Introduction
Lipopolysaccharide (LPS), a component of
Gram-negative bacteria, induces the production of nitric oxide (NO)
in monocytes and macrophages. NO plays a vital role in the
regulation of inflammatory responses. In the acute inflammatory
response, the increase in NO production represents the upregulation
of the immune defense against pathogens. As NO induction enhances
pro-inflammatory responses, in this manner, external invaders can
be effectively excluded (1,2).
To produce NO during inflammation, the activation of inducible NO
synthase (iNOS) is required. iNOS is a member of the NOS family,
including endothelial NOS (eNOS) and neuronal NOS (nNOS). In
inflammatory responses, LPS and various cytokines stimulate iNOS
expression in macrophages. In particular, interleukin (IL)-4 with
anti-CD23, interferon (IFN)-γ with LPS and IFN-α/β activates iNOS,
producing NO during the inflammatory response through the uptake of
L-arginine (3,4). Thus, the regulation of iNOS may
prove to be a therapeutic strategy for the treament of inflammatory
disorders (5).
During NO production, class IA
phosphoinositide 3-kinase (PI3K) is associated with the regulation
of iNOS expression. PI3Ks are divided into 4 classes according to
their structure and substrate specificity. Class IA PI3K
is composed of the p85 regulatory subunit and the p110 catalytic
subunit, and is a component of receptor-mediated signaling in the
immune system (6). In
receptor-mediating signaling, class IA PI3K catalyzes
the conversion of PI(4,5)P2 into PI(3,4,5)P3.
In turn, PI(3,4,5)P3 induces the phosphorylation of
3-phosphoinositide dependent protein kinase-1 (PDK-1) and the
upregulation of Akt phosphorylation. The phosphorylation of Akt can
be inhibited by the activation of phosphatase and tensin homologue
(PTEN), converting PIP3 into PIP2 (7). It has been reported that PI3K is
associated with the regulation of iNOS expression. PI3K/Akt
signaling plays a role in the suppression of iNOS expression. The
inhibition of PI3K in C6 glial cells enhances iNOS
expression under LPS stimulation comparable with the LPS
stimulation in wild-type C6 glial cells, and LPS-induced
iNOS amplification following the inhibition of PI3K is not mediated
by mitogen-activated protein kinase (MAPK) and nuclear factor
(NF)-κB (8,9). By contrast, the PI3K/Akt pathway
upregulates iNOS expression. Treatment of hepatocytes with IL-1β
has been shown to accentuate iNOS expression, but does not induce
iNOS expression when PI3K/Akt is inhibited (10).
Histone acetyltransferases (HATs) and histone
deacetylases (HDACs) are enzymes that mediate acetylation and
deacetylation at lysine residues of various proteins, including
histones (11). HATs are divided
into 5 families: the GCN5-related N-acetyltransferase (GNAT)
family, represented by general control non-derepressible 5 (GCN5)
and p300/CREB-binding protein (CBP)-associated factor (PCAF); the
p300/CBP family, including p300 and CBP; the MYST family, including
TAT-interacting protein 60 (Tip60); general transcription factor
HATs; and nuclear hormone-related HATs (12). HDACs are classified into 4 classes
containing a total of 18 enzymes. There is sequence similarity
between classical HDACs (class I, II and IV) whose activity is
dependent on Zn2+. Class III includes the family of
sirtuins. In contrast to classical HDACs, the activity of class III
sirtuins is dependent on NAD+ (13). The acetylation of histones by HATs
and the tight package of chromatin structure by HDACs are critical
to the control of gene expression. In resting cells, DNA is wound
around an octomer with 2 molecules each of the core histone
proteins, including H2A, H2B, H3 and H4. This chromatin structure
suppresses gene expression as the basal transcription complex is
unable to bind to DNA. Conversely, once the cells are activated by
exogenous stimulation, lysine residues of a long terminal of each
core histone are acetylated by HATs, including CBP, p300 and
CBP/p300 co-activators, reducing the electrical charge of core
histones. Thereby, the chromatin structure is transformed from the
closed form to the opened conformation, allowing the binding of RNA
polymerase II and the initiation of transcription (14). By contrast, removal of acetyl
group by HDAC repacks chromatin and causes gene silencing (15). Thus, histone acetylation is
closely linked to the induction of pro-inflammatory gene
expression, and is considered a promising target for the treatment
of inflammatory diseases.
Veratric acid is a phenolic compound derived from
Sparassis crispa and has been reported to have antioxidant
properties (16,17). In our previous study, we proved
that veratric acid inhibited iNOS expression through the
inactivation of MAPKs and NF-κB in LPS-stimulated RAW264.7 cells
(18). In the present study, we
demonstrate that the inhibitory effects of veratric acid on the
PI3K/Akt pathway, HATs and HDACs lead to the downregulation of iNOS
expression in LPS-stimulated RAW264.7 cells.
Materials and methods
Chemicals and reagents
Veratric acid, LPS (Escherichia coli
0111:B4), Griess reagent and Tween-20 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Skim milk powder was purchased
from Bioshop Canada Inc. (Burlinton, ON, Canada). Dulbecco’s
modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and
penicillin/streptomycin were purchased from Cellgro.com, Mediatech
Inc. (Manassas, VA, USA). LY294002, a PI3K inhibitor, was purchased
from Cayman Chemical Co. (Ann Arbor, MI, USA).
Animal experiments
All animal experiments were approved by the
Institutional Animal Care and Use Committe of Dong-eui University,
Busan, Korea. Female Balb/c mice (4–5 weeks of age, weighing 25–30
g) were purchased from Samtako Bio Korea Co. (Gyeonggi-do, Korea).
These mice were housed in a specific pathogen-free facility with
appropriate temperature and humidity and allowed free access to
food and water. Skin inflammation was induced topically with the
application of 12-O-tetradecanoylphorbol-13-acetate (TPA) on
the dorsal skin of the mice; hair had been previously removed with
an animal clipper. Briefly, the mice (5 per group) were either
untreated, exposed to 200 μl acetone, TPA alone or treated with 0.5
and 1 mg (20, 40 and 80 mg/kg) veratric acid (VA) in acetone 1 h
prior to exposure to TPA. Following exposure and treatment as
described above for 24 h, the mice were sacrificed by cervical
dislocation, and the dorsal skin was collected and snap-frozen in
liquid nitrogen.
Cell culture
The RAW264.7 cell line was purchased from the
American Tissue Culture Collection (ATCC, Manassas, VA, USA). The
BV-2 microglial cell line was a generous gift from Professor
Jong-Hwan Lee of Dong-eui University, Busan, Korea. The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin, and were incubated under humidified
conditions of 5% CO2 at 37°C. For the experiments, the
cells were washed with warm DMEM and treated with with 100 or 200
μM of veratric acid for 1 h prior to LPS stimulation. For the
inhibition of PI3K, the cells were treated with the PI3K inhibitor,
LY294002, for 1 h prior to the addition of veratric acid and
LPS.
NO production
NO production in the RAW264.7 and BV-2 cell culture
medium was measured using Griess reagent by reaction with nitrite.
The cells (5×105 cells/well) were seeded in 24-well
plates and incubated for 24 h. Following incubation, the cells were
pre-treated with veratric acid for 1 h prior to LPS stimulation for
24 h. The cultured supernatant (100 μl) was reacted with the same
volume of Griess reagent [1% sulfanilamide/0.1%
N-(1-naphthyl)-ethylenediamine dihydrochloride/2.5%
H3PO4] for 10 min at room temperature.
Nitrite was measured at 540 nm using an ELISA microplate reader
(Molecular Devices, Sunnyvale, CA, USA).
