Introduction
Skin aging occurs through two independent, complex
biological processes in the human skin, specifically chronological
or intrinsic aging and photo-aging (extrinsic aging) (1). The main events involved in intrinsic
aging are enhanced matrix metalloproteinase (MMP)-1 activity and
decreased collagen production (2). The symptoms of intrinsic aging are
the wrinkling and sagging of the skin, ultimately resulting in the
loss of flexibility and elasticity (3,4).
The other type of aging is caused by environmental factors, such as
ultraviolet (UV) irradiation, gravity and smoking (5). This also plays an important role in
wrinkle formation as the synthesis of MMPs accumulates through UV
(6).
Human skin is composed of the epidermis and dermis;
the dermis is made up of not only collagen, but also elastin.
Dermal fibroblasts are produced by the extracellular matrix (ECM),
which generates collagen and elastin fiber in tissue and represents
the extracellular part of multicellular structure (7). Collagen and elastin from the dermal
ECM and have been shown to play a role in regeneration and
remodeling (8). Collagen is the
most abundant structural protein. Its degradation can be induced by
MMPs, which remodel the ECM; several MMPs are known to regulate
collagen proteolysis (9). The
MMPs are produced by several cell types, including epithelial
cells, fibroblasts, neutrophills and mast cells, as well as a
family of zinc-dependent endopeptidases that play critical roles in
inflammation, tumor invasion and skin aging (10). MMPs have been classified into
diverse groups in human skin in vivo and in vitro,
including MMP-1 (collagenases-1), MMP-3 (stromelysin 1 and
progelatinase) and MMP-9 (gelatinase B) (11,12). They are inhibited by chelating
agents and tissue inhibitors of matrix metalloproteinases (TIMPs),
which are known as endogenous inhibitors of the ECM, particularly
in the case TIMP-1 and TIMP-2 (13). Elastic fibers consist of two
morphologically and chemically distinct components, elastin and
microfibrils (1). Elastin is
major component of elastic fibers, and greatly contributes to the
elastic recoil properties of skin; it is composed of granular
amorphous elastin structures, and is also involved in the
inhibition or repair of wrinkle formation. However, elastase is a
degrading enzyme present in the dermis, and plays a role in the
degradation of the ECM, which contains elastin (14,15). For that reason, it has been
reported that it affects the degradation of collagen and elastin in
dermal fibroblasts, and that its proteolysis contributes to
decreased winkle formation.
The mycelium of Tricholoma matsutake (T.
matsutake), a high-class edible mushroom which has bioactive
components, grows throughout late autumn in pine forests, and is
widely distributed in Asian countries. It has been reported that
polysaccharide extracts from the mycelium of T. matsutake
exhibit regulatory activity, with immunomodulatory effects in
macrophages (16,17). The mycelium of T. matsutake
has also been reported to have excellent biological activities; the
extract from the natural biomaterial of this mushroom is rich in
polysaccharides, such as β-glucan. Moreover, β-glucan is presently
available as an ingredient in cosmetic formulations and is found in
extracts from mushroom, yeasts, various fungi, cereals and seaweed
(18). The extract of the
mycelium of T. matsutake also has strongly bioactive
properties, exerting antioxidant, cholesterol-lowering, heart
disease prevention, and antitumoral, anti-aging, anti-wrinkle and
anti-acne effects (16,19,20). The most promising treatments in
skin aging include herbal extracts, several vitamins and
antioxidant food supplements, which have been widely accepted to
enhance collagen and elastin production in the ECM and reduce the
levels of dermal enzymes, as MMPs and elastase, thereby restoring
skin elasticity and delaying the process of wrinkle formation.
Therefore, natural anti-wrinkle or anti-aging formulations can be
developed to reverse the effects of skin aging. In this study, we
demonstrate that treatment with extract of the mycelium of T.
matsutake inhibits 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced MMP-1 gene expression. We also investigated the
anti-aging properties of the mycelium of T. matsutake and
whether they are mediated through the expression of TIMP-1 and
tropoelastin.
Materials and methods
Materials
Antibodies against MMP-1 (ab53142), MMP-3 (ab52915),
TIMP-1 (ab61224), tropoelastin (ab21600), and collagen I (ab292)
were purchased from Abcam, Inc. (Cambridge, MA, USA). The antibody
to GAPDH (sc-20357) was obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The antibody to tropoelastin purchased
from Elastin Products Company Inc. (Owensville, MO, USA). Reagents
including thiazolyl blue tetrazolium bromide (MTT), TPA, Tris-HCl,
sodium dodecyl sulfate (SDS), β-mercaptoethanol,
phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate
(Na3VO4), sodium fluoride (NaF) and
ethylenediaminetetraacetic acid (EDTA) were purchased from
Sigma-Aldrich Co. (St. Louis, MO, USA). MMP inhibitor I (#444250)
and p38 inhibitor (SB203580) were obtained from Calbiochem (San
Diego, CA, USA). The synthetic substrate for
N-succinyl-tri-alanyl-p-nitroaniline (STANA) was purchased from the
Peptide Institute Inc. (Osaka, Japan). Phosphoramidon was obtained
from Boeringer Mannheim (Mannheim, Germany).
Preparation of extract of the mycelium of
T. matsutake
The extract of the mycelium of T. matsutake
was purchased from NovaCell Technology Inc. (Seoul, Korea). The
dried plant material (20 g) was cut into small sections and
extracted using a shaking incubator at 37°C for 24 h. The extract
was then centrifuged at 6,000 × g for 10 min and processed through
filter paper and freeze-dried to yield a powdered extract.
Cell isolation and culture of
fibroblasts
Human fibroblasts were isolated from human donors
aged 30 to 40 years, obtained during plastic surgical procedures.
Skin specimens were processed according to the methods described in
the study by Rheinwald and Green (21), and the procedure was modified by
the use of thermolysin (Sigma-Aldrich Co.). Enzyme activity was
inactivated using Dulbecco’s modified Eagle’s medium (DMEM; WelGENE
Inc., Daegu, Korea) with 10% fetal bovine serum (FBS; HyClone Inc.,
Logan, UT, USA) and centrifuged at 1,200 × g for 10 min to obtain a
pellet. The cell pellet was resuspended in medium and incubated at
room temperature, and the cellular remains were filtered through a
100 μm nylon mesh. The filtered cells were collected by
centrifugation at 1,200 × g for 10 min. The resuspended cells were
maintained in high-glucose (4.5 g/l) DMEM. Normal human fibroblasts
were cultured in DMEM, supplemented with 10% FBS, 1% penicillin
(10,000 U/ml) and 1% streptomycin (10,000 g/ml). The cultures were
maintained at 37°C in a humidified 5% CO2 incubator.
