Introduction
Autophagy, or cellular self-digestion, is an
important pathophysiological process of cell development,
differentiation, survival and homeostasis. It involves the
degradation of cellular proteins and organelles lying outside the
membrane, which originate from the outer membrane of the
mitochondria. Lysosomes participate in the formation of the
autophagosome, which engulfs small molecules and nutrients and then
recycles them for energy production (1–3).
A number of studies have demonstrated that autophagy
plays an essential role in a number of hepatic diseases (1–3).
It is known that autophagy can play a protective role during
nutrient starvation, and it has been shown that autophagy can be
induced in liver cells due to lack of nutrition or under hypoxic
conditions (1). Hypoxic cell
injury is aggravated in the liver that has suffered occlusion of
the portal triad and following the return of blood flow delivery
(4,5). It has also been found that autophagy
can alleviate cell injury by limiting necrosis through cellular
self-digestion, reducing oxygen consumption or regulating cell
apoptosis (6–8).
Zinc protoporphyrin (ZnPP) is a compound found in
red blood cells when heme production is inhibited by the lack of
iron or due to other reasons, and it is an intermediate of glycine
and succinyl-CoA that is produced during heme biosynthesis
(9). ZnPP inhibits heme oxygenase
(HO), the rate-limiting enzyme in the heme degradation pathway. It
has been demonstrated that the induction of heme oxygenase-1 (HO-1)
attenuates liver cell injury, and that preconditioning with
protoporphyrin IX zinc accentuates liver damage (10). A previous study reported that HO-1
attenuates liver injury by inducing autophagy and inhibiting cell
apoptosis, and that a decrease in HO-1 activity increases cell
apoptosis and thus accentuates liver injury (11). Therefore, it may be of interest to
investigate the role of ZnPP in liver ischemia/reperfusion (IR)
injury. ZnPP may play a role in hepatocyte injury by downregulating
HO-1 and reducing autophagy, or by inducing apoptosis. However, the
mechanisms involved, as well as the association between HO-1 and
autophagy and cell apoptotic pathways have not yet been fully
elucidated. Increased HO-1 expression may induce autophagy to
alleviate liver injury. We thus hypothesized that ZnPP, a hemin
inhibitor, may downregulate HO-1 expression, which may subsequently
reduce autophagy and induce apoptosis, aggravating hepatocyte
injury.
Materials and methods
Reagents and materials
Dulbecco’s modified Eagle’s medium (DMEM),
penicillin, streptomycin, 10% heat-inactivated fetal bovine serum
(FBS) and pancreatic enzymes were purchased from Gibco (Grand
Island, NY, USA). Protoporphyrin IX zinc, mineral oil and Hank’s
balanced salt solution (HBSS) were from Sigma-Aldrich (St. Louis,
MO, USA). Antibodies against β-actin, HO-1, caspase-3, caspase-8,
caspase-9, Bax, Bcl-2, poly(ADP-ribose) polymerase (PARP) and
cleaved PARP were from Abcam (Cambridge, UK). Cytochrome c
and light chain 3-II (LC3-II) antibodies were obtained from Novus
Biologicals (Littleton, CO, USA). The following reagents were also
used: the JC-1 mitochondrial membrane potential assay kit RIPA
lysis buffer (Beyotime, Haimen, China), enhanced chemiluminescence
(ECL), protease inhibitor (Pierce Biotechnology, Inc., Rockford,
IL, USA), polyvinylidene difluoride membranes (Millipore, Bedford,
MA, USA), horseradish peroxidase (HRP)-conjugated secondary
antibody (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China), TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), PrimeScript RT Master Mix and the SYBR Premix
Ex Taq™ real-time PCR kit (Takara Bio, Inc., Shiga, Japan).
Cell culture and treatment
We purchased the buffalo rat liver (BRL) cells from
the cell bank of the Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in DMEM plus 100 U/ml penicillin, 100 U/ml
streptomycin and 10% FBS. The cells were seeded in 6-well plates
for treatments, western blot analysis and cell viability
analysis.
The cells were divided into 6 groups: i) the control
group, in which the cells were cultured in DMEM without any
treatment; ii) the IR (IR simulation) group, in which the cells
were treated with mineral oil (1 ml/well) for 1 h initially, which
simulated ischemia, and then the cells were washed with PBS before
the return of nutritional and oxygen supply by the replacement of
DMEM for 3, 6 and 12 h, as previously described (12,13); iii) the ZIR (ZnPP treatment prior
to IR simulation) group, in which the cells were pre-treated with
DMEM without 10% heat-inactivated FBS in the presence of 20 μmol/l
ZnPP for 12 h, then the cells were washed twice with PBS, immersed
in mineral oil, and cultured in DMEM for 3, 6 and 12 h, as
previously described (14); iv)
the SIR (starvation prior to IR simulation) group, in which the
cells were cultured in HBSS medium with Ca2+ and
Mg2+ supplemented with 10 mM HEPES (1 ml/well) for 0.5 h
to induce autophagy, then the cells were washed with PBS before
being immersed in mineral oil, and then the cells were finally
washed twice with PBS and cultured in DMEM for 3, 6 and 12 h, as
previously described (15); v)
the HIR (hemin treatment prior to IR simulation) group, in which
the cells were pre-treated with DMEM without 10% heat-inactivated
FBS in the presence of 20 μmol/l hemin (HO-1 inducer) for 12 h,
then washed twice with PBS, immersed in mineral oil and cultured in
DMEM for 3, 6 and 12 h, as previously described (14); and vi) the ZSIR (ZnPP treatment
then starvation prior to IR simulation), in which the cells were
pre-treated with DMEM without 10% heat-inactivated FBS in the
presence of 20 μmol/l ZnPP for 12 h, and washed twice with PBS,
then the cells were cultured in HBSS medium with Ca2+
and Mg2+ supplemented with 10 mM HEPES (1 ml/well) for
0.5 h to induce autophagy, and then the cells were washed with PBS
before being immersed in mineral oil, and finally washed twice with
PBS and cultured in DMEM for 3, 6 and 12 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells and purified
using TRIzol reagent. cDNA was synthesized using the PrimeScript RT
Master Mix in a 10-μl reaction mixture. According to the SYBR
Premix Ex Taq real-time PCR kit, we used cDNA (2 μl) as a template
in a 20-μl reaction. Primers were synthesized by Invitrogen
(Shanghai, China). The primers for HO-1 were
5′-GTCAAGCACAGGGTGACAGA-3′ (sense) and 5′-CTGCAGCTCCTCAAACAGC-3′
(antisense). The following primers were used for the detection of
LC3-II expression: 5′-GAGCTTCGAACAAA GAGTGGA-3′ (sense) and
5′-CTTCTCACCCTTGTATCG CTCTA-3′ (antisense). β-actin was used as the
reference gene in our experiment, and the primers for β-actin were
5′-TCACCCACACTGTGCCC ATCTACGA-3′ (sense) and 5′-CAGCGGAACCGCTCATTG
CCAATGG-3′ (antisense). For RT-PCR, we used the following cycles:
95°C for 30 sec, 40 cycles of 95°C for 5 sec, 60°C for 31 sec and
the dissociation stage: 95°C for 15 sec, 60°C for 1 min and 95°C
for 15 sec.