RNA isolation and RT-PCR
The RAW264.7 cells were seeded on 12-well plates at
a density of 2×105 cells/well and incubated 24 h. The
cells were cultured with or without 200 μM of veratric acid for 1 h
prior to LPS stimulation. Total RNA was isolated from the cells
using the RNAeasy kit (Qiagen, Hilden, Germany, USA). One microgram
of total RNA was used for cDNA synthesis using
AccuPower® RT PreMix (Bioneer Corp., Daejeon, Korea)
containing M-MLV reverse transcriptase. The iNOS and GAPDH genes
were amplified from the cDNA by PCR. The PCR primers used were as
follows: iNOS (5′-ATGTCCGAAGCAAACATCAC-3′ and 5′-TAATGTCC
AGGAAGTAGGTG-3′); GAPDH (5′-AGGCCGGTGCT GAGTATGTC-3′ and
5′-TGCCTGCTTCACCACCTTCT-3′); HDAC1 (5′-CGCATGACTCACAATTTGCT-3′ and
5′-AAAC ACCGGACAGTCCTCAC-3′); HDAC2 (5′-AGACTGCAGTT GCCCTTGAT-3′
and 5′-TTTGAACACCAGGTGCATGT-3′); and HDAC3
(5′-CACCTTTTCCAGCCAGTCAT-3′ and 5′-GTA GCCACCACCTCCCAGTA-3′). The
amplified DNA was electrophoresed on an agarose gel.
Nuclear extraction
The nuclear extraction was conducted using NE-PER
nuclear and cytoplasmic extraction reagents according to the
manufacturer’s instructions (Pierce, Rockford, IL, USA). Briefly,
the RAW264.7 cells were plated in 100-mm dishes, treated with
veratric acid, stimulated with LPS for 1 h, scraped into 3 ml of
cold PBS and pelleted by centrifugation. The cell pellets were
resuspended in ice-cold CER I buffer and incubated on ice for 10
min. The cells were then mixed with ice-cold CER II buffer, and
centrifuged at 12,000 × g for 5 min. The supernatant was
transferred to a pre-chilled tube. The insoluble fraction was
resuspended in ice-cold NER buffer, incubated on ice for 40 min,
and centrifuged at 12,000 × g for 10 min. The supernatant fraction
was used for analyzing the nuclear protein expression.
Western blot analysis
For preparing cell lysates, the RAW264.7 cells were
treated with veratric acid for 1 h and then stimulated with LPS for
the indicated periods of time. Following stimulation, the cells
were washed twice with cold PBS and scraped off. The scraped cells
were centrifuged and lysed with lysis buffer [50 mM Tris-Cl (pH
7.5), 150 mM NaCl, 1 mM DTT, 0.5% NP-40, 1% Triton X-100, 1%
deoxycholate, 0.1% SDS, 1 mM PMSF, 1 mM EDTA, 1 μM aprotinin, 1 μM
leupeptin and 1 μM pepstatin A] (Intron Biotechnology, Inc.,
Seongnam, Korea). For obtaining skin tissue lysates, at the end of
the desired treatments, tissue lysates were prepared in denaturing
buffer. Insoluble materials were discarded by centrifugation at
14,000 rpm for 20 min at 4°C. Protein content was measured using
the Bradford method. Equal amounts of protein were separated on 12%
SDS-polyacrylamide minigel. Proteins were transferred onto
nitrocellulose membranes (Pall Corp., Ann Arbor, MI, USA) and
subsequently blocked with 5% skim milk and phosphate-buffered
saline with Tween-20 (PBST; 135 μM NaCl, 2.7 mM KCl, 4.3 mM
NAPO4, 1.4 mM KH2PO4 and 0.5%
Tween-20) for 1 h at room temperature and probed overnight at 4°C
with primary antibodies (1:1,000). Primary antibodies, including
iNOS (#2977), phosphorylted (p-)p85 (#4228), p85 (#4257), p110α
(#4249), 110γ (#5405), p-PDK1 (#3238), PDK1 (#3062), p-Akt at
Thr308 (#2965), p-Akt at Ser473 (#4058), Akt (#9272), Ac-p300
(#4771), p-activating transcription factor 2 (ATF-2; #5112), PCAF
(#3378), HDAC1 (#5356), HDAC2 (#5513), HDAC3 (#3949), Ac-H3
(#9649), H3 (#4499), Ac-H4 (#2594), H4 (#2935), lamin A (#2032),
GAPDH (#2118) and β-actin (#4967), were purchased from Cell
Signaling Technology (Beverly, MA, USA). Primary antibodies to 110β
(sc-602), 110δ (sc-7176), p300 (sc-585), and ATF-2 (sc-187) were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Primary antibodies were rabbit-derived antibodies apart from the
primary antibodies to HDAC1, HDAC2 and HDAC3 that were detected by
anti-mouse IgG. After washing 3 times with PBST, the membranes were
incubated with horseradish peroxidase-conjugated anti-rabbit IgG as
secondary antibodies (1:10,000; Cell Signaling Technology) and
washed 3 times with PBST. Immunoreactive bands were detected using
an Enhanced chemiluminescence system detection system (Young In
Frontier Co., Ltd., Seoul, Korea) and exposed to X-ray film.
Densiometric analysis was conducted using ImageJ software [National
Institutes of Health (NIH), Bethesda, MD, USA].
Immunofluorescence staining
The expression of p-AKT in the RAW264.7 cells was
detected by indirect immunofluorescence assay. The RAW264.7
macrophages were seeded on coverglass bottom dishes for 24 h. The
cells were cultured with or without 200 μM of veratric acid for 1 h
prior to stimulation with LPS for 3 h. Following stimulation, the
cells were stained with 4, 6-diamidino-2-phenylindole (DAPI) (Roche
Diagnostics Corp., Indianapolis, IN, USA) for 15 min and fixed with
4% paraformaldehyde (Junsei Chemical Co., Ltd., Tokyo, Japan). The
fixed cells were blocked with 5% mouse and rabbit serum (Santa Cruz
Biotechnology Inc.) and polyclonal antibodies against p-AKT (1
μg/well) and 0.3% Triton X-100 were then applied for 1 h. The cells
were then incubated with anti-rabbit IgG tagged with Alexa Fluor
488 (Cell Signaling Technology) for 1 h and washed with PBS. The
cells were embedded with ProLong Antifade Reagent (Invitrogen,
Eugene, OR, USA). The samples were observed with an Nikon ECLIPSE
50i microscope equipped with a charged-coupled device (CCD) camera
(Nikon, Tokyo, Japan). To determine subcellular regions of protein
co-localization, individual red-, blue- and green-stained images
derived from the same field were merged using High Content Analysis
Software (Cambridge Healthtech Institute, Needham, MA, USA). The
pixel intensities of the nucleus were measured as a percentage area
of immunoreactivity with ImageJ software (NIH).
