Introduction
Arthrodial cartilage degradation, subchondral bone
remodeling and osteophyte formation are involved in the development
of osteoarthritis (OA) and are the result of mechanical and
biological factors (1). OA is
divided into 2 categories, primary and secondary OA. The prevalence
of primary OA in women increases with age. Glucocorticoids and
non-steroidal anti-inflammatory drugs are the recommended
treatments for OA; however, these drugs are associated with
elevated risks, such as gastrointestinal disorders and osteoporosis
and have thus failed to obstruct the progression of OA. Due to the
economic burden and limited efficacy of these drugs, safer and more
well tolerated agents are required for the treatment of OA.
Although the exact mechansisms involved in the
pathogenesis of OA remain to be elucidated, cartilage degradation
and subchondral bone remodeling are the most commonly observed
changes. Both of these processes are involved in the progression of
OA and are interconnected and cannot be separated from each other;
however, there is controversy as to which of these processes is the
initial etiological factor in OA (2,3).
In particular, OA is characterized by an imbalance in bone
metabolism that results in subchondral bone osteoporosis at the
early stages of OA and bone sclerosis at the late stages (2,4,5),
in which the osteoprotegerin (OPG)-receptor activator of nuclear
factor kappa-B ligand (RANKL)-receptor activator of nuclearfactor
kappa-B (RANK) system has been proven to play a critical role
(6). OPG, as a secreted member of
the tumor necrosis factor receptor family, inhibits bone resorption
through competitive binding to RANKL and prevents it from binding
to its receptor, RANK, on the surface of osteoclasts. Therefore,
the OPG/RANKL ratio is a decisive factor in bone metabolism
(7). It has also been
demonstrated that OPG and RANKL are detected in the superficial
zone of normal articular cartilage, but extend to the middle zone
of OA cartilage (8). Previous
studies have found that OPG/RANKL and OPG levels are significantly
decreased in chondrocytes in knee OA and in patients with OA, but
are increased at the late stages of OA (8–11).
Generally, OPG exerts dual effects based on its ability to suppress
chondrocyte apoptosis (12) and
alleviate growth plate cartilage destruction (13); it stimulates the production fo 2
catabolic factors, protease-activated receptor-2 (PAR-2) and matrix
metalloproteinase (MMP)-13 (11);
on the contrary, RANKL aggravates cartilage degradation (14). Thus, maintaining the OPG/RANKL
ratio within a normal range represents a strategy for protecting
cartilage. The OPG-RANKL-RANK system has been shown to be a common
downstream target in OA and is inappropriately activated by
interleukin (IL)-1β (15) during
OA and downregulated by 1α,25(OH)2D3
(16,17). In addition, pro-inflammatory
cytokines, most prominently IL-1β and
1α,25(OH)2D3, have been shown to contribute
to the progression of OA by inducing the expression of OPG, RANKL
and RANK. Various pathways have also been shown to mediate the
expression of OPG, RANKL and RANK during the progression of OA, and
these include mitogen-activated protein kinases (MAPKs) (18,19), EP2/EP4 receptor (20) and the Wnt/β-catenin signaling
pathway (7).
Plant-derived agents have received considerable
attention for their potential for use as substitute therapies for
the treatment of OA in recent years, as they have fewer
complications and multiple curative effects (21,22). For instance, icariin, a prenylated
flavonol glycoside, is the cardinal active constituent of the crude
extract of the herb Epimedium pubescens which has been
traditionally used in the treatment of age-related diseases in Asia
(23). Icariin exhibits both
proliferative and anti-inflammatory (24,25) properties. It has also been
reported that icariin protects chondrocytes from inflammatory and
apoptotic responses and promotes extracellular matrix synthesis
(22,26,27), during OA, thus preventing bone
loss (28,29). Taken together, these data suggest
that icariin has therapeutic potential for use in the treatment of
OA.
In the present study, we aimed to determine the
effects of icarrin on the expression of OPG, RANKL and RANK in
SW1353 chondrosarcoma cells stimulated with IL-1β. We decided to
use the SW1353 cell line in our experiments as SW1353 cells have a
similar phenotype with human chondrocytes and they have been
successfully used in previous scientific studies (30–33). The expression of transcription
factors possibly involved in the upregulation of OPG, RANKL and
RANK during the development of OA, including phosphorylated p38
(p-p38) and extracellular signal-regulated kinase (ERK)1/2 was also
analyzed.
Materials and methods
Cell culture and study design
SW1353 human chondrosarcoma cells (Institute of
Biochemistry and Cell Biology, Shanghai, China) were cultivated in
Dulbecco’s modified Eagle’s medium/nutrient (Gibco BRL, Rockville,
MD, USA) containing 10% (v/v) fetal bovine serum (Gibco BRL) and
100 U/ml penicillin-streptomycin solution (Beyotime Institute of
Biotechnology, Jiangsu, China) at 37°C in a humidified atmosphere
under 5% CO2. The cells were seeded at a density of
106 cells in one tissue culture flask and pre-treated in
the presence or absence of p38 inhibitor (SB203580; 10 μmol/l),
ERK1/2 inhibitor (PD98059; 20 μmol/l) (both from Beyotime Institute
of Biotechnology), or icariin (12 μg/ml) (Cayman Chemical Co., Ann
Arbor, MI, USA) for 1 h, then stimulated with IL-1β (10 ng/ml)
(Pepro Tech, Rocky Hill, NJ, USA) for 48 h.
MTT assay
The SW1353 cells were cultured in 96-well plates
(1×104 cells/well; 3 plates) with icariin (0, 1.5, 3, 6,
12 and 24 μg/ml) and incubated for 24, 48 and 72 h. Subsequently,
MTT solution was added to each well for another 4 h at the final
concentration of 5 mg/ml. The supernatants were then removed, and
dimethylsulphoxide was added to the wells to dissolve the formazan
crystals. The A-values of each well were recorded at 570 nm using
an enzyme-labeled meter (Thermo Fisher Scientific, Waltham, CA,
USA) after the 96-well plates were shaken for 10 min.
