Introduction
Acute myeloid leukemia (AML) is characterized by the
rapid growth of abnormal white blood cells that accumulate in the
bone marrow and interfere with the production of normal blood
cells. With the increasing understanding of its pathogenesis,
cytotoxic chemotherapy with or without follow-up with hematopoietic
cell transplantation is the primary treatment for AML. Although
much effort has been made regarding AML treatment, the prognosis
remains dismal. The development of novel therapies is thus highly
desirable (1).
The bark of Magnolia officinalis (cortex
Magnoliae officinalis; M. officinalis) has been widely
used as a folk remedy for gastrointestinal disorders, cough,
anxiety and allergies. Several compounds have been isolated from
M. officinalis including magnolol, honokiol and obovatol
(2,3). Magnolol, a low molecular weight
lignan (4,5), has been shown to possess
anti-platelet aggregation, anxiolytic, anti-fungal, anti-bacterial
(3,6,7),
anti-viral, anti-carcinogenic (8), and anti-metastatic properties
(9). It is also worth noting that
honokiol has demonstrated anti-angiogenic, anti-invasive and
anti-proliferate effects in a variety of cancer cells, including
squamous cell lung cancer (10),
leukemia (11–13) and multiple myeloma (14). Obovatol is the major biphenolic
component of Magnolia obovata (M. obovata) leaves and
is known to have anti-inflammatory and anti-tumor effects through
the inhibition of nuclear factor-κB (NF-κB) (15).
Previous studies have reported that obovatol
inhibits cell growth through the induction of apoptotic cell death
in solid cancers by blocking the NF-κB or mammalian target of
rapamycin (mTOR) signaling pathways. However, to the best of our
knowledge, the effects of obovatol in leukemia have not yet been
reported. Thus, the aim of this study was to investigate the
underlying mechanisms of action of obovatol using a leukemia cell
line and to explore the possibility of its use as a therapeutic
agent in the treatment of leukemia.
Materials and methods
Cell culture and compounds
Human AML cells, such as Jurkat, MM6, THP-1 and U937
cells (all purchased from ATCC, Manassas, VA, USA) were maintained
in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (Gibco-BRL, Grand Island, NY, USA). The
cells were incubated at 37°C in a humidified atmosphere of 5%
CO2. Solutions (100 mM) of obovatol were prepared with
dimethyl sulfoxide (DMSO), stored at −20°C, and then diluted as
needed in the cell culture medium. Obovatol (purity >98%) was
kindly provided by the Korea Research Institute of Bioscience and
Biotechnology (Daejeon, Korea).
Assessment of cell viability and
caspase-3/caspase-7 expression
The cells were seeded at a density of
3×104 cells/well in a 96-well microtiter plate. After 24
h, the medium in the wells was replaced with fresh complete medium
containing various concentrations of obovatol (0–80 μM) in 0.1%
DMSO for 48 h, or the medium was replaced with fresh complete
medium containing 60 μM of obovatol and maintained for different
periods of time (3–48 h). Cell viability was determined using the
CCK-8 kit (Dojindo, Kumamoto, Japan). The cells (1×104)
were seeded in 96-well plates and then treated with 60 μM obovatol
for 1, 4, 7 and 10 h, and caspase activity was determined with the
Caspase-Glo 3/7 assay (Promega, Madison, WI, USA), according to the
manufacturer’s instructions. The intensity of luminescence in a
plate-reading luminometer was measured (Perkin Elmer Victor3
multilabel counter; PerkinElmer, Inc., Waltham, MA, USA).