Statistical analyses
The data are expressed as the means ± standard
errors of the mean (SEM) of triplicate experiments. Each untreated
control group and LPS-stimulated group was measured for
statistically significant values by t-tests. To evaluate
statistically significant values between the LPS-stimulated group
and the veratric acid/LPS-treated group, ANOVA post hoc tests and
Dunnett’s multiple comparison tests were used. A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
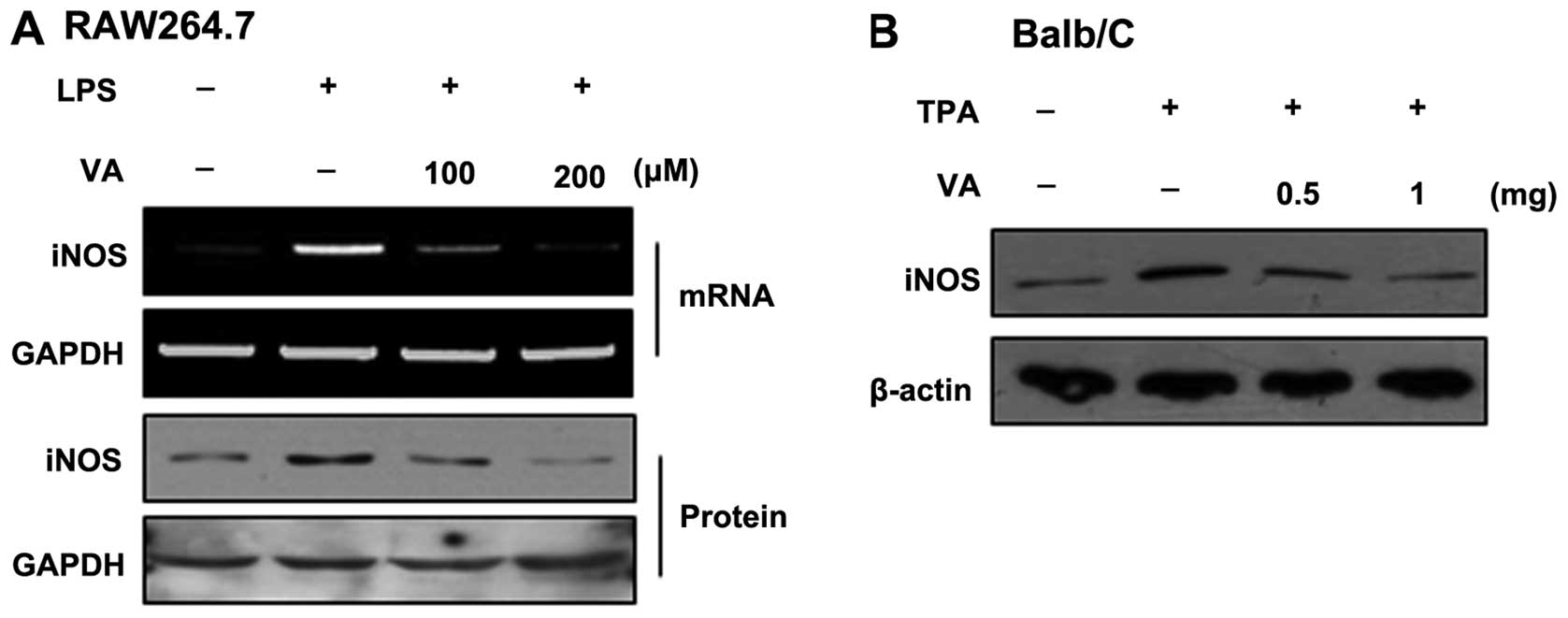
Effects of veratric acid on iNOS
expression in inflammatory cells and tissue
To evaluate the regulatory effects of veratric acid
on iNOS expression, LPS-stimulated RAW264.7 cells and Balb/C mice
with skin inflammation induced by TPA were treated with veratric
acid. iNOS protein expression was then measured. In the RAW264.7
cells, the LPS-induced iNOS expression was suppressed by veratric
acid in a dose-dependent manner (Fig.
1A). As shown by western blot analysis of the skin tissue
lysates, the topical application of TPA onto mice transiently
increased the iNOS protein level. However, treatment with veratric
acid decreased the iNOS protein level in comparison with the
TPA-treated mice (Fig 1B).
Collectively, veratric acid exerts a regulatory effect on iNOS
expression in inflammatory conditions.
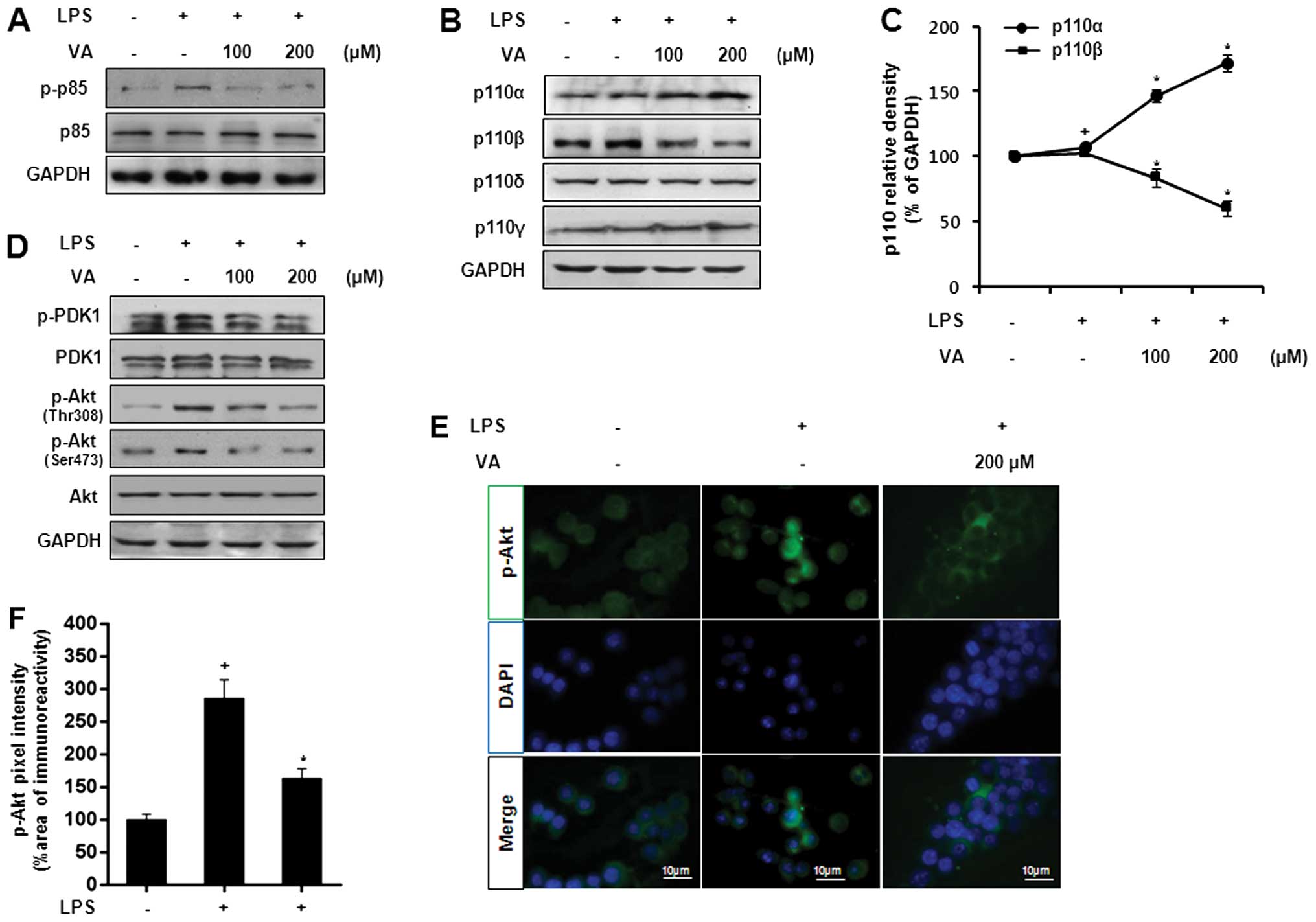
Effects of veratric acid on the
LPS-induced activation of the PI3K/Akt pathway
Previously, it has been demonstrated that the
increase in iNOS expression in macrophages is mediate through the
activation of PI3K and Akt (6).