Western blot analysis
Total proteins from the cells were extracted using a
phosphorylated protein extraction kit (Keygen Biotech Co., Ltd.,
Nanjing, China). The protein concentration was determined using the
bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology) and separated using sodium dodecyl sulfate
polyacrylamide gel electrophoresis; subsequently, the proteins were
transferred onto polyvinylidene difluoride (PVDF) membranes by
electroblotting. The membranes were sealed at room temperature with
5% bull serum albumin (Sigma, San Antonio, TX, USA) for 2 h and
then washed 3 times with Tris-buffered saline and Tween-20 (TBST)
(Tris, 4.84 g; NaCl, 17.6 g; HCl, 2 ml; 0.1% Tween-20, 2 ml). The
PVDF membranes were probed with primary antibodies against the
p-p38 (1:1,000), p-ERK1/2 (1:2,000) (Cell Signaling Technology,
Inc., Beverly, MA, USA) and glyceraldehyde 3-phosphate
dehydrogenase (ImmunoWay, Biotechnology Co., Newark, DE, USA) at
4°C overnight. The membranes were then washed with TBST and
incubated with peroxidase-conjugated second antibody (1:2,000)
(Cell Signaling Technology) at 37°C for 2 h. These protein bands
were subsequently analyzed using an enhanced chemiluminescence
(ECL) Plus kit (Beyotime Institute of Biotechnology).
Quantitative reverse
transcriptionpolymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Burlington, ON, Canada) according to the
recommendations of the manufacturer. Reverse transcription was
performed using the PrimeScript RT Reagent kit (Promega Madison,
WI, USA) and first-strand cDNA was synthesized using the RNA PCR
kit (Takara Bio, Inc., Shiga, Japan). The cDNA mixtures were
diluted 1:10 in sterile distilled water (dH2O), and 5 μl
aliquots were subjected to quantitatvie (real-time) PCR using 2X
SYBR-Green I dye (Takara Bio) in 20 μl reactions containing
dH2O 4 μl, 2X SYBR-Green I 10 μl and 0.5 μl primers
(sense and antisense), as shown in Table I. The PCR primers were designed by
Geneseed Biotech (Guangzhou, China). Quantitative (real-time) PCR
was performed using an ABI Prism 7500 Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA) with 40 cycles of 95°C for 5 min,
95°C for 15 sec and 60°C for 30 sec; measurements were made at the
end of a 60°C annealing step. Data were analyzed using SmartCycler
software (version 2.0). The 2−ΔΔCT method was used to
calculate relative fold changes in mRNA expression. All real-time
PCR experiments were performed in triplicate. The calculated values
for gene expression were normalized against the levels of Homo
sapiens RNA, 18S ribosomal 1 (RN18S1) ribosomal RNA.
 | Table IPrimers used for PCR analysis. |
Table I
Primers used for PCR analysis.
| Target | Primer sequence
(5′→3′) | GenBank accession
no. |
|---|
| RANKL | F:
GGATCACAGCACATCAGAGCAGAG
R: CCAGATGGGATGTCGGTGGCATT | NM_003701.3 |
| RANK | F:
GGCACTGGATCAATGAGGCTTGT
R: CATGCTCCCTGCTGACCAAAGT | NM_003839.3 |
| OPG | F:
CGGGAAAGAAAGTGGGAGCAG
R: CTTCAAGGTGTCTTGGTCGCCAT | U94332.1 |
| 18SrRNA | F:
CCTGGATACCGCAGCTAGGA
R: GCGGCGCAATACGAATGCCCC | NR_003286 |
ELISA
For the quantification of OPG and RANKL protein
levels in the culture medium, the SW1353 cells were treated as
described above. After 48 h, the supernatants were collected and
stored at −80°C until analysis with ELISA. Human OPG and RANKL
ELISA kits were purchased from Uscn Life Science Inc. (Wuhan,
China). The experiments were performed in accordance with the
manufacturer’s instructions.
Statistical analysis
Data are expressed as the means ± standard
deviation. All data were analyzed with variance analysis.
Statistical significance was assessed by one-way analysis of
variance (ANOVA) or the Games-Howell test according to the
homogeneity of variance, and a value of P<0.05 was considered to
indicate a statistically significant difference.