Flow cytometric analysis
For cell cycle analysis, the cells were treated with
60 μM of obovatol for 24–48 h. The cells were then washed with
phosphate-buffered saline (PBS) and fixed with ice-cold 70% ethanol
for 30 min. The fixed cells were resuspended in PBS (100
μl/1×105 cells) and treated with 100 μg/ml of RNase A
and stained with 100 μg/ml of propidium iodide (PI) for 40 min. The
DNA content was analyzed on a FACScalibur flow cytometer (BD
Biosciences, Bedford, MA, USA). To analyze apoptosis, the cells
were collected at 24–48 h following treatment with obovatol, washed
with PBS, and stained with Alexa Fluor® 488-conjugated
Annexin V and PI (Invitrogen, Carlsbad, CA, USA). The stained cells
were analyzed by flow cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cells were treated with obovatol for 24 h and
total RNA was extracted using the RNeasy Mini kit (Qiagen,
Valencia, CA, USA) according to the instructionsof the
manufacturer. cDNA synthesis was performed using M-MLV Reverse
Transcriptase according to the insructions of the manufacturer
(Invitrogen). RT-qPCR was performed with StepOnePlus (Applied
Biosystems, Foster City, CA) using SYBR Premix Ex Taq (Takara Bio,
Inc., Shiga, Japan) with the following primers: ACTB sense,
5′-TGAGATGCGTTGTT ACAGGAAGTC-3′ and antisense, 5′-GACTGGGCCATT
CTCCTTAGAGA-3′; Bak sense, 5′-CAGCACCCTAAG AGATGGGACTA-3′ and
antisense, 5′-CCTGCTCC TGGGACACATG-3′; Bax, 5′-GCCGCCGTGGACACA-3′
and antisense, 5′-TTGCCGTCAGAAAACATGTCA-3′; Bim, sense,
5′-TTCGGGTCCTGGTATTTCCA-3′ and antisense,
5′-GGCATCAAACACACACTTCATCA-3′; Puma sense,
5′-GGGCCCAGACTGTGAATCCT-3′ and antisense,
5′-CGTGCTCTCTCTAAACCTATGCAA-3′; Bcl-2 sense,
5′-TGGTACGACCTTTAGATTCCAGAGA-3′ and antisense,
5′-CCCATTAGACATATCCAGCTTGAA-3′).
Western blot analysis
The cells were cultured with obovatol for the
indicated periods of time. Following treatment, the cells were
harvested, washed twice with cold PBS and lysed on ice. Western
blot analysis with antibodies to Akt, p38, phosphoryalted (p-)p38,