Western blot analysis
The cells were treated as described above. The cells
were then cultured and homogenized in RIPA lysis buffer in the
presence of 1% (v/w) protease inhibitor. Subsequently, we shook the
mixture at 4°C for 1 h and removed the insoluble matter by
centrifugation at 40,000 × g at 4°C for 1 h. For the analysis of
cytochrome c (16), we
cultured the cell pellets in the HEPES buffer containing 250 mM
sucrose, homogenization by a 22-gauge needle, and then the
homogenate was centrifuged at 800 × g at 4°C for 15 min. The
supernatants were centrifuged at 10,000 × g for 15 min at 4°C.
Finally, we collected the mitochondrial pellets and aliquots of the
supernatant (cytosolic fraction). The total protein concentration
was determined using the bicinchoninic acid protein assay kit. All
proteins were mixed with loading buffer before being resolved on a
12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel,
and this gel was subsequently transferred onto polyvinylidene
difluoride membranes at 250 mA for 2 h. Subsequently, the membranes
were blocked in 5% milk for 1 h and incubated overnight with
primary antibodies (HO-1, LC3-II, β-actin, caspase-3, caspase-8,
caspase-9, cytochrome c, Bax, Bcl-2, PARP and cleaved PARP)
in 5% milk. β-actin protein was used as a control. The membranes
were washed the following day in TBS Tween-20 (TBST) for 30 min,
and were then incubated with HRP-conjugated secondary antibody
(1:4,000) for 1 h and washed in TBST again before being visualized
using an ECL detection kit. Each experiment was repeated at least 3
times, and the gray value of each protein was measured using
Image-Pro Plus 6.0 software for statistical analysis.
Electron microscopic analysis
The cells were plated on 6-well plates, and were
harvested with pancreatic enzymes before being fixed in 2%
glutaraldehyde with 0.1 mol/l PBS (pH 7.4). For ultrastructural
examination, the cells were post-fixed with 2% osmium tetroxide
(OsO4) and were dehydrated through a graded series of
ethanol; the cells were then embedded in molds and incubated at
37°C overnight. Subsequently, ultrathin sections were made using an
ultramicrotome and stained with uranyl acetate and lead citrate.
The sections were viewed udner an JEOL JEM 1010 transmission
electron microscope (The basic medical laboratory of Nanjing
Medical University, Nanjing, China).
Immunofluorescence
The BRL cells were plated on 6-well plates and then
following treatment, were fixed on coverslips with 4%
paraformaldehyde for 20 min. After rinsing with PBS 4 times (10 min
each time), the cells were blocked in 10% BSA for 1 h and incubated
with primary antibody (LC3-II in 10% BSA) overnight. The following
day, the cells were rinsed with PBS again before being cultured
with secondary antibody (1:100) for 1 h at 37°C. Subsequently, the
nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) and
autophagy was monitored. Ten visual fields were randomly selected,
and the optical density of each visual field was measured and a
semi-quantitative analysis using Image-Pro Plus 6.0 software was
performed.
Analysis of apoptosis
The apoptosis of the BRL cells was determined by
double staining with Annexin V-fluorescein isothiocyanate (FITC)
and propidium iodide (PI). The cells (5×105) were washed
twice with ice-cold PBS, and were then resuspended in 400 μl of
binding buffer. Subsequently, 5 μl of Annexin V-FITC and 5 μl of PI
were added followed by incubation for 5–15 min in the dark at 4°C.
The samples were analyzed using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) using Cell Quest software (BD
Biosciences).
Mitochondrial membrane potential
assay
A JC-1 mitochondrial membrane potential assay kit
was used. Briefly, after the cells were washed with PBS, JC-1
liquid dye was added to each well (1 ml/well), and the cells were
incubated at 37°C for 20 min. Subsequently, the cells were washed
with 1× dyeing buffer twice, and were cultured in DMEM. Afterwards,
the cells were observed under a fluorescence microscope (The basic
medical laboratory of Nanjing Medical University).
Statistical analysis
Data are expressed as the means ± standard
deviation. The parameters were analyzed by analysis of variance
(ANOVA) and a Q test and Student’s t-test. A value of P<0.05 was
considered to indicate a statistically significant difference. SPSS
11.0 software was used in all statistical analyses.
Results
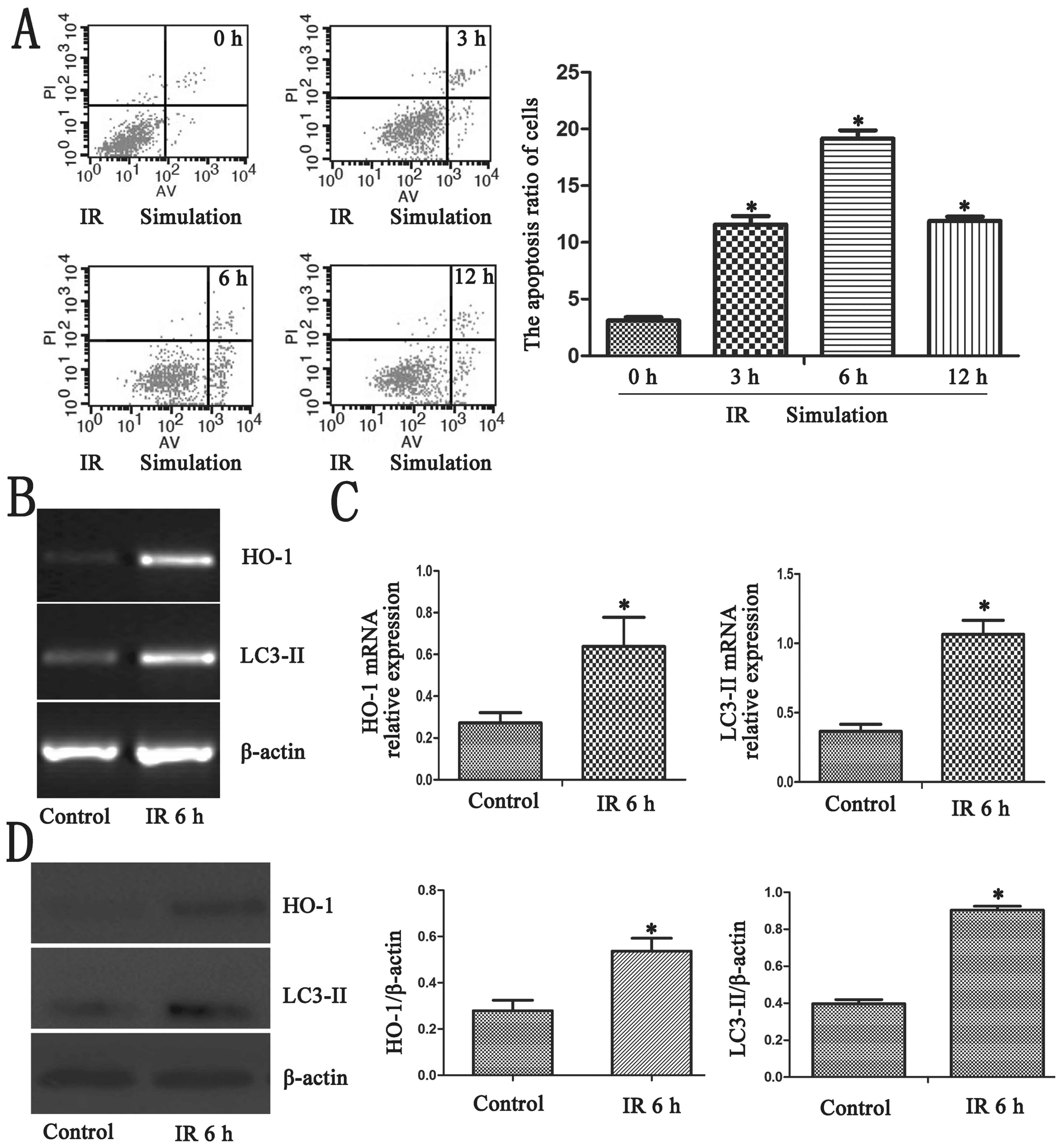
IR simulation in BRL cells increases
hepatocyte apoptosis, and induces HO-1 expression and
autophagy
We detected hepatocyte apoptosis in the different IR
simulation groups. The highest apoptotic rates were observed in the
6-h group (Fig. 1A; P<0.05).
