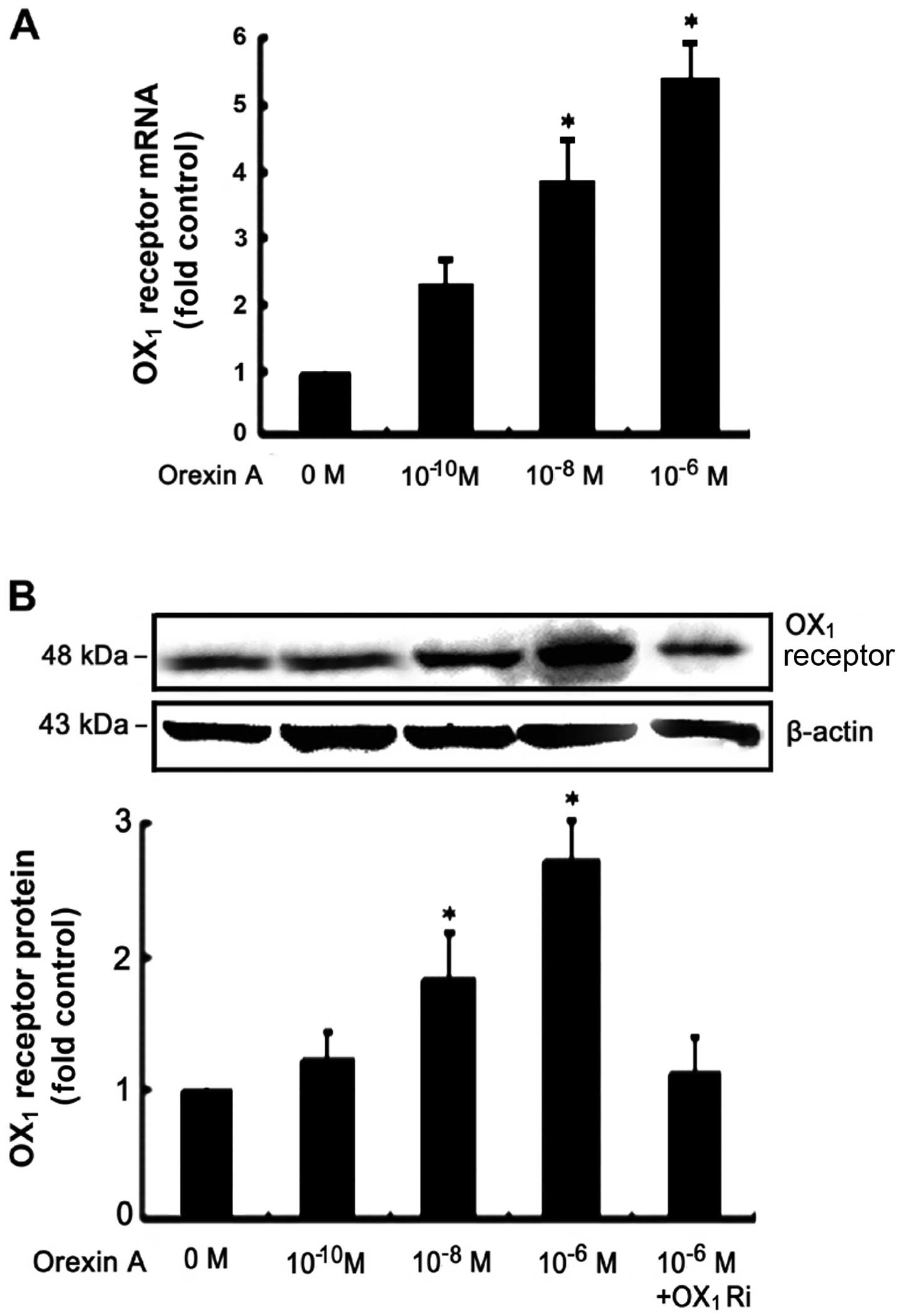
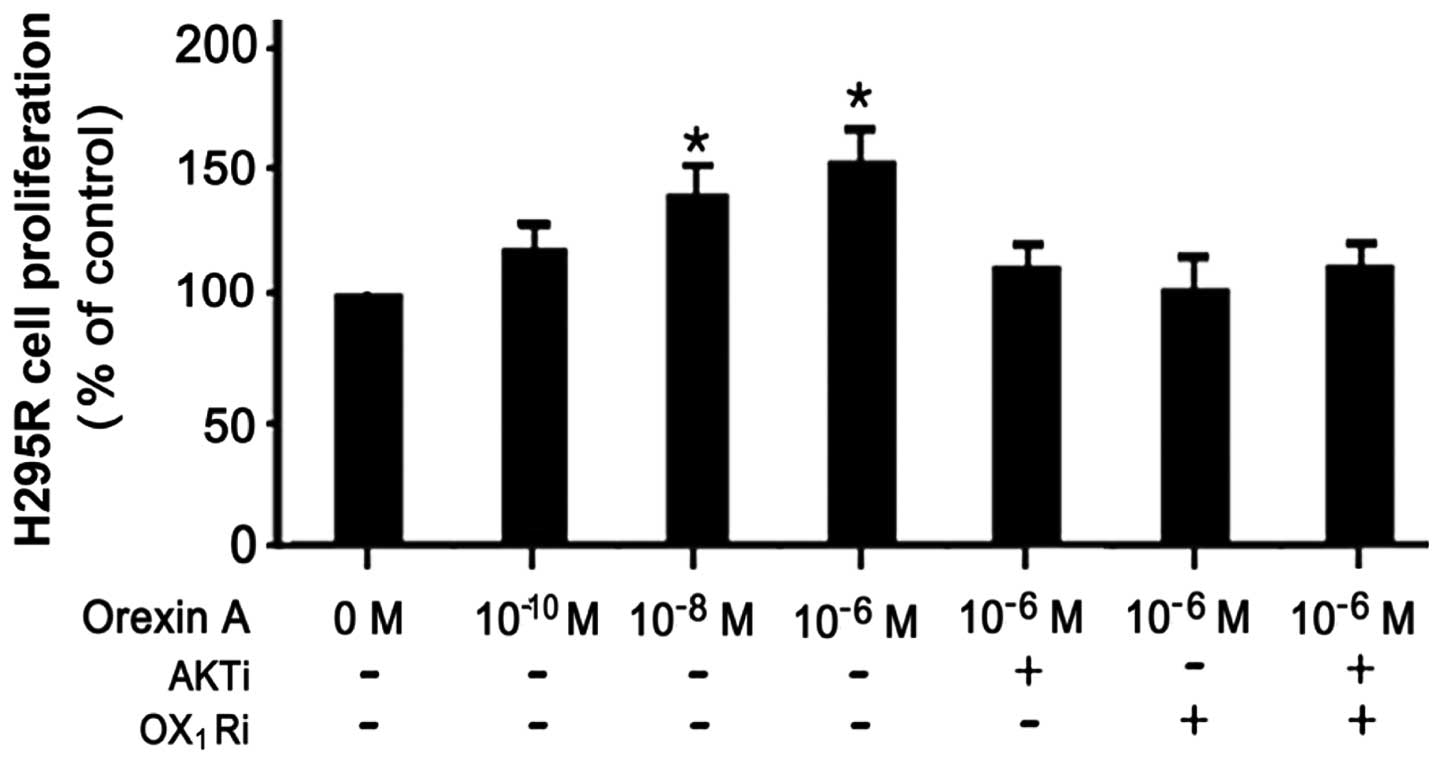
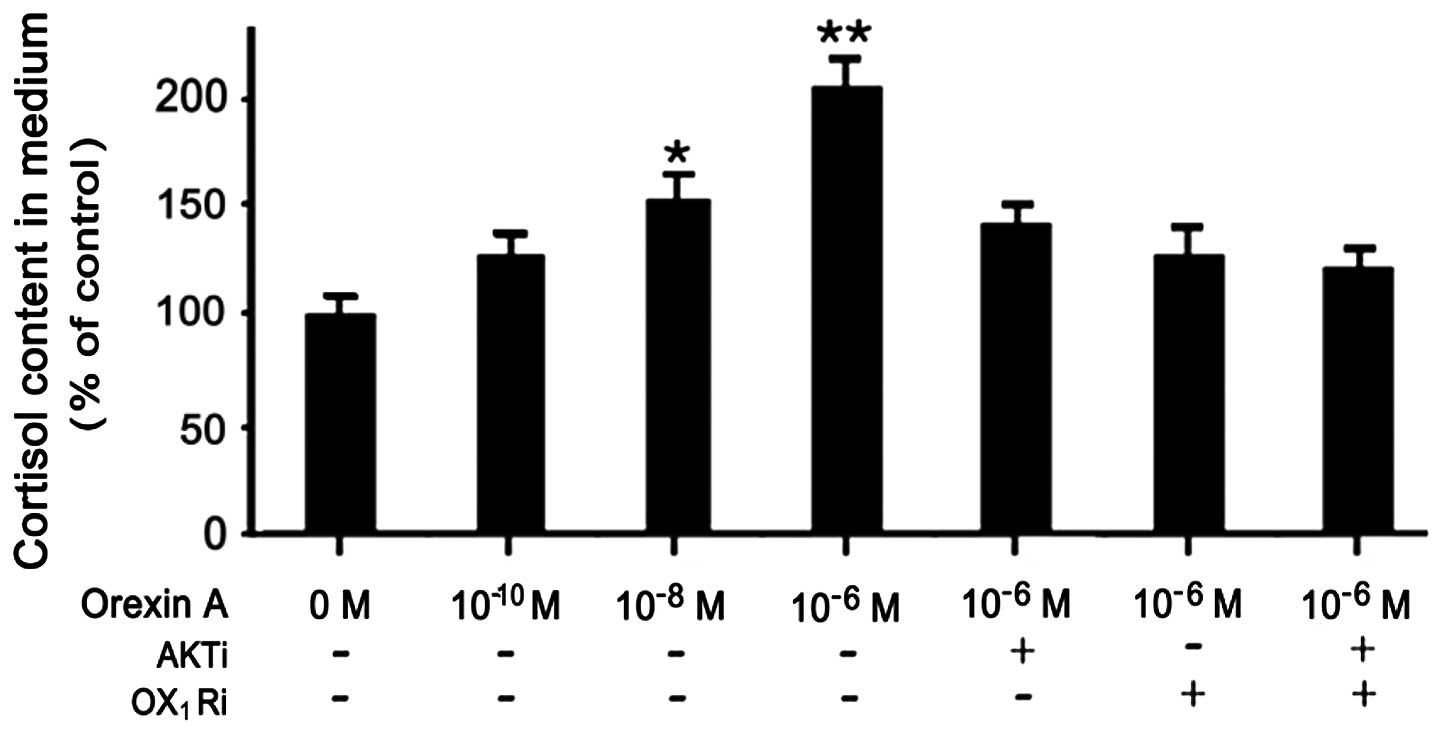
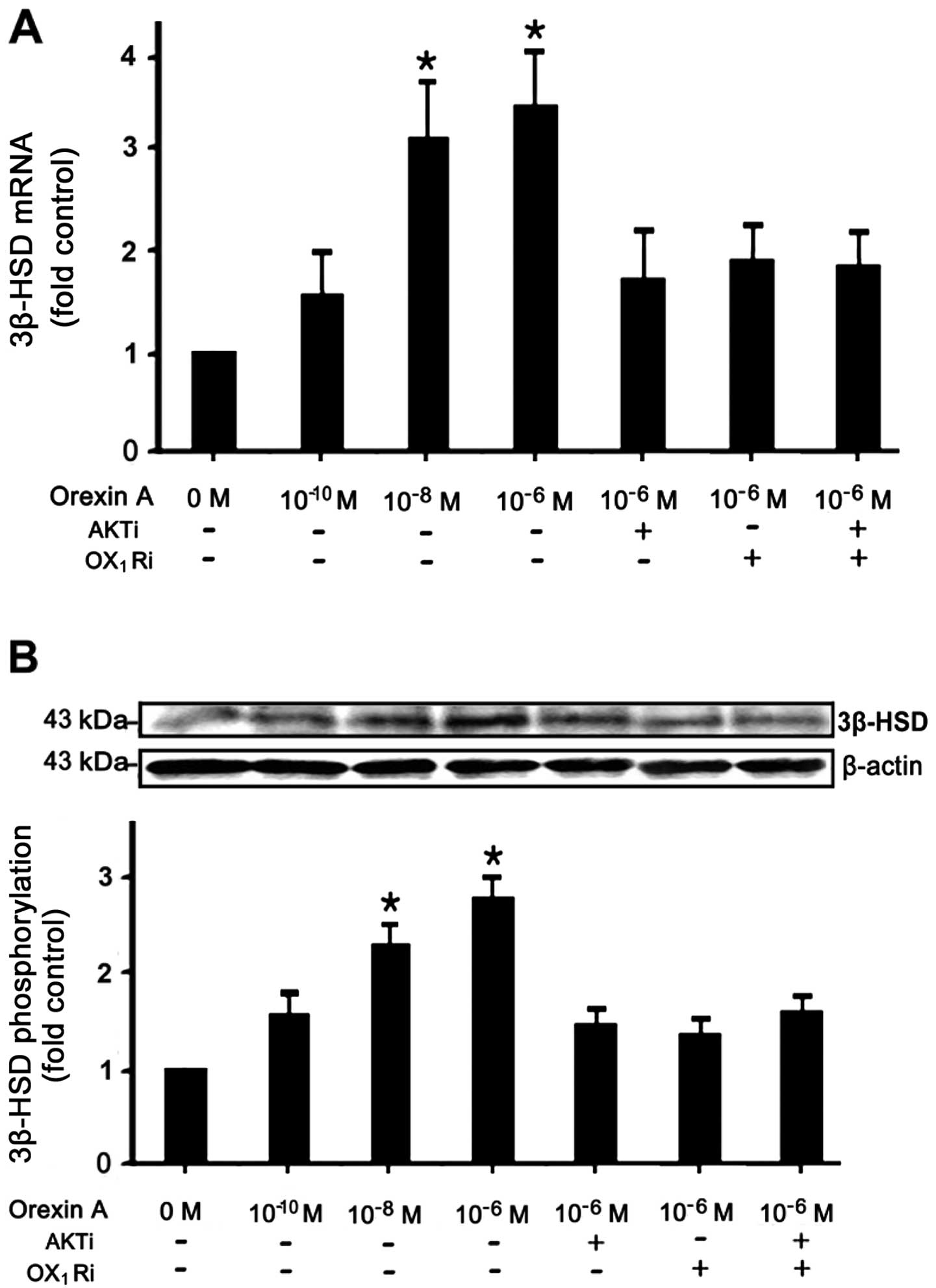
|
1
|
de Lecea L, Kilduff TS, Peyron C, Gao X,
Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT,
Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM
and Sutcliffe JG: The hypocretins: hypothalamus-specific peptides
with neuroexcitatory activity. Proc Natl Acad Sci USA. 95:322–327.
1998.PubMed/NCBI
