Introduction
Human mesenchymal stem cells (MSCs) are widely used
adult stem cells which have self-renewal potential and multipotent
cell differentiation capacity. In particular, human bone
marrow-derived mesenchymal stem cells (hBM-MSCs) have been used
experimentally in a number of cell therapies and in regenerative
medicine due to their abundant and non-tumorigenic properties
(1). hBM-MSCs have the ability to
differentiate into various types of cells, such as fibroblasts,
chondrocytes, adipocytes and osteoblasts (2). In addition, hBM-MSCs play an
essential role in the repair of bone (3).
Previous studies have indicated that bone
regeneration occurs following an electrical stimulation and leads
to bone development (4,5). Based on this knowledge, the effect
of electromagnetic fields (EMFs) on osteogenesis has been
investigated. Recently, it has been reported that an extremely low
frequency of EMF stimulation plays an essential role in the
osteogenic differentiation and proliferation of hBM-MSCs. Tsai
et al (6) demonstrated
that exposure to an extremely low frequency of EMFs (7.5 Hz) played
a modulatory role in the osteogenic differentiation of hBM-MSCs,
with an increase in alkaline phosphatase (ALP) levels. Sun et
al (7) demonstrated that a
frequency of 15 Hz EMFs increased the expression of
osteogenesis-related genes in hBM-MSCs.
Nanomagnetic particles (MPs) have been widely used
in biomedicine. MPs may be applied to apply drug delivery,
biological labels and the detection of proteins due to their
controllable size and magnetic properties (8). Cartmell et al (9) reported that the mechanical
stimulation of primary human osteoblasts by magnetic particle
technology affected osteoblastic activity. In the present study,
iron oxide (Fe3O4) MPs were encapsulated with
a polyethylene glycol (PEG)-phospholipid shell for enhanced
biocompatibility.
In the present study, we investigated the effects of
EMF exposure and Fe3O4 MP treatment on the
osteogenic differentiation of hBM-MSCs. hBM-MSCs were treated with
50 μg/ml of Fe3O4 MPs or exposed to a
frequency of 45 Hz at an intensity of 1 mT EMF twice every 8 h per
day for 7 days. We also examined whether treatment with
Fe3O4 MPs in conjunction with exposure to
EMFs is more effective in enhancing osteogenic differentiation. The
osteogenic differentiation was analyzed by immunohistochemical
staining, western blot analysis, quantitative reverse
transcription-polymerase chain reaction (RT-qPCR) and by measuring
ALP activity. Lactate dehydrogenase (LDH) activity in the 4
experimental groups was similar; this suggests that treatment did
not affect LDH secretion and did not induce damage to the cell
membrane.
Materials and methods
Cell culture
hBM-MSCs were purchased from Lonza (Basel,
Switzerland) and maintained in culture in Dulbecco’s modified
Eagle’s medium (DMEM; Welgene, Daejeon, Korea) supplemented with
10% fetal bovine serum (FBS; Lonza), 1% penicillin/streptomycin
(PS; Welgene), 25 μM L-ascorbic acid 2-phosphate (Sigma-Aldrich,
St. Louis, MO, USA) in a 37°C incubator in a humidified atmosphere
of 5% CO2. The hBM-MSCs were used from passages 4 to 7
with similar results obtained throughout.
Osteogenic differentiation of
hBM-MSCs
Osteogenic differentiation medium consisted of DMEM
(Welgene) supplemented with 10% fetal bovine serum (FBS; Lonza), 1%
penicillin/streptomycin (PS; Welgene), 10 mM β-glycerophosphate
(Sigma-Aldrich), 50 μM L-ascorbic acid 2-phosphate (Sigma-Aldrich)
and 100 nM dexamethasone (Sigma-Aldrich). The medium was changed
every 2–3 days.
Exposure to EMFs
A schematic representation of the sinusoidal EMF
device is presented in Fig. 1.
The EMF device was placed in a 37°C incubator in a humidified
atmosphere of 5% CO2.
The stimulation unit is designed to handle a pair of
identical coils of 30 cm in diameter assembled in a Helmholtz
configuration. The pair of coils operates on alternating current,
generating an EMF. The current in the coil is controlled by a
generator. The applied magnetic field consisted of 45 Hz frequency
and an intensity of 1 mT twice every 8 h per day for 7 days. The
cells were seeded into a culture plate or dish.
Preparation and characterization of
Fe3O4 MPs
Water- dispersible and biocompatible
Fe3O4 MPs were prepared using a method
previously described with some modifications (10). The monodispersed
Fe3O4 MPs were dispersed in a non-polar
organic solvent and synthesized using a high-temperature organic
solution phase reaction. Iron (III) acetylacetonate (Fe (acac)3, 2
mmol; 99.9%), 1,2-hexadecanediol (10 mmol; 90%), oleic acid (6
mmol; 99%), oleylamine (6 mmol; 70%) and 1-octadecene (20 ml; 90%)
(all from Sigma-Aldrich) were mixed and magnetically stirred under
a nitrogen atmosphere. The mixture was heated to 200°C for 2 h and
then heated to reflux (~300°C) for an additional hour. The
black-colored mixture was cooled down to room temperature. Ethanol
(40 ml) was added to the mixture under ambient conditions, and a
black material was precipitated and separated via centrifugation
(12,000 rpm, 30 min). The black product was redispersed in hexane
in the presence of oleic acid (~0.05 ml) and oleylamine (~0.05 ml).
Centrifugation (6,000 rpm, 10 min) was applied to remove any
undispersed residue. The product was then precipitated with
ethanol, centrifuged (10,000 rpm, 20 min) to remove the solvent and
redispersed in organic solvents, such as n-hexane and chloroform.
The resulting Fe3O4 MPs dispersed in
chloroform were encapsulated with a PEG-phospholipid shell to make
them biocompatible. Typically, 2 ml of the organic dispersible 12
nm sized Fe3O4 MPs in chloroform (5 mg/ml)
was mixed with 1 ml of chloroform solution containing 10 mg
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(PEG)-2000]
(mPEG-2000 PE) (Avanti Polar Lipids, Inc.) at a ratio of 5:1. After
complete evaporation of the chloroform, the residue was incubated
at 80°C in a vacuum for 1 h. Five milliliters of water were added,
which produced a clear and dark-brown suspension containing PEG-PE
micelles. As this suspension contained both empty micelles and
micelles containing MPs, the empty micelles were removed by
ultracentrifugation. The micelles containing MPs formed a pellet,
whereas the empty micelles remained suspended. The supernatant was
discarded, and the MP micelles were resuspended in
phosphate-buffered saline (PBS). In the present study, 50 μg/ml of
Fe3O4 MPs were added to the osteogenic
differentiation medium.
Immunohistochemical staining
The cells cultured on the cover slide were fixed for
20 min at 4°C using 10% neutral-buffered formalin and subsequently
washed 3 times with PBS (pH 7.2). These cover slides were then
incubated with anti-osteocalcin (predilution, AM 386; BioGenex, San
Ramon, CA, USA), anti-osteopontin (1:1,000 dilution) and
anti-osteonectin (1:500 dilution; AB 1858, Chemicon, Carlsbad, CA,
USA) antibodies for 24 h, followed by development using EnVision
Plus reagent (Dako, Carpinteria, CA, USA), diaminobenzidine as a
chromogen and Mayer’s hematoxylin as a counterstain.
von Kossa staining
The mineralized matrix of the cells was assessed
using 5% silver nitrate (Sigma-Aldrich) under ultra-violet light
for 60 min, followed by the addition of 3% sodium thiosulphate
(Sigma-Aldrich) for 5 min and then counterstaining with Van Gieson
(Sigma-Aldrich) for 5 min. With this staining method, the mineral
matrix is stained black and the osteoid (unmineralized matrix) is
stained red.