Thus, the 6-h group was used for our research. RT-qPCR was peformed
using cDNA synthesized from different groups of BRL cells as
mentioned above. IR simulation enhanced the mRNA expression of HO-1
and LC3-II in the 6-h group (Fig. 1B
and C; P<0.05). As shown by western blot analysis, the
highest protein expression of HO-1 and LC3-II was observed in the
6-h group, (Fig. 1D;
P<0.05).
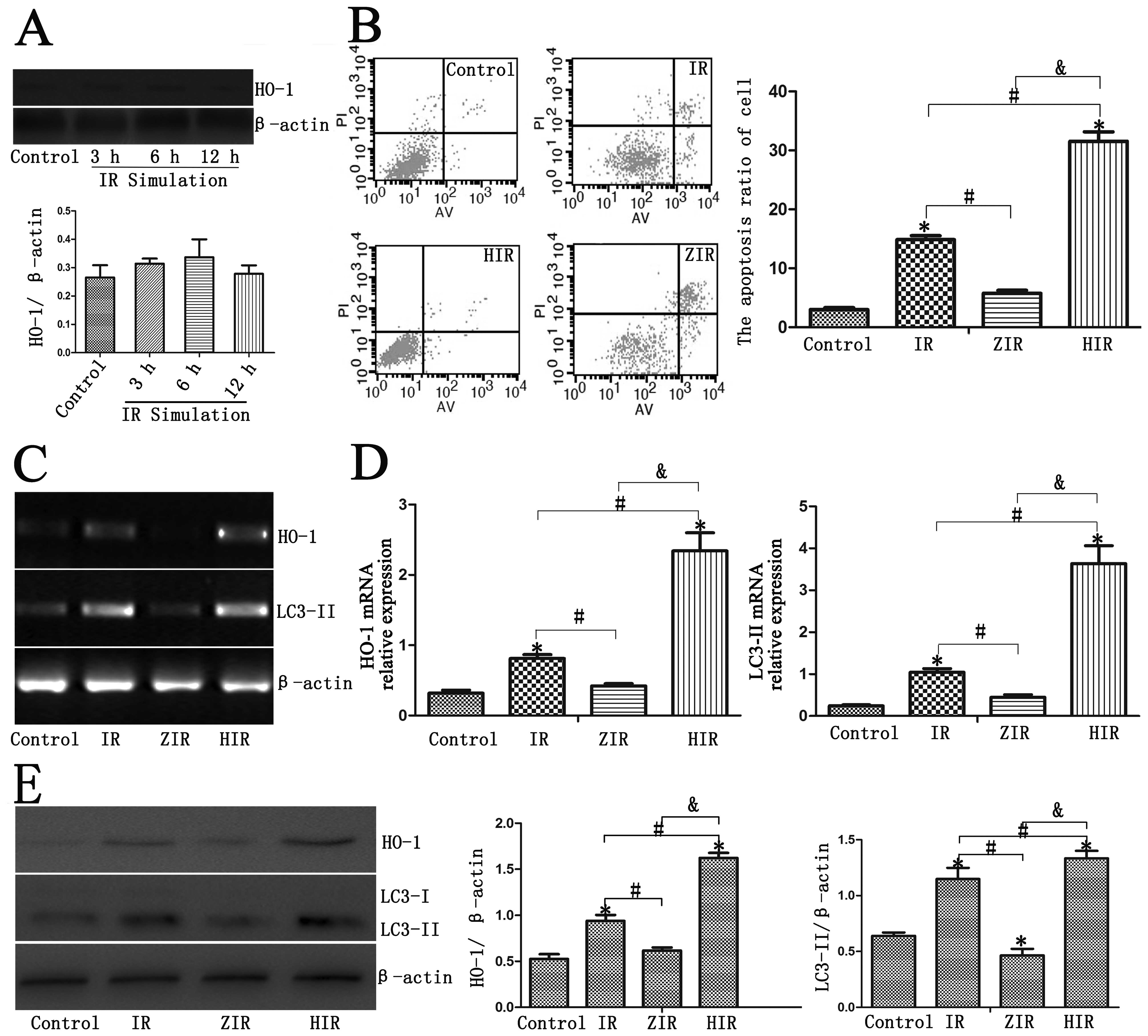
ZnPP treatment prior to IR simulation
increases hepatocyte apoptosis, inhibits HO-1 expression and
reduces autophagy
Treatment with ZnPP prior to IR simulation (ZIR
group) increased the cell apoptotic rates compared with the control
and IR groups (Fig. 2B;
P<0.05). We found that ZnPP inhibited the expression of HO-1 in
the BRL cells (Fig. 2A).
Treatment with ZnPP prior to IR simulation (ZIR group) did not
induce any significant differences compared with the control group
(Fig. 2B; P>0.05), and this
result is in agreement with the results of a previous study
(31). Treatment with ZnPP prior
to IR simulation in the BRL cells induced a decrease in the mRNA
expression of HO-1 and LC3-II (Fig.
2C and D; P<0.05). HO-1 and LC3-II protein expression was
inhibited in the ZIR group (Fig.
2E; P<0.05).