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK), IKKβ, IκBα, p-IκBα (Ser32/36), p-p65 (Ser536), Bax,
Bcl-2 (Cell Signaling Technology, Beverly, MA, USA), p-Akt
(Thr308), p-JNK, p-ERK (Bioworld Technology, Saint Louis Park, MN,
USA), homeobox A9 (HOXA9), p-retinoblastoma protein (Rb; Ser780)
and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
was performed.
Data analysis and statistics
Data are presented as the means ± standard deviation
(SD). All the experiments were performed a minimum of 3 times.
Statistical analyses were performed using the unpaired sample
two-tailed Student’s t-test. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Obovatol inhibits cell proliferation and
cell cycle progression
To determine the biological activity of obovatol in
leukemia cells, we investigated whether the obovatol inhibits the
proliferation of leukemia cells (Jurkat, MM6, THP-1 and U937)
following treatment with various concentrations of obovatol (0–80
μM) for different periods of time (3, 6, 12, 24 and 48 h). DMSO was
used as a negative control in most of the experiments. We measured
the cytotoxicity of obovatol indirectly using the CCK-8 assay. The
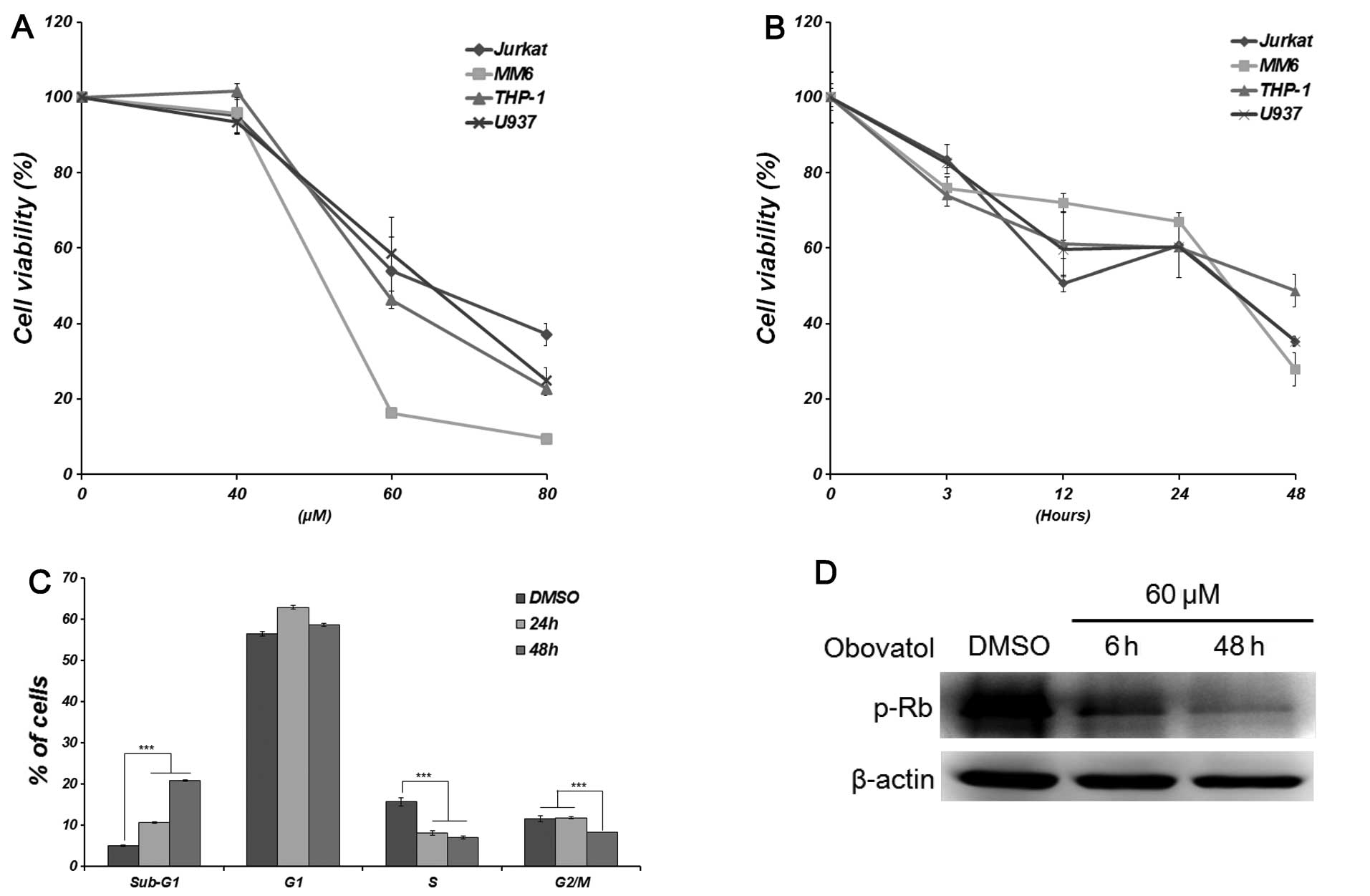
viability of all the leukemia cells decreased in a concentration-
and a time-dependent manner (Fig. 1A
and B). In particular, the viability of the MM6 cells was
affected the greatest by obovatol treatment at the same
concentrations. To determine the effects of obovatol on the cell
cycle, the cell cycle distribution was examined by FACS analysis.
The treatment of MM6 cells with obovatol led to an accumulation of
cells in the sub-G1 phase compared with the control cells, coupled
with a concomitant decrease in the proportion of cells in the S and
G2/M phases in a time-dependent manner (Fig. 1C). In addition, obovatol
significantly decreased the phosphorylation of Rb in a
time-dependent manner (Fig. 1D).
Based on these results, we hypothesized that obovatol inhibits cell
proliferation and cell cycle progression in either non- or
mixed-lineage leukemia (MLL)-rearranged human leukemia cells.