Results
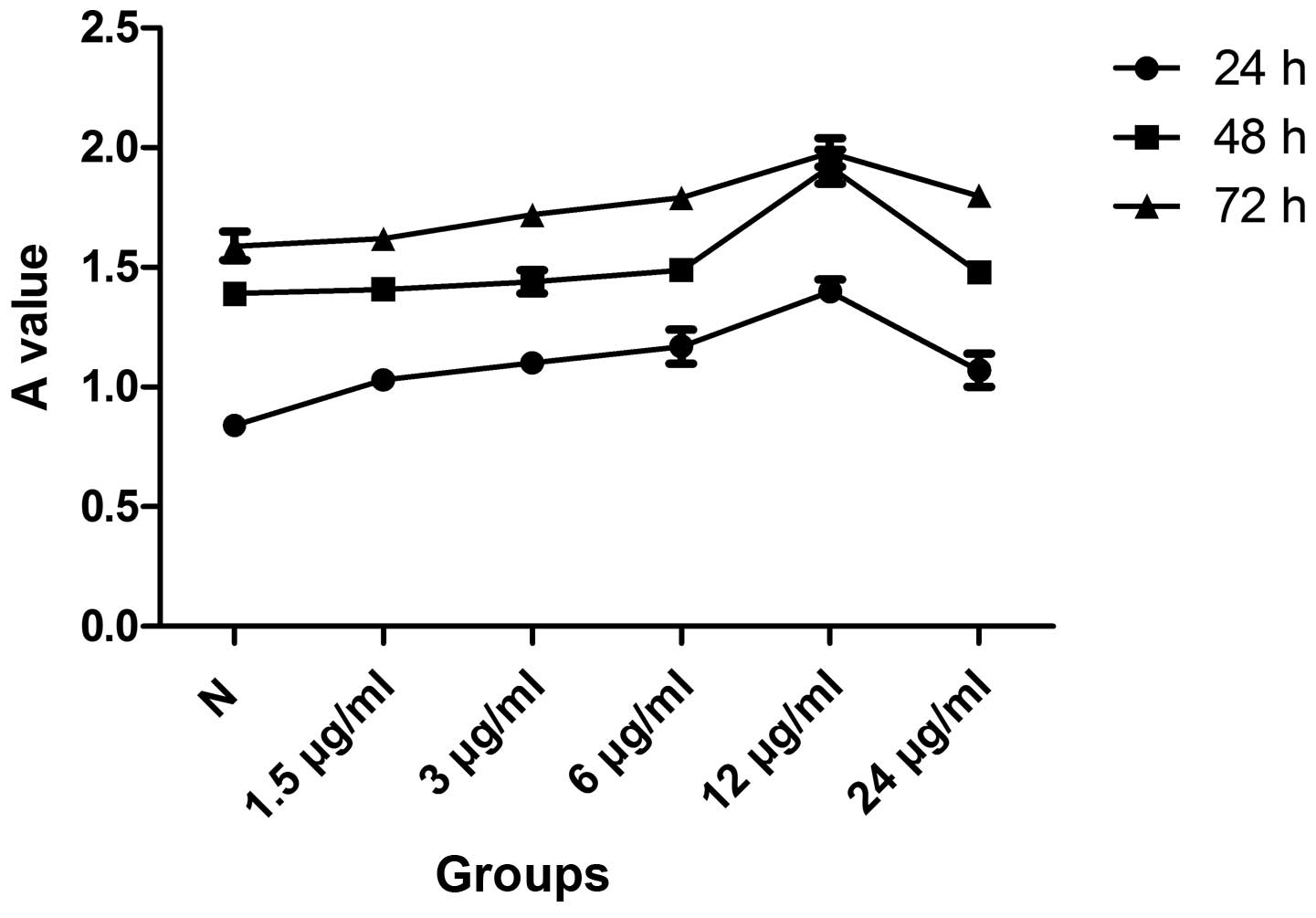
Effects of icariin on cell viability
As shown by MTT assay, treatment of the SW1353 cells
with icariin (1.5, 3, 6 and 12 μg/ml) had a dose-dependent
proliferative effect. However, the treatment of the SW1353 cells
with 24 μg/ml icariin was associated with adverse effects. In a
subsequent experiment, SW1353 cells cultured with icariin for 3
consecutive days revealed a time-dependent increase in
proliferation. According to our results, the pro-mitogenic effect
of 12 μg/ml of icariin at 48 h was superior to the effect of other
doses at other time points and, therefore, this concentration and
time period were used in subsequent experiments (Fig. 1).
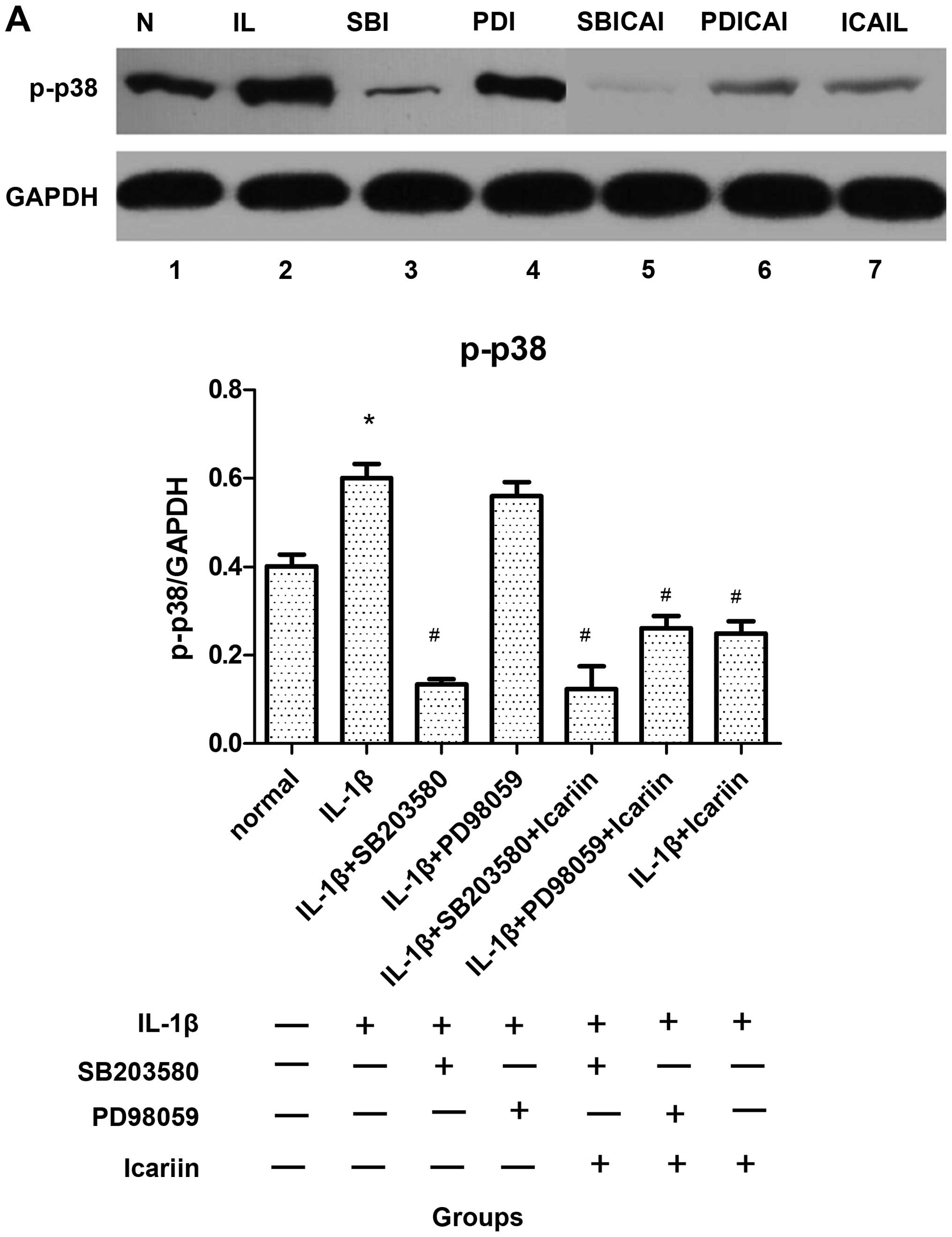
Effects of icariin treatment on p-p38 and
p-ERK1/2 expression in IL-1β-stimulated SW1353 cells
To analyze the effects of icariin on the activation
of p38 and ERK1/2, the SW1353 cells were incubated in the presence
or absence of 12 μg/ml of icariin, in addition to a p38 inhibitor
(SB203580, 10 μM) and a ERK1/2 inhibitor (PD98059, 20 μM). After 1
h, the cells were stimulated with 10 ng/ml IL-1β. After 48 h, p-p38
and p-ERK1/2 were extracted and analyzed by western blot analysis.