RT-qPCR
Total RNA was isolated from the cells using 500 μl
TRIzol reagent (Sigma-Aldrich). Subsequently, 100 μl of chloroform
were added and the solution was mixed and incubated for 3 min.
Following centrifugation (12,000 rpm, 4°C for 15 min), the upper
phase was transferred to a new tube and 500 μl of isopropanol were
added. After an incubation period of 10 min and another
centrifugation step (14,000 rpm, 4°C for 10 min), the supernatant
was discarded. The pellet was washed with 1 ml of 70% ethanol and
centrifuged (9,500 rpm, 4°C for 5 min). The supernatant was
discarded and the pellet was dried. Following the addition of 20 μl
of diethylpyrocarbonate (DEPC)-treated water, the pellet was
dissolved for 10 min on ice. The amount and purity of total RNA
were determined using a nanodrop spectrophotometer (Hankyong
National University). Reverse transcriptase (RT) reactions were
used to synthesize the cDNA from 1 μg of total RNA using an
Advantage RT-for-RCR kit (Clontech Laboratories, Inc., Palo Alto,
CA, USA). RT-PCR was routinely performed. The sample was cooled to
−20°C and stored until further analysis. For PCR, the primers were
purchased from Bioneer Corp. (Deajeon, Korea). The primer sequences
used for quantitative PCR are listed in Table I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Genes | Upstream primer
sequence | Downstream primer
sequence |
|---|
| GAPDH | 5′-ACC ACA GTC CAT
GCC ATC AC-3′ | 5′-TCC ACC ACC CTG
TTG CTG TA-3′ |
| Collagen I | 5′-GAA AAC ATC CCA
GCC AAG AA-3′ | 5′-CAG GTT GCC AGT
CTC CTC AT-3′ |
| Collagen III | 5′-CAG GTG AAC GTG
GAG CTG C-3′ | 5′-TGC CAC ACG TGT
TTC CGT GG-3′ |
| Osteonectin | 5′-CCA GAA CCA CCA
CTG CAA AC-3′ | 5′-GGC AGG AAG AGT
CGA AGG TC-3′ |
| Osteocalcin | 5′-AGG GGA AGA GGA
AAG AAG GG-3′ | 5′-CCA GGC GCT ACC
TGT ATC AA-3′ |
| Osteopontin | 5′-TCG CAG ACC TGA
CAT CCA GT-3′ | 5′-TCG GAA TGC TCA
TTG CTC TC-3′ |
| BMP-2 | 5′-GTC CAG CTG TAA
GAG ACA CC-3′ | 5′-GTA CTA GCG ACA
CCC ACA AC-3′ |
| Runx-2 | 5′-CTC ACT ACC ACA
CCT ACC TG-3′ | 5′-TCA ATA TGG TCG
CCA AAC AGA TTC-3′ |
| BSP | 5′-CAC AGC CTC ATC
TTC ATG G-3′ | 5′-GCA TCT CAT AGT
GCA TCT GG-3′ |
| CACNA1C | 5′-ACA GTG ACC AGT
GTG GTG GA-3′ | 5′-CGT AGC CTC TGG
AGA ACC TG-3′ |
| CACNA1E | 5′-GTT CGG CCG CGA
TCA CCT TTG T-3′ | 5′-GGC GGC CAA TCG
ATG AGC TTC T-3′ |
| CACNA1G | 5′-CGG CAA CTAC GTG
CTC TTC A-3′ | 5′-GTG ACT TCA TCT
CGT GGG CC-3′ |
| CACNA1I | 5′-CGT TGT CAT AGC
GAC CCA GTT-3′ | 5′-CAC AGC TCT CTT
CCC CGA GTG A-3′ |
Western blot analysis
After 10 days of cell culture, the cells were lysed
with RIPA buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% SDS (Sigma-Aldrich), protease
inhibitors (Complete™; Roche Diagnostics, Mannheim, Germany).
Protein (30 μg) was then separated by SDS-polyacrylamide gel
electrophoresis and blotted onto nitrocellulose membranes, which
were then blocked with 5% skim milk in phosphate-buffered saline
(PBS) containing 0.2% Tween-20. Western blot analysis was performed
according to the manufacturer’s instructions (Abcam, Cambridge, MA,
USA) with the following primary antibodies: anti-bone sialoprotein
(BSP, ab52128; Abcam), anti-bone morphogenetic protein 2 (BMP-2,
ab17885; Abcam), anti-osteopontin (ab63856; Abcam),
anti-osteonectin (ab14174; Abcam), anti-phosphorylated
extracellular signal-regulated kinase (p-ERK, #9101; Cell Signaling
Technology, Danvers, MA, USA) and anti-actin (ab3280; Abcam). The
blots were incubated with the primary antibodies at a dilution of
1:5,000 and then further incubated with horseradish
peroxidase-conjugated secondary antibody (#7076; Cell Signaling
Technology).
Cell surface antigen analysis by
fluorescence-activated cell sorting (FACS)
Antibodies against the human antigens, CD73 and
CD90, were purchased from BD Biosciences (San Jose, CA, USA) and
the antibody against CD105 was purchased from Ancell Corp.
(Bayport, MN, USA). A total of 5×105 cells were
resuspended in 200 μl of PBS and incubated with fluorescein
isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies
for 20 min at room temperature (or for 45 min at 4°C). The
fluorescence intensity of the cells was evaluated using a flow
cytometer (FACScan; BD Biosciences), and the data were analyzed
using CellQuest software (BD Biosciences).
Proliferation and activity assay of
hBM-MSCs
Cell proliferation and activity were measured using
a cell counter (Scepter™; Millipore Corp., Billerica, MA, USA) and
a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT; Sigma) assay. For the MTT assay, the cells were cultured in a
6-well plate, and each well was supplemented with MTT (3 mg/ml)
(n=4). The plates were then incubated in the dark at 37°C in an
atmosphere containing 5% CO2 for 2 h and the supernatant
was aspirated. Dimethylsulfoxide (DMSO) was added and the 6-well
plate was shaken slowly for 5 min. The absorption was measured at
570 nm.
LDH assay
LDH activity was measured using an LDH-LQ kit (Asan
Pharmaceutical Inc., Seoul, Korea). Briefly, after 7 days of
culture, 20-μl aliquots of medium and 50-μl of working solution
were mixed and incubated in the dark at room temperature for 30
min. The reaction was terminated by the addition of stop solution
(1 N HCl) and the absorbance was measured at 570 nm.
ALP assay
ALP deposition was measured using a
SensoLyte® pNPP Alkaline Phosphatase Assay kit
(AnaSpec Inc., Fremont, CA, USA). Following sample preparation, the
samples were mixed with pNPP substrate solution. The
mixtures were incubated for 30–60 min and stop solution was added.
The amount of ALP was quantified by ELISA at 405 nm.