Thus, in this study, we analyzed regulatory effects of veratric
acid on the LPS-induced activation of PI3K/Akt. Initially, the
phosphorylation of p85, a regulatory subunit of PI3K, was measured
at the protein level. As shown by western blot analysis, veratric
acid decreased the LPS-induced p85 phosphorylation in a
dose-dependent manner (Fig. 2A).
In the same manner, veratric acid enhanced p110α expression and
decreased p110β expression (Fig. 2B
and C). As veratric acid regulated LPS-induced PI3K activation,
we investigated the regulatory effects of veratric acid on PDK1 and
Akt. As shown in Fig. 2D,
veratric acid decreased the LPS-induced phosphorylation of PDK-1
and Akt. Immunofluorescenct staining revealed the translocation of
Akt from the cytosol to the nucleus in response to LPS, and this
translocation was abolished by treatment with veratric acid
(Fig. 2E and F). Taken together,
our results indicate that veratric acid exerts inhibitory effects
on the LPS-induced activation of the PI3K/Akt pathway in RAW264.7
cells.
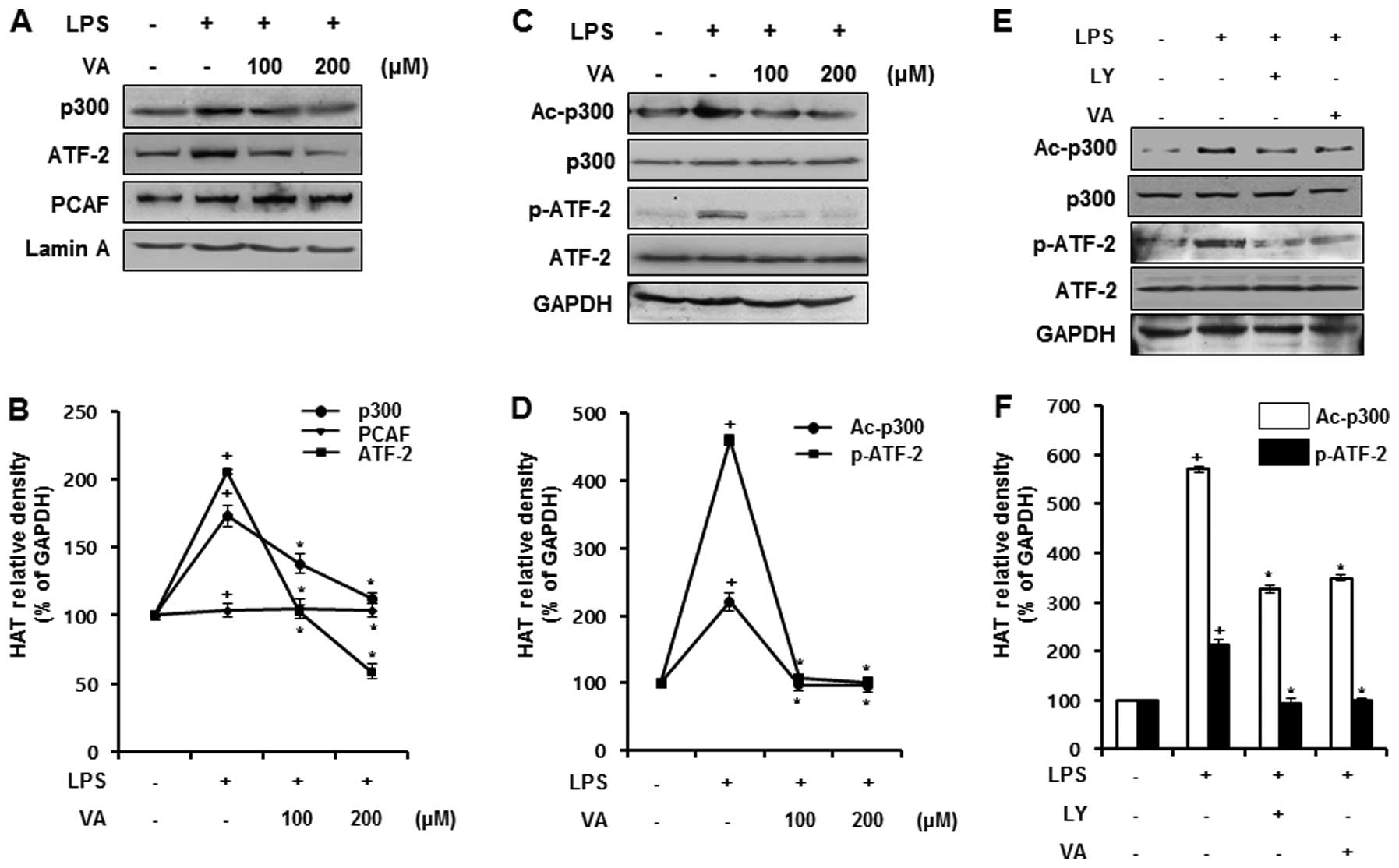
Effects of veratric acid on the
expression and activation of HATs in LPS-stimulated RAW264.7
cells
Akt activation promotes p300 HAT activity through
its association with PCAF. The acetylation of p300 at lysine 1499
is crucial to enhancing its HAT activity, resulting in an increase
in the acetylation of histone H3 or H4 (19,20). The phosphorylation of ATF-2 at
threonine (Thr)71 is regulated by PI3K/Akt signaling, and induces
its HAT activity (21,22). As we found that the LPS-induced
activation of the PI3K/Akt pathway was regulated by veratric acid,
we examined whether veratric acid regulates the activation and
expression of HATs, including p300, ATF-2 and PCAF in the
LPS-stimulated RAW264.7 cells. To examine the expression of the
HATs, RAW264.7 cells were treated with veratric acid for 1 h prior
to LPS stimulation for 12 h. At the protein level, the expression
of p300 and ATF-2 was increased by LPS stimulation, and reduced by
veratric acid in a dose-dependent manner. By contrast, PCAF
expression was not significantly altered by LPS and veratric acid
(Fig. 3A). As the expression of
ATF-2 and p300 were sensitively regulated by veratric acid, we
evaluated the regulatory effects of veratric acid on the
acetylation of p300 and the phosphorylation of ATF-2. The cells
were treated with veratric acid for 1 h, and then stimulated with
LPS for 1 h. As shown in Fig. 3B,
p300 acetylation and ATF-2 phosphorylation by LPS was observed, and
this effect was inhibited by treatment with veratric acid in a
concentration-dependent manner. As veratric acid inhibited the
LPS-induced activation of the PI3K/Akt pathway and HAT activation,
we wished to determine whether the inhibition of the PI3K/Akt
pathway is associated with the inactivation of p300 and ATF-2 in
LPS-stimulated RAW264.7 cells. To reveal the correlation between
the PI3K/Akt pathway and the activation of p300 and ATF-2, the
PI3K/Akt pathway was inhibited by a specific inhibitor, LY294002,
and the activity of HAT p300 and ATF-2 was examined by western blot
analysis. In comparison with the LY294002-treated cells, the
acetylation of p300 and the phosphorylation of ATF-2 were similarly
downregulated in the veratric acid-treated cells (Fig. 3C). Thus, our results suggest that
the LPS-induced activity of HAT p300 and ATF-2 is negatively
regulated by veratric acid, induced by the suppression of the
LPS-induced activation of the PI3K/Akt pathway.