The expression levels of these 2 proteins were significantly
elevated when the SW1353 cells were only treated with IL-1β
(Fig. 2). By contrast, the levels
of p-p38 decreased (Fig. 2A),
while the p-ERK1/2 levels increased following treatment with
icariin (Fig. 2B). In addition,
western blot analysis revealed that the levels of these 2 proteins
were inhibited to a greater extent by treatment with the
corresponding signaling pathway-specific inhibitors than with
icariin (Fig. 2). Furthermore,
the difference in the expression levels was statistically
significant (P<0.05).
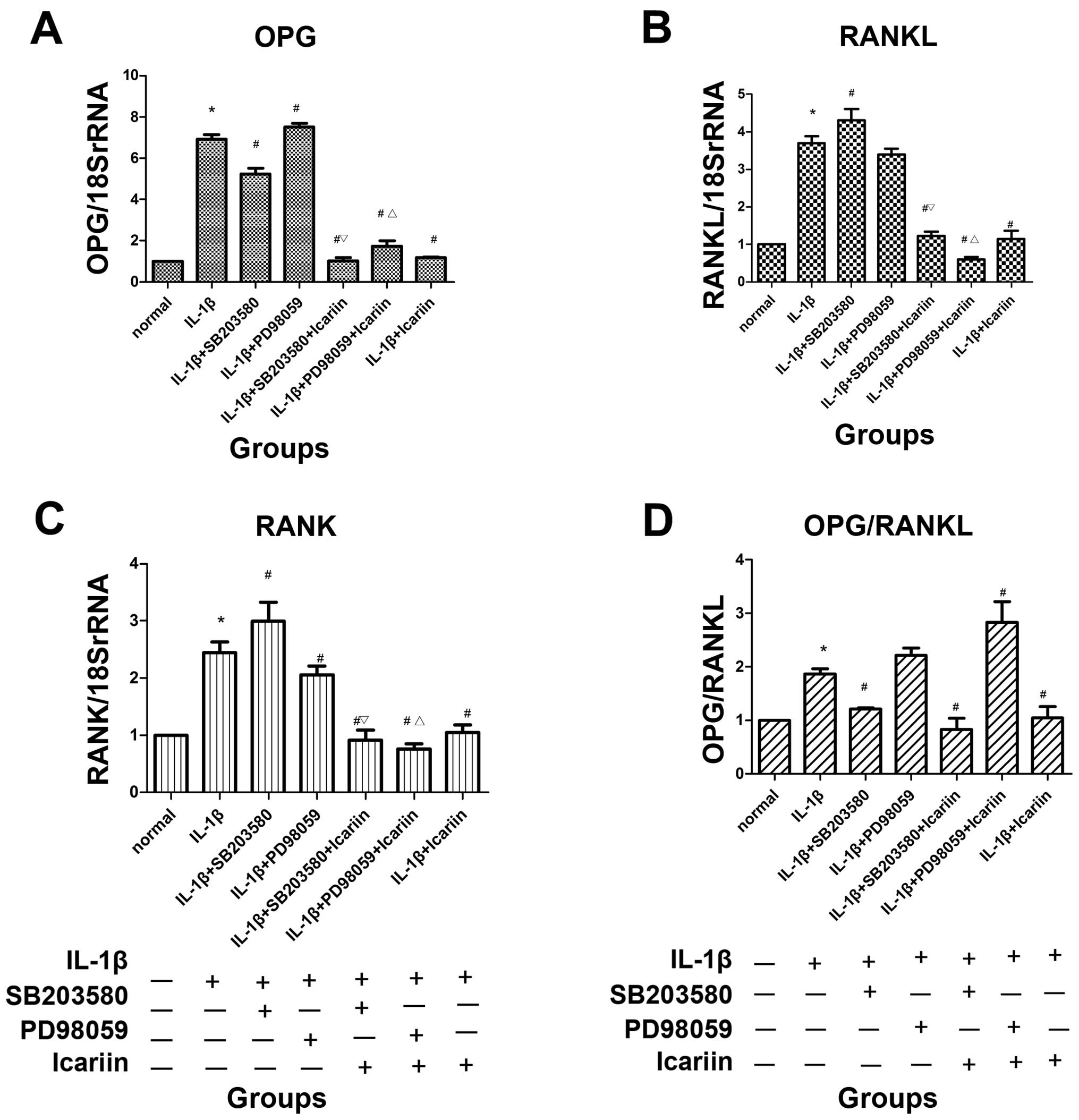
mRNA expression of OPG, RANKL and RANK
following treatment with signaling pathway inhibitors and/or
icariin in IL-1β-stimulated SW1353 cells
In order to investigate the effects of icariin on
OPG, RANKL and RANK expression, the SW1353 cells were treated with
or without 12 μg/ml icariin for 1 h and then stimulated with 10
ng/ml IL-1β. Total RNA and cell extracts were collected 48 h later
and the mRNA expression levels of OPG, RANKL and RANK levels
determined detected in the cell extracts by RT-qPCR. Treatment with
icariin inhibited the increase in the mRNA expression of OPG, RANKL
and RANK and the OPG/RANKL ratio which was induced in response to
pre-treatment with IL-1β. To further investigate the possible
regulatory mechanisms, inhibitors of MAPK signaling pathways were
used. Treatment with icariin induced the most significant decrease
in the mRNA expression of OPG, RANKL and RANK and in the OPG/RANKL
ratio, followed by the p38 inhibitor (SB203580) and ERK1/2
inhibitor (PD98059). Treatment with icariin was associated with a
more prominent inhibition of OPG, RANKL, RANK expression and the
OPG/RANKL ratio than treatment with the p38 signaling
pathway-specific inhibitor alone (Fig. 3).