Results and Discussion
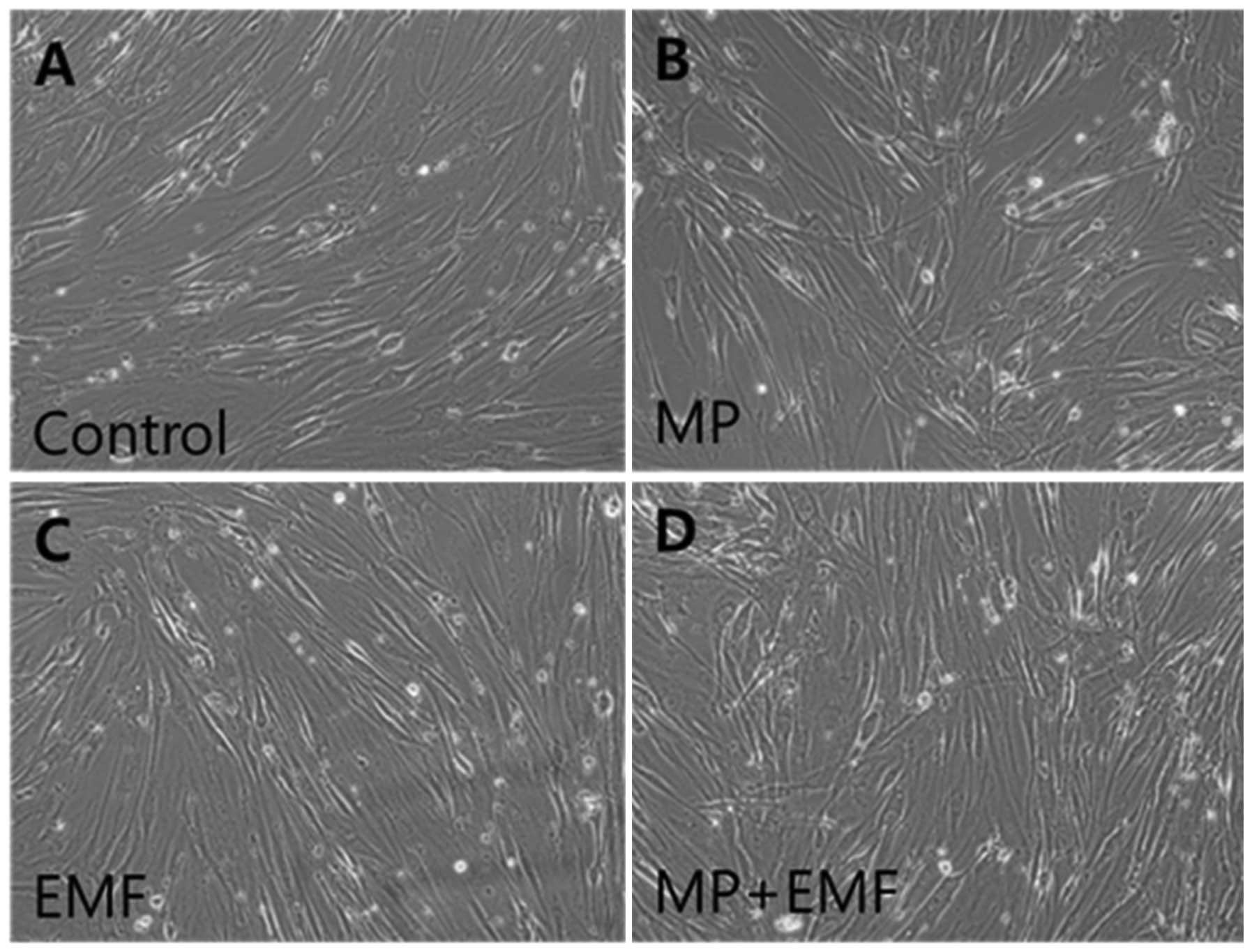
Morphology and characterization of
hBM-MSCs
The hBM-MSCs were cultured in osteogenic
differentiation medium and were treated with MPs or exposed to EMFs
over a period of 7 days. The morphology of the hBM-MSCs during
osteogenesis under the different experimental conditions [control
(no treatment), MP incorporation, EMF exposure, MP incorporation
and exposure to EMF] is shown in Fig.
2. No morphological changes or necrosis of the hBM-MSCs were
observed following the induction of osteogenic differentiation
(Fig. 2). Therefore, MP
incorporation, EMF exposure and MP incorporation in conjunction
with exposure to EMF did not induce any cytotoxic effects.
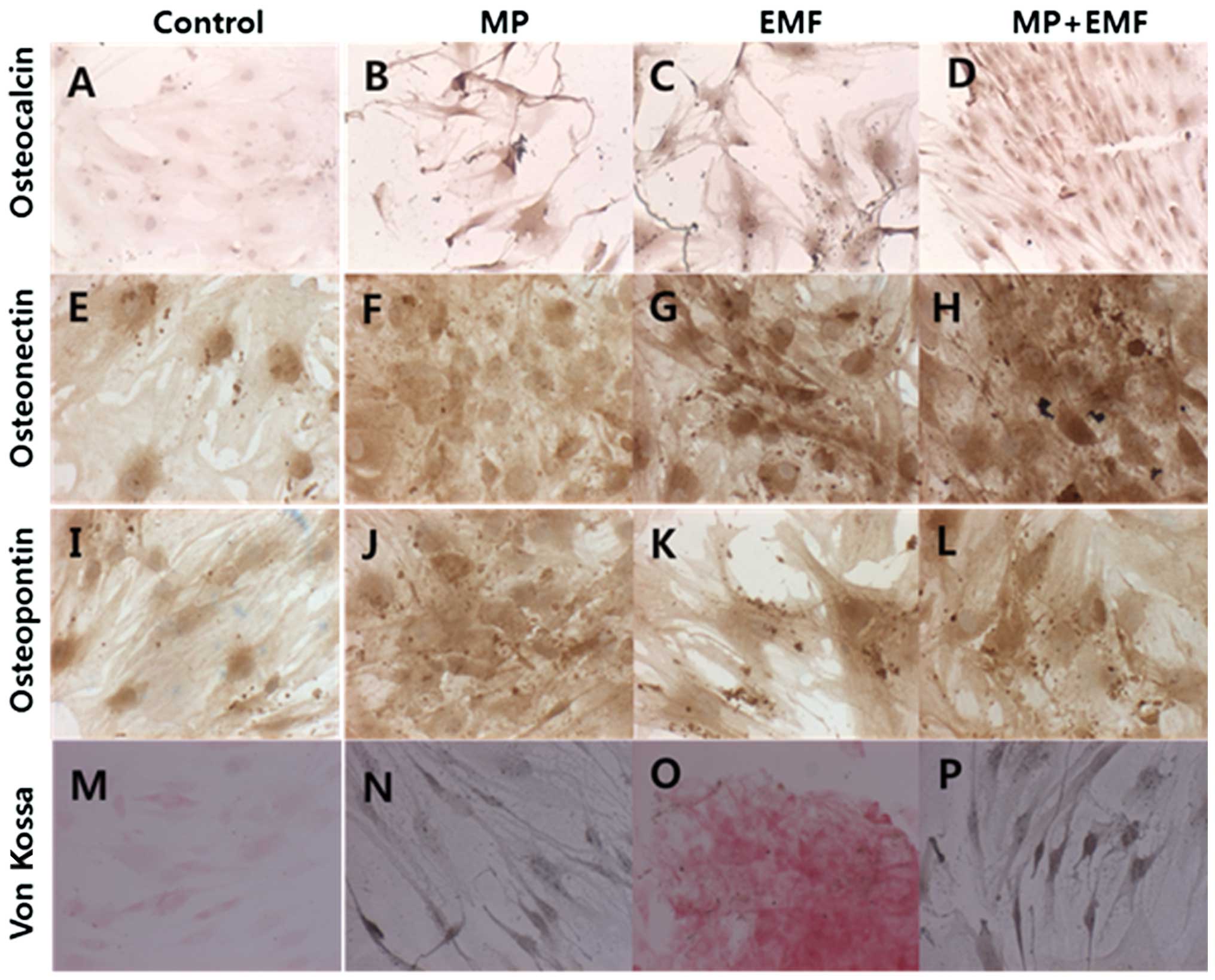
Immunohistochemical staining
To evaluate the protein expression levels of
osteogenic markers and mineralization, immunohistochemical staining
was performed. Osteocalcin protein is specifically synthesized by
osteoblasts and is a marker of osteoblast differentiation during
the later stages of bone formation (11). Osteonectin is a glycoprotein in
the bones which binds sodium. It is secreted by osteoblasts during
bone formation, initiating mineralization and promoting mineral
crystal formation (12).
