indicate that
the downregulation of TRAF4 may be associated with the
downregulation of NF-κB, and that the downregulation of NF-κB may
be further responsible for the induced apoptotic ability. However,
its exact mechanism requires further research.
These results indicate that the knockdown of
TRAF4 may play an important role in carcinogenesis and the
development of osteosarcoma. Additional studies are required to
investigate the specific mechanisms underlying the effects of TRAF4
in the tumorigenesis, xenograft tumor growth, cell cycle arrest and
apoptosis of osteosarcoma. These results suggest that TRAF4 is a
good molecular target for the prevention and treatment of
osteosarcoma.
References
|
1
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion,
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimbo K, Miyaki S, Ishitobi H, Kato Y,
Kubo T, Shimose S and Ochi M: Exosome-formed synthetic microRNA-143
is transferred to osteosarcoma cells and inhibits their migration.
Biochem Biophys Res Commun. 445:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pierz KA, Womer RB and Dormans JP:
Pediatric bone tumors: osteosarcoma ewing’s sarcoma, and
chondrosarcoma associated with multiple hereditary
osteochondromatosis. J Pediatr Orthop. 21:412–418. 2001.
|
|
4
|
Yang C, Hou C, Zhang H, Wang D, Ma Y,
Zhang Y, Xu X, Bi Z and Geng S: miR-126 functions as a tumor
suppressor in osteosarcoma by targeting Sox2. Int J Mol Sci.
15:423–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie ZG, Xie Y and Dong QR: Inhibition of
the mammalian target of rapamycin leads to autophagy activation and
cell death of MG63 osteosarcoma cells. Oncol Lett. 6:1465–1469.
2013.PubMed/NCBI
|
|
6
|
Xie P: TRAF molecules in cell signaling
and in human diseases. J Mol Signal. 8:72013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wajant H, Henkler F and Scheurich P: The
TNF-receptor-associated factor family: scaffold molecules for
cytokine receptors, kinases and their regulators. Cell Signal.
13:389–400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha H, Han D and Choi Y: TRAF-mediated
TNFR-family signaling. Curr Protoc Immunol. Chapter 11(Unit
11.9D)2009.PubMed/NCBI
|
|
9
|
Arron JR, Walsh MC and Choi Y:
TRAF-mediated TNFR-family signaling. Curr Protoc Immunol. Chapter
11(Unit 11.9D)2002.PubMed/NCBI
|
|
10
|
Tomasetto C, Regnier CH and Rio MC: TRAF-4
expression in breast carcinomas. Am J Pathol. 153:2007–2008. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camilleri-Broët S, Cremer I, Marmey B,
Comperat E, Viguié F, Audouin J, Rio MC, Fridman WH, Sautès-Fridman
C and Régnier CH: TRAF4 overexpression is a common characteristic
of human carcinomas. Oncogene. 26:142–147. 2007.PubMed/NCBI
|
|
12
|
Marinis JM, Homer CR, McDonald C and
Abbott DW: A novel motif in the Crohn’s disease susceptibility
protein, NOD2, allows TRAF4 to down-regulate innate immune
responses. J Biol Chem. 286:1938–1950. 2011.PubMed/NCBI
|
|
13
|
Qin GQ, He HC, Han ZD, Liang YX, Yang SB,
Huang YQ, Zhou L, Fu H, Li JX, Jiang FN and Zhong WD: Combined
overexpression of HIVEP3 and SOX9 predicts unfavorable biochemical
recurrence-free survival in patients with prostate cancer. Onco
Targets Ther. 7:137–146. 2014.PubMed/NCBI
|
|
14
|
Tayarani-Najaran Z, Asili J, Aioubi E and
Emami SA: Growth inhibition and apoptosis induction of Salvia
chloroleuca on MCF-7 breast cancer cell line. Iran J Pharm Res.
12:789–799. 2013.
|
|
15
|
Zhang B, Lu Z, Hou Y, Hu J and Wang C: The
effects of STAT3 and Survivin silencing on the growth of human
bladder carcinoma cells. Tumour Biol. 35:5401–5407. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: TRAF6 regulates proliferation, apoptosis,
and invasion of osteosarcoma cell. Mol Cell Biochem. 371:177–186.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Synergistic apoptotic effect of crocin and cisplatin on
osteosarcoma cells via caspase induced apoptosis. Toxicol Lett.
221:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo F, Sun A, Wang W, He J, Hou J, Zhou P
and Chen Z: TRAF1 is involved in the classical NF-κB activation and
CD30-induced alternative activity in Hodgkin’s lymphoma cells. Mol
Immunol. 46:2441–2448. 2009.
|
|
19
|
Zheng H, Liu C, Ou Y, Zhang Y and Fu X:
Total saponins of Panax notoginseng enhance VEGF and
relative receptors signals and promote angiogenesis derived from
rat bone marrow mesenchymal stem cells. J Ethnopharmacol.
147:595–602. 2013.
|
|
20
|
Yang WT and Zheng PS: Promoter
hypermethylation of KLF4 inactivates its tumor suppressor function
in cervical carcinogenesis. PLoS One. 9:e888272014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garrington GE, Scofield HH, Cornyn J and
Hooker SP: Osteosarcoma of the jaws. Analysis of 56 cases. Cancer.
20:377–391. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geller DS and Gorlick R: Osteosarcoma: a
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
23
|
Tomasetto C, Régnier C, Moog-Lutz C,
Mattei MG, Chenard MP, Lidereau R, Basset P and Rio MC:
Identification of four novel human genes amplified and
overexpressed in breast carcinoma and localized to the q11-q21.3
region of chromosome 17. Genomics. 28:367–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleckenstein DS, Dirks WG, Drexler HG and
Quentmeier H: Tumor necrosis factor receptor-associated factor
(TRAF) 4 is a new binding partner for the p70S6 serine/threonine
kinase. Leuk Res. 27:687–694. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sax JK and El-Deiry WS: Identification and
characterization of the cytoplasmic protein TRAF4 as a
p53-regulated proapoptotic gene. J Biol Chem. 278:36435–36444.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esparza EM and Arch RH: TRAF4 functions as
an intermediate of GITR-induced NF-κB activation. Cell Mol Life
Sci. 61:3087–3092. 2004.PubMed/NCBI
|
|
27
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|