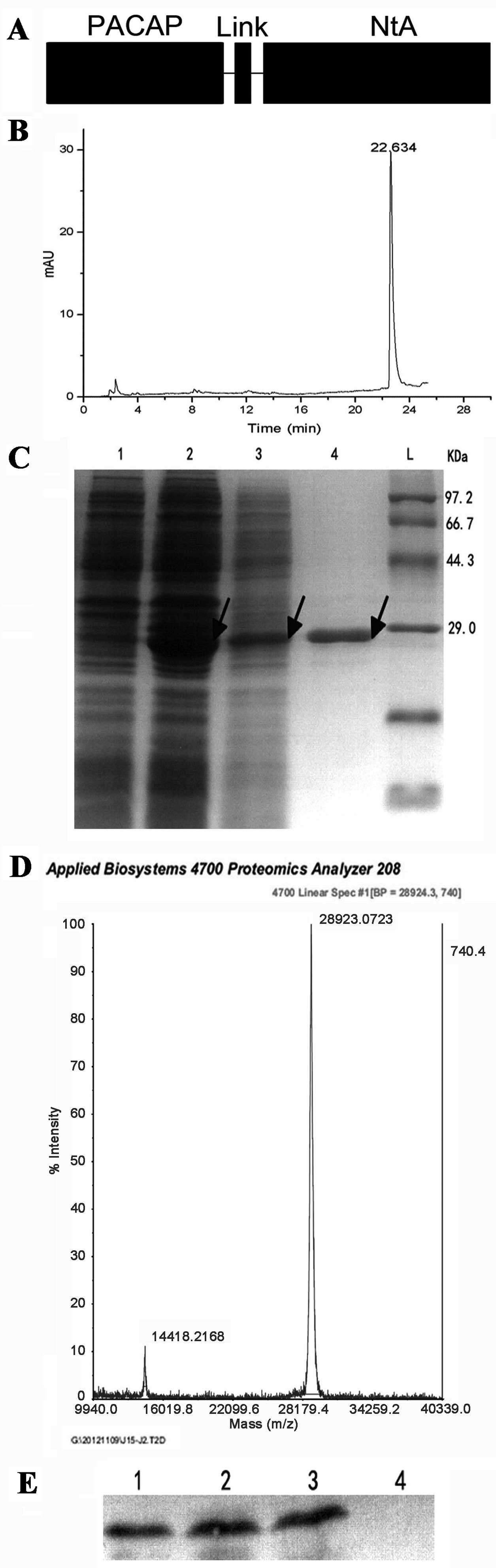
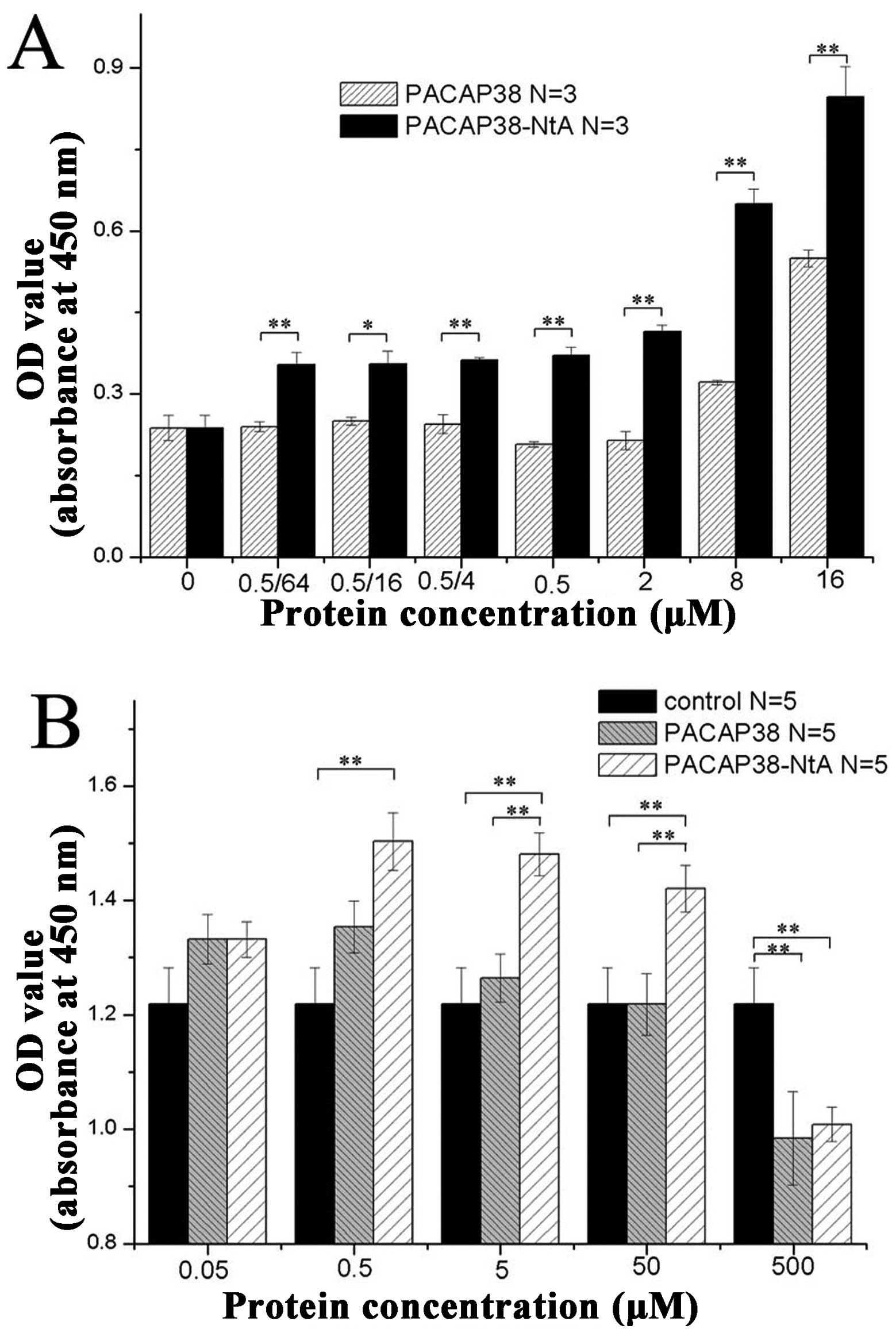
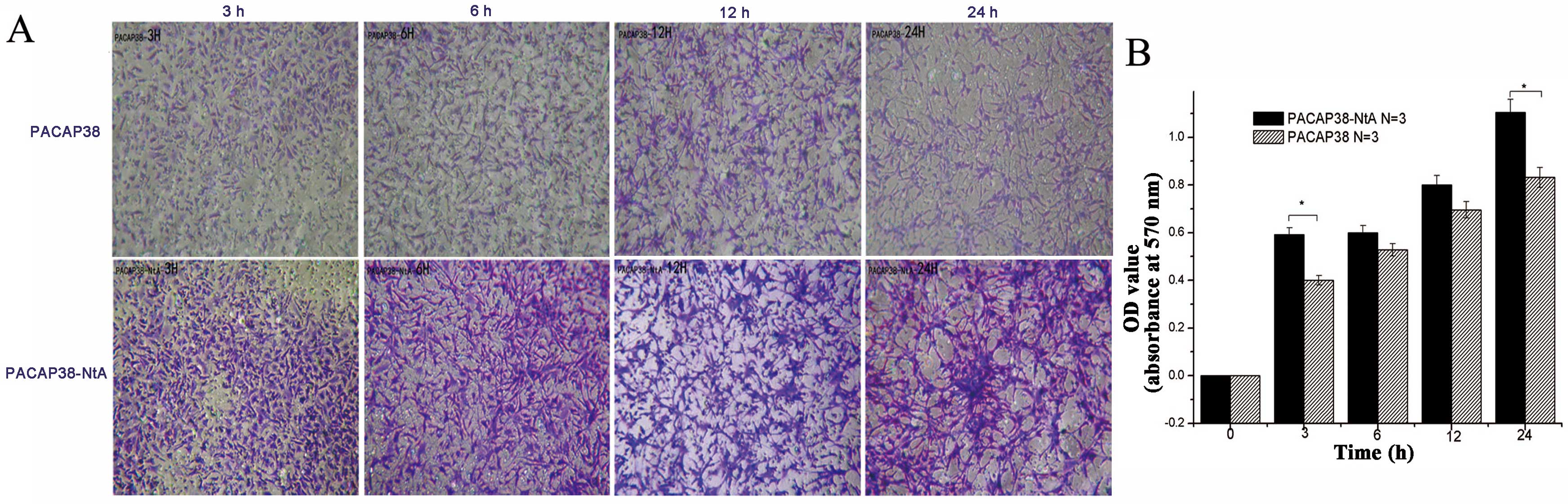
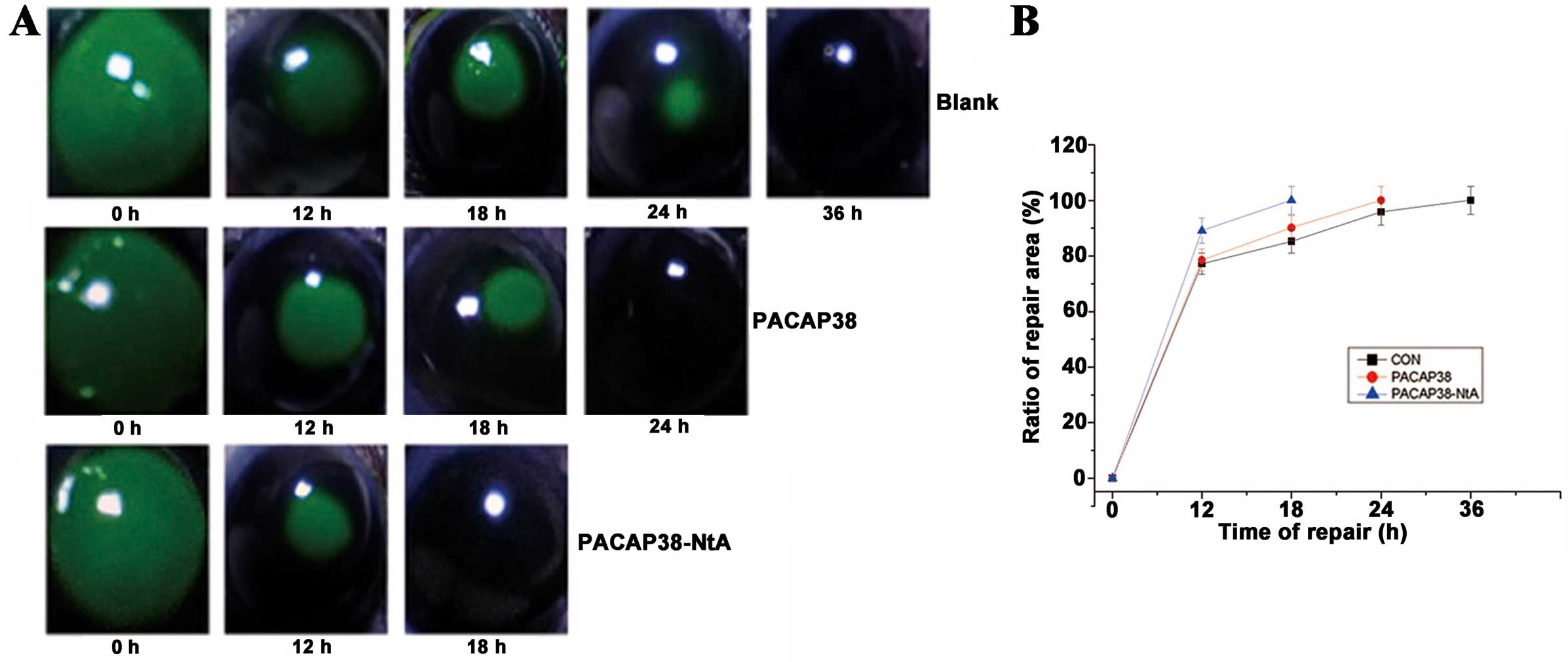
|
1
|
Miyata A, Arimura A, Dahl RR, et al:
Isolation of a novel 38 residue-hypothalamic polypeptide which
stimulates adenylate cyclase in pituitary cells. Biochem Biophys
Res Commun. 164:567–574. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wray V, Kakoschke C, Nokihara K and Naruse
S: Solution structure of pituitary adenylate cyclase activating
polypeptide by nuclear magnetic resonance spectroscopy.