Osteopontin is a highly phosphorylated sialoprotein, which is a
prominent component of the mineralized extracellular matrices of
bones (13). The osteogenic
markers (osteocalcin, osteopontin and osteonectin) were strongly
expressed in the cells treated with MPs, in those exposed to EMFs
and in the cells treated with MPs and exposed to EMFs compared to
the control group (Fig. 3A–L).
The results from immunohistochemical staining of the expression
levels of the osteogenic markers are presented in Table II.
 | Table IIStaining results of the osteogenic
markers. |
Table II
Staining results of the osteogenic
markers.
| Control | MP | EMF | MP + EMF |
|---|
| Osteocalcin | − | + | ++ | ++ |
| Osteonectin | + | ++ | +++ | +++ |
| Osteopontin | + | +++ | ++ | +++ |
| von Kossa | − | ++ | + | ++ |
von Kossa staining is widely used to quantify
mineralization (14–16). It is generally believed that, in
von Kossa’s technique, silver cations react with phosphates and
carbonates in calcium deposits (17). We used von Kossa staining for the
investigation of the mineralization of hBM-MSCs during osteogenesis
(Fig. 3M–P). The untreated
hBM-MSCs (control) or those exposed to EMFs growing in osteogenic
medium exhibited a small amount of matrix mineralization; however,
the hBM-MSCs treated with MPs and those treated with MPs and
exposed to EMFs exhibited a stronger matrix mineralization compared
to the untreated controls on day 7. The amount of mineralization in
each treatment group is shown in Table II.
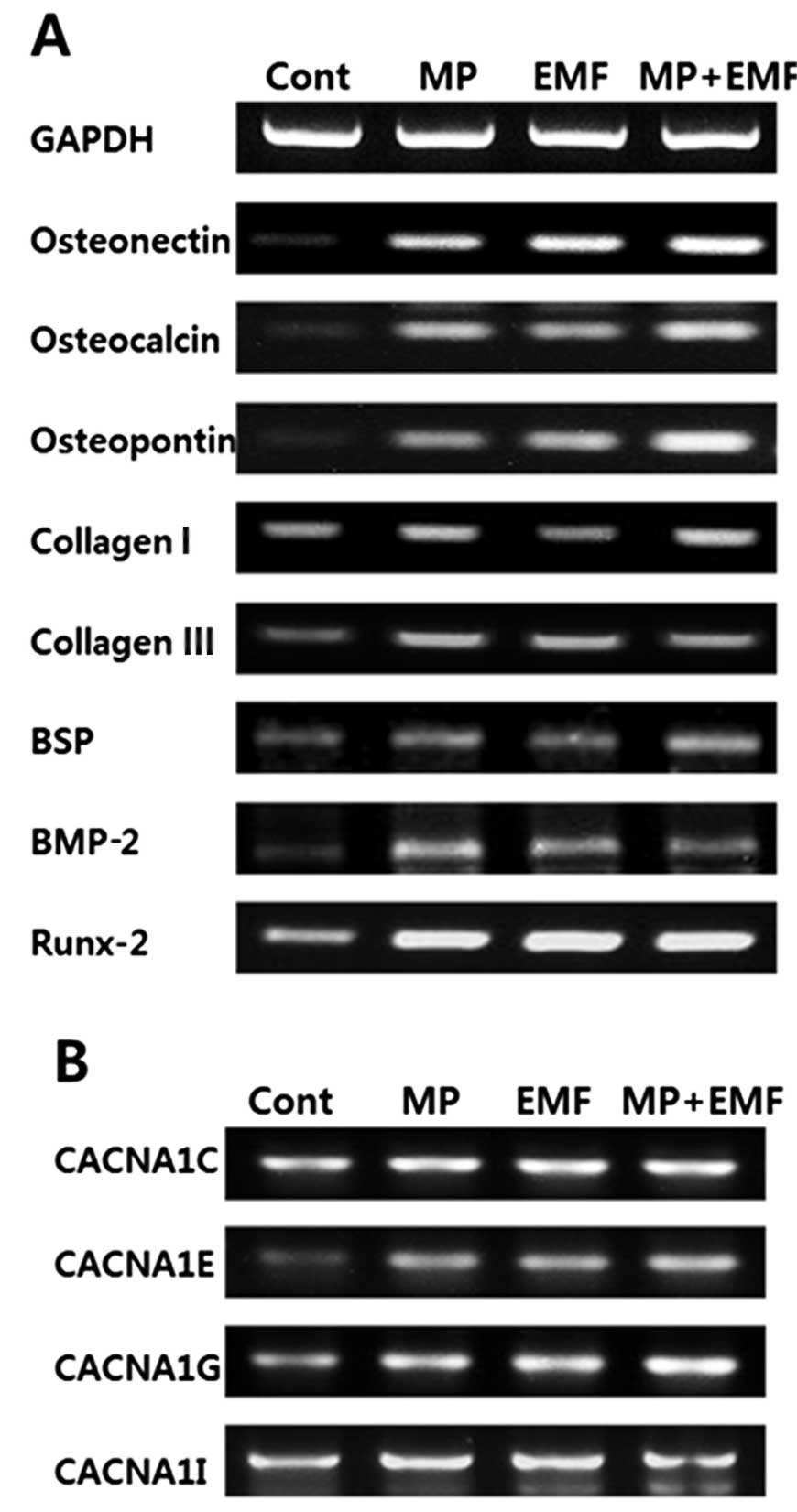
RT-qPCR
The mRNA expression levels of osteogenesis-related
genes from the hBM-MSCs following the induction of osteogenic
differentiation for 3 days is shown in Fig. 4A. The osteoblast markers,
osteocalcin, osteopontin and osteonectin were highly expressed in
the hBM-MSCs treated with MPs and exposed to EMFs. We also examined
the expression levels of major bone matrix protein genes, such as
collagen I, collagen III, BMP-2 and BSP. BMP-2 induces osteoblastic
differentiation by acting directly on MSCs and is clinically used
to induce bone formation although high doses are required (18). BSP is a highly
post-translationally modified acidic phosphorus protein normally
expressed in mineralized tissue, such as bone and dentin (19). The representative
osteogenesis-related genes were highly expressed in the hBM-MSCs
treated with MPs, those exposed to EMFs and in those treated with
MPs and exposed to EMFs. The results of our experiments indicated
that the expression levels of BSP, OPN, OCN, and collagen I were
significantly increased in the EMF with MP exposure group.
Osteogenic gene expression can be used as an early index during
osteogenesis. BSP is a late stage marker of osteoblast
differentiation and an early stage marker of matrix mineralization.
OPN is expressed throughout matrix maturation, followed first by
BSP and finally by OCN, which characterizes the post-proliferate
phase. The OCN protein is specifically synthesized by osteoblasts
and is a marker of osteoblast differentiation during the later
stages of bone formation. Collagen I is the most abundant protein
in the bone matrix and is an early marker of osteoblastic
differentiation and the major organic component of the mineralized
bone matrix. Additionally, the mRNA expression of the transcription
factor Runx-2 was measured by RT-qPCR. Runx-2 is involved in the
production of bone matrix proteins as it is able to upregulate the
expression of major bone matrix protein genes leading to an
increase in immature osteoblasts from pluripotent stem cells; the
immature osteoblasts form immature bone (20–25). Runx-2 was strongly expressed in
the hBM-MSCs treated with MPs, those exposed to EMFs and in those
treated with MPs and exposed to EMFs compared to the untreated
control.