MTT assay for cell proliferation
Cell proliferation was determined using the MTT
reduction assay. To measure cell proliferation, human fibroblasts
(1×104 cells/well) seeded in 24-well plates for 24 h
were incubated in DMEM containing extract of the mycelium of T.
matsutake for 72 h at 37°C in 5% CO2. Following
serum starvation for 24 h, the cells were incubated with the test
substances for the indicated periods of time at 37°C in 5%
CO2. Subsequently, 100 μl of MTT at 5 mg/ml were added
to each well, and incubation was continued for 4 h. The
supernatants were removed and formazan crystals resulting from
mitochondrial enzymatic activity on MTT substrate were solubilized
with dimethylsulfoxide (DMSO; Sigma-Aldrich Co.). Absorbance was
measured at 540 nm using an ELISA reader (VERSAMax; Molecular
Devices, Sunnyvale, CA, USA).
Measurement of elastase activity
Elastase activity was evaluated using isolated
elastase from human fibroblasts at passage 4 and measured using the
substrate, STANA (Peptide Institute Inc.), as previously described
in the study by Tsuji et al (22). Fibroblast-derived elastase
activity was measured following treatment of the fibroblasts with
the extract of the mycelium of T. matsutake at various
concentrations (0.1–100 μg/ml) in DMSO. The cells were dissolved in
0.1 M phosphate buffer (pH 6.8) and the supernatant was then used
as the enzyme source. To measure the elastase activity, 200 μl of
enzyme solution were distributed into each well of a 96-well plate
and then pre-incubated for 15 min at 37°C with or without
inhibitors. Following the addition of 2 μl of 62.5 mM STANA, the
plates were further incubated for 1 h at 37°C. The release of
p-nitroaniline was measured by the absorbance at 405 nm, and the
enzymatic activity was expressed as units per milligram of protein.
Each assay was carried out in triplicate.
Western blot analysis
Human fibroblasts were prepared in cell lysis buffer
[62.5 mM Tris-HCl (pH 6.8), 2% SDS, 5% β-mercaptoethanol, 2 mM
phenylmethylsulfonyl fluoride, 1 mM Na3VO4,
50 mM NaF, and 10 mM EDTA and protease inhibitors (Roche
Diagnostics Inc., Indianapolis, IN, USA)]. SDS-polyacrylamide gel
electrophoresis was performed using 10 μg of protein per lane. The
gels were blotted onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA) that were then saturated with 5%
dried milk in Tris-buffered saline with 0.5% Tween-20
(Sigma-Aldrich Co.) The blots were incubated with the appropriate
primary antibodies against collagen I, MMP-1, MMP-3, TIMP-1 and
GAPDH overnight at 4°C and then further incubated with horseradish
peroxidase-conjugated secondary antibodies after washing. Bound
antibodies were detected using a Super Signal West Pico
chemiluminescence substrate (Pierce Biotechnology Inc., Rockford,
IL, USA).
Immunofluorescence staining
The cells (5×104) were seeded onto glass
coverslips that were pre-coated with poly-L-lysine (0.01%;
Sigma-Aldrich Co.) and incubated for 24 h. The cells used for
immunefluorescence staining were fixed with 4% paraformaldehyde for
30 min at room temperature. After 30 min, the cells were washed
with PBS, and then treated for 10 min in 0.01% Triton X-100
(Sigma-Aldrich Co.) The cells were blocked for 30 min with 5%
bovine serum albumin (BSA; Sigma-Aldrich Co.) and incubated
overnight at 4°C with an antibody against collagen I at 1:100 in 5%
BSA. After washing, the cells were further incubated with
FITC-labeled secondary antibody (Santa Cruz Biotechnology, Inc.)
for 2 h and then stained with 4,6-diamino-2-phenylidole (DAPI;
Pierce Biotechnology Inc.) at 1 μg/ml for 10 min. Cell morphology
was observed under a a DP70 fluorescence microscope and DP
Controller software (Olympus Optical Co., Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cultured human
fibroblasts using an RNeasy Mini kit (Qiagen Inc., Valencia, CA,
USA). Subsequently, 1 μg of RNA was reverse transcribed using
AccuPower RT-PCR Premix (Bioneer Inc., Daejeon, Korea). cDNA
obtained was amplified using a CFX96™ Real-Time System (Takara
Inc., Otsu, Japan) with the following primers: collagen I forward,
5′-TCCCCAGCCACAAAGAGTCTACA-3′ and reverse,
5′-GTGATTGGGTGGGATGTCTTCGTC-3′; tropoelastin forward,
5′-AAAGCAGCAGCAAAGTTCGG-3′ and reverse, 5′-ACCTGGGACAACTGGAATCC-3′;
MMP-1 forward, 5′-GGAGGGGATGCTCATTTTGATG-3′ and reverse,
5′-TAGGGAAGCCAAAGGAGCTGT-3′; MMP-3 forward,
5′-CCTGCTTTGTCCTTTGATGC-3′ and reverse, 5′-TGAG
TCAATCCCTGGAAAGTC-3′; TIMP-1 forward, 5′-TTCGT
GGGGACACCAGAAGTCAAC-3′ and reverse, 5′-TGGACA CTGTGCAGGCTTCAGTTC
-3′; and GAPDH forward, 5′-GAG TCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGG AGGGATCTCG-3′.
The PCR profiles were as follows: 94°C for 5 min, 30
cycles for 1 min at 94°C, 1 min at 55–60°C, and 1 min at 72°C, with
a final extension step of 72°C for 10 min. The resulting PCR
products were visualized by electrophoretic separation on 1.5%
agarose gels containing 1 μg ethidium bromide/ml. The gel samples
were prepared by mixing 20 μl of reaction mixture with loading
buffer and were then separated by electrophoresis for 20 min at 100
V before being visualized with a ChemiDoc XRS system (Bio-Rad
Laboratories Inc., Hercules, CA, USA). Each band was
densitometrically quantified by image analysis and controlled vs.
GAPDH intensity.
Statistical analysis
One-way ANOVA followed by Dunnett’s T3 test was used
to assess statistical significance with thresholds of P<0.05,
P<0.01 and P<0.001 indicating significant and highly
significant differences, respectively.