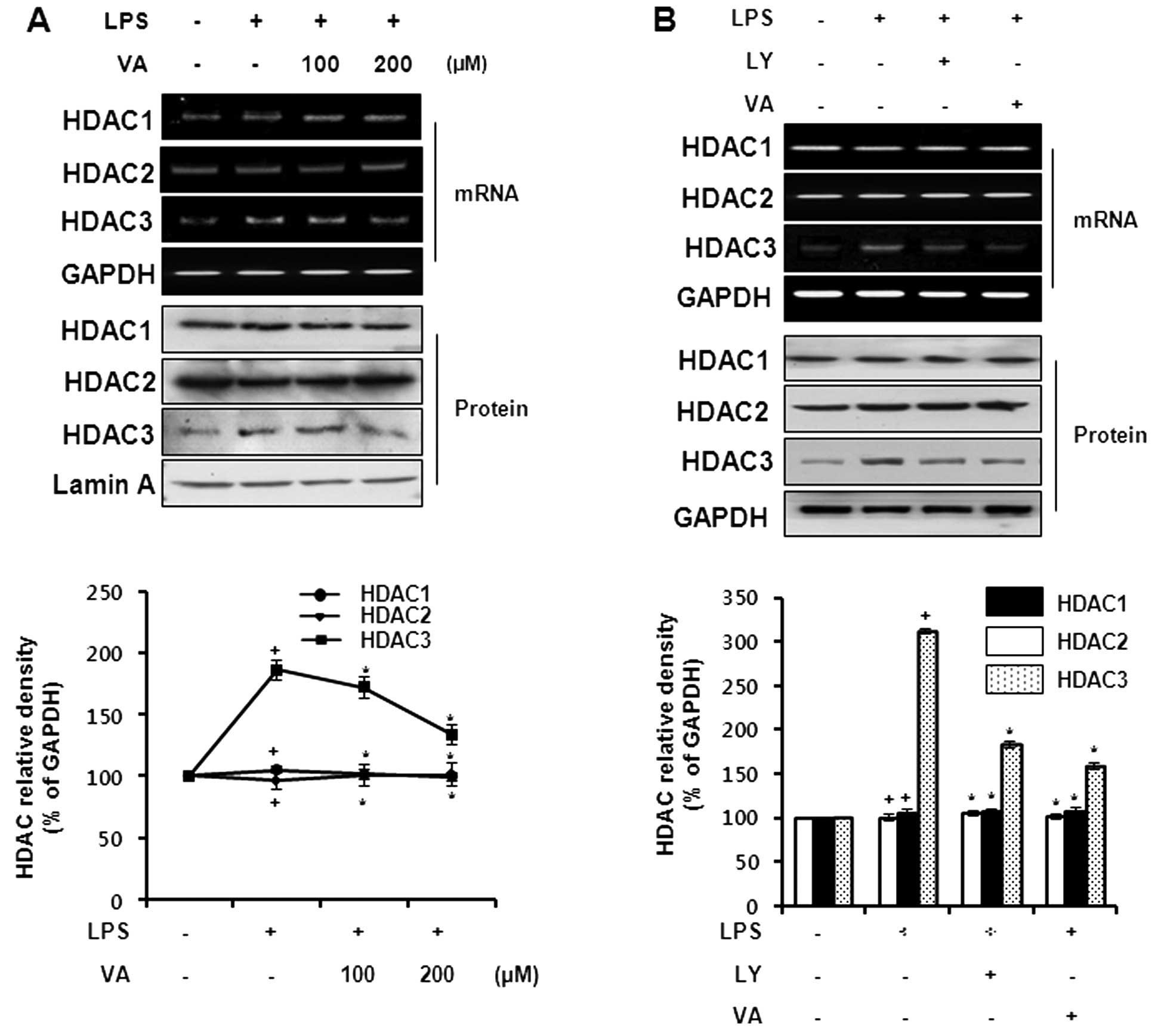
Effects of veratric acid on HDAC
expression in LPS-stimulated RAW264.7 cells
Chromatin remodeling events by HDAC are closely
related to inflammatory gene transcription (23). To evaluate the effects of veratric
acid on HDAC expression, we measured HDAC expression at the mRNA
and protein level. The cells were treated with veratric acid for 1
h prior to LPS stimulation for 8 h. As shown in Fig. 4A and B, the mRNA expression of
HDAC3 was increased by LPS stimulation, and was reduced to the
basal level following treatment with veratric acid. Neither HDAC1
nor HDAC2 expression was influenced by LPS and veratric acid
treatment. In accordance with the mRNA expression of HDACs, only
HDAC3 was significantly regulated by veratric acid at the protein
level. We also compared the expression of HDACs in veratric
acid-treated cells with that in LY294002-treated cells. The cells
were treated with veratric acid or LY294002 for 1 h, and in turn
stimulated with LPS for 8 h. The LPS-induced expression of HDAC3
was downregulated by LY294002 and veratric acid; however, the
expression of HDAC1 and HDAC2 was not affected in the same manner
(Fig. 4A and B). As suggested by
our results, the inhibitory effects of veratric acid on the
LPS-stimulated HDAC3 expression are mediated through the PI3K/Akt
signaling pathway.
Effects of veratric acid on LPS-induced
histone acetylation
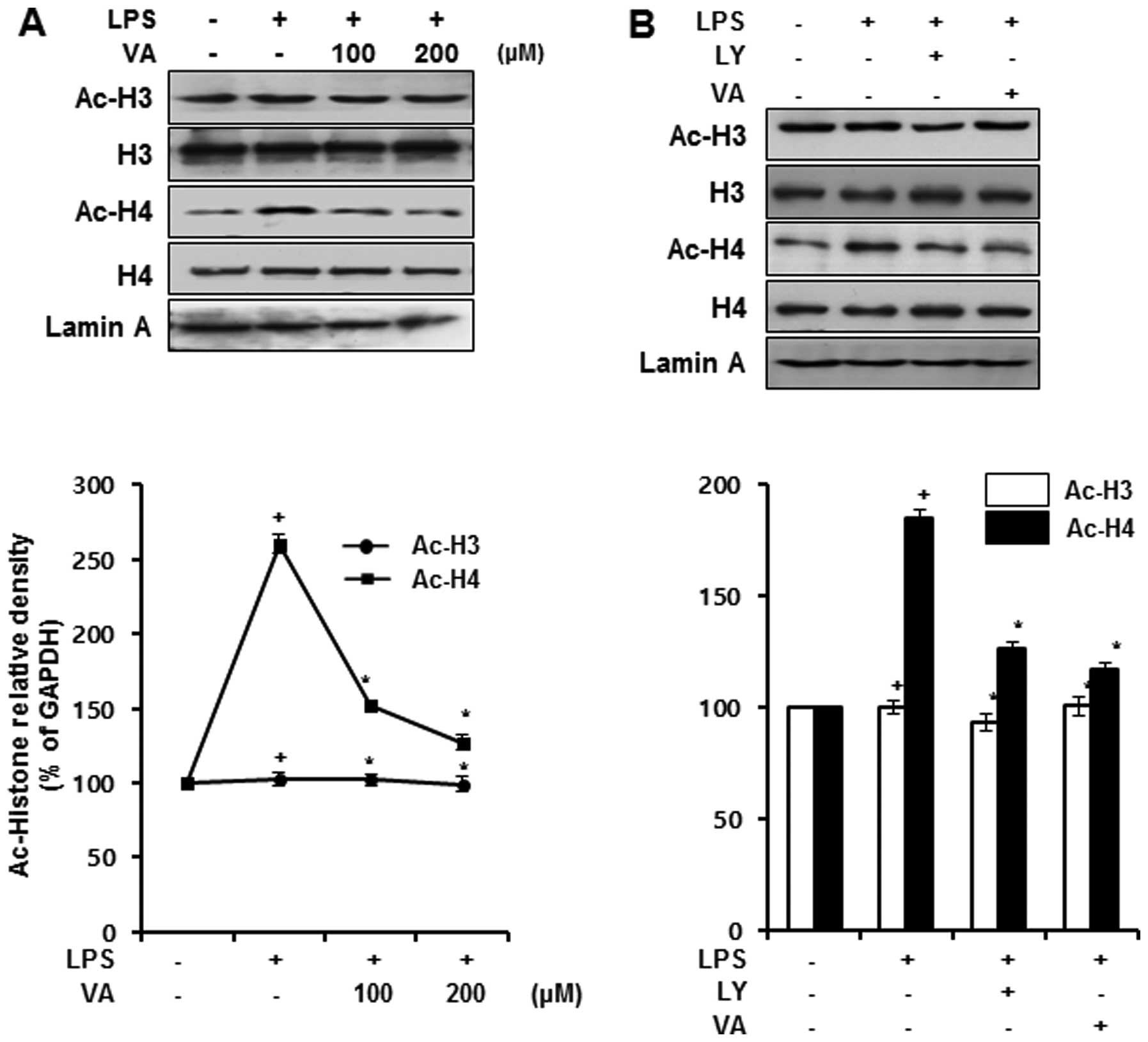
To confirm whether the regulation of HATs and HDACs
by veratric acid is associated with histone acetylation, we
measured the overall level of acetylation of histones at the
protein level. The nuclear proteins were extracted from the cells
treated with veratric acid for 1 h prior to LPS stimulation for 1
h. LPS stimulated the acetylation of histone H4; this acetylation
was downregulated by veratric acid in a dose-dependent manner. By
contrast, no changes were observed in the acetylation levels of
histone H3 following treatment with LPS and veratric acid (Fig. 5A). As treatment with LY294002
downregulated the acetylation of p300 and the phosphorylation of
ATF-2, we hypothesized that LY294002 may attenuate the activity of
HAT p300 and ATF-2. To demonstrate our hypothesis, we compared the
acetylation levels of histone H3 and H4 in the LY294002-treated
cells and those treated with veratric acid by western blot
analysis. LPS promoted the acetylation of histone H4, and this
acetylation was downregulated by LY294002, which coincided with the
acetylation levels of histone H4 in the veratric acid-treated cells
(Fig. 5B). Hence, our results
indicated that veratric acid suppressed the LPS-induced histone H3
acetylation, and suggested that the suppression of the LPS-induced
histone H4 acetylation by veratric acid may be accompanied by the
regulation of the PI3K/Akt pathway.