|
|
2
|
Heinonen MV, Purhonen AK, Mäkelä KA and
Herzig KH: Functions of orexins in peripheral tissues. Acta Physiol
(Oxf). 192:471–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuki T and Sakurai T: Orexins and
orexin receptors: from molecules to integrative physiology. Results
Probl Cell Differ. 46:27–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakurai T1, Amemiya A, Ishii M, Matsuzaki
I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP,
Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS,
McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ and
Yanagisawa M: Orexins and orexin receptors: a family of
hypothalamic neuropeptides and G protein-coupled eceptors that
regulate feeding behavior. Cell. 92:573–585. 1998. View Article : Google Scholar
|
|
5
|
Voisin T, Rouet-Benzineb P, Reuter N and
Laburthe M: Orexins and their receptors: structural aspects and
role in peripheral tissues. Cell Mol Life Sci. 60:72–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kagerer SM and Jöhren O: Interactions of
orexins/hypocretins with adrenocortical functions. Acta Physiol
(Oxf). 198:361–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malendowicz LK, Hochol A, Ziolkowska A,
Nowak M, Gottardo L and Nussdorfer GG: Prolonged orexin
administration stimulates steroid-hormone secretion, acting
directly on the rat adrenal gland. Int J Mol Med. 7:401–404.