Results
Extract of the mycelium of T. matsutake
does not alter the expression of type I collagen in human
fibroblasts
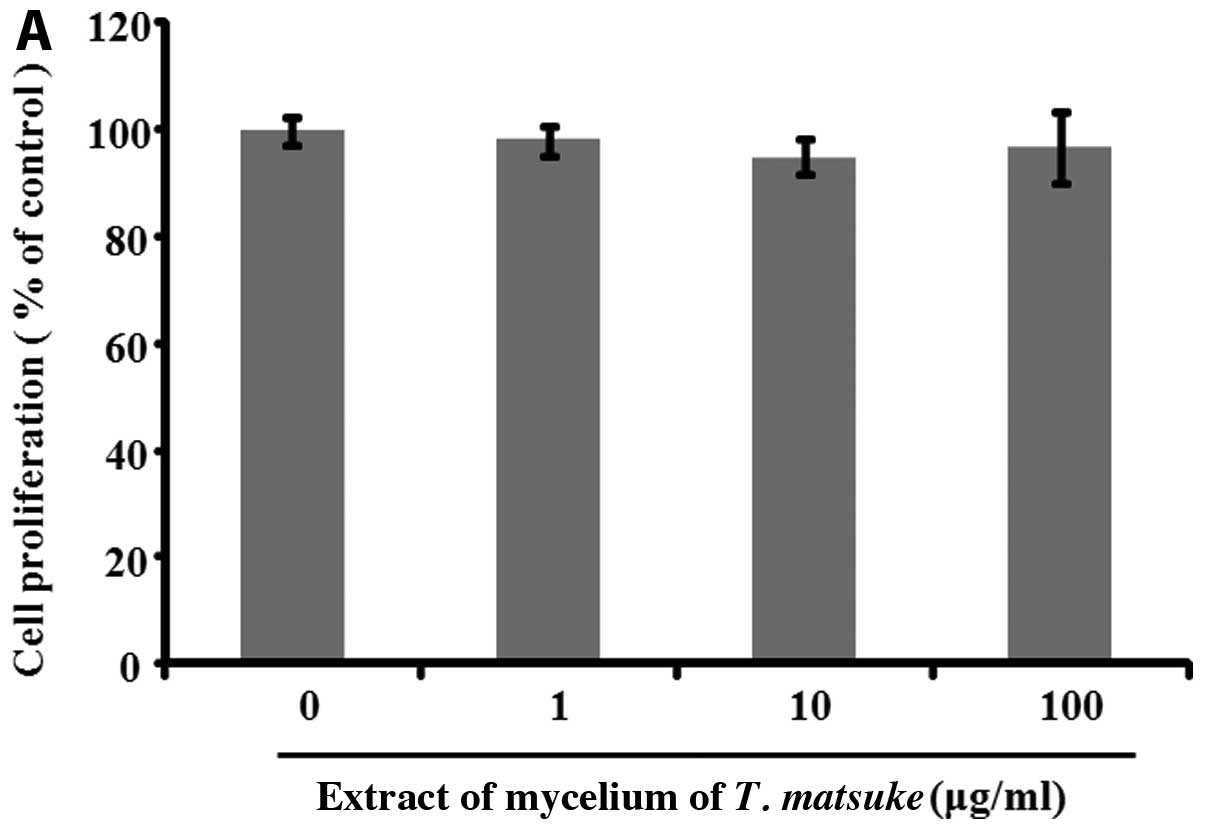
To determine whether the extract of the mycelium of
T. matsutaket exerts a proliferative effect on human
fibroblasts, we treated human fibroblasts with extract of the
mycelium of T. matsutake at concentrations of 1–100 μg/ml
for 72 h. Cell proliferation was determined by MTT assay; the
results revealed that the extract of the mycelium of T.
matsutake did not increase cell proliferation (Fig. 1A). Collagens are necessary
proteins found abundantly in the dermis and are normally produced
by fibroblasts. Thus, we investigated the expression of collagen I
in fibroblasts treated with extract of the mycelium of T.
matsutake. As shown in Fig.
1B, the mRNA and protein expression of collagen I remained
unaltered following treatment with various doses of the extract of
the mycelium of T. matsutake treatment. This result suggests
that the mycelium of T. matsutake does not have the
potential to promote the synthesis of collagen I.
Extract of the mycelium of T. matsutake
decreases the mRNA and protein expression of MMP-1 and MMP-3 in
human fibroblasts
We analyzed the expression of MMPs in fibroblasts
treated with extract of the mycelium of T. matsutake, and
found that it decreased the mRNA and protein expression of MMP-3 in
the cells treated with 100 μg/ml of the extract. Furthermore, we
found that treatment with extract of the mycelium of T.
matsutake clearly decreased the mRNA and protein expression of
MMP-1 in a dose-independent manner (Fig. 1C).
Extract of the mycelium of T. matsutake
increases the expression of TIMP-1 and tropoelastin in human
fibroblasts
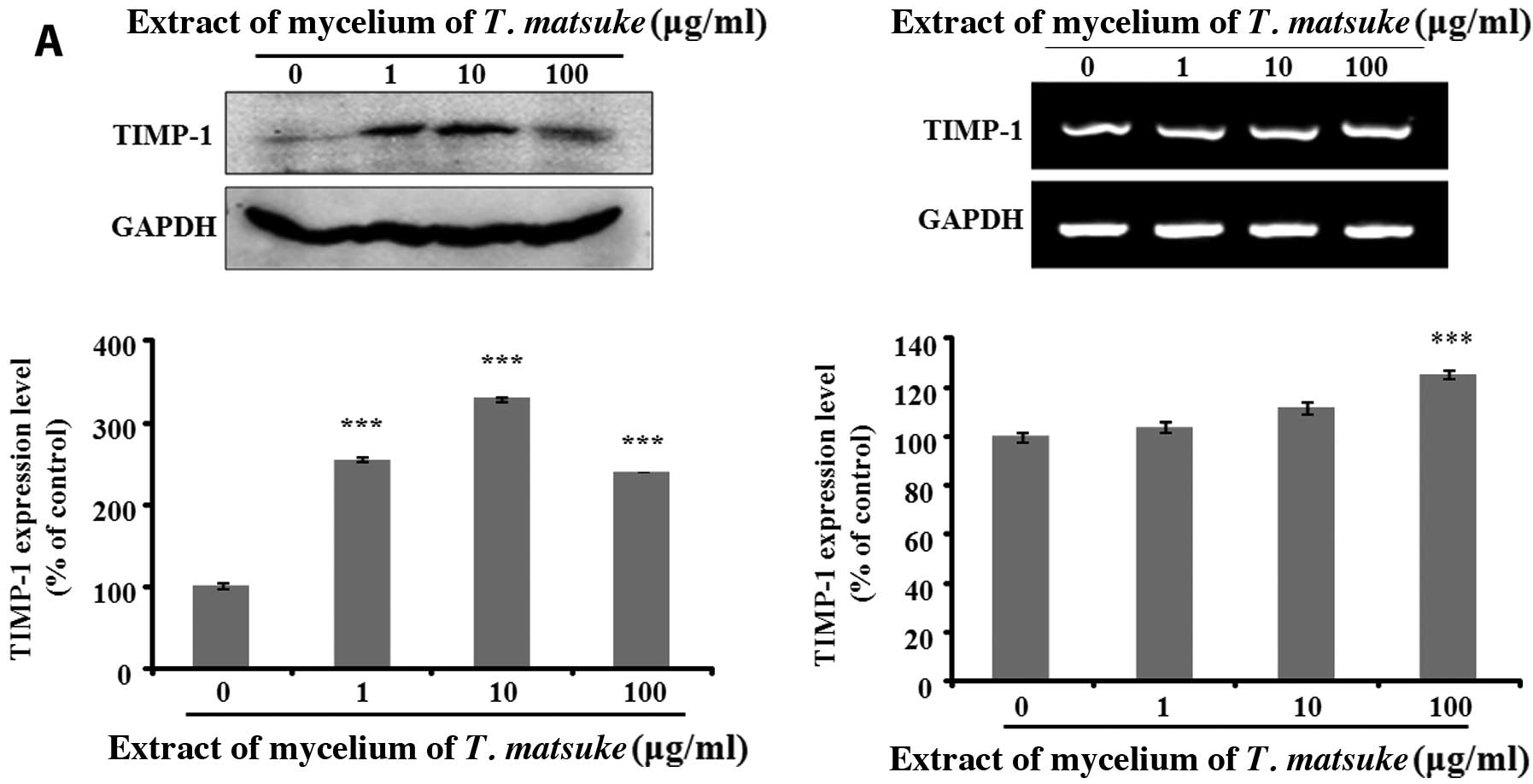
Our results demonstrated that the extract of the
mycelium of T. matsutake decreased the expression of MMP-1.