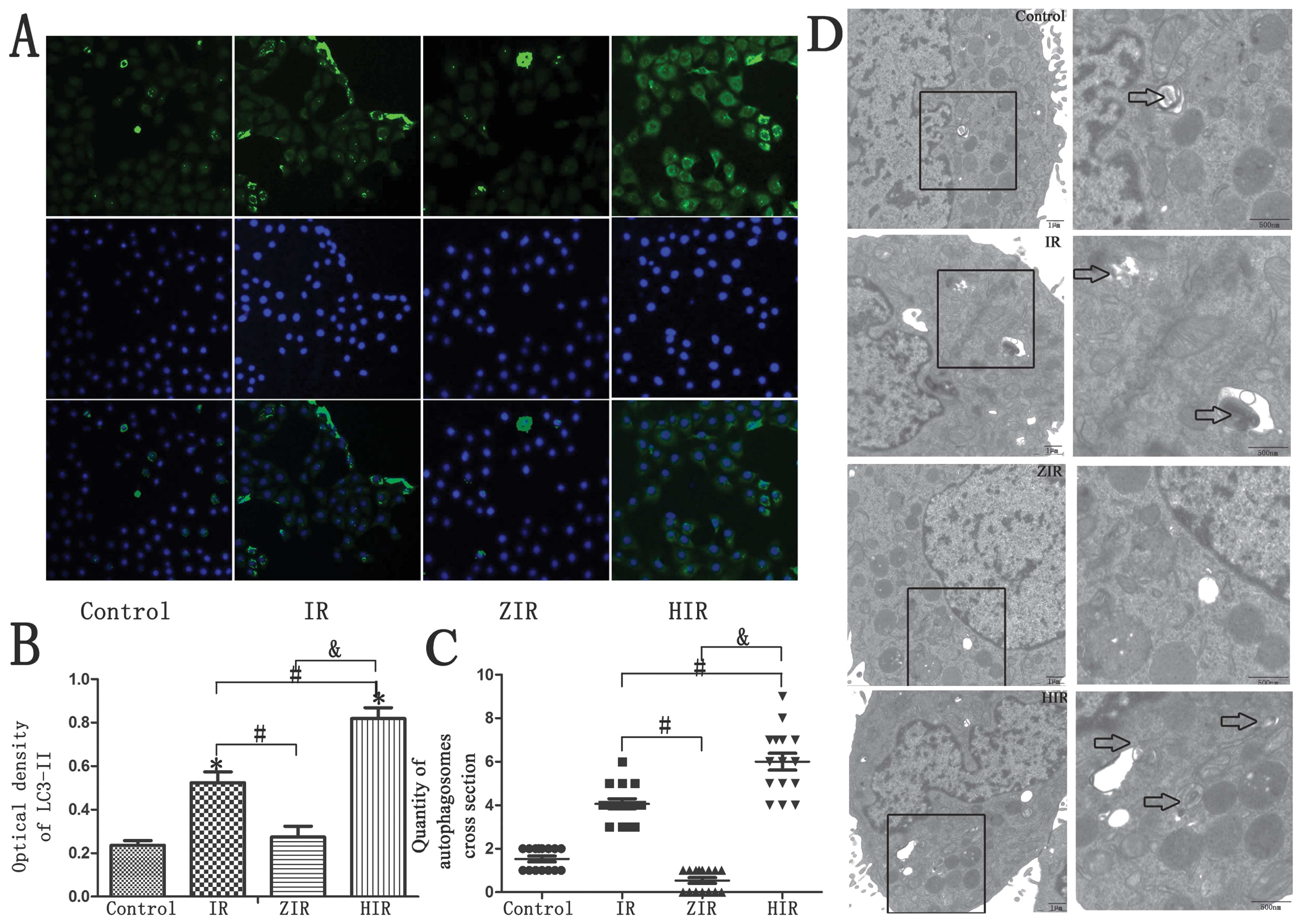
Immunofluorescence staining revealed decreased
LC3-II staining in the ZIR group (Fig. 3A and B; P<0.05). The
ultrastructure of the BRL cells, which was examined by transmission
electron microscopy (TEM), revealed few autophagosomes in the ZIR
group compared with the IR group (Fig. 3C and D; P<0.05).
Upregulation of HO-1 induces autophagy
and reduces hepatocyte apoptosis
Hemin was used in the BRL cells to induce HO-1
expression. An increase in the expression of autophagy-related
genes (LC3-II) was detected by RT-qPCR and western blot analysis
(Fig. 2C–E; P<0.05).
Furthermore, the intensity of immunostaining for LC3-II was also
significantly increased in the HIR group (Fig. 3A and B; P<0.05). A greater
number of autophagosomes was detected in the HIR group; this number
was much greater than that detected in the control, IR and ZIR
group (Fig. 3C and D;
P<0.05).
Annexin V/PI staining revealed that treatment with
hemin decreased the early and late apoptotic rats of the BRL cells
(Fig. 2B; P<0.05).
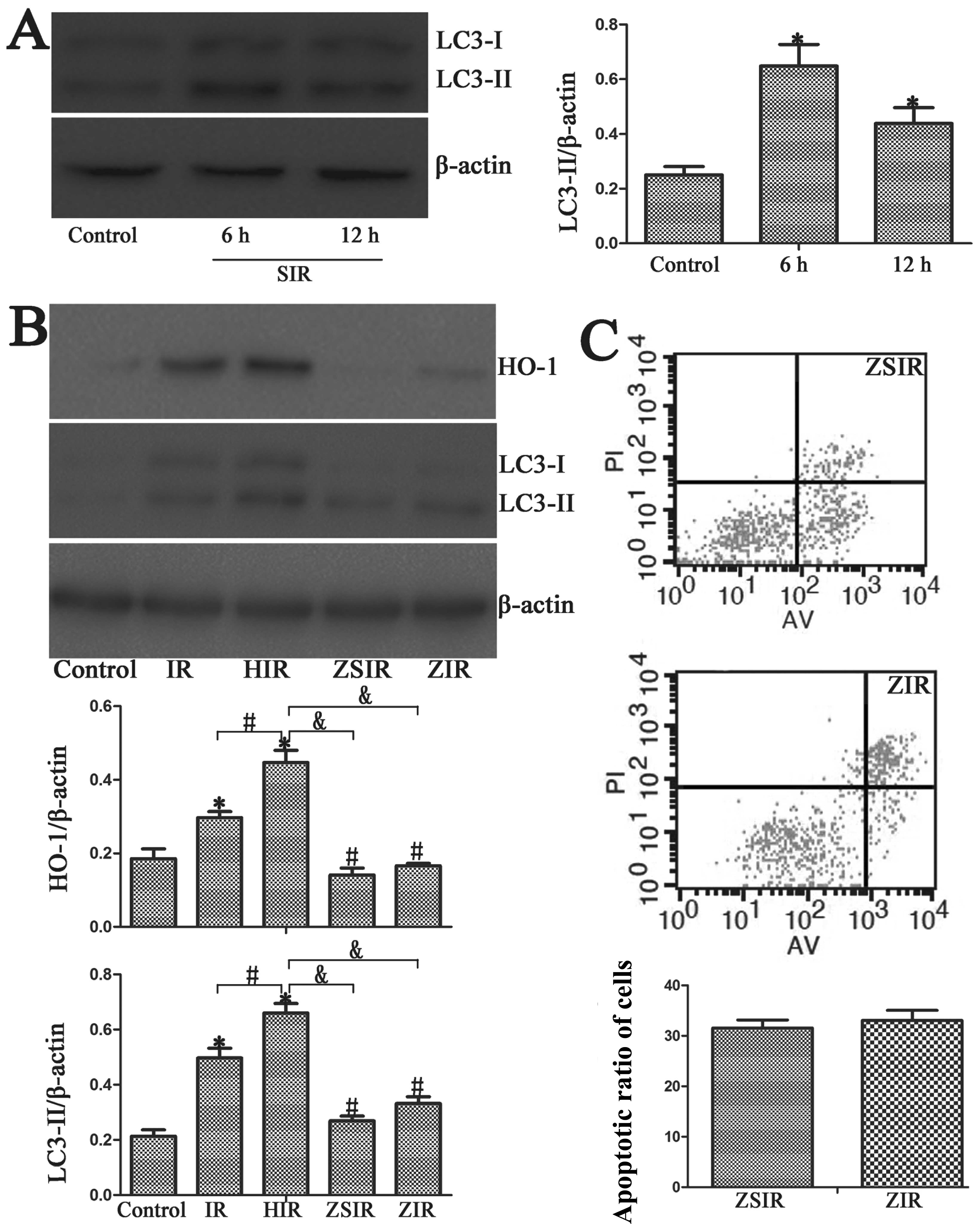
The protective role of autophagy in BRL
cells may be related to HO-1 expression
In order to fiurther dertermine the role of HO-1 and
autophagy, we used HBSS medium with Ca2+ and
Mg2+ supplemented with 10 mM HEPES to induce autophagy.