Discussion
In a previous study, we proved that veratric acid
downregulates the LPS-induced expression of iNOS in RAW264.7 cells
by inhibiting the activation of MAPKs and transcription factors,
including signal transducer and activator of transcription (STAT)1,
STAT3 and NF-κB (18). In the
present study, we demonstrated that treatment with veratric acid
suppressed the LPS-induced effects on p85, p110β and Akt in a
dose-dependent manner. The acetylation levels of histone H4 were
increased by LPS stimulation; however, following treatment with
veratric acid, the acetylation levels returned to basal levels. The
results from western blot analysis for HATs and HDACs provided
additional evidence of the inhibitory effects of veratric acid on
histone H4.
Class IA PI3K forms a heterodimer,
composed of catalytic and regulatory subunits. There are 3
catalytic subunits, including p110α, β and δ, and 5 regulatory
subunits, including p85α, p85β, p55α, p55γ and p50α. Class
IB PI3K is expressed preferentially in leukocytes, and
is also composed of catalytic and regulatory subunits, p110γ and
p101, respectively (24,25). PI3K is recruited to the inner
surface of the plasma membrane, and converts PI(4,5)P2
into PI(3,4,5)P3, which results in the recruitment and
activation of PDK-1 at the plasma membrane. Akt is then
phosphorylated by PDK-1 at Thr308 and activated (26). Our findings indicated that LPS
stimulated the phosphorylation of p85, PDK-1 and Akt, and increased
p110β expression. However, treatment with veratric acid reversed
the LPS-induced effects on these proteins (Fig. 2A–C). It has been reported that
p85, p110 and Akt are tightly linked to the regulation of iNOS
expression. The catalytic subunits of PI3K, including p110α and
p110β, regulate Akt phosphorylation in LPS-stimulated macrophages.
In p110α-deficient macrophages, Akt is strikingly phosphorylated by
LPS stimulation, whereas the LPS-induced Akt phosphorylation is not
properly induced in p110β-deficient macrophages (27). Sheu et al (28) demonstrated that PI3K is associated
with the regulation of iNOS expression in mesangial cells. The LPS
and IFN-γ-induced iNOS expression in mesangial cells was
downregulated by the PI3K/Akt inhibitor, LY294002. Another recent
study demonstrated that psoralidin suppresses the LPS-induced
expression of p85 and Akt in RAW264.7 cells, resulting in the
decrease of iNOS expression (29). Our findings indicated that the
treatment of the skin of mice with inflammation induced by TPA and
LPS-stimulated RAW264.7 cells with veratric acid attenuated iNOS
expression (Fig. 1). Taken
together, these observations suggested that veratric acid inhibited
the LPS-induced Akt activation through the regulation of the
LPS-induced effects on p85, p110α and p110β, thereby suppressing
iNOS expression.
It has been reported that PI3K/Akt signaling is
required to maintain the stability and HAT function of p300,
closely related to iNOS expression. In LY294002-treated HeLa cells,
p300 is not sufficiently expressed, and its transcriptional
function related to retinoic acid receptor signaling is impaired,
while stable cells expressing constitutive active Akt maintain the
steady-state level and function of p300 in a similar manner
(30). Likewise, Akt activation
by suberoylanilide hydroxamic acid (SAHA)-induced HDAC inhibition
stimulates p300 phosphorylation, resulting in the augmentation of
NF-κB acetylation. Activated Akt, p300 and NF-κB are recruited to
the NF-κB-regulated promoters, including cIAP-2 and Bfl-1/A1
promoters. However, LY294002 treatment downregulates the
SAHA-induced p300 phosphorylation, and in turn suppresses NF-κB
acetylation (31). In
macrophages, acetylated p300 elicits the acetylation of p50 in the
nucleus, accentuating iNOS expression by increasing NF-κB binding
to its corporate sites. Roscovitine, an inhibitor of cyclin E-CdK2,
abrogates p300 HAT activity, and then impedes iNOS promoter
activity induced by LPS/IFN-γ (32). These findings underscore the
distinct roles of Akt-mediated p300 acetylation in the modulation
of iNOS expression. In this study, in accordance with the
acetylation levels of p300 in the LY294002-treated cells, p300
acetylation in the veratric acid-treated cells returned to levels
comparable to those observed in the control cells, suggesting that
veartric acid blocks the LPS-induced p300 acetylation through the
suppression of PI3K/Akt signaling (Fig. 3). Not only p300 acetylation, but
also ATF-2 phosphorylation is associated with iNOS expression
through its HAT function. It has been reported that ATF-2
phosphorylation at Thr71 induces HAT function. Under conditions of
leucine deprivation, ATF-2 is rapidly phosphorylated; histone H4
and H2B are markedly acetylated, promoting the transcriptional
activation of CHOP, a CCAAT/enhancer-binding protein-related gene
(33). ATF-2 phosphorylation at
Thr71 enhances iNOS expression by advanced glycation end products
(AGEs), while SB203580, a specific inhibitor of p38, suppresses the
AGE-induced ATF-2 activation, resulting in a decrease in iNOS
expression (34). PI3K/Akt
signaling also affects ATF-2 phosphorylation. In regenerating gene
(Reg)-overexpressed β-cells, ATF-2 phosphorylation is
markedly amplified, increasing cyclin D1 promoter activity.
However, LY294002 treatment returned the amplified ATF-2
phosphorylation to the basal level observed in the control group
(21). Our findings demonstrated
that the LPS-induced ATF-2 phosphorylation was attenuated by
veratric acid and LY294002 (Fig.