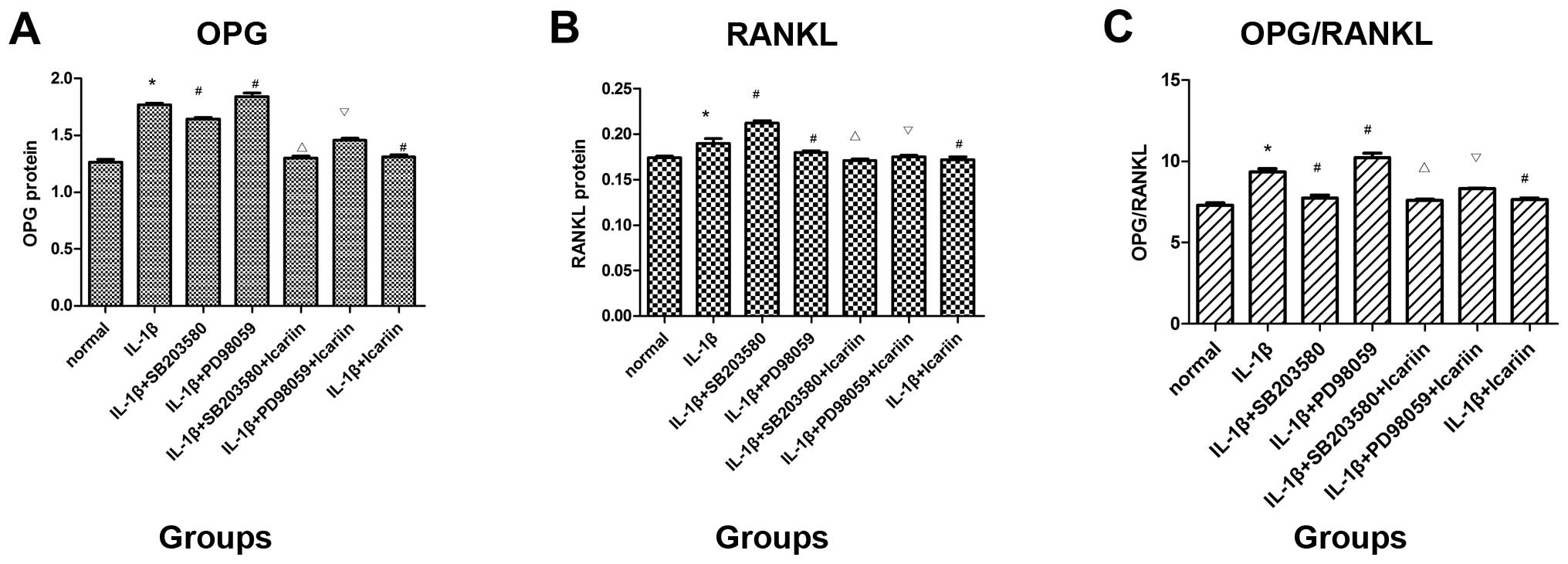
Protein expression of OPG and RANKL
following treatment with signaling pathway inhibitors and icariin
in IL-1β-stimulated SW1353 cells
To investigate the effects of icariin on OPG and
RANKL protein expression, the supernatants were collected and
analyzed using the ELISA kit. Treatment with SB203580 (p38
inhibitor) markedly decreased OPG expression and the OPG/RANKL
ratio and increased RANKL protein expression, whereas treatment
with PD98059 (ERK inhibitor) had the opposite effect. Consistent
with the aforementioned findings, it was demonstrated that
treatment with icariin inhibited the increase in OPG and RANKL
protein expression and the OPG/RANKL ratio which was induced in
response to pre-treatment with IL-1β. In addition, icariin
treatment was associated with a more prominent inhibition of OPG
expression and the OPG/RANKL ratio than treatment with the p38
signaling pathway-specific inhibitor alone (Fig. 4).
Discussion
In the present study, the effects of treatment with
icariin on OPG, RANKL and RANK expression in IL-1β-stimulated
SW1353 chondrosarcoma cells were investigated. In addition, these
effects were associated with those on the p38 and ERK1/2 signaling
pathways induced by specific signaling pathway inhibitors. As a
result, it was shown that the inhibition of the increase in the
expression of OPG, RANKL and RANK and the OPG/RANKL ratio by
icariin treatment was partly mediated by the downregulation in p38
and the upregulation of ERK1/2.