2001.PubMed/NCBI
|
|
8
|
Malendowicz LK, Jedrzejczak N, Belloni AS,
Trejter M, Hochol A and Nussdorfer GG: Effects of orexins A and B
on the secretory and proliferative activity of immature and
regenerating rat adrenal glands. Histol Histopathol. 16:713–717.
2001.PubMed/NCBI
|
|
9
|
Malendowicz LK, Tortorella C and
Nussdorfer GG: Orexins stimulate corticosterone secretion of rat
adrenocortical cells, through the activation of the adenylate
cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol.
70:185–188. 1999. View Article : Google Scholar
|
|
10
|
Mazzocchi G, Malendowicz LK, Gottardo L,
Aragona F and Nussdorfer GG: Orexin A stimulates cortisol secretion
from human adrenocortical cells through activation of the adenylate
cyclase-dependent signaling cascade. J Clin Endocrinol Metab.
86:778–782. 2001. View Article : Google Scholar
|
|
11
|
Nanmoku T, Isobe K, Sakurai T, Yamanaka A,
Takekoshi K, Kawakami Y, Goto K and Nakai T: Effects of orexin on
cultured porcine adrenal medullary and cortex cells. Regul Pept.
104:125–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spinazzi R, Rucinski M, Neri G,
Malendowicz LK and Nussdorfer GG: Preproorexin and orexin receptors
are expressed in cortisol-secreting adrenocortical adenomas, and
orexins stimulate in vitro cortisol secretion and growth of tumor
cells. J Clin Endocrinol Metab. 90:3544–3549. 2005. View Article : Google Scholar
|
|
13
|
Ziolkowska A, Spinazzi R, Albertin G,
Nowak M, Malendowicz LK, Tortorella C and Nussdorfer GG: Orexins
stimulate glucocorticoid secretion from cultured rat and human
adrenocortical cells, exclusively acting via the OX1
receptor. J Steroid Biochem Mol Biol. 96:423–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wenzel J, Grabinski N, Knopp CA, Dendorfer
A, Ramanjaneya M, Randeva HS, Ehrhart-Bornstein M, Dominiak P and
Jöhren O: Hypocretin/orexin increases the expression of
steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J
Physiol Regul Integr Comp Physiol. 297:R1601–R1609. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayes HM Jr and Wilson GP:
Hormone-dependent neoplasms of the canine perianal gland. Cancer
Res. 37:2068–2071. 1977.PubMed/NCBI
|
|
16
|
Sauer LA, Chapman JC and Dauchy RT:
Topology of 3 beta-hydroxy-5-ene-steroid dehydrogenase/delta
5-delta 4-isomerase in adrenal cortex mitochondria and microsomes.
Endocrinology. 134:751–759. 1994.PubMed/NCBI
|
|
17
|
Zhao HF, Labrie C, Simard J, de Launoit Y,
Trudel C, Martel C, Rhéaume E, Dupont E, Luu-The V, Pelletier G and
Labriel F: Characterization of rat 3 beta-hydroxysteroid
dehydrogenase/delta 5- delta 4 isomerase c DNAs and differential
tissue specific expression of the corresponding mRNAs in
steroidogenic and peripherial tissues. J Biol Chem. 266:583–593.
1991.
|
|
18
|
van Blitterswijk WJ and Verheij M:
Anticancer mechanisms and clinical application of
alkylphospholipids. Biochim Biophys Acta. 1831:663–674.
2013.PubMed/NCBI
|
|
19
|
Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial
S, Khuri FR and Sun SY: Perifosine inhibits mammalian target of
rapamycin signaling through facilitating degradation of major
components in the mTOR axis and induces autophagy. Cancer Res.
69:8967–8976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
21
|
Richardson CJ, Schalm SS and Blenis J:
PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol.