3). Thus, the regulatory effects of veratric acid on CBP/p300
acetylation and ATF-2 phosphorylation have important implications
for the PI3K/Akt-mediated regulation of iNOS expression in
LPS-stimulated macrophages.
We found that the LPS-induced expression of HDAC1
and HDAC3 was reduced following treatment with veratric acid, and
there was no apparent change in HDAC2 expression folloiwng
treatment with LPS and veratric acid (Fig. 4A). It has been reported that class
I HDACs, including HDAC1, HDAC2 and HDAC3, closely correlate with
iNOS expression. The specific knockdown of HDAC1, HDAC2 and HDAC3
in INS cells significantly reduces cytokine-induced iNOS
expression, while the knockdown of only HDAC3 modulates the binding
of NF-κB to the iNOS promoter (35). HDAC3 physically interacts with a
NF-κB p65 subunit in the nucleus, and deacetylates tumor necrosis
factor (TNF)-α-induced p65 acetylation, promoting the export of p65
from the nucleus to the cytoplasm (36). HDAC1 and HDAC2 also directly
interact with the p65 protein of NF-κB, exerting its corepressor
function through the interaction and enhancing cytokine induction
of iNOS promoter (37,38). HDAC3 is closely related to the
regulation of Akt phosphorylation. HDAC3 depletion inhibits
TGF-β-induced Akt phosphorylation in murine fibroblasts (39), and reduces Akt phosphorylation in
Drosophila which antagonizes PI3K-induced tissue overgrowth
(40). These data suggest that
HDAC1 and HDAC3 activity is required for iNOS expression through
the regulation of the activation of Akt and NF-κB. Our results,
therefore, suggest that the inhibitory effects of veratric acid on
the LPS-induced HDAC1 and HDAC3 expression may elicit the decrease
in iNOS expression associated with the negative regulation of Akt
phosphorylation.
Upon Toll-like receptor 4 (TLR4) activation, the
HATs, GCN5 and PCAF, are required for the inducible acetylation of
H4K5/K8/K12, resulting in the expression of primary response genes
(PRGs) via the prompt activation of transcription factors, such as
NF-κB. In contrast to the inducible acetylation of histone H4
lysine residues by PCAF and GCN5, CBP/p300 are recruited to many
PRG promoters in resting cells, and constitutively maintain low
levels of H3K9 acetylation at the promoters (41). The activation of HAT p300
amplifies the acetylation of histone H3 and H4 at the ICAM-1
promoter in response to TNF-α, facilitating PCAF recruitment to
p300 and histone H3 acetylation (20). In the case of ATF-2, it
selectively acetylates histone H2B and H4 through phosphorylation
at Thr71 and K5, K8 and K16 residues in histone H4 are specifically
acetylated by ATF-2 HAT activity (22). Studies have demonstrated the
differential histone acetylation at lysine residues under
inflammatory conditions. In a mouse model of mastitis and in a rat
model of colitis induced by Escherichia coli, significant
hyperacetylation at the histone H4K8 residue in inflammatory cells
was observed. The histone H4K8 hyperacetylation is caused by the
autoacetylation of p300, facilitating the expression of iNOS, MAPKs
and inflammatory cytokines. Histone H3K9 acetylation levels were
not significantly altered in the mastitis model, but the total
acetylation levels of histone H3 in the colitis model were
augmented (42,43). The reason why the acetylation
levels of histone H3 are differentially regulated in inflammatory
diseases is that histone H3 acetylation does not markedly and
rapidly responded to inflammatory signals comparable to histone H4
acetylation (44). Histone H4 is
promptly acetylated by inflammatory stimuli, and the
hyperacetylation of histone H4 at the iNOS promoter is associated
with cytokine-induced iNOS expression. For the transcription of the
iNOS gene, NF-κB should be bound to its promoter region,
placed in −971 to −962 and −85 to −76 from the transcriptional
start site of the murine iNOS gene. The region −978 to −710
of the murine iNOS promoter contains a κB enhancer element, which
is wound by histone H4. In murine mesangial cells, the inhibition
of PI3K and HDACs augments histone H4 acetylation at the iNOS
promoter, inducing the reduction of cytokine-induced iNOS
expression (45,46). In this study, we detected a
decrease in the LPS-induced H4K8 acetylation by veratric acid, and
there was no significant alteration in H3K9 acetylation by LPS and
veratric acid (Fig. 5),
suggesting that the suppression of H4K8 acetylation may be involved
in the inhibitory effects of veratric acid on iNOS expression.
Although we evaluated the alteration in LPS-induced overall H4K8
acetylation by veratric acid, further studies are required to
examine the veratric acid-mediated changes in the acetylation
levels of histone H4 at the iNOS promoter.
In conclusion, veratric acid plays an inhibitory
role in iNOS expression by inflammatory stimuli. Our results
suggest that it suppresses the LPS-induced effects on p85, p110α
and p110β, leading to Akt inactivation. The LPS-induced activity of
HAT p300 and ATF-2 and the expression of HDAC3 are downregulated by
veratric acid, thereby decreasing histone H4K8 acetylation. In
addition, the regulation of protein activation and expression by
veratric acid is consistent with that observed with treatment with
LY294002, a PI3K inhibitor. The inhibitory effects of veratric acid
on the PI3K/Akt pathway may thus elicit the suppression of histone
H4 acetylation through the inactivation of HATs and HDACs,
resulting in the downregulation of iNOS expression. Our data
suggest that veratric acid has potential for use as a therapeutic
agent for the transcriptional regulation of iNOS expression and may
contribute to alleviating inflammatory diseases through the
prevention of excessive NO production.
Acknowledgements
The present study was supported by the
Next-Generation BioGreen 21 Program (SSAC, NO. PJ009615) from the
Rural Development Administration.
References
|
1
|
Swantek JL, Christerson L and Cobb MH:
Lipopolysaccharide-induced tumor necrosis factor-α promoter
activity is inhibitor of nuclear factor-κB kinase-dependent. J Biol
Chem. 274:11667–11671. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma J, Al-Omran A and Parvathy S: Role
of nitric oxide in inflammatory diseases. Inflammopharmacology.
15:252–259. 2007. View Article : Google Scholar
|
|
3
|
Bogdan C: Nitric oxide and the immune
response. Nat Immunol. 2:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogdan C, Röllinghoff M and Diefenbach A:
The role of nitric oxide in innate immunity. Immunol Rev.
173:17–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamora R, Vodovotz Y and Billiar TR:
Inducible nitric oxide synthase and inflammatory diseases. Mol Med.
6:347–373. 2000.PubMed/NCBI
|
|
6
|
Sakai K, Suzuki H, Oda H, et al:
Phosphoinositide 3-kinase in nitric oxide synthesis in macrophage:
critical dimerization of inducible nitric-oxide synthase. J Biol
Chem. 281:17736–17742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luyendyk JP, Schabbauer GA, Tencati M,
Holscher T, Pawlinski R and Mackman N: Genetic analysis of the role
of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and
tissue factor gene expression in monocytes/macrophages. J Immunol.
180:4218–4226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pahan K, Raymond JR and Singh I:
Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide
synthase in lipopolysaccharide-or cytokine-stimulated C6 glial
cells. J Biol Chem. 274:7528–7536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pahan K, Liu X, Wood C and Raymond JR:
Expression of a constitutively active form of phosphatidylinositol
3-kinase inhibits the induction of nitric oxide synthase in human
astrocytes. FEBS Lett. 472:203–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teshima S, Nakanishi H, Nishizawa M, et
al: Up-regulation of IL-1 receptor through PI3K/Akt is essential
for the induction of iNOS gene expression in hepatocytes. J
Hepatol. 40:616–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choudhary C, Kumar C, Gnad F, et al:
Lysine acetylation targets protein complexes and co-regulates major
cellular functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghizzoni M, Haisma HJ, Maarsingh H and
Dekker FJ: Histone acetyltransferases are crucial regulators in
NF-κB mediated inflammation. Drug Discov Today. 16:504–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang WM, Tsai SC, Wen YD, Fejér G and Seto
E: Functional domains of histone deacetylase-3. J Biol Chem.