Obovatol induces apoptosis through the
caspase-dependent pathway
In order to further elucidate the effects of
obovatol-induced growth inhibition, we investigated whether the
increased accumulation of cells in the sub-G1 phase was due to
apoptosis or necrosis. FACS analysis was carried out after staining
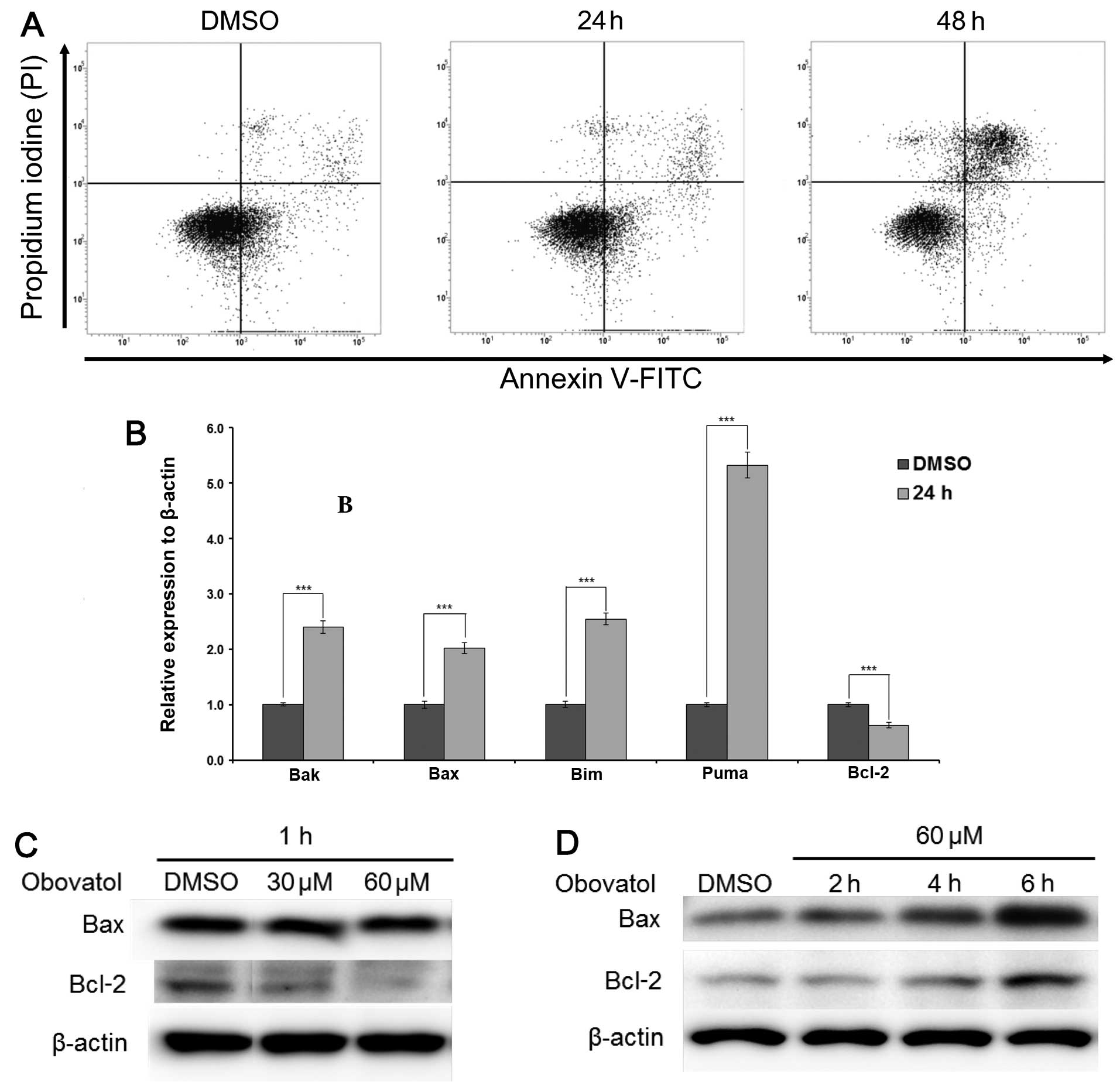
the cells with Annexin V (FITC) and PI. Treatment with a
concentration of 60 μM of obovatol for 24 and 48 h increased the
number of apoptotic cells (Fig.