Western blot analysis revealed the increased expression of
autophagy-related genes (LC3-II) in the SIR group (Fig. 4A). In the ZSIR group, the cells
were pre-treated with ZnPP prior to starvation in HBSS medium. Our
results revealed that there were no significant differences in
LC3-II expression between the ZIR and the ZSIR group (Fig. 4B). Additionally, there was no
significant difference in the cell apoptotic rates between the ZIR
and the ZSIR group (Fig. 4C).
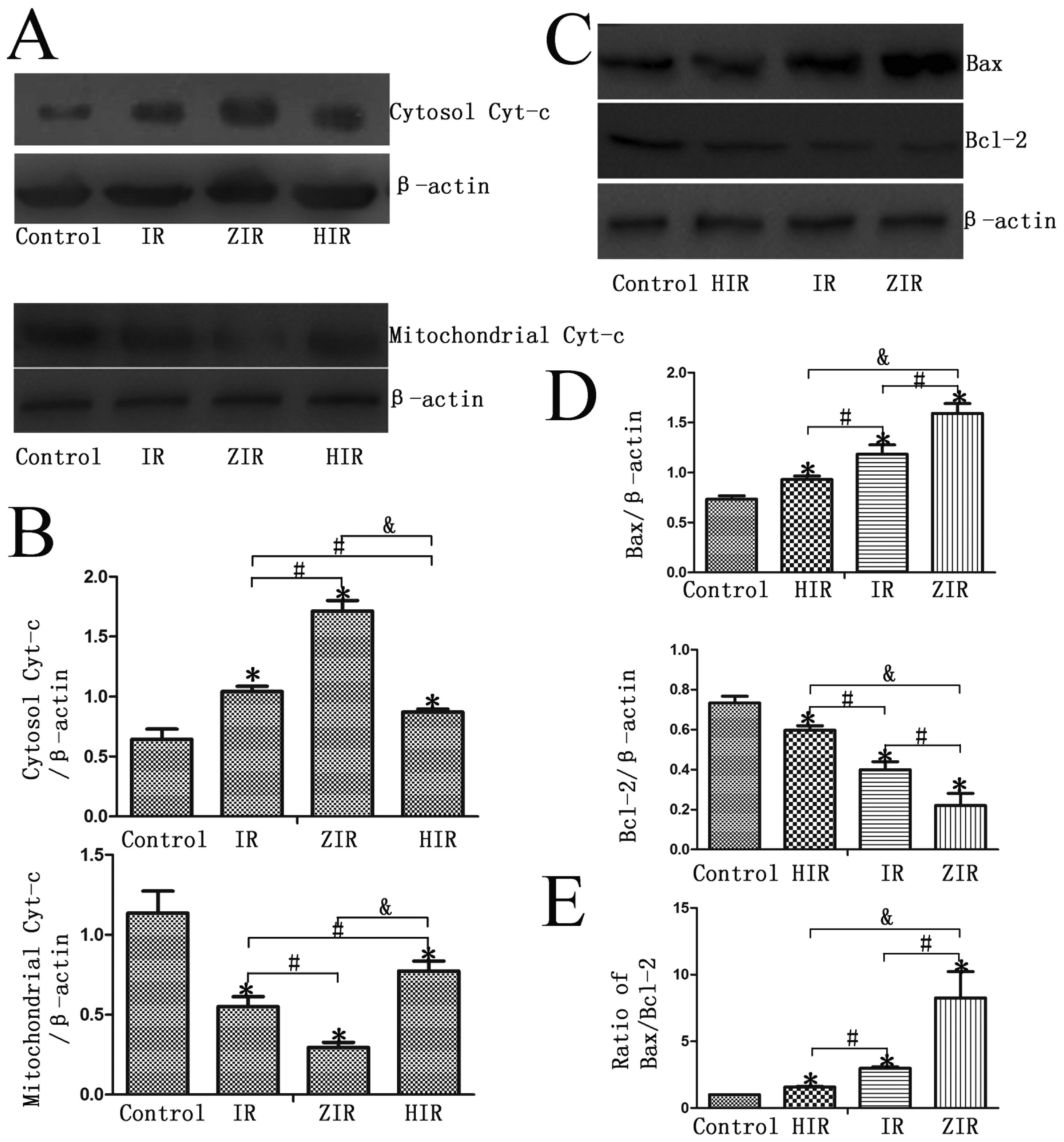
Pre-treatment with ZnPP induces Bax,
cytochrome c, caspase-3 and caspase-9 protein expression, and
reduces Bcl-2 expression
As shown in Fig.
5, following treatment with mineral oil (IR group), the protein
expression of Bax and cytosolic cytochrome c in the BRL
cells increased compared with the control group. When the cells
were treated with ZnPP prior to IR simulation (ZIR group), the
protein expression of Bax and cytosolic cytochrome c
significantly increased; however, this increase was abrogated when
the cells were treated with hemin prior to IR simulation (HIR
group) (P<0.05). The expression of mitochondrial cytochrome
c and Bcl-2 protein was downregulated in the ZIR group
compared with the IR and HIR groups (P<0.05).