277:9447–9454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roth SY, Denu JM and Allis CD: Histone
acetyltransferases. Annu Rev Biochem. 70:81–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin HY and Chen CS, Lin SP, Weng JR and
Chen CS: Targeting histone deacetylase in cancer therapy. Med Res
Rev. 26:397–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raja B: Veratric acid, a phenolic acid
attenuates blood pressure and oxidative stress in l-NAME induced
hypertensive rats. Eur J Pharmacol. 671:87–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MY, Seguin P, Ahn JK, et al: Phenolic
compound concentration and antioxidant activities of edible and
medicinal mushrooms from Korea. J Agr Food Chem. 56:7265–7270.
2008. View Article : Google Scholar
|
|
18
|
Choi WS, Shin PG, Lee JH and Kim GD: The
regulatory effect of veratric acid on NO production in
LPS-stimulated RAW264.7 macrophage cells. Cell Immunol.
280:164–170. 2012. View Article : Google Scholar
|
|
19
|
Stiehl DP, Fath DM, Liang D, Jiang Y and
Sang N: Histone deacetylase inhibitors synergize p300
autoacetylation that regulates its transactivation activity and
complex formation. Cancer Res. 67:2256–2264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang WC and Chen CC: Akt phosphorylation
of p300 at Ser-1834 is essential for its histone acetyltransferase
and transcriptional activity. Mol Cell Biol. 25:6592–6602. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takasawa S, Ikeda T, Akiyama T, et al:
Cyclin D1 activation through ATF-2 in Reg-induced pancreatic β-cell
regeneration. FEBS Lett. 580:585–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawasaki H, Schiltz L, Chiu R, et al:
ATF-2 has intrinsic histone acetyltransferase activity which is
modulated by phosphorylation. Nature. 405:195–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao F, Gonzalo IG, Lanting L and
Natarajan R: In vivo chromatin remodeling events leading to
inflammatory gene transcription under diabetic conditions. J Biol
Chem. 279:18091–18097. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geering B, Cutillas PR, Nock G, Gharbi SI
and Vanhaesebroeck B: Class IA phosphoinositide 3-kinases are
obligate p85–p110 heterodimers. Proc Natl Acad Sci USA.
104:7809–7814. 2007. View Article : Google Scholar
|
|
25
|
Koyasu S: The role of PI3K in immune
cells. Nat Immunol. 4:313–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monick MM, Carter AB, Robeff PK, Flaherty
DM, Peterson MW and Hunninghake GW: Lipopolysaccharide activates
Akt in human alveolar macrophages resulting in nuclear accumulation
and transcriptional activity of β-catenin. J Immunol.
166:4713–4720. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukamoto K, Hazeki K, Hoshi M, et al:
Critical roles of the p110β subtype of phosphoinositide 3-kinase in
lipopolysaccharide-induced Akt activation and negative regulation
of nitrite production in RAW 264.7 cells. J Immunol. 180:2054–2061.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheu ML, Chao KF, Sung YJ, Lin WW,
Lin-Shiau SY and Liu SH: Activation of phosphoinositide 3-kinase in
response to inflammation and nitric oxide leads to the
up-regulation of cyclooxygenase-2 expression and subsequent cell
proliferation in mesangial cells. Cell Signal. 17:975–984. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiou WF, Don MJ, Liao JF and Wei BL:
Psoralidin inhibits LPS-induced iNOS expression via repressing
Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Eur J
Pharmacol. 650:102–109. 2011. View Article : Google Scholar
|
|
30
|
Chen J, Halappanavar S, St-Germain J,
Tsang B and Li Q: Role of Akt/protein kinase B in the activity of
transcriptional coactivator p300. Cell Mol Life Sci. 61:1675–1683.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Denlinger CE, Rundall BK, Smith PW
and Jones DR: Suberoylanilide hydroxamic acid induces Akt-mediated
phosphorylation of p300, which promotes acetylation and
transcriptional activation of RelA/p65. J Biol Chem.
281:31359–31368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng WG and Wu KK: Regulation of inducible
nitric oxide synthase expression by p300 and p50 acetylation. J
Immunol. 171:6581–6588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bruhat A, Chérasse Y, Maurin AC, et al:
ATF2 is required for amino acid-regulated transcription by
orchestrating specific histone acetylation. Nucleic Acid Res.
35:1312–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang PC, Chen TH, Chang CJ, Hou CC, Chan
P and Lee HM: Advanced glycosylation end products induce inducible
nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent
pathway. Kidney Int. 65:1664–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lundh M, Christensen D, Nielsen MD, et al:
Histone deacetylases 1 and 3 but not 2 mediate cytokine-induced
beta cell apoptosis in INS-1 cells and dispersed primary islets
from rats and are differentially regulated in the islets of type 1
diabetic children. Diabetologia. 55:2421–2431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen LF, Fischle W, Verdin E and Greene
WC: Duration of nuclear NF-κB action regulated by reversible
acetylation. Science. 293:1653–1657. 2001. View Article : Google Scholar
|
|
37
|
Yu Z, Zhang W and Kone BC: Histone
deacetylases augment cytokine induction of the iNOS gene. J Am Soc
Nephrol. 13:2009–2017. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ashburner BP, Westerheide SD and Baldwin
AS: The p65 (RelA) subunit of NF-κB interacts with the histone
deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively
regulate gene expression. Mol Cell Biol. 21:7065–7077. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barter MJ, Pybus L, Litherland GJ, et al:
HDAC-mediated control of ERK-and PI3K-dependent TGF-β-induced
extracellular matrix-regulating genes. Matrix Biol. 29:602–612.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv WW, Wei HM, Wang DL, Ni JQ and Sun FL:
Depletion of histone deacetylase 3 antagonizes PI3K-mediated
overgrowth of Drosophila organs through the acetylation of histone
H4 at lysine 16. J Cell Sci. 125:5369–5378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hargreaves DC, Horng T and Medzhitov R:
Control of inducible gene expression by signal-dependent
transcriptional elongation. Cell. 138:129–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Modak R, Mitra SD, Krishnamoorthy P, et
al: Histone H3K14 and H4K8 hyperacetylation is associated with
Escherichia coli-induced mastitis in mice. Epigenetics. 7:492–501.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsaprouni LG, Ito K, Powell JJ, Adcock IM
and Punchard N: Differential patterns of histone acetylation in
inflammatory bowel diseases. J Inflamm. 8:12011. View Article : Google Scholar
|
|
44
|
Ito K, Barnes PJ and Adcock IM:
Glucocorticoid receptor recruitment of histone deacetylase 2
inhibits interleukin-1β-induced histone H4 acetylation on lysines 8
and 12. Mol Cell Biol. 20:6891–6903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lowenstein CJ, Alley EW, Raval P, et al:
Macrophage nitric oxide synthase gene: two upstream regions mediate
induction by interferon gamma and lipopolysaccharide. Proc Natl
Acad Sci USA. 90:9730–9734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu Z and Kone BC: Targeted histone H4
acetylation via phosphoinositide 3-kinase-and
p70s6-kinase-dependent pathways inhibits iNOS induction in
mesangial cells. Am J Physiol Renal Physiol. 290:F496–F502. 2006.
View Article : Google Scholar
|