2A). As Bcl-2 family proteins are key factors in controlling
the mitochondrial-dependent apoptotic pathway (16), we further examined the expression
levels of anti- and pro-apoptotic molecules in obovatol-treated and
untreated MM6 cells. The increased transcriptional expression of
Bak, Bax, Bim and Puma (pro-apoptotic proteins) and the decreased
expression of Bcl-2 (anti-apoptotic protein) was observed in the
obovatol-treated MM6 cells after 24 h (Fig. 2B). The protein expression levels
of Bax increased after 4 h, while Bcl-2 expression decreased after
1 h in a concentration-dependent manner, but started to increase
after 4 h of treatment with 60 μM obovatol (Fig. 2C and D). Moreover, since the
caspase-dependent pathway plays a pivotal role in apoptosis
(17) and a previous study
demonstrated that obovatol induced apoptosis by regulating the
caspase-dependent pathway in prostate and colon cancer cells
(18), we examined the expression
of proteins associated with caspase-related mitochondrial-dependent
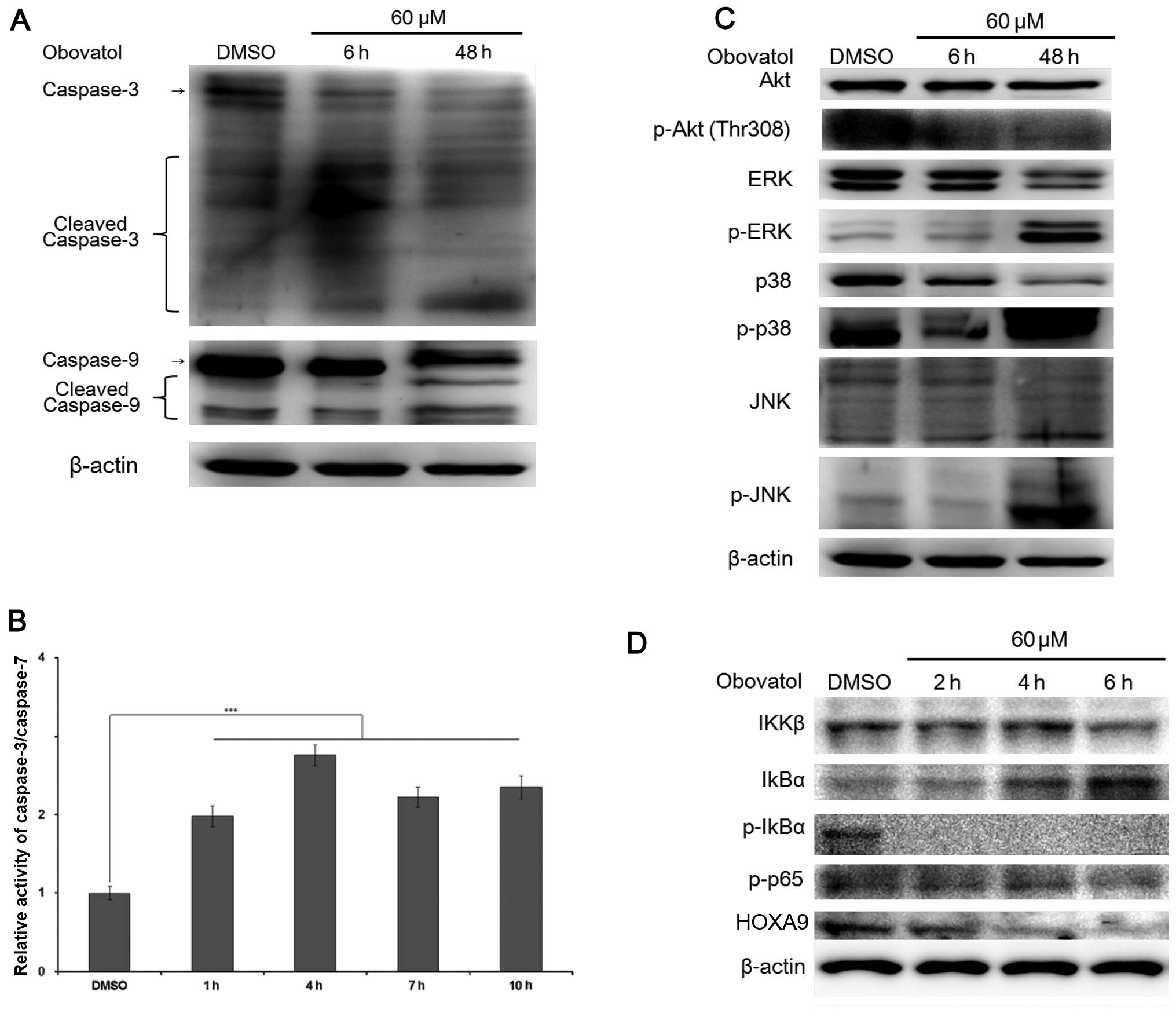
apoptosis in the obovatol-treated MM6 cells. As expected, the
active fragments of caspase-3 and caspase-9 were observed following
treatment with obovatol for 6–48 h (Fig. 3A). Additionally, there was a
sustained increase in the relative activity of caspase-3/caspase-7
after 1 h (Fig. 3B). These
results collectively suggest that obovatol induces apoptosis
rapidly through the mitochondrial apoptotic pathway.