To evaluate the anti-wrinkle effects of the mycelium of T.
matsutake in human fibroblasts, we determined the protein
expression of TIMP-1 in the fibroblasts treated with various
concentrations of extract of the mycelium of T. matsutake.
TIMPs are known as inhibitors of MMP and are produced by
fibroblasts. We found that the expression of TIMP-1 increased in
the fibroblasts treated with extract of the mycelium of T.
matsutake; the increase in the expression of TIMP-1 was clearly
evident at the concentration of 1 μg/ml of the extract (Fig. 2A). In addition, we evaluated the
effects of the extract of the mycelium of T. matsutake on
the mRNA expression of TIMP-1 in human fibroblasts. As shown in
Fig. 2A, extract of the mycelium
of T. matsutake markedly increased the mRNA expression of
TIMP-1 in the cells treated with 100 μg/ml of the extract.
We then examined the expression of tropoelastin in
fibroblasts treated with the extract of the mycelium of T.
matsutake. Tropoelastin is a soluble precursor of elastin. To
quantitatively assess tropoelastin expression, tropoelastin mRNA
expression was analyzed by RT-PCR. The results revealed that the
extract of the mycelium of T. matsutake increased the mRNA
expression of tropoelstin compared to the untreated cells (Fig. 2B). To verify our results, we
evaluated tropoelastin expression by immunofluorescence staining.
We found that the expression of tropoelastin was increased in the
cells treated with extract of the mycelium of T. matsutake
compared to the untreated cells (Fig.
2C). Thus, these results support our observation of the
increased expression of TIMP-1 and tropoelastin in fibroblasts
treated with extract of the mycelium of T. matsutake; the
increase in the expression of TIMP-1 was mediated by the
downregulation in the expression of MMPs in fibroblasts.
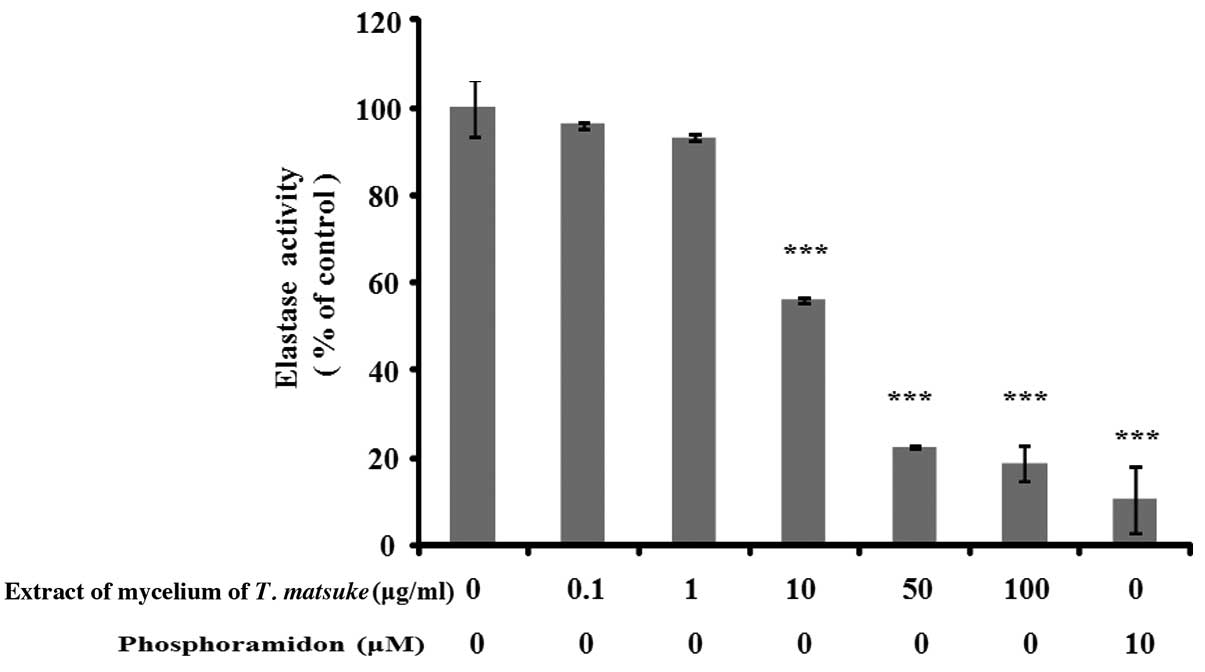
Extract of the mycelium of T. matsutake
inhibits elastase activity in human fibroblasts
Fibroblast elastase is a metalloproteinase that has
been reported to play a role in the degradation of elastin
(23). Elastase is inhibited by
metal chelating agents, such as EDTA, as well as
1,10-phenanthroline and phosphoramidon as a metalloproteinase
inhibitor (22). To determine
whether the extract of the mycelium of T. matsutake exerts
an inhibitory effect on human fibroblast-derived elastase, we
treated the cells with various concentrations (0.1–100 μg/ml) of
extract of the mycelium of T. matsutake. We determined
elastase activity in human fibroblasts using STANA as a substrate
and phosphoramidon. As a result, the extract of the mycelium of
T. matsutake exerted an inhibitory effect on human
fibroblast-derived elastase in a dose-dependent manner; the most
potent inhibitory effects of the extract of the mycelium of T.
matsutake (81.4±3.92%) were observed at a concentration of 100
μg/ml, and those of phophoramidon (positive control, 89.6±7.74%) at
a concentration of 10 μM (Fig.
3). These results indicate that the mycelium of T.
matsutake prevented the degradation of elastin by inhibiting
elastase activity.