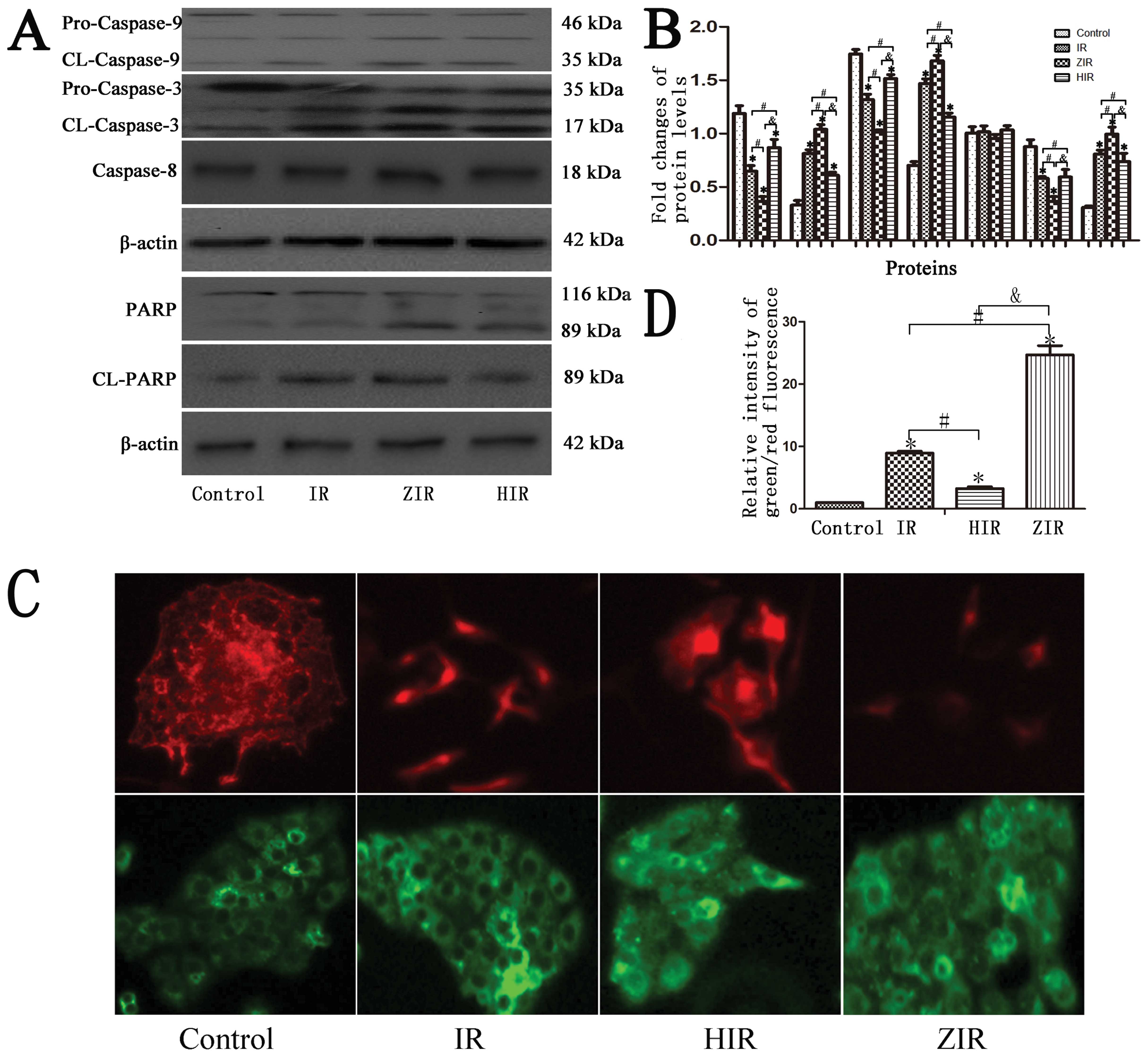
Subsequently, the activity of cleaved caspase-3,
caspase-8 and caspase-9 was detected. The expression of cleaved
caspase-3 and caspase-9 in the cytosolic fraction at 6 h following
IR simulation markedly increased compared with the control group
(Fig. 6A and B; P<0.05). In
the ZIR group, the activity of cleaved caspase-3 and capsase-9 was
increased compared to the IR group (Fig. 6A and B; P<0.05). However, there
was no significant change in caspase-8 protein expression in any of
the groups. Furthermore, treatment with hemin attenuated the
increase in the protein expression of caspase-3 and caspase-9
(Fig. 6A and B; P<0.05).
Higher cleaved PARP protein expression
and disruption of mitochondrial membrane potential in IR and ZIR
groups
Pre-treatment with ZnPP prior to IR simulation
promoted the cleavage of PARP protein from its full-length form to
its cleaved form. However, this was was not observed in the control
and HIR group (Fig. 6A and B;
P<0.05).
As shown in Fig.
6C, more areas of red fluorescence were observed in the control
and HIR groups. In the IR and ZIR groups however, there were more
areas of green fluorescence. Greater areas of green fluorescence in
cells denote a greater disruption of the mitochondrial membrane
potential (Fig. 6C and D;
P<0.05).
Discussion
Our results demonstrated that reduced autophagy was
detected in an in vitro model of IR (mineral oil treatment),
in which the cells were pre-treated with ZnPP. Treatment with hemin
however, induced HO-1 expression in the cells and increased
autophagy and alleviated hepatocyte apoptosis. Thus, autophagy
induced by HBSS may be abrogated by pre-treatment with ZnPP.
Moreover, the protein expression of cytochrome
c, Bax, Bcl-2, caspase-3, caspase-8 and caspase-9 was
detected. The inhibition of HO-1 expression by ZnPP increased
hepatocyte apoptosis, increased the protein expression of cytosolic
cytochrome c and Bax, and promoted the cleavage of caspase-3
and caspase-9. The expression of mitochondrial cytochrome c
and Bcl-2 protein expression were decreased in the ZIR group. Based
on these findings (11), as well
as those of previous studies, we hypothesized that the decrease in
HO-1 expression induced by ZnPP may reduce autophagy, thus
increasing liver cell injury, and that this may partly occur
through the activation of the mitochondrial apoptotic pathway.
Hepatic IR injury is an important phenomenon in
hepatic transplantation, hepatic resection and trauma. Previous
studies have identified a number of key factors associated with
liver IR injury, such as Toll-like receptor (TLR), HO, leukocyte
cascades and oxygen-free radicals (OFRs) (17,18). Understanding the mechanism of IR
injury is crucial to reducing liver injury during liver surgery.
Pharmacological strategies, ischemic preconditioning or the
application of new technologies have been shown to reduce liver IR
injury (19,20). Our results, as well as those of a
previous study have shown that ischemic preconditioning induces
HO-1, alleviating IR liver injury (21). As an important factor to reducing
liver damage, HO-1 is a hot topic of investigation in liver IR
injury.
ZnPP is a general metabolite formed in the process
of heme biosynthesis. The lack of iron or decreased iron
utilization may lead to increased ZnPP formation in the blood.
Evidence suggests that increased levels of ZnPP in the blood play a
role in the inhibition of HO, which is the rate-limiting enzyme in
the heme degradation pathway (9).
HO is the rate-limiting enzyme in the process of the decomposition
of heme metabolism, which may catabolize heme into 3 products:
carbon monoxide (CO), biliverdin and free iron. There are 3 types
of HO: HO-1, which mainly plays a role in the abnormal or stress
state; HO-2, which is mainly distributed in the central nervous
system and testes and HO-3, whose role is not known (22). Among these types, HO-1 plays a
very important role in a number of organs and cells. It serves as a
protective factor by as it has anti-inflammatory, anti-apoptotic
and anti-proliferative properties. These beneficial effects are due
to the metabolites of HO-1 (CO, biliverdin and free iron) (23–25).
Previous studies have shown that HO-1 plays a very
important anti-inflammatory role in a number of inflammatory
diseases, and it may act as an anti-apoptotic and
anti-proliferative mediator for many damaged organs or cells under
stress conditions (26–28). In liver cell injury, induced HO-1
preconditioning may lead to adaptive stress reaction, and may occur
in response to organ ischemic insults, protecting the cells from
injury (29,30). It has been suggested that ZnPP
regulates heme catabolism through the inhibition of HO-1, and thus
aggravates organ damage and cell apoptosis (31). In our study, HO-1 was
downregulated in the ZIR group, and higher cell apoptotic ratios
were detected in this group. However, when the cells were treated
with hemin prior to IR simulation, less hepatocyte apoptosis was
detected. Our results are in accordance with those of previous
studies (11). The specific
mechanisms involved require further investigation.
An increasing number of studies have reported that
autophagy is a primarily protective factor for cells. Autophagy
(self-eating) is known as a process through which cytoplasmic
macromolecules or organelles are delivered to lysosomes for
degradation to maintain the energy balance in the cell. Previous
studies have indicated that the induction of autophagy exerts a
protective effect against tissue and cell injury (32–34). Studies have demonstrated the
protective effects of autophagy against liver injury (1,6,11);
however, the mechanisms through which autophagy prevents liver
injury are not yet completely understood. Researchers have always
supported the idea that one of the major functions of autophagy is
to keep cells alive under stressful ‘life-threatening’ conditions
(6,34). In our study, the lack of nutrition
and oxygen to BRL cells by treatment with mineral oil increased
autophagy, and this was verified by the results of RT-qPCR, western
blot analsyis, electron microscopic analysis and immunofluorescence
(Figs. 1–3). Thus, autophagy is an adaptive
response in cells that are subjected to stress or are damaged.