15:147–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh AC, Truitt ML and Ruggero D:
Oncogenic AKTivation of translation as a therapeutic target. Br J
Cancer. 105:329–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sale EM and Sale GJ: Protein kinase B:
signalling roles and therapeutic targeting. Cell Mol Life Sci.
65:113–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blandino-Rosano M, Chen AY, Scheys JO,
Alejandro EU, Gould AP, Taranukha T, Elghazi L, Cras-Méneur C and
Bernal-Mizrachi E: mTORC1 signaling and regulation of pancreatic
β-cell mass. Cell Cycle. 11:1892–1902. 2012.
|
|
25
|
Ramanjaneya M, Conner AC, Chen J, Kumar P,
Brown JE, Jöhren O, Lehnert H, Stanfield PR and Randeva HS:
Orexin-stimulated MAP kinase cascades are activated through
multiple G-protein signalling pathways in human H295R
adrenocortical cells: diverse roles for orexins A and B. J
Endocrinol. 202:249–261. 2009. View Article : Google Scholar
|
|
26
|
Ramanjaneya M, Conner AC, Chen J,
Stanfield PR and Randeva HS: Orexins stimulate steroidogenic acute
regulatory protein expression through multiple signaling pathways
in human adrenal H295R cells. Endocrinology. 149:4106–4115. 2008.
View Article : Google Scholar
|
|
27
|
Rainey WE, Saner K and Schimmer BP:
Adrenocortical cell lines. Mol Cell Endocrinol. 228:23–38. 2004.
View Article : Google Scholar
|
|
28
|
Blanco M, García-Caballero T, Fraga M,
Gallego R, Cuevas J, Forteza J, Beiras A and Diéguez C: Cellular
localization of orexin receptors in human adrenal gland,
adrenocortical adenomas and pheochromocytomas. Regul Pept.
104:161–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rouet Benzineb P, Rouyer Fessard C, Jarry
A, Avondo V, Pouzet C, Yanagisawa M, Laboisse C, Laburthe M and
Voisin T: Orexins acting at native OX(1) receptor in colon cancer
and neuroblastoma cells or at recombinant OX(1) receptor suppress
cell growth by inducing apoptosis. J Biol Chem. 279:45875–45886.
2004.PubMed/NCBI
|
|
31
|
Biegańska K, Sokołowska P, Jöhren O and
Zawilska JB: Orexin A suppresses the growth of rat C6 glioma cells
via a caspase-dependent mechanism. J Mol Neurosci. 48:706–712.
2012.PubMed/NCBI
|
|
32
|
Alessandro R and Kohn EC: Signal
transduction targets in invasion. Clin Exp Metastasis. 19:265–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sachdev P, Jiang YX, Li W, Miki T, Maruta
H, Nur-E-Kamal MS and Wang LH: Differential requirement for Rho
family GTPases in an oncogenic insulin-like growth factor-I
receptor-induced cell transformation. J Biol Chem. 276:26461–26471.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen KT, Wang WJ, Chan JL and Wang LH:
Differential requirements of the MAP kinase and PI3 kinase
signaling pathways in Src- versus insulin and IGF-1
receptors-induced growth and transformation of rat intestinal
epithelial cells. Oncogene. 19:5385–5397. 2000. View Article : Google Scholar
|
|
35
|
Sachdev LP, Zeng and Wang LH: Distinct
role of phosphatidylinositol 3-kinase and Rho family GTPases in
Vav3-induced cell transformation, cell motility, and morphological
changes. J Biol Chem. 277:17638–17648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nguyen KT, Zong CS, Uttamsingh S, Sachdev
P, Bhanot M, Le MT, Chan JL and Wang LH: The role of
phosphatidylinositol 3-kinase, rho family GTPases, and STAT3 in
Ros-induced cell transformation. J Biol Chem. 277:11107–11115.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
38
|
Göncz E, Strowski MZ, Grötzinger C, Nowak
KW, Kaczmarek P, Sassek M, Mergler S, El-Zayat BF, Theodoropoulou
M, Stalla GK, Wiedenmann B and Plöckinger U: Orexin-A inhibits
glucagon secretion and gene expression through a Foxo1-dependent
pathway. Endocrinology. 149:1618–1626. 2008.PubMed/NCBI
|