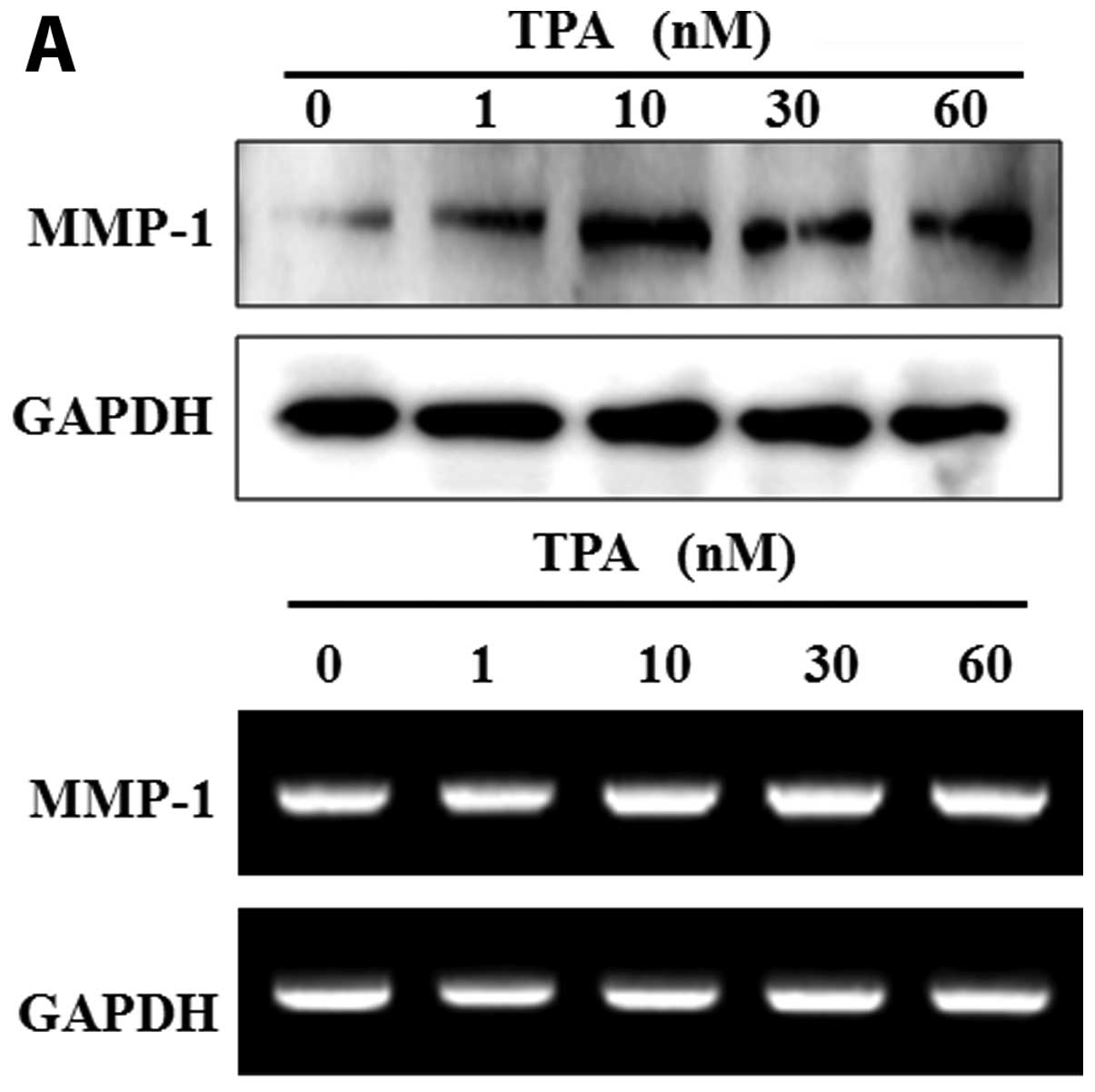
Treatment with extract of the mycelium of
T. matsutake inhibits TPA-induced MMP-1 expression in human
fibroblasts
To confirm the inhibitory effects of the extract of
the mycelium of T. matsutake (100 μg/ml) on MMP-1 expression
in human fibroblasts, we examined the expression of MMP-1 in
fibroblasts treated with TPA prior to treatment with extract of the
mycelium of T. matsutake. TPA induced an increase in the
mRNA and protein expression of MMP-1 in a dose-dependent manner
(Fig. 4A). The increase in the
mRNA and protein expression of MMP-1 induced by TPA (60 nM) was
abrogated by MMP inhibitor I (decrease of 39.7% compared to the
level observed with 60 nM TPA). In addition, treatment with extract
of the mycelium of T. matsutake (100 μg/ml) significantly
decreased MMP-1 protein expression by 26.5% compared to the level
observed with 60 nM TPA (Fig.
4B). As shown in Fig. 4C,
treatment with extract of the mycelium of T. matsutake (100
μg/ml) inhibited TPA-induced MMP-1 mRNA expression in human
fibroblasts by 17.2% compared to the level observed with 60 nM
TPA.