Of note, in our study, the HO-1 and LC3-II proteins
were simultaneously upregulated following IR simulation; when the
cells were treated with ZnPP (inhibitor of HO-1) prior to IR
simulation, both proteins were simultaneously downregulated
(Figs. 2 and 3). Based on the aforementioned results,
we hypothesized that HO-1 may be related to autophagy. It has been
demonstrated that HO-1 induces autophagy in a mouse model of liver
IR injury; however, the specific mechanisms involved require
further investigation (35). It
has also been previously demonstrated that HO-1 protein expression
is induced in injured cells or cells under stess (26–28). In our study, this was achieved by
treatment with mineral oil, which caused damage to the liver cells
due to the lack of nutrients and oxygen. This was also associated
with the process of autophagy.
A previous study demonstrated that the
phloretin-induced expression of HO-1 may contribute to the cellular
defense mechanisms against cisplatin-induced apoptosis (36). Another study found that the
induction of HO-1 ameliorated hepatocyte apoptotic activity and
oxidative damage in rats with hyperthyroidism, and decreased the
expression of cytochrome c, caspase-3, caspase-8 and Bax.
Liver injury was aggravated by the administration of ZnPP (HO-1
inhibitor) (37). In addition, it
has been demonstrated that mitophagy, which is the selective
removal of mitochondria by autophagy, plays a crucial role in IR
injury (38). Thus, in the
present study, we focused on HO-1, autophagy and the mitochondrial
apoptotic pathway. We demonstrated the adverse effects of ZnPP on
autophagy induced by treatment with mineral oil and the increase in
apoptotis, which was due to the inhibition of HO-1 (Fig. 4).
There are two classical apoptosis pathways, the
intrinsic pathway, which is also known as the
mitochondrial-mediated pathway and the extrinsic or death
receptor-mediated pathway (39).
It has also been reported that the Bcl-2 family of proteins,
including the anti-apoptotic protein, Bcl-2, and the pro-apoptotic
protein, Bax, are crucial in initiating the mitochondrial death
cascade. When cells are under stress, Bax protein translocates to
the outer mitochondrial membrane and promotes the release of
cytochrome c from the mitochondria to the cytosol. By
contrast, Bcl-2 protein disrupts this process and decreases
apoptosis (40). It has also been
demonstrated that Bcl-2 expression is reduced while that of Bax is
increased in HECI-OC1 cells pre-treated with ZnPP (36). Thus, we hypothesized that
pre-treatment with ZnPP would promote the release of cytochrome
c and the activation of the mitochondrial death cascade. The
upregulation of Bax and the downregulation of Bcl-2 was observed in
the ZIR group, while the induction of HO-1 prevented this event.
The expression of cytosolic cytochrome c was induced in the
cells pre-treated with ZnPP and the expression of mitochondrial
cytochrome c was decreased. When mitochondrial membrane
potential was examined, more areas of red fluorescence were
observed in the control and the HIR group; however, more areas of
green fluorescence were observed in the IR and ZIR group. Higher
levels of green fluorescence indicate a greater disruption of
mitochondrial membrane potential. A previous study found that the
activation of caspase-3 and caspase-9 was important in the
intrinsic pathway (41). In our
study, there was an increase in the expression of cleaved caspase-3
and caspase-9 in the IR group and the group treated with ZnPP prior
to IR simulation; however, this increase was not observed in the
control and HIR group.
PARP protein, another important protein associated
with apoptosis, is cleaved by activated caspase-3. It is regarded
as the symbol of the activation of caspase-3 (42). In our study, the cleavage of PARP
from the full-length 116 kDa form to its cleaved 89 kDa form was
observed in the IR and ZIR groups (Fig. 6). Based on this result, we
hypothesized that the inhibition of HO-1 by ZnPP increased BRL cell
apoptosis and that it may play a role in the mitochondrial-mediated
apoptotic pathway.
In conclusion, in the present study, we demonstrate
that ZnPP reduces HO-1 expression and subsequently inhibits
autophagy, thus aggravating BRL cell IR injury and has a
pro-apoptotic effect via the mitochondrial apoptotic pathway. In
the present study, we hypothesized that the reduction of autophagy
by HO-1 inhibition may lead to the activation of the mitochondrial
apoptotic pathway. However, the association between autophagy and
the mitochondrial apoptotic pathway, as well as the role of
autophagy in ZnPP-induced apoptosis require further
investigation.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (81170415), Nanjing Medical
University (2012NJMU087) and Xuzhou City Central Hospital
(XZS2013018).
Abbreviations:
|
IR
|
ischemia/reperfusion
|
|
HO-1
|
heme oxygenase-1
|
|
ZnPP
|
zinc protoporphyrin
|
|
LC3-II
|
light chain 3-II
|
|
BRL
|
buffalo rat liver
|
|
HBSS
|
Hank’s balanced salt solution
|
References
|
1
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar
|
|
2
|
Nivon M, Richet E, Codogno P, Arrigo AP
and Kretz-Remy C: Autophagy activation by NFkappaB is essential for
cell survival after heat shock. Autophagy. 5:766–783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teoh NC: Hepatic ischemia reperfusion
injury: contemporary perspectives on pathogenic mechanisms and
basis for hepatoprotection-the good, bad and deadly. J
Gastroenterol Hepatol. 26(Suppl 1): S180–S187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walsh KB, Toledo AH, Rivera-Chavez FA,
Lopez-Neblina F and Toledo-Pereyra LH: Inflammatory mediators of
liver ischemia-reperfusion injury. Exp Clin Transplant. 7:78–93.
2009.PubMed/NCBI
|
|
6
|
Esposti DD, Domart MC, Sebagh M, Harper F,
Pierron G, Brenner C and Lemoine A: Autophagy is induced by
ischemic preconditioning in human livers formerly treated by
chemotherapy to limit necrosis. Autophagy. 6:172–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng
P, Hogan RN, Gilpin C and Levine B: Autophagy gene-dependent
clearance of apoptotic cells during embryonic development. Cell.
128:931–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara T, Nakamura K, Matsui M, et al:
Suppression of basal autophagy in neural cells causes
neurodegenerative disease in mice. Nature. 441:885–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Labbé RF, Vreman HJ and Stevenson DK: Zinc
protoporphyrin: a metabolite with a mission. Clin Chem.
45:2060–2072. 1999.PubMed/NCBI
|
|
10
|
Kim SJ, Eum HA, Billiar TR and Lee SM:
Role of heme oxygenase 1 in TNF/TNF receptor-mediated apoptosis
after hepatic ischemia/reperfusion in rats. Shock. 39:380–388.
2013. View Article : Google Scholar
|
|
11
|
Carchman EH, Rao J, Loughran PA, Rosengart
MR and Zuckerbraun BS: Heme oxygenase-1-mediated autophagy protects
against hepatocyte cell death and hepatic injury from
infection/sepsis in mice. Hepatology. 53:2053–2062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meldrum KK, Burnett AL, Meng X, Misseri R,
Shaw MB, Gearhart JP and Meldrum DR: Liposomal delivery of heat
shock protein 72 into renal tubular cells blocks nuclear
factor-kappaB activation, tumor necrosis factor-alpha production,
and subsequent ischemia-induced apoptosis. Circ Res. 92:293–299.