Recent studies have suggested that calcium
activation affects bone formation. Klar et al (26) reported that the spontaneous
induction of bone formation is initiated by a local peak of
calcium-activating stem cell differentiation and the induction of
bone formation. Moreover, Wen et al (27) mentioned that the inhibition of the
L-type voltage-dependent calcium channel (VDCCL) downregulates the
proliferation and osteogenic differentiation of rat MSCs (rMSCs),
but promotes apoptosis. These results suggest that VDCCL plays a
crucial role in the proliferation and osteogenic differentiation of
rMSCs. Based on this knowledge, we investigated the effects of MPs
and EMF exposure on the expression of calcium channel-related genes
in the hBM MSCs during osteogenesis. After 3 days of osteogenesis
and treatment with MPs and EMF exposure, the mRNA expression levels
of CACNA1C and CACNA1I in the cells treated with MPs, those exposed
to EMFs and in those treated with MPs and exposed to EMFs were
slightly higher compared to the control group (Fig. 4B). In addition, the expression
levels of CACNA1E and CACNA1G were significantly higher in the
cells treated with MPs and exposed to EMFs (Fig. 4B). This suggested that the hBM-MSC
calcium channel was activated during osteogenic
differentiation.
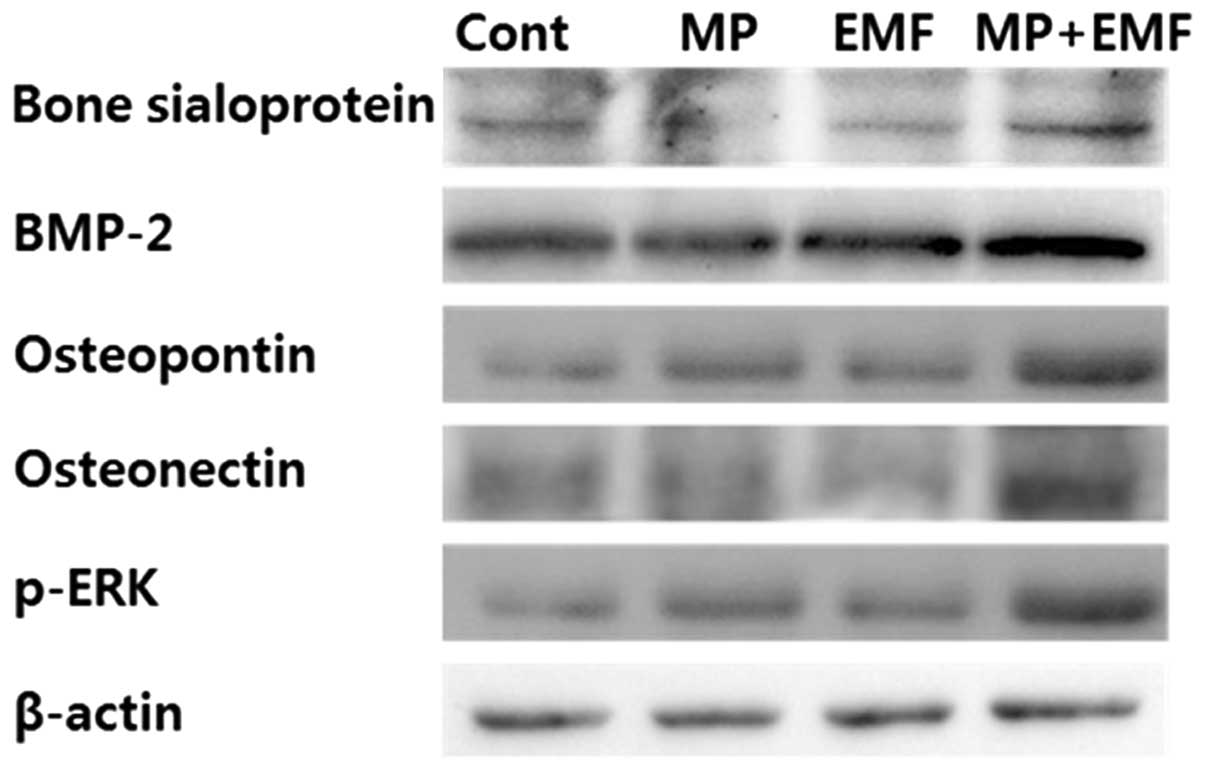
Western blot analysis
To examine osteogenic differentiation,
osteogenesis-related proteins were detected by western blot
analysis at day 7 of induction. As shown in Fig. 5, the expression levels of
osteogenesis-related proteins (BSP, BMP-2, osteopontin and
osteonectin) were increased in the cells treated with MPs, those
exposed to EMFs and in those treated with MPs and exposed to EMFs
compared to the control group.
To examine the potential p-ERK activation during
osteogenesis, western blot analysis was performed. Several studies
have reported that p-ERK activation is an essential mediator of
growth factor-induced cell proliferation and differentiation in
various cell types, including osteoblasts (28–31). Kapur et al (32) demonstrated that the activation of
ERK1/2 by mechanical stimuli is involved in collagen synthesis and
osteopontin production. In the present study, after 7 days of
osteogenesis with MP incorporation and EMF exposure, the expression
of p-ERK was increased and it was highly expressed in the cells
treated with MPs and exposed to EMFs (Fig. 5).
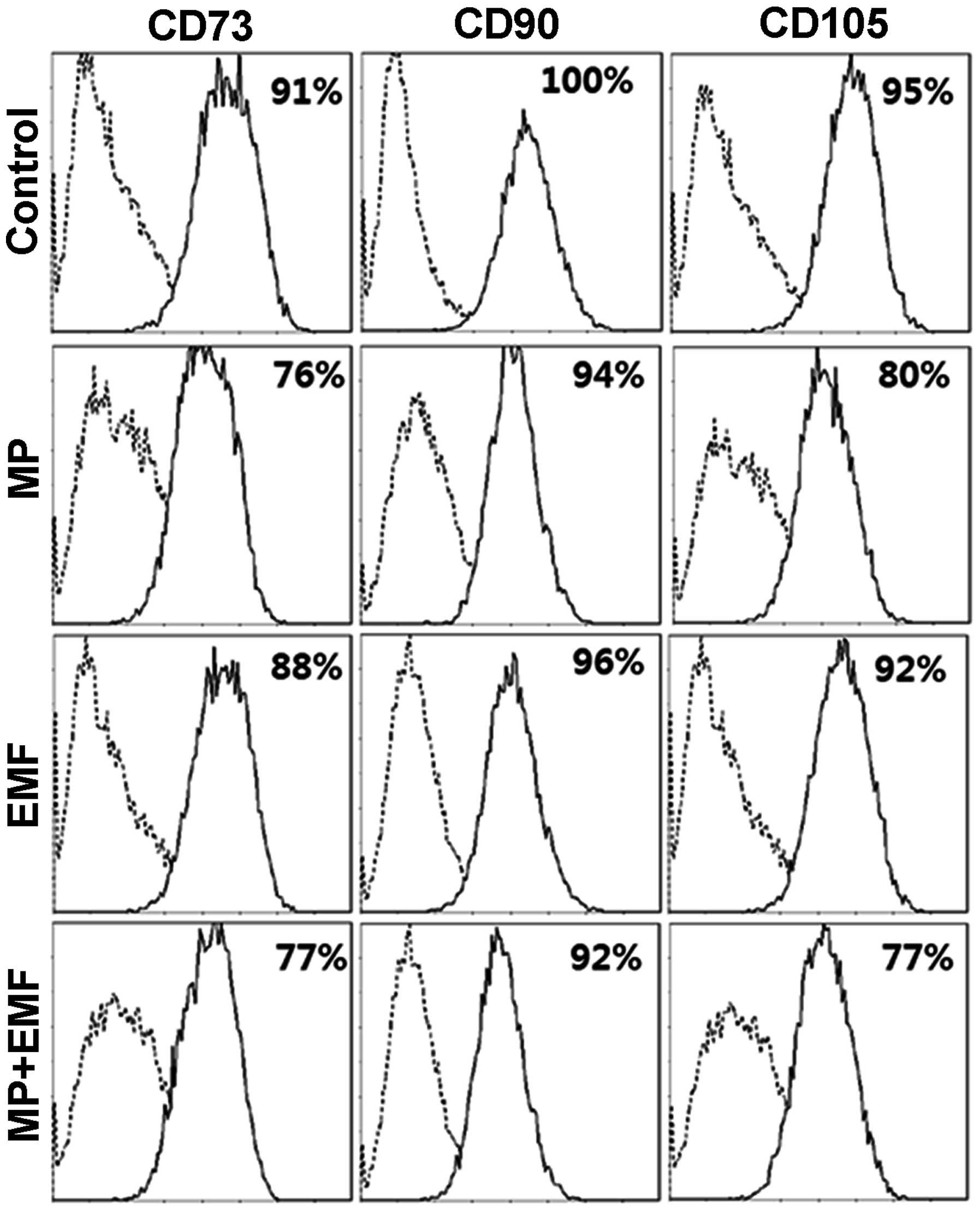
FACS analysis
Several antigens are known as MSC surface markers.