OA is a gradual and degenerative disease,
characterized by inflammation and erosion of articular cartilage
and subchondral bone remodeling. Moreover, available
anti-osteoarthritic drug treatments seem to be limitedly effective
in treating OA, apart from surgical therapie. One reason for this
failure may be the multi-factorial and organic pathogenesis of this
disease (34,35). It has been previously demonstrated
that agents with multiple properties derived from natural resources
(36) offer new treatment
opportunities and are recommended for the treatment of OA (37).
Epimedium pubescens is commonly used in
traditional Chinese medicine for the nourishment of bone and the
stimulation of gonadal functions. Icariin, as the main active
compound, has been associated with anti-inflammatory,
anti-apoptotic (22,26,27), anti-arthritic (in chondrocytes)
(38) and anti-bone resorption
(28,29) properties. It is widely accepted
that icariin has estrogen-like and anti-osteoporotic activity and
can be potentially used for the treatment of osteoporosis during
the onset of primary OA (39). In
a previous study, it was demonstrated that icariin protected
chondrocytes from damage by reducing the activity of nuclear factor
(NF)-κB and increasing the expression of nuclear factor of κ light
polypeptide gene enhancer in B-cells inhibitor, α (IκBα) (38). However, although it has been
demonstrated that icariin suppresses the loss of bone mass and
elevates the OPG/RANKL ratio (40), the regulation of OPG, RANKL and
RANK expression by icariin in chondrocytes is not yet fully
understood.
IL-1β, known as a pro-inflammatory cytokine and
critical catabolic factor, induces the expression of other
cytokines, such as IL-8 and prostaglandin E2, and plays an
important role in OA (41). The
pathological microenvironment of OA chondrocytes is usually
mimicked in vitro with IL-1β (42). In previous studies, IL-1β was
applied in order to create a cellular model of OA using SW1353
cells, and it was found to significantly increase the expression of
OPG, RANKL and RANK and the OPG/RANKL ratio (15,43). By contrast, treatment with icariin
was proved effective in conserving the chondrocytes in vitro
by inhibiting the excessive increase in the expression of OPG,
RANKL and RANK and the OPG/RANKL ratio.
It is well known that the OPG-RANKL-RANK system
plays important roles in OA, with each factor appropriately
expressed. The role of these these 3 factors in OA largely involves
their ability to regulate bone metabolism. Their expression has
have been found in chondrocytes (11). In particular, the overexpression
of OPG induces an increase in MMP-13 and PAR-2 expression (11) rather than cell pro-proliferation
under appropriate levels and induces OA with chondrocalcinosis
(44). Additionally, RANKL
stimulates osteoclastogenesis in growth plates (45). Of these factors, the
OPG-RANKL-RANK system has been the most commonly studied, but OPG-
and RANKL-specific inhibitors have not gained attention as regards
cartilage and chondrocytes. Therefore, therapeutic strategies
focusing on prophylactic agents applied for the regulation of the
OPG-RANKL-RANK system in chondrocytes are required. The present
study suggests that icariin treatment inhibits the expression of
OPG, RANKL and RANK and the OPG/RANKL ratio induced by IL-1β in
chondrocytes. Furthermore, this suppressive effect demonstrates the
therapeutic potential of icariin for use in the treatment of
OA.
The mechanisms through which icariin downregulates
the expression of OPG, RANKL and RANK and the OPG/RANKL ratio
remains unclear. However, an increasing number of studies has
demonstrated that MAPK signaling pathways and the Wnt/β-catenin
signaling pathway are involved in the IL-1β-induced expression of
OPG, RANKL, and RANK and the OPG/RANKL ratio (7,46).
Therefore, in the present study, the molecular mechanisms mediated
by icariin that result in the inhibition of OPG, RANKL and RANK
expression and the OPG/RANKL ratio were investigated. It was
confirmed that MAPK signaling pathways are involved in chondrocytes
stimulated with IL-1β and treated with icariin.
Consequently, the inhibition of cartilage
degeneration and subchondral bone remodeling is a priority for the
effective treatment of OA. Additionally, maintaining the OPG/RANKL
ratio within the normal range is a main issue. In the present
study, the p38 inhibitor, SB203580, was found to have a suppressive
effect on elevated OPG levels and the OPG/RANKL ratio, but it
increased RANK and RANK levels in IL-1β-stimulated SW1353 cells,
while the ERK1/2 inhibitor, PD98059, had the opposite effect.
However, while the inhibition of each pathway alone partly affected
the expression of OPG, RANKL and RANK and the OPG/RANKL ratio,
other pathways also participate in this process. Although treatment
with icariin resulted in the marked activation of the ERK1/2
pathway and the inactivation of the p38 pathway, it also had a
suppressive effect on the increased RANKL and RANK levels in
IL-1β-stimulated SW1353 cells cultured with icariin. Therefore,
apart from the p38 and ERK1/2 pathways, other pathways, such as the
c-Jun N-terminal kinase (JNK) and Wnt/β-catenin signaling pathways,
are involved in this process. The ability of icariin to modulate
multiple pathways suggests that icarrin has synergistic effects,
thus rendering it effective as an anti-arthritic herb extract
compared to other specific inhibitors of signaling pathways.
Furthermore, icariin has become an effective agent for
pharmacological intervention against the progression of OA.
The results of this study indicate that icariin
decreases the expression of OPG, RANKL and RANK and the OPG/RANKL
ratio in IL-1β-stimulated chondrocytes. This is partly achieved by
inhibiting the activation of the p38 and enhancing the activation
of the ERK1/2 pathways. These findings provide valuable insight
into the mechanistic details of OA, which may facilitate the
development of original therapeutic strategies for this
disease.
Acknowledgements
The authors would like to thank Professor Weixue
Tang, (Pathophysiology, Chongqing Medical University, Chongqing,
China) for providing technical assistance.