Biochemistry. 32:5832–5841. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jüppner H, Schipani E, Bringhurst FR, et
al: The extracellular amino-terminal region of the parathyroid
hormone (PTH)/PTH-related peptide receptor determines the binding
affinity for carboxyl-terminal fragments of PTH (1-34).
Endocrinology. 134:879–884. 1994.
|
|
4
|
Wei Y and Mojsov S: Tissue specific
expression of different human receptor types for pituitary
adenylate cyclase activating polypeptide and vasoactive intestinal
polypeptide: implications for their role in human physiology. J
Neuroendocrinol. 8:811–817. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gou rlet P and Va nder me ers A:
Vasoactive intestinal peptide (VIP) and pituitary adenylate
cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation
by the neutral endopeptidase EC 3.4.24.11. Biochem Pharmacol.
54:509–515. 1997. View Article : Google Scholar
|
|
6
|
Ohkubo S, Kimura C, Ogi K, et al: Primary
structure and characterization of the precursor to human pituitary
adenylate cyclase activating polypeptide. DNA Cell Biol. 11:21–30.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaudry D, Gonzalez BJ, Basille M, Yon L,
Fournier A and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: from structure to functions.
Pharmacol Rev. 52:269–324. 2000.PubMed/NCBI
|
|
8
|
Inooka H, Ohtaki T, Kitahara O, et al:
Conformation of a peptide ligand bound to its G-protein coupled
receptor. Nat Struct Biol. 8:161–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimizu N, Guo J and Gardella TJ:
Parathyroid hormone (PTH)-(1-14) and -(1-11) analogs
conformationally constrained by alpha-aminoisobutyric acid mediate
full agonist responses via the juxtamembrane region of the PTH-1
receptor. J Biol Chem. 276:49003–49012. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tibaduiza EC, Chen C and Beinborn M: A
small molecule ligand of the glucagon-like peptide 1 receptor
targets its amino-terminal hormone binding domain. J Biol Chem.
276:37787–37793. 2001.PubMed/NCBI
|
|
11
|
Miyata A, Jiang L, Dahl RD, et al:
Isolation of a neuropeptide corresponding to the N-terminal 27
residues of the pituitary adenylate cyclase activating polypeptide
with 38 residues (PACAP38). Biochem Biophy Res Commun. 170:643–648.
1990. View Article : Google Scholar
|
|
12
|
Inooka H and Shirakawa M: Conformation of
a peptide ligand bound to its G-protein coupled receptor and its
implication for ligand transportation. Tanpakushitsu Kakusan Koso.
47:787–793. 2002.In Japanese. PubMed/NCBI
|
|
13
|
Laburthe M and Couvineau A: Molecular
pharmacology and structure of VPAC Receptors for VIP and PACAP.
Regul Pept. 108:165–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laburthe M, Couvineau A and Marie JC: VPAC
receptors for VIP and PACAP. Receptors Channels. 8:137–153. 2002.
View Article : Google Scholar
|
|
15
|
Runge S, Wulff BS, Madsen K,
Brauner-Osborne H and Knudsen LB: Different domains of the glucagon
and glucagon-like peptide-1 receptors provide the critical
determinants of ligand selectivity. Br J Pharmacol. 138:787–794.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shioda S, Shuto Y, Somogyvari-Vigh A, et
al: Localization and gene expression of the receptor for pituitary
adenylate cyclase-activating polypeptide in the rat brain. Neurosci
Res. 28:345–354. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nowak JZ and Zawilska JB: PACAP in avians:
origin, occurrence, and receptors-pharmacological and functional
considerations. Curr Pharm Des. 9:467–481. 2003. View Article : Google Scholar
|
|
18
|
Bourgault S, Vaudry D, Segalas-Milazzo I,
et al: Molecular and conformational determinants of pituitary
adenylate cyclase-activating polypeptide (PACAP) for activation of
the PAC1 receptor. J Med Chem. 52:3308–3316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linden A, Hansson L, Andersson A, et al:
Bronchodilation by an inhaled VPAC (2) receptor agonist in patients
with stable asthma. Thorax. 58:217–221. 2003. View Article : Google Scholar
|
|
20
|
Yuhara A, Nishio C, Abiru Y, Hatanaka H
and Takei N: PACAP has a neurotrophic effect on cultured basal
forebrain cholinergic neurons from adult rats. Brain Res Dev Brain
Res. 131:41–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laburthe M, Couvineau A and Tan V: Class
II G protein-coupled receptors for VIP and PACAP: structure, models
of activation and pharmacology. Peptides. 28:1631–1639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hautmann M, Friis UG, Desch M, et al:
Pituitary adenylate cyclase-activating polypeptide stimulates renin
secretion via activation of PAC1 receptors. J Am Soc Nephrol.