Induction of MMP-1 expression by TPA is
mediated through p38, and is inhibited by extract of the mycelium
of T. matsutake
Previous studies have elucidated the roles of
distinct mitogen-activated protein kinase (MAPK) pathways in the
regulation of MMP-1 (24). In
addition, it has been reported that the induction of MMP-1
expression by okadaic acid requires the simultaneous activation of
the extracellular signal-regulated kinase (ERK)1/2, Jun N-terminal
kinase (JNK) and/or p38 pathways (24,25). To determine the possible signaling
pathway through which TPA increases the expression of MMP-1, we
examined whether the induction of MMP-1 expression by TPA occurs
through the phosphorylation of p38 (p-p38). The fibroblasts were
treated with TPA alone or in combination with p38 inhibitor or
extract of the mycelium of T. matsutake, and the levels of
p-p38 were determined by western blot analysis. The expression of
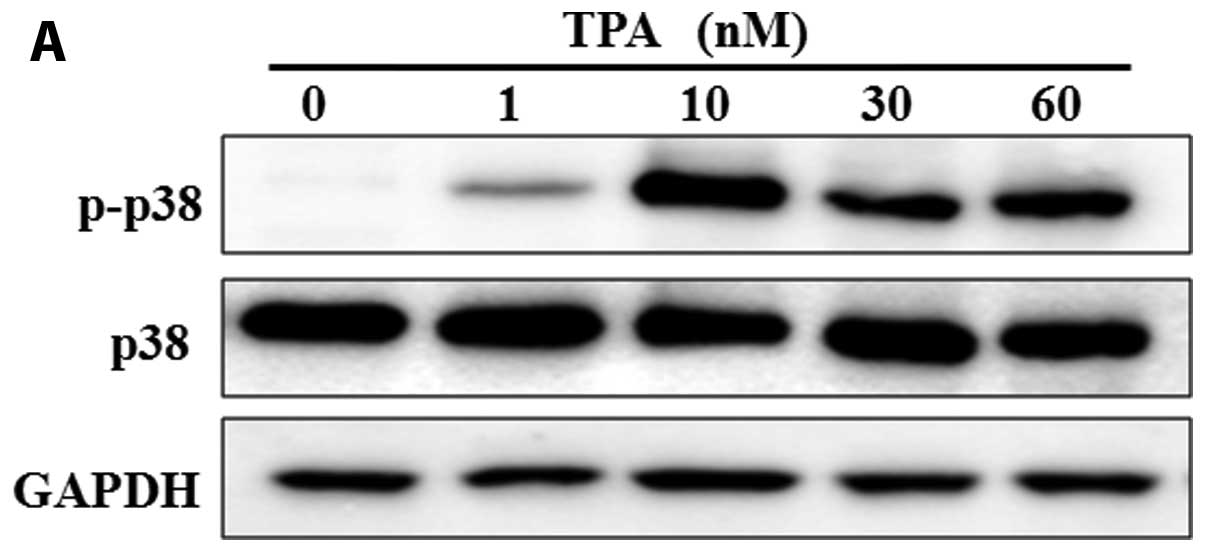
p-p38 protein was increased in the TPA-treated cells (Fig. 5A). We evaluated the role of p38 in
the TPA-induced expression of MMP-1 in human fibroblasts by
blocking p-p38 using inhibitors. It has been reported that the
upregulation of MMP-1 expression by p38 is activated by diverse
stimuli (26). As shown in
Fig. 5B, the increase in the
expression of p-p38 induced by TPA (60 nM) was inhibited by
SB203580 (SB), a specific inhibitor of p38. Of note, the extract of
the mycelium of T. matsutake had an inhibitory effect on
p-p38 expression induced by TPA. In parallel, the expression of
p-p38 induced by TPA was potently inhibited by pre-treatment with
extract of the mycelium of T. matsutake in combination wtih
SB, indicating that the activation of p-p38 may have equal effects
on MMP-1 expression (Fig. 5C). In
addition, the induction of MMP-1 expression by TPA was potently
inhibited by pre-treatment with extract of the mycelium of T.
matsutake in combination with SB, whereas the expression of
TIMP-1 which was inhibited by TPA was restored by treatment with
the extract (Fig. 5C). These
results demonstrated that SB and the extract of the mycelium of
T. matsutake decreased the efficacy of TPA in increasing
MMP-1 expression. This finding suggests that the TPA-induced
expression of MMP-1 may be regulated through the p38 pathway, and
that the mycelium of T. matsutake has an abrogating effect
on p38 activation induced by TPA.
Discussion
The extract of the mycelium of T. matsutake,
a biopharmaceutical polysaccharide component, has a wide range of
medicinal effects, including reduced oxidation, inhibition of
inflammation and anticancer properties (16,19,27,28). However, the anti-aging effects of
the mycelium of T. matsutake in human fibroblasts are not
yet well known, and to the best of our knowledge, its effects on
MMP-1 and TIMP-1 expression have not been reported to date. In this
study, we investigated the potent wrinkle inhibitory effects of the
extract of the mycelium of T. matsutake and its effects on
the basal and TPA-induced expression of MMP-1 in human fibroblasts.
We demonstrated that the extract of the mycelium of T.
matsutake is an anti-wrinkle agent that acts by inhibiting the
basal and TPA-induced expression of MMP-1 and increasing the
expression of TIMP-1 and tropoelastin.
The remodeling of the extracellular matrix is
essential for processes, such as skin aging, wound healing and
fibrosis. Collagen and elastic fibers in the ECM network are
related to skin aging symptoms, such as wrinkles, sagging and
looseness. The degradation of collagen and elastin fibers in the
skin is mainly caused by the expression of MMP-1, MMP-3 and
elastase (29,30). MMP activity is regulated by TIMPs.
TIMPs are naturally produced proteins that are important regulators
of ECM turnover (30,31). The ratio between MMPs and TIMPs
plays an essential role leading to ECM remodeling. An imbalance in
the MMP/TIMP ratio has been shown to be involved in various
diseases in humans (13,29). In this study, treatment of
fibroblasts with extract of the mycelium of T. matsutake
suppressed the basal levels of MMP-1 and MMP-3 expression (Fig. 1C). MMP-1 expression decreased
significantly by 10±3.21, 23±0.68 and 39±0.46% in relation to the
controls (untreated cells) following treatment with extract of the
mycelium of T. matsutake at doses of 1, 10 and 100 μg/ml,
respectively. On the other hand, the basal level of TIMP-1
expression increased in a dose-dependent manner following treatment
with the extract (Fig. 2A). The
basal level of TIMP-1 expression significantly increased by
154±2.42, 218±2.66 and 139±0.41% compared to the controls following
treatment with extract of the mycelium of T. matsutake at
doses of 1, 10 and 100 μg/ml, respectively. The effects of the
extract of the mycelium of T. matsutake occurred in a
dose-dependent manner and were not cytotoxic. The progression of
skin aging caused by harmful agents in the environment is
accompanied by both inflammatory reactions and oxidative damage
(16,32). Previously, the extract of the
mycelium of T. matsutake was shown to exert antioxidant
effects, including an antioxidant component that enhances skin
resistance to external factors and inhibits skin oxidation
(33). In our study, we
demonstrated that the decrease in MMP-1 expression and the increase
in TIMP-1 expression may be mediated by the anti-inflammatory and
antioxidant effects of the mycelium of T. matsutake in human
fibroblasts.
The general age-associated characteristic in both
internal and external processes is the loss of normal elastic fiber
functions through the degradation of elastin, as exhibited in the
wrinkling and sagging of the skin (3,4).
The proteolytic degradation of elastin results in the formation of
elastase as MMP-1 is the endogenous inhibitor present in the
dermis. It has been reported that skin fibroblast elastase is
involved in the metabolism of elastic fiber during aging (6,34).
Enhanced skin fibroblast elastase has been demonstrated to cause
the loss of skin elasticity and is the primary component of
phosphoramidon, which is known to be a typical elastase inhibitor,
and significantly inhibits fibroblast elastase activity by more
than 89.6% (35). However,
phosphoramidon exhibits poor penetration through the skin,
including the hydrophilic rhamnose residue. Researchers have
focused on overcoming these issues, and have investigated
N-phenetylphosphonyl-leucyl-tryptophane (NPLT), which has similar
potency to the inhibitory effects of phosphoramidon (22,36). On the other hand, tropoelastin, a
soluble elastin molecule and major structural component of
microfibrils, is produced by fibroblasts and is a protein of the
ECM (2). The proteolysis of
microfibrils is caused by degradation emzymes, such as elastases
and MMPs (10). It has previously
been reported that ellagic acid and tannic acid, which are known
polyphenols, may be useful in preventing the proteolytic
degradation of dermal elastic fibers and in enhancing the
expression of tropoelastin (37).
