Introduction
The pituitary adenylate cyclase-activating
polypeptide (PACAP) is a polypeptide with various biological
activities that was discovered in the study by Miyata et al
(1), which was investigating the
hypothalamic hypophysiotropic hormone in 1989 (1–5)
and it is also a new member in the family of
secretin/glucagon/vasoactive intestinal peptide (VIP) (6–10).
According to the previous studies, PACAP exists in the body in two
forms; PACAP38 with 38 types of amino acid and PACAP27 with 11
types of amino acid but without the C-terminus (11–15). PACAP and its receptor are
distributed in the central and peripheral nervous systems, as well
as the surrounding tissues and organs, such as the pancreas,
pancreas islet, digestive tract and genital glands (7,16,17).
PACAP mainly has three types of receptors, which are
PAC1R, VPAC1R and VPAC2R. The first is the main acceptor of PACAP,
whereas the other two are the common acceptors of PACAP and VIP
(3,7,18).
The three types of receptors are widely spread in the
cardiovascular, respiratory, genital and nervous systems (3,7,19)
The content of PAC1R in the central nervous system is higher
compared to VPAC1R and VPAC2R, and PAC1R spreads wider than the
other two in central nervous system (7,20,21). Studies have shown that PACAP
promotes the repairing of injured nervous tissue (20,22), as well as the differentiation of
embryonic stem cells into neurons (23). During this process, PAC1R acceptor
expression increases and PAC1R also improves the survival amount of
neurons following spinal cord compression (24–28). In the eyes, PACAP and its acceptor
are mainly distributed in the cornea, iris, Schacher’s ganglion,
choroid membranes and retina (7,29).
The study by Wang et al (30) discovered that in the inflammatory
responses of the ocular surface, PACAP, as the neurotransmitter of
C-fibers, plays a positive feedback regulation role in the release
of inflammatory factors from C-fibers. PACAP and its main acceptor
PAC1 are widely spread in the eyes. PACAP is nutritious, and it can
repair the nerve and also promote the repair of the corneal
epithelium and regulation of the ocular inflammatory reaction.
Therefore, it plays a vital role in the neural restoration and the
recovery of cornea sensory following corneal flap surgery.
N-terminal agrin domain (NtA) is the receptor
protein of laminin, which is a type of proteoglycan that exists in
the extracellular matrix, (ECM) and was firstly obtained following
separation from the ECM of the Torpedo electric organ synapses by
Fu and Gordon (31). The
C-terminal structural domain of agrin covers all the regions that
may interact with the surface of the skeletal muscle, particularly
the three independent spherical G-shaped structural regions, and it
can also induce the congregation of acetylcholine receptors,
similar to the full-length agrin (32). Therefore, only acting as an
‘anchoring region’, the NtA of agrin allows its functional region,
which is the combination of C-terminal with other specific parts,
to perform its biological function. In 2009, Sun et al
(33) reported that a fusion
protein [known as LBD-nerve growth factor (NGF)] had been
constructed by combining the NtA of agrin with NGF, and the fusion
protein promotes the effect of repairing nerve regeneration.
In the present study, the C-terminal of PACAP38 and
NtA were connected through a linker peptide (including 16 types of
amino acid), constructing the fusion protein PACAP38-NtA.
Theoretically, the recombination polypeptide has the biological
activity of PACAP, and it can be combined with laminin to improve
the remediation efficiency of the PACAP polypeptide. Furthermore,
it can avoid the cutting of the C-terminal from PACAP38 by
carboxypeptidase, thus preventing the decrease of receptor-binding
capacity. In addition, it shows that the Schwann cells aggregate in
the nerve injury, and significantly express laminin, indicating
that the polypeptide can combine the cells in the neural injury and
improve the effect of injury repair.
Materials and methods
Expression and purification
The structure of the recombinant DNA segment of
PACAP38-NtA that was applied for the patent of invention (ID:
201310057657.7) is shown in (Fig.
1A). The DNA segment was chemically synthesized by GenScript,
Inc. (Piscataway, NJ, USA). Following PCR amplification, the target
segment was transferred into the carrier pET-3c, obtaining the
cloning vector bacteria of the recombinant DNA segment.