2003. View Article : Google Scholar
|
|
13
|
Meldrum KK, Meldrum DR, Hile KL, et al:
p38 MAPK mediates renal tubular cell TNF-alpha production and
TNF-alpha-dependent apoptosis during simulated ischemia. Am J
Physiol Cell Physiol. 281:C563–C570. 2001.PubMed/NCBI
|
|
14
|
Choi BM, Pae HO, Kim YM and Chung HT:
Nitric oxide-mediated cytoprotection of hepatocytes from glucose
deprivation-induced cytotoxicity involvement of heme oxygenase-1.
Hepatology. 37:810–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Settembre C, Di Malta C, Polito VA, et al:
TFEB links autophagy to lysosomal biogenesis. Science.
332:1429–1433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis JS, Meeke K, Osipo C, et al:
Intrinsic mechanism of estradiol-induced apoptosis in breast cancer
cells resistant to estrogen deprivation. J Natl Cancer Inst.
97:1746–1759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serizawa A, Nakamura S, Suzuki S, Baba S
and Nakano M: Involvement of platelet-activating factor in cytokine
production and neutrophil activation after hepatic
ischemia-reperfusion. Hepatology. 23:1656–1663. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vardanian AJ, Busuttil RW and
Kupiec-Weglinski JW: Molecular mediators of liver ischemia and
reperfusion injury: a brief review. Mol Med. 14:337–345. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Selzner N, Rudiger H, Graf R and Clavien
PA: Protective strategies against ischemic injury of the liver.
Gastroenterology. 125:917–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao CH, Chen SL, Dong TF, et al:
Transplantation of bone marrow-derived mesenchymal stem cells after
regional hepatic irradiation ameliorates thioacetamide-induced
liver fibrosis in rats. J Surg Res. 186:408–416. 2014. View Article : Google Scholar
|
|
21
|
Wang CF, Wang ZY, Tao SF, Ding J, Sun LJ,
Li JY and Quan ZW: Preconditioning donor liver with Nodosin
perfusion lessens rat ischemia reperfusion injury via heme
oxygenase-1 upregulation. J Gastroenterol Hepatol. 27:832–840.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuchihashi S, Fondevila C and
Kupiec-Weglinski JW: Heme oxygenase system in ischemia and
reperfusion injury. Ann Transplant. 9:84–87. 2004.PubMed/NCBI
|
|
23
|
Otterbein LE, Soares MP, Yamashita K and
Bach FH: Heme oxygenase-1: unleashing the protective properties of
heme. Trends Immunol. 24:449–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu FC, Yu HP, Hwang TL and Tsai YF:
Protective effect of tropisetron on rodent hepatic injury after
trauma-hemorrhagic shock through P38 MAPK-dependent hemeoxygenase-1
expression. PLoS One. 7:e532032012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu FC, Hwang TL, Lau YT and Yu HP:
Mechanism of salutary effects of astringinin on rodent hepatic
injury following trauma-hemorrhage: Akt-dependent hemeoxygenase-1
signaling pathways. PLoS One. 6:e259072011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mandal P, Pritchard MT and Nagy LE:
Anti-inflammatory pathways and alcoholic liver disease: role of an
adiponectin/interleukin-10/heme oxygenase-1pathway. World J
Gastroenterol. 16:1330–1336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Öllinger R and Pratschke J: Role of heme
oxygenase-1 in transplantation. Transpl Int. 23:1071–1081.
2010.
|
|
28
|
Mandal P, Park PH, McMullen MR, Pratt BT
and Nagy LE: The anti-inflammatory effects of adiponectin are
mediated via a heme oxygenase-1-dependent pathway in rat Kupffer
cells. Hepatology. 51:1420–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devey L, Ferenbach D, Mohr E, Sangster K,
Bellamy CO, Hughes J and Wigmore SJ: Tissue-resident macrophages
protect the liver from ischemia reperfusion injury via a heme
oxygenase-1-dependent mechanism. Mol Ther. 17:65–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mashreghi MF, Klemz R, Knosalla IS, et al:
Inhibition of dendritic cell maturation and function is independent
of heme oxygenase 1 but requires the activation of STAT3. J
Immunol. 180:7919–7930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu SY, Li MH, Ko FC, Wu GC, Huang KL and
Chu SJ: Protective effect of hypercapnic acidosis in
ischemia-reperfusion lung injury is attributable to upregulation of
heme oxygenase-1. PLoS One. 8:e747422013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan W, Zhang H, Bai X, Lu Y, Dong H and
Xiong L: Autophagy activation is involved in neuroprotection
induced by hyperbaric oxygen preconditioning against focal cerebral
ischemia in rats. Brain Res. 1402:109–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reed M, Morris SH, Jang S, Mukherjee S,
Yue Z and Lukacs NW: Autophagy-inducing protein beclin-1 in
dendritic cells regulates CD4 T cell responses and disease severity
during respiratory syncytial virus infection. J Immunol.
191:2526–2537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen YY, Sun LQ, Wang BA, Zou XM, Mu YM
and Lu JM: Palmitate induces autophagy in pancreatic β-cells via
endoplasmic reticulum stress and its downstream JNK pathway. Int J
Mol Med. 32:1401–1406. 2013.PubMed/NCBI
|
|
35
|
Wang Y, Shen J, Xiong X, et al: Remote
ischemic preconditioning protects against liver
ischemia-reperfusion injury via heme oxygenase-1-induced autophagy.
PLoS One. 9:e988342014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi BM, Chen XY, Gao SS, Zhu R and Kim
BR: Anti-apoptotic effect of phloretin on cisplatin-induced
apoptosis in HEI-OC1 auditory cells. Pharmacol Rep. 63:708–716.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giriş M, Erbil Y, Depboylu B, Mete O,
Türkoğlu U, Abbasoğlu SD and Uysal M: Heme oxygenase-1 prevents
hyperthyroidism induced hepatic damage via an antioxidant and
antiapoptotic pathway. J Surg Res. 164:266–275. 2010.PubMed/NCBI
|
|
38
|
Kim I and Lemasters JJ: Mitophagy
selectively degrades individual damaged mitochondria after
photoirradiation. Antioxid Redox Signal. 14:1919–1928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie SQ, Zhang YH, Li Q, Xu FH, Miao JW,
Zhao J and Wang CJ: 3-Nitro-naphthalimide and nitrogen mustard
conjugate NNM-25 induces hepatocellular carcinoma apoptosis via
PARP-1/p53 pathway. Apoptosis. 17:725–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qi F, Inagaki Y, Gao B, et al: Bufalin and
cinobufagin induce apoptosis of human hepatocellular carcinoma
cells via Fas- and mitochondria-mediated pathways. Cancer Sci.
102:951–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nicholson DW: From bench to clinic with
apoptosis-based therapeutic agents. Nature. 407:810–816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scovassi AI and Poirier GG: Poly
(ADP-ribosylation) and apoptosis. Mol Cell Biochem. 199:125–137.
1999. View Article : Google Scholar : PubMed/NCBI
|