The antibodies against CD73 (membrane-bound
ecto-5′-nucleotisidase), CD90 (Thy-1), CD105 (endoglin) have been
reported to react with undifferentiated MSCs (33–35).
To investigate whether MP incorporation and exposure
to EMFs affects hBM-MSC differentiation, FACS analysis was
performed for the hBM-MSC markers, CD73, CD90 and CD105 (Fig. 6). The expression level of CD73 was
91% in the control group, 76% in the MP incorporation group, 88% in
the EMF-exposed group and 77% in the MP incorporation with exposure
to EMF group after 7 days. The expression level of CD90 was 100% in
the control group, 94% in the MP incorporation group, 96% in the
EMF-exposed group and 77% in the MP incorporation with exposure to
EMF group. The expression of CD105 was 95% in the control group,
80% in the MP incorporation group, 92% in the EMF-exposed group and
77% in the MP incorporation with exposure to EMF group. The
expression levels of hBM-MSC surface antigens were decreased in the
cells treated with MPs, those exposed to EMFs and in those treated
with MPs and exposed to EMFs compared to the control group. In
conclusion, our data indicate that MP incorporation and EMF
exposure alter MSC surface antigen expression, suggesting that the
hBM-MSCs differentiate into a specific cell type, thus changing
their cell fate.
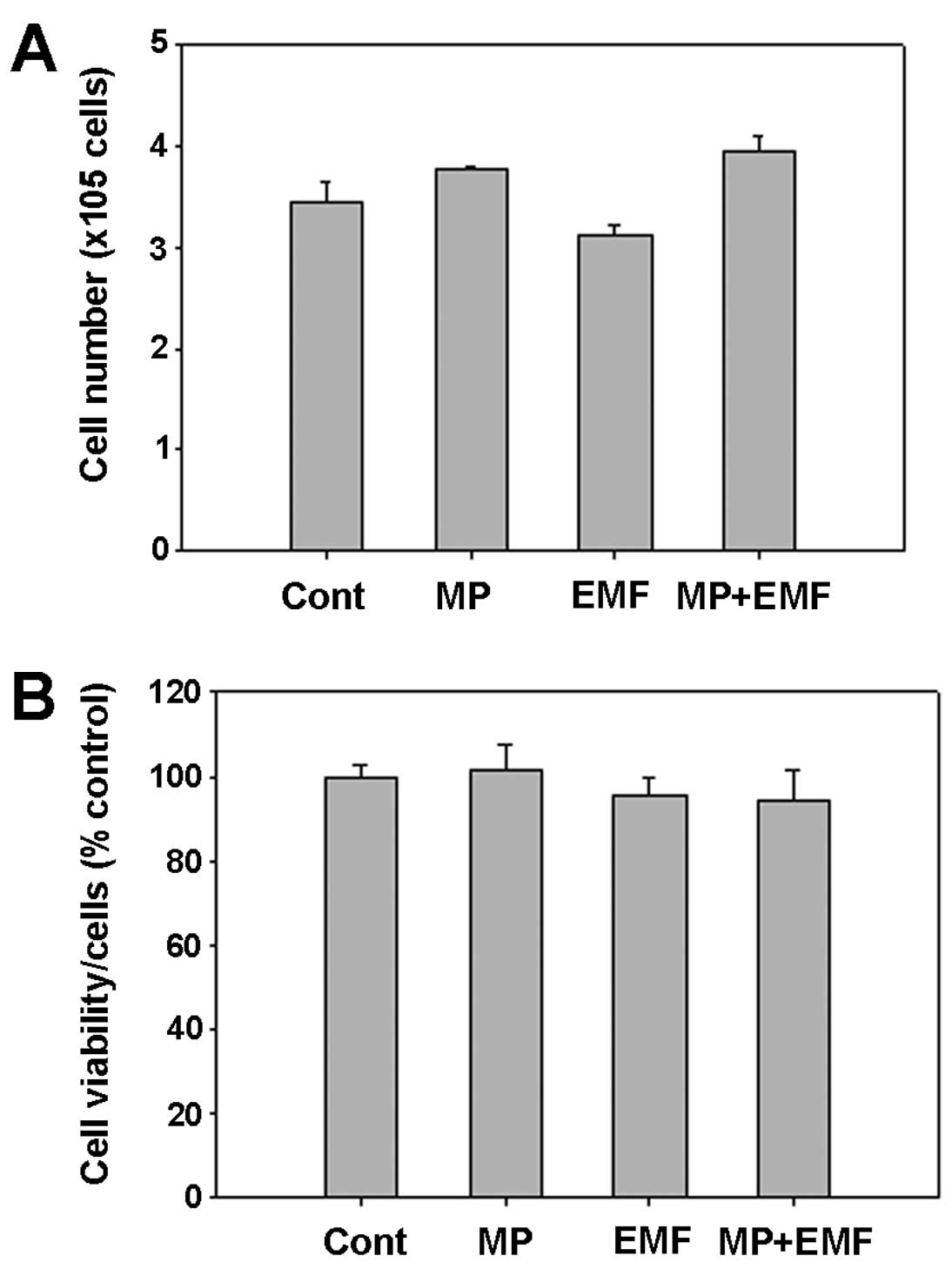
Cell proliferation and activity
assay
The cell number was determined to be approximately
3.4×105 cells in the control group, 3.7×105
cells in the MP incorporation group, 3.1×105 cells in
the EMF-exposed group and 3.9×105 cells in the MP
incorporation with exposure to EMF group after 7 days (Fig. 7A).
As shown by the cell counting results, the cells
treated with MPs and those treated with MPs and exposed to EMFs
exhibited slightly accelerated cell growth compared to the control
group, while the group exposed to EMFs showed a more decelerated
cell growth compared to the control group (Fig. 7A).