Subsequently, it was transferred to expression bacteria BL21 (DE3),
achieving enough expression bacteria with the protein of interest
after inducing with 1 mmol/l isopropylthiogalactoside (IPTG) at
30°C for 4 h. The protein of interest was obtained through crushing
bacteria and centrifugation was purified by the two-step
purification; anion-exchange chromatography and Ni-column affinity
chromatography. The purity was tested with SDS-PAGE analysis,
western blotting detection for the PACAP antibody [rabbit
anti-human PACAP38, 1:1000, Sigma, St. Louis, MO, USA; goat
anti-rabbit immunoglobulin G (IgG) (heavy and light), 1:3000, Cell
Signaling Technology, Danvers, MA, USA] and high-performance liquid
chromatography (HPLC), and its molecular weight was tested with
mass spectrometry.
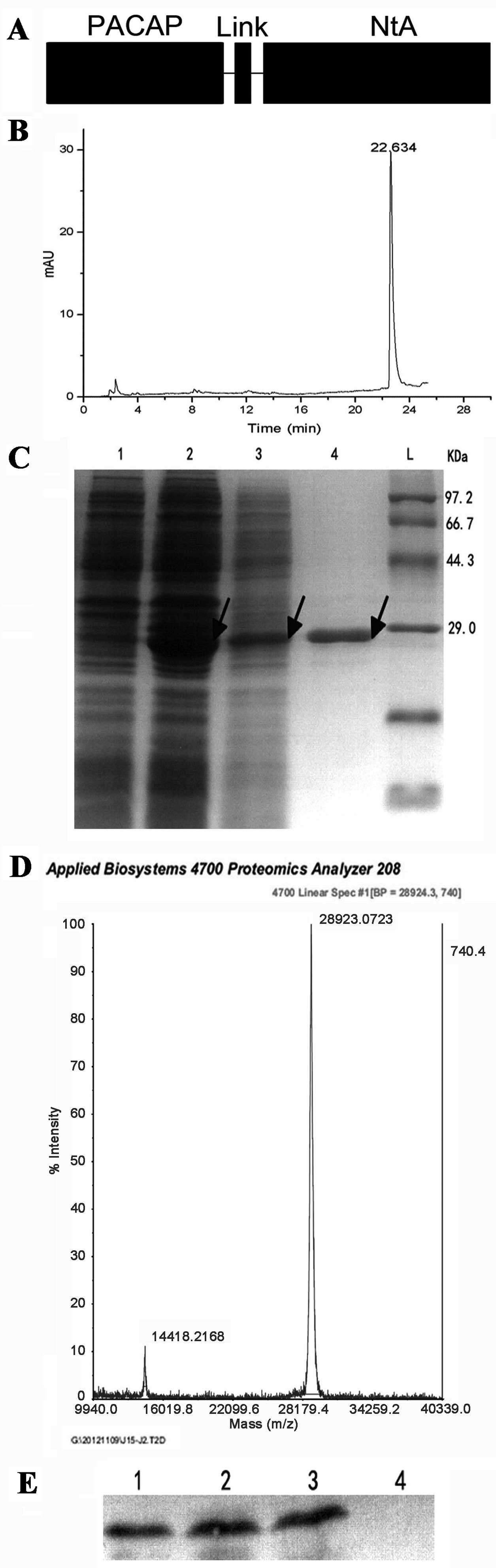 | Figure 1Expression, purification and
identification of PACAP38-NtA. (A) Structural diagram of the
recombinant protein. (B) The results of PACAP38-NtA expression and
purification. L, protein ladder; 1, the expressed result of
PACAP38-NtA without IPTG; 2, the expressed result of PACAP38-NtA by
IPTG; 3, the supernatant containing PACAP38-NtA prior to
purification; 4, the result of PACAP38-NtA subsequent to
purification. (C) The high-performance liquid chromatography
results of PACAP38-NtA. (D) The results of PACAP38-NtA at mass
spectrometric analysis. (E) Detection of PACAP38-NtA by
western-blotting analysis. 1, the expressed PACAP38-NtA by IPTG; 2,
the supernatant containing PACAP38-NtA prior to purification; 3,
NtA-APCAP subsequent to purification; 4, the expressed E. coli.