References
|
1
|
Jiao K, Niu LN, Wang MQ, et al:
Subchondral bone loss following orthodontically induced cartilage
degradation in the mandibular condyles of rats. Bone. 48:362–371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prasadam I, van Gennip S, Friis T, Shi W,
Crawford R and Xiao Y: ERK-1/2 and p38 in the regulation of
hypertrophic changes of normal articular cartilage chondrocytes
induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum.
62:1349–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wenham CY and Conaghan PG: New horizons in
osteoarthritis. Age Ageing. 42:272–278. 2013. View Article : Google Scholar
|
|
4
|
Shimizu M, Tsuji H, Matsui H, Katoh Y and
Sano A: Morphometric analysis of subchondral bone of the tibial
condyle in osteoarthrosis. Clin Orthop Relat Res. 229–239.
1993.PubMed/NCBI
|
|
5
|
Bailey AJ, Mansell JP, Sims TJ and Banse
X: Biochemical and mechanical properties of subchondral bone in
osteoarthritis. Biorheology. 41:349–358. 2004.PubMed/NCBI
|
|
6
|
Kearns AE, Khosla S and Kostenuik PJ:
Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and
disease. Endocr Rev. 29:155–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silva I and Branco JC: Rank/Rankl/opg:
literature review. Acta Reumatol Port. 36:209–218. 2011.PubMed/NCBI
|
|
8
|
Komuro H, Olee T, Kuhn K, et al: The
osteoprotegerin/receptor activator of nuclear factor
kappaB/receptor activator of nuclear factor kappaB ligand system in
cartilage. Arthritis Rheum. 44:2768–2776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Upton AR, Holding CA, Dharmapatni AA and
Haynes DR: The expression of RANKL and OPG in the various grades of
osteoarthritic cartilage. Rheumatol Int. 32:535–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwan Tat S, Pelletier JP, Lajeunesse D,
Fahmi H, Lavigne M and Martel-Pelletier J: The differential
expression of osteoprotegerin (OPG) and receptor activator of
nuclear factor kappaB ligand (RANKL) in human osteoarthritic
subchondral bone osteoblasts is an indicator of the metabolic state
of these disease cells. Clin Exp Rheumatol. 26:295–304. 2008.
|
|
11
|
Kwan Tat S, Amiable N, Pelletier JP, et
al: Modulation of OPG, RANK and RANKL by human chondrocytes and
their implication during osteoarthritis. Rheumatology (Oxford).
48:1482–1490. 2009.PubMed/NCBI
|
|
12
|
Feng ZY, He ZN, Zhang B and Chen Z:
Osteoprotegerin promotes the proliferation of chondrocytes and
affects the expression of ADAMTS-5 and TIMP-4 through MEK/ERK
signaling. Mol Med Rep. 8:1669–1679. 2013.PubMed/NCBI
|
|
13
|
Sagar DR, Ashraf S, Xu L, et al:
Osteoprotegerin reduces the development of pain behaviour and joint
pathology in a model of osteoarthritis. Ann Rheum Dis.
73:1558–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim N, Odgren PR, Kim DK, Marks SC Jr and
Choi Y: Diverse roles of the tumor necrosis factor family member
TRANCE in skeletal physiology revealed by TRANCE deficiency and
partial rescue by a lymphocyte-expressed TRANCE transgene. Proc
Natl Acad Sci USA. 97:10905–10910. 2000. View Article : Google Scholar
|
|
15
|
Watanabe Y, Namba A, Aida Y, et al:
IL-1beta suppresses the formation of osteoclasts by increasing OPG
production via an autocrine mechanism involving celecoxib-related
prostaglandins in chondrocytes. Mediators Inflamm. 2009:3085962009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mandelin J, Li TF, Hukkanen M, et al:
Interface tissue fibroblasts from loose total hip replacement
prosthesis produce receptor activator of nuclear factor-kappaB
ligand, osteoprotegerin, and cathepsin K. J Rheumatol. 32:713–720.
2005.
|
|
17
|
Palmqvist P, Persson E, Conaway HH and
Lerner UH: IL-6, leukemia inhibitory factor, and oncostatin M
stimulate bone resorption and regulate the expression of receptor
activator of NF-kappa B ligand, osteoprotegerin, and receptor
activator of NF-kappa B in mouse calvariae. J Immunol.
169:3353–3362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kusumi A, Sakaki H, Kusumi T, et al:
Regulation of synthesis of osteoprotegerin and soluble receptor
activator of nuclear factor-kappaB ligand in normal human
osteoblasts via the p38 mitogen-activated protein kinase pathway by
the application of cyclic tensile strain. J Bone Miner Metab.
23:373–381. 2005. View Article : Google Scholar
|
|
19
|
Wang YD, Tao MF, Wang L, Cheng WW and Wan
XP: Selective regulation of osteoblastic OPG and RANKL by
dehydroepiandrosterone through activation of the estrogen receptor
β-mediated MAPK signaling pathway. Horm Metab Res. 44:494–500.
2012.PubMed/NCBI
|
|
20
|
Moreno-Rubio J, Herrero-Beaumont G, Tardio
L, Alvarez-Soria MA and Largo R: Nonsteroidal antiinflammatory
drugs and prostaglandin E(2) modulate the synthesis of
osteoprotegerin and RANKL in the cartilage of patients with severe
knee osteoarthritis. Arthritis Rheum. 62:478–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ying X, Chen X, Cheng S, Shen Y, Peng L
and Xu HZ: Piperine inhibits IL-β induced expression of
inflammatory mediators in human osteoarthritis chondrocyte. Int
Immunopharmacol. 17:293–299. 2013.