Obovatol regulates the mitogen-activated
protein kinase (MAPK) pathway and suppresses the expression of MLL
target genes
Studies have shown that the phosphorylation of the
MAPK pathway can positively or negatively regulate cell mitosis,
proliferation and apoptosis. In order to determine the effects of
obovatol on the activation of Akt and the MAPK pathway, we
evaluated the phosphorylation of Akt, JNK, ERK1/2 and p38 in
obovatol-treated MM6 cells. We found that the total protein
expression level of Akt was sustained, but the phosphorylation of
Akt decreased significantly after 6 h. The expression levels of
p-JNK, p-ERK and p-p38 proteins following treatment with DMSO and
obovatol for 6 h were very low, but increased significantly
following treatment with obovatol (60 μM) for 48 h (Fig. 3C). These findings suggest that
obovatol activates the phosphorylation of JNK, p38 and ERK proteins
to induce apoptosis in MM6 cells.
A recent study demonstrated that IKK/NF-κB signaling
is a crucial factor in regulating the expression of MLL target
genes and to maintain stem cell ability in MLL-rearranged leukemia
(19). As MM6 is a leukemia cell
line harboring the MLL-AF9 translocation, we investigated whether
the expression levels of NF-κB-associated proteins were affected by
obovatol treatment. Treatment with obovatol decreased the protein
expression levels of total IKKβ in a time-dependent manner
(Fig. 3D) and significantly
inhibited the phosphorylation of IκBα and p65, following the
increased expression of total IκBα (Fig. 3D). In addition, the protein
expression of HOXA9, a MLL target gene, decreased due to its
dependency on the activity of p-p65. These results suggest that
obovatol suppresses the activity of the NF-κB signaling pathway and
the expression of MLL target genes by hindering the phosphorylation
of IκBα.
Discussion
Leukemia signifies a pathological condition
characterized by the dysplasia of hematopoietic tissues, often with
evidence of widespread metastases and tumors in distant organs, in
addition to the striking feature of leukocytosis of immature cells
in the peripheral blood (20).
There is no single known cause for all the different types of
leukemia. The few known causes, which are generally factors outside
the control of the average individual, account for relatively few
cases.
Several drugs have been developed for the treatment
of leukemia, such as imatinib, nilotinib and dasatinib; however,
there is no optimized treatment available to date for AML. Thus,
the induction of apoptosis in leukemia cells may be a possible
therapeutic strategy for AML. Numerous studies have suggested that
obovatol induces apoptosis in prostate and colon cancer cells and
thus, it may also be capable of inducing apoptosis in leukemia
cells (11–15,18). Based on these data, in this study,