BL21 (DE3) by IPTG, as control. PACAP, pituitary adenylate
cyclase-activating polypeptide; NtA, N-terminal agrin domain; IPTG,
isopropylthiogalactoside. |
Cell culture and research methods
PC12 cell (Committee on Type Culture Collection of
Chinese Academy of Sciences, Shanghai, China) was cultured in
RPMI-1640 medium with 10% fetal bovine serum (FBS) in a humidified
atmosphere containing 5% CO2. In total, 8×103
cells/holes were added to a 96-hole plate, and the peripheral 36
holes were sealed with phosphate-buffered saline (PBS). Starvation
medium with 0.5% FBS was employed after the cells were 50–60%
confluent, and subsequently 100 nM of PACAP38-NtA protein were
added and sterilized with a 0.22-μm filter. The hole without
PACAP-NtA was the negative control, while the wild-PACAP was taken
as the positive control. After cultivating for 24 h, 10 μl
cell counting kit-8 (CCK8) reagent was added into each hole. After
cultivating for 1 h, the absorbance value was measured with 450/630
nm dual wavelength on the enzyme mark instrument (Bio-Rad,
Hercules, CA, USA), and the growth curve was created with
concentration and absorbance on the X and Y axis, respectively.
Quantitative analysis experiment of
neurite
Smit et al (34) reported the experiment of analyzing
the nervous processes for PC12 cells with quantitative analysis.
Assays were performed in 24-well dishes (Corning Life Sciences,
Tewksbury, MA, USA). Fresh transwell cell culture inserts were
placed in wells containing ECM protein solutions (propagation, 10
μg/ml) and incubated for 2 h at 37°C. The insert was removed
and transferred to a new well containing PACAP and PACAP-NtA with
different concentrations. After 24 h, the nervous processes were
dyed purple by crystal violet subsequent to crossing the transwell
insert. Images of 100 nM polypeptide at different time points (0,
3, 6, 12 and 24 h) were captured to observe the dyeing effect, and
subsequently the nervous processes that were dyed in purple were
dissolved with glacial acetic acid, and the absorbance value at 570
nm was detected.
ELISA
The ELISA detection of the laminin protein was
purchased from Sigma to test the combination ability of
PACAP38-NtA. An appropriate amount of laminin was covered on 96
ELISA plates (PerkinElmer, Inc., Waltham, MA, USA), maintaining at
4°C for 24 h and discarding the liquid. Subsequently, the plate was
dried and washed with PBS (pH 7.3) three times. Bovine serum
albumin [2.5% (w/v)], including 0.1% (v/v) Tween 20, was added into
each hole (200 μl/hole) and incubated at 37°C for 2 h for the
blocking reaction. PACAP38-NtA at a concentration of 100 nM was
added into the enzyme labeling hole (100 μl/hole) at 37°C
for 2 h. PBS was used as the negative control, while 100 nM
wild-PACAP was the positive control. The proteins that did not
combine were eliminated subsequent to washing with PBS and Tween 20
(PBST) three times. The rabbit anti-human PACAP38 antibody (1:1000;
Sigma) and goat anti-rabbit IgG-HRP (1:1000) were added and
maintained at 37°C for 1 h, washing with PBST three times. The
3,3′,5,5′-tetramethylbenzidine substrate was added (200
μl/hole) and incubated at room temperature for 10 min; 2 M
H2SO4 stopped coloration and absorbance was
detected at 450 nm.
Western blot analysis
Following electrophoresis, the protein blots were
transferred to a PVDF membrane. The membrane was blocked with 5%
skimmed milk in TBST and incubated with primary antibody in TBST
containing 2% skimmed milk overnight at 4°C. After washing three
times with TBST, the membrane was incubated at room temperature for
1 h with secondary antibody diluted with TBST containing 2% skimmed
milk. The detected protein signals were visualized by an
electrochemiluminescence system.