18:1150–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cazillis M, Gonzalez BJ, Billardon C, et
al: VIP and PACAP induce selective neuronal differentiation of
mouse embryonic stem cells. Eur J Neurosci. 19:798–808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Yu R, Liu X, Guo X and Zeng Z:
The expression of PAC1 increases in the degenerative thymus and low
dose PACAP protects female mice from cyclophosphamide induced
thymus atrophy. Peptides. 38:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harmar AJ, Fahrenkrug J, Gozes I, et al:
Pharmacology and functions of receptors for vasoactive intestinal
peptide and pituitary adenylate cyclase-activating polypeptide:
IUPHAR review 1. B J Pharmacol. 166:4–17. 2012. View Article : Google Scholar
|
|
26
|
Racz B, Gasz B, Borsiczky B, et al:
Protective effects of pituitary adenylate cyclase activating
polypeptide in endothelial cells against oxidative stress-induced
apoptosis. Gen Comp Endocrinol. 153:115–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horvath G, Brubel R, Kovacs K, et al:
Effects of PACAP on oxidative stress-induced cell death in rat
kidney and human hepatocyte cells. J Mol Neurosci. 43:67–75. 2011.
View Article : Google Scholar
|
|
28
|
Horvath G, Reglodi D, Opper B, et al:
Effects of PACAP on the oxidative stress-induced cell death in
chicken pinealocytes is influenced by the phase of the circadian
clock. Neurosci Lett. 484:148–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukiage C, Nakajima T, Takayama Y,
Minagawa Y, Shearer TR and Azuma M: PACAP induces neurite outgrowth
in cultured trigeminal ganglion cells and recovery of corneal
sensitivity after flap surgery in rabbits. Am J Ophthalmol.
143:255–262. 2007. View Article : Google Scholar
|
|
30
|
Wang ZY, Alm P and Hakanson R:
Distribution and effects of pituitary adenylate cyclase-activating
peptide in the rabbit eye. Neuroscience. 69:297–308. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu SY and Gordon T: The cellular and
molecular basis of peripheral nerve regeneration. Mol Neurobiol.
14:67–116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daggett DF, Cohen MW, Stone D, Nikolics K,
Rauvala H and Peng HB: The role of an agrin-growth factor
interaction in ACh receptor clustering. Mol Cell Neurosci.
8:272–285. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun W, Sun C, Zhao H, et al: Improvement
of sciatic nerve regeneration using laminin-binding human NGF-beta.
PloS One. 4:e61802009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smit M, Leng J and Klemke RL: Assay for
neurite outgrowth quantification. Biotechniques. 35:254–256.
2003.PubMed/NCBI
|
|
35
|
Li Z, Burns AR, Han L, Rumbaut RE and
Smith CW: IL-17 and VEGF are necessary for efficient corneal nerve
regeneration. Am J Pathol. 178:1106–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SJ, Kim JK, Seo KY, Kim EK and Lee HK:
Comparison of corneal nerve regeneration and sensitivity between
LASIK and laser epithelial keratomileusis (LASEK). Am J Ophthalmol.
141:1009–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waschek JA: Multiple actions of pituitary
adenylyl cyclase activating peptide in nervous system development
and regeneration. Dev Neurosci. 24:14–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Somogyvári-Vigh A and Reglodi D: Pituitary
adenylate cyclase activating polypeptide: a potential
neuroprotective peptide. Curr Pharma Des. 10:2861–2889. 2004.
View Article : Google Scholar
|
|
39
|
Li M, Maderdrut JL, Lertora JJ and Batuman
V: Intravenous infusion of pituitary adenylate cyclase-activating
polypeptide (PACAP) in a patient with multiple myeloma and myeloma
kidney: a case study. Peptides. 28:1891–1895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mentlein R: Dipeptidyl-peptidase IV (CD26)
- role in the inactivation of regulatory peptides. Regul Pept.
85:9–24. 1999. View Article : Google Scholar : PubMed/NCBI
|