We hypothesized that the extract of the mycelium of T.
matsutake would have an effect on cellular elastase activity
and used it as an inhibitor of fibroblast elastase to examine this
possibility. In addition, we investigated the expression of
tropoelastin in fibroblasts treated with extract of the mycelium of
T. matsutake. Our results revealed that the extract of the
mycelium of T. matsutake significantly decreased elastase
activity in a dose-dependent manner. Moreover, as shown in Fig. 2C, treatment with extract of the
mycelium of T. matsutake increased tropoelastin expression.
It is clear that the elastase inhibitory effects of extract of the
mycelium of T. matsutake in fibroblasts are more potent than
those of phosphoramidone. This suggests that the extract of the
mycelium of T. matsutake may be a reliable anti-aging agent
for use in the cosmetics industry.
Increased MMP-1 levels have been shown to be
involved in the metastasis of several types of tumor (38); moreover, they facilitate skin
wrinkling following exposure to UV irradiation (39). It has also been reported that
MMP-1 expression is increased by several stimuli, such as
cytokines, growth factors, tumor-promoting agents and UV
irradiation (40). TPA, a
well-known tumor-promoting agent, is a protein kinase C (PKC)
activator and can increase MMP-1 production in various types of
cells through the activation of PKC-dependent and/or -independent
signaling pathways (41–43). The molecular mechanisms
responsible for TPA-induced MMP-1 expression involve the
stress-activated MAPK pathway, as well as c-Jun N-terminal
kinase/stress-activated protein kinase (JNK/SAPK) and p38 (44). In this study, to investigate the
correlation between p38 inhibition and MMP-1 reduction, fibroblasts
were treated with SB203580 (a p38 inhibitor) prior to treatment
with extract of the mycelium of T. matsutake or TPA and the
expression of p-p38 was evaluated by western blot analysis. Our
results revealed that the level p-p38 was increased in the
TPA-treated fibroblasts (Fig.
5A). Our results also demonstrated that pre-treatment with
SB203580 and extract of the mycelium of T. matsutake alone
or in combination inhibited the activation of p38 and decreased
TPA-induced MMP-1 expression levels (Fig. 5C). However, the expression of
TIMP-1 was increased under the same conditions (Fig. 5C): the inhibition of p38 and the
suppression of MMP-1 expression in the human fibroblasts was
observed concurrently, indicating a possible mechanism of action of
the extract of the mycelium of T. matsutake.
The increased expression of MMP-1 has been shown to
correlate with several other factors, such as the ERK1/2, JNK,
Raf-1, MKK3, AP-1 and ETS transcription factors (45,46). Previous studies have found that
these pathways are involved in the regulation of MMP-1 expression
in fibroblasts (45,47). However, we did not examine the
correlation between MMP-1 expression and phosphorylated PKC levels,
phosphorylated ERK levels or phosphorylated JNK levels following
treatment with extract of the mycelium of T. matsutake and
specific inhibitors, such as staurosporine (PKC inhibitor), PD98059
(MEK inhibitor), or SP600125 (JNK inhibitor). Therefore, to fully
elucidate the role of these pathways in the inhibition of the
TPA-induced production of MMP-1 by the extract of the mycelium of
T. matsutake in fibroblasts, further studies are
required.
In conclusion, our results suggest that the
treatment of human fibroblasts with extract of the mycelium of
T. matsutake decreases the basal levels and the TPA-induced
MMP-1 expression in a dose-dependent manner. In addition, extract
of the mycelium of T. matsutake decreased elastase activity
in a dose-dependent manner. These data suggest that extract of the
mycelium of T. matsutake may be used as an effective
biomaterial in-aging treatments that can obstruct the degradation
of the dermal ECM by inhibiting elastase activity and MMP-1
expression.
Acknowledgements
This study was supported by Biomedical Science
Scholarship Grants, Department of Medicine, Chung-Ang University in
2012.
References
|
1
|
Naylor EC, Watson RE and Sherratt MJ:
Molecular aspects of skin ageing. Maturitas. 69:249–256. 2011.
View Article : Google Scholar
|
|
2
|
Jenkins G: Molecular mechanisms of skin
ageing. Mech Ageing Dev. 123:801–810. 2002. View Article : Google Scholar
|
|
3
|
Iida I and Noro K: An analysis of the
reduction of elasticity on the ageing of human skin and the
recovering effect of a facial massage. Ergonomics. 38:1921–1931.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seite S, Zucchi H, Septier D,
Igondjo-Tchen S, Senni K and Godeau G: Elastin changes during
chronological and photo-ageing: the important role of lysozyme. J
Eur Acad Dermatol Venereol. 20:980–987. 2006.PubMed/NCBI
|
|
5
|
Mukherjee PK, Maity N, Nema NK and Sarkar
BK: Bioactive compounds from natural resources against skin aging.
Phytomedicine. 19:64–73. 2011.PubMed/NCBI
|
|
6
|
Imokawa G, Takema Y, Yorimoto Y, Tsukahara
K, Kawai M and Imayama S: Degree of ultraviolet-induced tortuosity
of elastic fibers in rat skin is age dependent. J Invest Dermatol.
105:254–258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teti A: Regulation of cellular functions
by extracellular matrix. J Am Soc Nephrol. 2:S83–S87.
1992.PubMed/NCBI
|
|
8
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enjoji M, Kotoh K, Iwamoto H, Nakamuta M
and Nawata H: Self-regulation of type I collagen degradation by
collagen-induced production of matrix metalloproteinase-1 on
cholangiocarcinoma and hepatocellular carcinoma cells. In Vitro
Cell Dev Biol Anim. 36:71–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Philips N and Devaney J: Beneficial
regulation of type I collagen and matrixmetalloproteinase-1
expression by estrogen, progesterone, and its combination in skin
fibroblasts. J Am Aging Assoc. 26:59–62. 2003.PubMed/NCBI
|
|
11
|
Fisher GJ, Datta SC, Talwar HS, et al:
Molecular basis of sun-induced premature skin ageing and retinoid
antagonism. Nature. 379:335–339. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rittie L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): an ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shapiro SD, Endicott SK, Province MA,
Pierce JA and Campbell EJ: Marked longevity of human lung
parenchymal elastic fibers deduced from prevalence of D-aspartate
and nuclear weapons-related radiocarbon. J Clin Invest.