we evaluated the effects of obovatol on various leukemia cells and
selected the MM6 cells for further analysis. MM6 cells have a
MLL-AF9 fusion gene, which can affect the inhibition of
proliferation. Based on the cell cycle analysis and PI/FITC dual
staining results, we confirmed that obovatol inhibited cell growth
and induced apoptosis in MM6 cells (Fig. 1). In addition, we observed an
increase in activated caspase-3, caspase-9, and the expression of
pro-apoptotic proteins (Bax, Bak, Bim and Puma) and a decrease in
the expression of anti-apoptotic proteins (Bcl-2) (Fig. 2). The Bcl-2 family of proteins
plays an important role regulating apoptosis. The Bcl-2 proteins
reside at a critical point upstream of cellular damage and modulate
apoptosis through the regulation of mitochondrial pathways
(21). These results are in
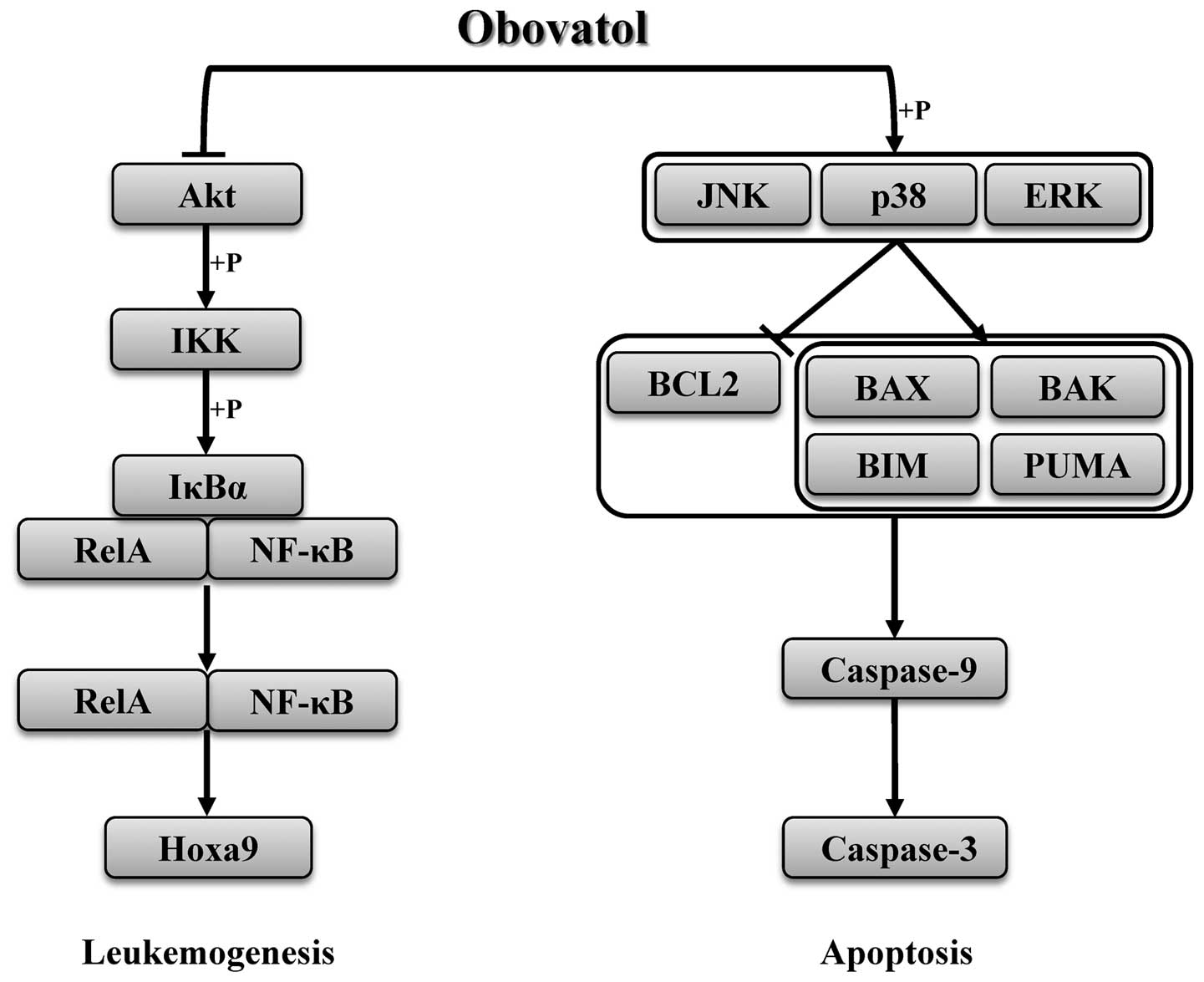
accordance with those of previous studies (10,14) and suggest that obovatol induces
apoptosis in leukemia cells through the mitochondrial apoptotic
pathway (Fig. 4).
There are three major MAPK families, the ERK family,
the JNK family and the p38 MAPK family. The MAPK signaling pathway
is important for cell growth, proliferation and metabolism
(22). Thus, studies on the
function of the MAPK pathway in cancer cells continue to confirm
the effects of obovatol as an anticancer agent (15,22). Our results indicated that the
increase in the phosphorylation of ERK, p38 and JNK, depending on
the length of obovatol treatment, resulted in apoptosis upstream of
anti- and pro-apoptotic genes (Fig.
3C). In addition, obovatol is known to suppress the NF-κB
signaling pathway in solid cancers (15). The phosphorylation of Akt triggers
the activation of the NF-κB signaling through activated IKKα/β
(23). The crucial target genes
(HOXA9 and MEIS1) in leukemia stem cells and the maintenance of
histone modifications are regulated by the NF-κB subunit, RelA
(p65) in MLL leukemia (19). We
evaluated the possibility that obovatol acts as an anti-leukemic
agent by suppressing the activation of the NF-κB signaling pathway.
Treatment with obovatol inhibited not only the expression of IKKβ,
but also the phosphorylation of Akt, IκBα and p65 (Fig. 3C and D). Additionally, HOXA9
protein expression was significantly diminished depending on the
concentration of obovatol.
In conclusion, our data confirm that obovatol
inhibits cell growth, induces apoptosis by regulating the MAPK
signaling pathway, and suppresses the expression of MLL target
genes by decreasing the phosphorylation of NF-κB
signaling-associated proteins (Fig.
4). The present study may aid in better understanding the
effects of obovatol in MLL leukemia and may lead to the development
of novel therapeutic approaches using obovatol as an anti-leukemic
agent.