In addition, cell viability and toxicity to hBM-MSCs
were measured by MTT assay 7 days after osteogenesis. MTT assay
provides valuable information as to the potential cell viability
and cytotoxic effects. Although the cell numbers were decreased
following exposure to EMFs and increased following treatment with
MPs and treatment with MPs in conjunction with EMF exposure, the
cell mitochondrial activity of the 4 experimental groups was
similar (Fig. 7B). Morevoer,
these results demonstrated that no cytotoxic effects were detected
in the hBM-MSCs treated with MPs and in those exposed to EMFs
during osteogenesis.
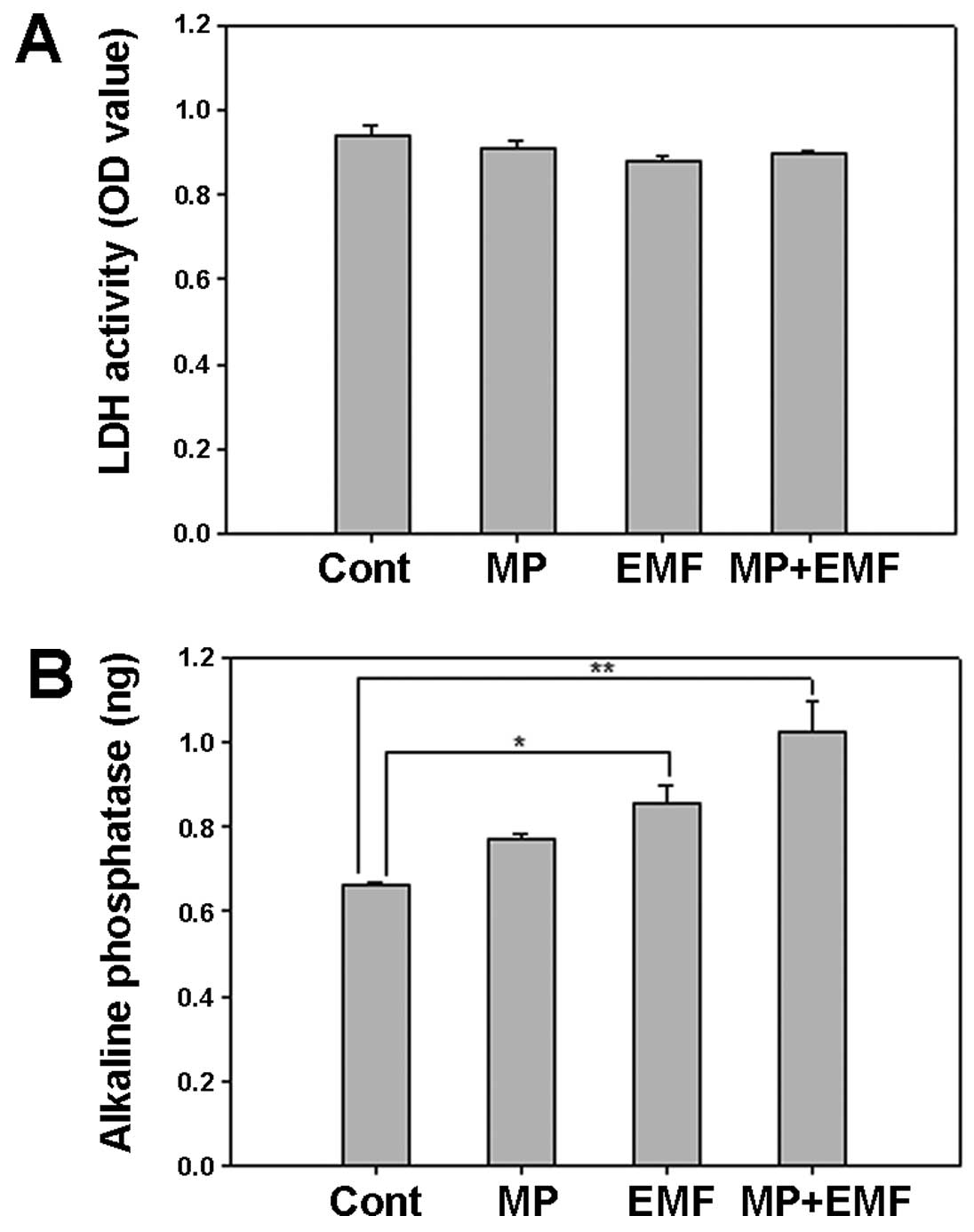
LDH and ALP activity
LDH is a cytoplasmic catalytic enzyme related to the
reversible conversion between pyruvic and lactic acid. LDH is
released through the cell membrane when it is damaged (36). Therefore, less LDH release means
less cellular damage. Thus, the media were collected and analyzed
after 7 days in order to examine cellular damage.
In the cells treated with MPs and in those treated
with MPs and exposed to EMFs there was a decrease in LDH secretion
(Fig. 8A). The LDH activity of
the 4 experimental groups was similar, which suggests that
treatment with MPs and exposrue to EMFs did not affect LDH
secretion and did not induce damage to the cell membrane.
ALP is an early mineralization-related protein
marker for the osteogenesis of osteoblasts (37). The effects of MP incorporation and
exposure to EMFs on cellular ALP activity during osteogenesis were
examined by measuring the ALP reaction products in the untreated
cells (control), the cells treated with MPs, those exposed to EMFs,
and in those treated with MPs and exposed to EMFs over time.
Several ALP-positive cells were observed in the cells exposed to
EMFs and in those treated with MPs and exposed to EMFs (Fig. 8B). These results suggest that MP
incorporation in conjunction with exposure to EMFs increases ALP
activity in hBM-MSCs during osteogenesis.
In conclusion, in the present study, the effects of
treatment with MPs in conjunction with exposure to EMFs on cell
differentiation were investigated. We treated hBM-MSCs with 50
μg/ml of Fe3O4 MPs or exposed them to a
frequency of 45 Hz and an intensity of 1 mT EMF twice every 8 h per
day for 7 days. No morphological changes and no cytotoxic effects
were observed during osteogenesis. The expression of osteogenic
markers was detected by immunohistochemical staining, RT-qPCR and
western blot analysis, demonstrating an increase in expression.
FACS analysis indicated that treatment with MPs and EMF exposure
reduced the expression of MSC surface markers; this suggests the
possibility of hBM-MSC differentiation. The mineralization of
hBM-MSCs in the MP incorporation group or EMF-exposed group was
observed by ALP assay. Taken together, these results suggest that
the treatment of hBM-MSCs with MP or their exposure to EMFs
increases osteogenic differentiation, and that MP incorporation in
conjunction with EMF exposure is more effective in enhancing
osteogenic differentiation. The results of our study demonstrate
that MPs may be potentially used for medical instruments or
scaffold materials, and that EMFs can be used for the
rehabilitation of osteogenic wounds.
Acknowledgements
The present study was supported by the Pioneer
Research Center Program through the National Research Foundation of
Korea funded by the Ministry of Science, ICT and Future Planning,
Republic of Korea (grant number 2009-0082941).
References
|
1
|
Sadan O, Melamed E and Offen D:
Bone-marrow-derived mesenchymal stem cell therapy for
neurodegenerative diseases. Expert Opin Biol Ther. 9:1487–1497.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA
and Verfaillie C: Pluripotency of mesenchymal stem cells derived
from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bielby R, Jones E and McGonagle D: The
role of mesenchymal stem cells in maintenance and repair of bone.
Injury. 38(Suppl 1): S26–S32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukada E and Yasuda I: On the
piezoelectric effect of bone. J Phys Soc Japan. 12:1158–1162. 1957.