Surgical procedures and application of
PACAP38-NtA
Six eight-month-old C57 mice purchased from the
Laboratory Animal Center of Sun Yat-sen University in China were
injected with 7 μl 10% chloral hydrate through the abdomen for
anesthesia. A circular injury was created by a scratch on the
cornea with the mini-keratome system (MK-2000; Nidek, Inc.,
Fremont, CA, USA) under the anatomical lens, which was ~2 mm in
diameter. The injury was dyed green with 2% sodium fluorescein and
images were captured and labeled as 0 h. The mice were randomly
divided into three groups by adding 5 μl normal saline, 5
μl 100 nM PACAP38-NtA and 100 nM wild PACAP, respectively.
The repairing of the injury after 12, 18, 24 and 36 h,
respectively, was observed with the images. The images were
captured and the repairing time of corneal injury in the different
groups was recorded. All the animals were treated in accordance
with the ARVO Statement for the Use of Animals in Ophthalmic and
Vision Research.
Statistical analysis
Statistical analyses were performed using Excel 2003
software. Data obtained from three or more separate experiments are
expressed as the means ± standard deviation. Data were compared
using standard analysis of variance methodology for repeated
measurement and calculation of P-values. Differences were
considered to indicate statistical significance at the 5% level
(P<0.05).
Results
Obtaining the protein of interest
Following the construction of the PACAP38-NtA
prokaryotic expression vector with molecular cloning, the soluble
protein was obtained through IPTG inducible expression when the
bacteria OD600 was between 0.4 and 0.6. Subsequently, the
supernatant was collected after crushing the bacteria and
centrifugation, and the protein of interest was obtained with the
two-step purification method (Fig.
1C) and its purity was confirmed with HPLC (Fig. 1B). According to the western
blotting PACAP38-NtA analysis of the rabbit anti-human PACAP38
antibody (Fig. 1E), the protein
of interest was expressed, with a purity >90%, and its molecular
weight was confirmed by mass spectrometry as 28.9 kDa (Fig. 1D), which was in accordance with
the molecular weight of SDS-PAGE electrophoresis (Fig. 1C). These preliminary results
indicated that the target protein was expressed and purified, and
could be activated in the subsequent experiments.