87:1828–1834. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mecham RP, Broekelmann TJ, Fliszar CJ,
Shapiro SD, Welgus HG and Senior RM: Elastin degradation by matrix
metalloproteinases. Cleavage site specificity and mechanisms of
elastolysis. J Biol Chem. 272:18071–18076. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JY, Byeon SE, Lee YG, et al:
Immunostimulatory activities of polysaccharides from liquid culture
of pine-mushroom Tricholoma matsutake. J Microbiol
Biotechnol. 18:95–103. 2008.PubMed/NCBI
|
|
17
|
Ebina T: Activation of antitumor immunity
by intratumor injection of biological preparations. Gan To Kagaku
Ryoho. 30:1555–1558. 2003.(In Japanese).
|
|
18
|
Hoshi H, Yagi Y, Iijima H, Matsunaga K,
Ishihara Y and Yasuhara T: Isolation and characterization of a
novel immunomodulatory alpha-glucan-protein complex from the
mycelium of Tricholoma matsutake in basidiomycetes. J Agric
Food Chem. 53:8948–8956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi JJ, Jin M, Lee JK, et al: Control of
cytokine gene expression by PG101, a water-soluble extract prepared
from Lentinus lepideus. Biochem Biophys Res Commun.
339:880–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI
|
|
21
|
Rheinwald JG and Green H: Serial
cultivation of strains of human epidermal keratinocytes: the
formation of keratinizing colonies from single cells. Cell.
6:331–343. 1975. View Article : Google Scholar
|
|
22
|
Tsuji N, Moriwaki S, Suzuki Y, Takema Y
and Imokawa G: The role of elastases secreted by fibroblasts in
wrinkle formation: implication through selective inhibition of
elastase activity. Photochem Photobiol. 74:283–290. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonnaure-Mallet M, Godeau G, Tixier JM, et
al: Elastin derived peptides protect elastic fibres degradation by
human neutrophil elastase: in vitro and in vivo studies using a
mechanically induced rat gingival inflammatory model. J Periodontal
Res. 30:58–65. 1995. View Article : Google Scholar
|
|
24
|
Westermarck J, Holmstrom T, Ahonen M,
Eriksson JE and Kahari VM: Enhancement of fibroblast collagenase-1
(MMP-1) gene expression by tumor promoter okadaic acid is mediated
by stress-activated protein kinases Jun N-terminal kinase and p38.
Matrix Biol. 17:547–557. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reunanen N, Westermarck J, Hakkinen L, et
al: Enhancement of fibroblast collagenase (matrix
metalloproteinase-1) gene expression by ceramide is mediated by
extracellular signal-regulated and stress-activated protein kinase
pathways. J Biol Chem. 273:5137–5145. 1998. View Article : Google Scholar
|
|
26
|
Lim M, Martinez T, Jablons D, et al:
Tumor-derived EMMPRIN (extracellular matrix metalloproteinase
inducer) stimulates collagenase transcription through MAPK p38.
FEBS Lett. 441:88–92. 1998. View Article : Google Scholar
|
|
27
|
Kim GY, Lee JY, Lee JO, et al: Partial
characterization and immunostimulatory effect of a novel
polysaccharide-protein complex extracted from Phellinus
linteus. Biosci Biotechnol Biochem. 70:1218–1226. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou Y, Ding X, Hou W, et al:
Anti-microorganism, anti-tumor, and immune activities of a novel
polysaccharide isolated from Tricholoma matsutake.
Pharmacogn Mag. 9:244–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amalinei C, Caruntu ID and Balan RA:
Biology of metalloproteinases. Rom J Morphol Embryol. 48:323–334.
2007.PubMed/NCBI
|
|
30
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsukahara K, Moriwaki S, Hotta M, et al:
The effect of sunscreen on skin elastase activity induced by
ultraviolet-A irradiation. Biol Pharm Bull. 28:2302–2307. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volman JJ, Helsper JP, Wei S, et al:
Effects of mushroom-derived beta-glucan-rich polysaccharide
extracts on nitric oxide production by bone marrow-derived
macrophages and nuclear factor-kappaB transactivation in Caco-2
reporter cells: can effects be explained by structure? Mol Nutr
Food Res. 54:268–276. 2010. View Article : Google Scholar
|
|
33
|
Tong H, Liu X, Tian D and Sun X:
Purification, chemical characterization and radical scavenging
activities of alkali-extracted polysaccharide fractions isolated
from the fruit bodies of Tricholoma matsutake. World J
Microbiol Biotechnol. 29:775–780. 2013. View Article : Google Scholar
|
|
34
|
Godeau G and Hornebeck W: Morphometric
analysis of the degradation of human skin elastic fibres by human
leukocyte elastase (EC 3-4-21-37) and human skin fibroblast
elastase (EC 3-4-24). Pathol Biol (Paris). 36:1133–1138.
1988.PubMed/NCBI
|
|
35
|
Thorsett ED, Harris EE, Peterson ER, et
al: Phosphorus-containing inhibitors of angiotensin-converting
enzyme. Proc Natl Acad Sci USA. 79:2176–2180. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsukahara K, Takema Y, Moriwaki S, et al:
Selective inhibition of skin fibroblast elastase elicits a
concentration-dependent prevention of ultraviolet B-induced wrinkle
formation. J Invest Dermatol. 117:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jimenez F, Mitts TF, Liu K, Wang Y and
Hinek A: Ellagic and tannic acids protect newly synthesized elastic
fibers from premature enzymatic degradation in dermal fibroblast
cultures. J Invest Dermatol. 126:1272–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verma AK, Pong RC and Erickson D:
Involvement of protein kinase C activation in ornithine
decarboxylase gene expression in primary culture of newborn mouse
epidermal cells and in skin tumor promotion by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 46:6149–6155.
1986.
|
|
42
|
Fagot D, Asselineau D and Bernerd F:
Direct role of human dermal fibroblasts and indirect participation
of epidermal keratinocytes in MMP-1 production after UV-B
irradiation. Arch Dermatol Res. 293:576–583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang TS, Duyster J and Wang JY:
Biological response to phorbol ester determined by alternative G1
pathways. Proc Natl Acad Sci USA. 92:4793–4797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simon C, Simon M, Vucelic G, et al: The
p38 SAPK pathway regulates the expression of the MMP-9 collagenase
via AP-1-dependent promoter activation. Exp Cell Res. 271:344–355.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Westermarck J, Li SP, Kallunki T, Han J
and Kahari VM: p38 mitogen-activated protein kinase-dependent
activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2
activity and collagenase 1 (MMP-1) gene expression. Mol Cell Biol.
21:2373–2383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Westermarck J, Seth A and Kahari VM:
Differential regulation of interstitial collagenase (MMP-1) gene
expression by ETS transcription factors. Oncogene. 14:2651–2660.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Millward TA, Zolnierowicz S and Hemmings
BA: Regulation of protein kinase cascades by protein phosphatase
2A. Trends Biochem Sci. 24:186–191. 1999. View Article : Google Scholar : PubMed/NCBI
|