View Article : Google Scholar
|
|
5
|
Bassett CA and Pawluk RJ: Effects of
electric currents on bone in vivo. Nature. 204:652–654. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai MT, Li WJ, Tuan RS and Chang WH:
Modulation of osteogenesis in human mesenchymal stem cells by
specific pulsed electromagnetic field stimulation. J Orthop Res.
27:1169–1174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun LY, Hsieh DK, Lin PC, Chiu HT and
Chiou TW: Pulsed electromagnetic fields accelerate proliferation
and osteogenic gene expression in human bone marrow mesenchymal
stem cells during osteogenic differentiation. Bioelectromagnetics.
31:209–219. 2010.
|
|
8
|
Pankhurst QA, Connolly J, Jones S and
Dobson J: Applications of magnetic nanoparticles in biomedicine. J
Phys D Appl Phys. 36:R167–R181. 2003. View Article : Google Scholar
|
|
9
|
Cartmell SH, Dobson J, Verschueren SB and
El Haj AJ: Development of magnetic particle techniques for
long-term culture of bone cells with intermittent mechanical
activation. IEEE Trans Nanobioscience. 1:92–97. 2002. View Article : Google Scholar
|
|
10
|
Cho H, Choi YK, Lee DH, Park HJ, Seo YK,
Jung H, Kim SC, Kim SM and Park JK: Effects of magnetic
nanoparticle-incorporated human bone marrow-derived mesenchymal
stem cells exposed to pulsed electromagnetic fields on injured rat
spinal cord. Biotechnol Appl Biochem. 60:596–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ducy P, Desbois C, Boyce B, Pinero G,
Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C,
Bradley A and Karsenty G: Increased bone formation in
osteocalcin-deficient mice. Nature. 382:448–452. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelm RJ Jr, Hair GA, Mann KG and Grant BW:
Characterization of human osteoblast and megakaryocyte-derived
osteonectin (SPARC). Blood. 80:3112–3119. 1992.PubMed/NCBI
|
|
13
|
Sodek J, Ganss B and McKee M: Osteopontin.
Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
von Kossa J: Über die im Organismus
künstlich erzeugbaren Verkalkungen. Beit Path Anat. 29:163–202.
1901.(In German).
|
|
15
|
Bills C, Eisenberg H and Pallante SL:
Complexes of organic acids with calcium phosphate: the von Kossa
stain as a clue to the composition of bone mineral. Johns Hopkins
Med J. 128:194–207. 1971.PubMed/NCBI
|
|
16
|
Puchtler H and Meloan S: On the chemistry
of formaldehyde fixation and its effects on immunohistochemical
reactions. Histochemistry. 82:201–204. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meloan SN and Puchtler H: Chemical
mechanisms of staining methods: von Kossa’s technique: what von
Kossa really wrote and a modified reaction for selective
demonstration of inorganic phosphates. J Histotechnol. 8:11–13.
1985. View Article : Google Scholar
|
|
18
|
Schwartz Z, Simon B, Duran M, Barabino G,
Chaudhri R and Boyan B: Pulsed electromagnetic fields enhance BMP-2
dependent osteoblastic differentiation of human mesenchymal stem
cells. J Orthop Res. 26:1250–1255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz Z, Lohmann C, Oefinger J,
Bonewald L, Dean D and Boyan B: Implant surface characteristics
modulate differentiation behavior of cells in the osteoblastic
lineage. Adv Dent Res. 13:38–48. 1999. View Article : Google Scholar
|
|
20
|
Ogawa E, Inuzuka M, Maruyama M, Satake M,
Naito-Fujimoto M, Ito Y and Shigesada K: Molecular cloning and
characterization of PEBP2β, the heterodimeric partner of a novel
Drosophila runt-related DNA binding protein PEBP2α. Virology.
194:314–331. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyoshi H, Shimizu K, Kozu T, Maseki N,
Kaneko Y and Ohki M: t (8;21) breakpoints on chromosome 21 in acute
myeloid leukemia are clustered within a limited region of a single
gene, AML1. Proc Natl Acad Sci USA. 88:10431–10434. 1991.
View Article : Google Scholar
|
|
22
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson R, Gao YH, Inada M, Sato M,
Okamoto R, Kitamura Y, Yoshiki S and Kishimoto T: Targeted
disruption of Cbfa1 results in a complete lack of bone formation
owing to maturational arrest of osteoblasts. Cell. 89:755–764.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: a transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otto F, Thornell AP, Crompton T, Denzel A,
Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen
BR, Selby PB and Owen MJ: Cbfa1, a candidate gene for cleidocranial
dysplasia syndrome, is essential for osteoblast differentiation and
bone development. Cell. 89:765–771. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Komori T: Regulation of bone development
and extracellular matrix protein genes by RUNX2. Cell Tissue Res.
339:189–195. 2010. View Article : Google Scholar
|
|
26
|
Klar RM, Duarte R, Dix-Peek T, Dickens C,
Ferretti C and Ripamonti U: Calcium ions and osteoclastogenesis
initiate the induction of bone formation by coral-derived
macroporous constructs. J Cell Mol Med. 17:1444–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen L, Wang Y, Wang H, Kong L, Zhang L,
Chen X and Ding Y: L-type calcium channels play a crucial role in
the proliferation and osteogenic differentiation of bone marrow
mesenchymal stem cells. Biochem Biophys Res Commun. 424:439–445.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai CF, Chaudhary L, Fausto A, Halstead
LR, Ory DS, Avioli LV and Cheng SL: Erk is essential for growth,
differentiation, integrin expression, and cell function in human
osteoblastic cells. J Biol Chem. 276:14443–14450. 2001.PubMed/NCBI
|
|
29
|
Azuma N, Duzgun SA, Ikeda M, Kito H,
Akasaka N, Sasajima T and Sumpio BE: Endothelial cell response to
different mechanical forces. J Vasc Surg. 32:789–794. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oldenhof AD, Shynlova OP, Liu M, Langille
BL and Lye SJ: Mitogen-activated protein kinases mediate
stretch-induced c-fos mRNA expression in myometrial smooth muscle
cells. Am J Physiol Cell Physiol. 283:C1530–C1539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferraro JT, Daneshmand M, Bizios R and
Rizzo V: Depletion of plasma membrane cholesterol dampens
hydrostatic pressure and shear stress-induced mechanotransduction
pathways in osteoblast cultures. Am J Physiol Cell Physiol.
286:C831–C839. 2004. View Article : Google Scholar
|
|
32
|
Kapur S, Baylink DJ and Lau KH: Fluid flow
shear stress stimulates human osteoblast proliferation and
differentiation through multiple interacting and competing signal
transduction pathways. Bone. 32:241–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schieker M, Pautke C, Haasters F, Schieker
J, Docheva D, Böcker W, Guelkan H, Neth P, Jochum M and Mutschler
W: Human mesenchymal stem cells at the single-cell level:
simultaneous seven-colour immunofluorescence. J Anat. 210:592–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bobis S, Jarocha D and Majka M:
Mesenchymal stem cells: characteristics and clinical applications.
Folia Histochem Cytobiol. 44:215–214. 2007.PubMed/NCBI
|
|
36
|
Mitchell DB, Santone KS and Acosta D:
Evaluation of cytotoxicity in cultured cells by enzyme leakage. J
Tissue Cult Methods. 6:113–116. 1980. View Article : Google Scholar
|
|
37
|
Gundberg C, Looker A, Nieman S and Calvo
M: Patterns of osteocalcin and bone specific alkaline phosphatase
by age, gender, and race or ethnicity. Bone. 31:703–708. 2002.
View Article : Google Scholar
|