|
|
22
|
Zeng L, Wang W, Rong XF, et al:
Chondroprotective effects and multi-target mechanisms of Icariin in
IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat
osteoarthritis model. Int Immunopharmacol. 18:175–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schluesener JK and Schluesener H: Plant
polyphenols in the treatment of age-associated diseases: revealing
the pleiotropic effects of icariin by network analysis. Mol Nutr
Food Res. 58:49–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma HR, Wang J, Chen YF, Chen H, Wang WS
and Aisa HA: Icariin and icaritin stimulate the proliferation of
SKBr3 cells through the GPER1-mediated modulation of the EGFR-MAPK
signaling pathway. Int J Mol Med. 33:1627–1634. 2014.PubMed/NCBI
|
|
25
|
Zhang X, Liu T, Huang Y, Wismeijer D and
Liu Y: Icariin: does it have an osteoinductive potential for bone
tissue engineering? Phytother Res. 28:498–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu MH, Sun JS, Tsai SW, Sheu SY and Chen
MH: Icariin protects murine chondrocytes from
lipopolysaccharide-induced inflammatory responses and extracellular
matrix degradation. Nutr Res. 30:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Zhang X, Li KF, et al: Icariin
promotes extracellular matrix synthesis and gene expression of
chondrocytes in vitro. Phytother Res. 26:1385–1392. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li GW, Xu Z, Chang SX, Nian H, Wang XY and
Qin LD: Icariin prevents ovariectomy-induced bone loss and lowers
marrow adipogenesis. Menopause. 21:1007–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pei Z, Zhang F, Niu Z and Shi S: Effect of
icariin on cell proliferation and the expression of bone
resorption/formation-related markers in human periodontal ligament
cells. Mol Med Rep. 8:1499–1504. 2013.PubMed/NCBI
|
|
30
|
Wang JJ, Huan SK, Hsieh KH, et al:
Inhibitory effect of midazolam on MMP-9, MMP-1 and MMP-13
expression in PMA-stimulated human chondrocytes via recovery of
NF-κB signaling. Arch Med Sci. 9:332–339. 2013.PubMed/NCBI
|
|
31
|
Choi YS, Park JK, Kang EH, et al: Cytokine
signaling-1 suppressor is inducible by IL-1beta and inhibits the
catabolic effects of IL-1beta in chondrocytes: its implication in
the paradoxical joint-protective role of IL-1beta. Arthritis Res
Ther. 15:R1912013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furumatsu T, Matsumoto E, Kanazawa T, et
al: Tensile strain increases expression of CCN2 and COL2A1 by
activating TGF-β-Smad2/3 pathway in chondrocytic cells. J Biomech.
46:1508–1515. 2013.PubMed/NCBI
|
|
33
|
Jia P, Chen G, Zhou G, Zhong Y and Li R:
Fuyuan Decoction inhibits nitric oxide production via inactivation
of nuclear factor-κB in SW1353 chondrosarcoma cells. J
Ethnopharmacol. 146:853–858. 2013.PubMed/NCBI
|
|
34
|
van der Kraan PM: Understanding
developmental mechanisms in the context of osteoarthritis. Curr
Rheumatol Rep. 15:3332013.PubMed/NCBI
|
|
35
|
Naumov AV: Current possibilities of
correcting subchondral bone resorption as a major pathogenetic
factor for progressive osteoarthrosis. Ter Arkh. 86:60–65. 2014.(In
Russian).
|
|
36
|
Saller R and Rostock M: Multimorbidity and
multi-target-therapy with herbal drugs. Praxis (Bern 1994).
101:1637–1642. 2012.(In German).
|
|
37
|
Zheng CS, Xu XJ, Ye HZ, et al: Network
pharmacology-based prediction of the multi-target capabilities of
the compounds in Taohong Siwu decoction, and their application in
osteoarthritis. Exp Ther Med. 6:125–132. 2013.PubMed/NCBI
|
|
38
|
Zhang W, Li R, Wang S, Zhou X and Zhong Y:
Study of molecular mechanisms of fuyuan capsule, icariin and
arasaponin R1 in treatment of osteoarthritis. Zhongguo Zhong Yao Za
Zhi. 36:2113–2117. 2011.(In Chinese).
|
|
39
|
Yang L, Yu Z, Qu H and Li M: Comparative
effects of hispidulin, genistein, and icariin with estrogen on bone
tissue in ovariectomized rats. Cell Biochem Biophys. 70:485–490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mok SK, Chen WF, Lai WP, et al: Icariin
protects against bone loss induced by oestrogen deficiency and
activates oestrogen receptor-dependent osteoblastic functions in
UMR 106 cells. Br J Pharmacol. 159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daheshia M and Yao JQ: The interleukin
1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol.
35:2306–2312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roman-Blas JA, Contreras-Blasco MA, Largo
R, Alvarez-Soria MA, Castaneda S and Herrero-Beaumont G:
Differential effects of the antioxidant n-acetylcysteine on the
production of catabolic mediators in IL-1beta-stimulated human
osteoarthritic synoviocytes and chondrocytes. Eur J Pharmacol.
623:125–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haynes DR, Barg E, Crotti TN, et al:
Osteoprotegerin expression in synovial tissue from patients with
rheumatoid arthritis, spondyloarthropathies and osteoarthritis and
normal controls. Rheumatology (Oxford). 42:123–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ramos YF, Bos SD, van der Breggen R, et
al: A gain of function mutation in TNFRSF11B encoding
osteoprotegerin causes osteoarthritis with chondrocalcinosis. Ann
Rheum Dis. Apr 17–2014.(Epub ahead of print).
|
|
45
|
Usui M, Xing L, Drissi H, et al: Murine
and chicken chondrocytes regulate osteoclastogenesis by producing
RANKL in response to BMP2. J Bone Miner Res. 23:314–325. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kusumi A, Kusumi T, Miura J and Tateishi
T: Passage-affected competitive regulation of osteoprotegerin
synthesis and the receptor activator of nuclear factor-kappaB
ligand mRNA expression in normal human osteoblasts stimulated by
the application of cyclic tensile strain. J Bone Miner Metab.
27:653–662. 2009. View Article : Google Scholar
|