Acknowledgements
This study was supported by a Korea Science and
Engineering Foundation (KOSEF) grant funded by the government of
Korea (MEST) (2011-0011163).
References
|
1
|
Teng CL, Yu CT, Hwang WL, et al: Effector
mechanisms of sunitinib-induced G1 cell cycle arrest,
differentiation, and apoptosis in human acute myeloid leukaemia
HL60 and KG-1 cells. Ann Hematol. 92:301–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagase H, Ikeda K and Sakai Y: Inhibitory
effect of magnolol and honokiol from Magnolia obovata on
human fibrosarcoma HT-1080. Invasiveness in vitro. Planta Med.
67:705–708. 2001.
|
|
3
|
Park J, Lee J, Jung E, et al: In vitro
antibacterial and anti-inflammatory effects of honokiol and
magnolol against Propionibacterium sp. Eur J Pharmacol.
496:189–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang T, Chen F, Chen Z, et al: Honokiol
induces apoptosis through p53-independent pathway in human
colorectal cell line RKO. World J Gastroenterol. 10:2205–2208.
2004.PubMed/NCBI
|
|
5
|
Wang X, Wang Y, Geng Y, Li F and Zheng C:
Isolation and purification of honokiol and magnolol from cortex
Magnoliae officinalis by high-speed counter-current
chromatography. J Chromatogr A. 1036:171–175. 2004.PubMed/NCBI
|
|
6
|
Chang B, Lee Y, Ku Y, Bae K and Chung C:
Antimicrobial activity of magnolol and honokiol against
periodontopathic microorganisms. Planta Med. 64:367–369. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho KY, Tsai CC, Chen CP, Huang JS and Lin
CC: Antimicrobial activity of honokiol and magnolol isolated from
Magnolia officinalis. Phytother Res. 15:139–141. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palayoor ST, Youmell MY, Calderwood SK,
Coleman CN and Price BD: Constitutive activation of IkappaB kinase
alpha and NF-kappaB in prostate cancer cells is inhibited by
ibuprofen. Oncogene. 18:7389–7394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikeda K, Sakai Y and Nagase H: Inhibitory
effect of magnolol on tumour metastasis in mice. Phytother Res.
17:933–937. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SE, Hsieh MT, Tsai TH and Hsu SL:
Down-modulation of Bcl-XL, release of cytochrome c and sequential
activation of caspases during honokiol-induced apoptosis in human
squamous lung cancer CH27 cells. Biochem Pharmacol. 63:1641–1651.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Battle TE, Arbiser J and Frank DA: The
natural product honokiol induces caspase-dependent apoptosis in
B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood.
106:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hibasami H, Achiwa Y, Katsuzaki H, et al:
Honokiol induces apoptosis in human lymphoid leukemia Molt 4B
cells. Int J Mol Med. 2:671–673. 1998.PubMed/NCBI
|
|
13
|
Hirano T, Gotoh M and Oka K: Natural
flavonoids and lignans are potent cytostatic agents against human
leukemic HL-60 cells. Life Sci. 55:1061–1069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishitsuka K, Hideshima T, Hamasaki M, et
al: Honokiol overcomes conventional drug resistance in human
multiple myeloma by induction of caspase-dependent and -independent
apoptosis. Blood. 106:1794–1800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi MS, Lee SH, Cho HS, et al: Inhibitory
effect of obovatol on nitric oxide production and activation of
NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells.
Eur J Pharmacol. 556:181–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulukaya E, Acilan C and Yilmaz Y:
Apoptosis: why and how does it occur in biology? Cell Biochem
Funct. 29:468–480. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Yuk DY, Song HS, et al: Growth
inhibitory effects of obovatol through induction of apoptotic cell
death in prostate and colon cancer by blocking of NF-kappaB. Eur J
Pharmacol. 582:17–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuo HP, Wang Z, Lee DF, et al: Epigenetic
roles of MLL oncoproteins are dependent on NF-κB. Cancer cell.
24:423–437. 2013.PubMed/NCBI
|
|
20
|
Lucia SP: Leukemia: Evaluation of the
Therapy. Cal West Med. 55:119–123. 1941.
|
|
21
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogen-activated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
23
|
Kane LP, Shapiro VS, Stokoe D and Weiss A:
Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 9:601–604.
1999. View Article : Google Scholar : PubMed/NCBI
|