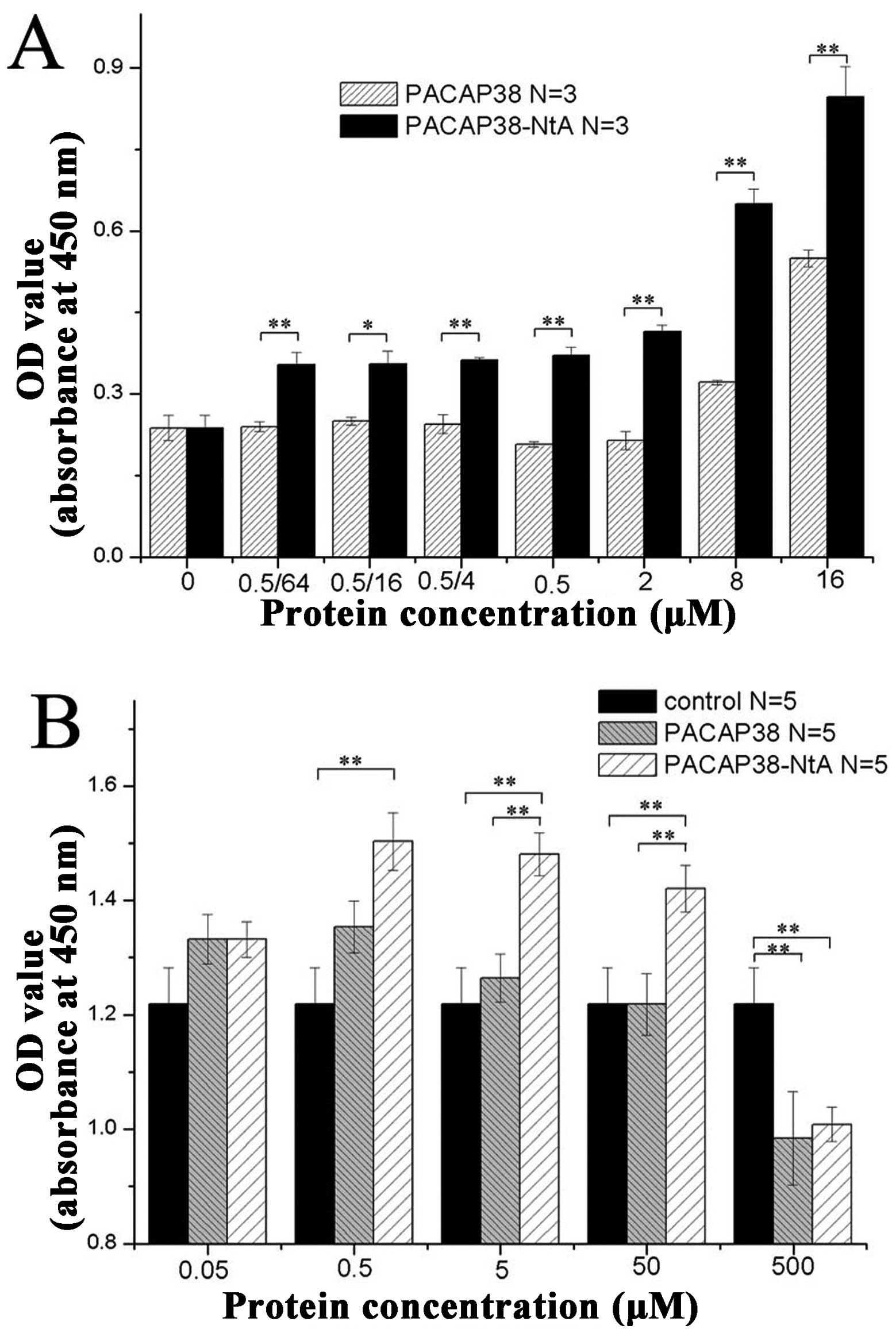
Binding capacity of PACAP39-NtA
The detection of the antigen antibody reaction with
ELISA had a high sensitivity and strong specificity. The relative
position of the PACAP38 and NtA structure is shown in Fig. 1A. In the present study, this
method was employed for comparing the binding capacity with laminin
between the PACAP38-NtA and wild-PACAP. The PACAP and recombinant
protein, PACAP38-NtA, were diluted equally according to 0.5/64,
0.5/16, 0.5/4, 0.5, 8 and 16 μM, and the binding capacity with
laminin was detected, respectively. According to the absorbance
value at OD450 nm, the laminin binding capacity with PACAP38-NtA
was higher compared to PACAP at various concentrations (Fig. 2A). The detection results showed
that the binding capacity of the two groups were significantly
different (P<0.01), indicating that the recombinant protein,
PACAP38-NtA, combined with the laminin protein. The C-terminal NtA
of the PACAP38-NtA protein purified with molecular cloning
maintains the original biological activity and it can target
laminin binding.
With the CCK8 method, the antagonistic effect of the
recombinant protein, PACAP38-NtA, and wild-type PACAP38 on the
starved PC12 injury apoptosis was studied. The detection results
are shown in Fig. 2B, from which
it is clear that PACAP38-NtA is more efficient compared to wild- on
the starved PC12 injury apoptosis when the protein concentration is
within 0.05-50 nM, and the two proteins are strong on
anti-apoptosis when the protein concentration is 0.5 nM. In
addition, it was found that with the increase of the concentration,
the antagonistic effect of recombinant protein PACAP38-NtA and
wild-type PACAP38 on the starved PC12 injury apoptosis was
weakened.
In the study, the promotion of the PC12 cell
processes and growth was compared for PACAP38-NtA and wild-PACAP38
quantitatively, as shown in Fig. 3A
and B. In Fig. 3A, the
density and length of the nerve processes across the transwell cell
under the microscope after 3, 6, 12 and 24 h are shown; whereas in
Fig. 3B, the absorbance value of
the nerve processes across the transwell cell at 570 nm are shown
subsequent to the addition of crystal violet to stain purple and
dissolving by glacial acetic acid. PACAP38-NtA and PACAP38 promote
the neuron differentiation, and there is no difference between the
PACAP38-NtA and PACAP38 proteins.
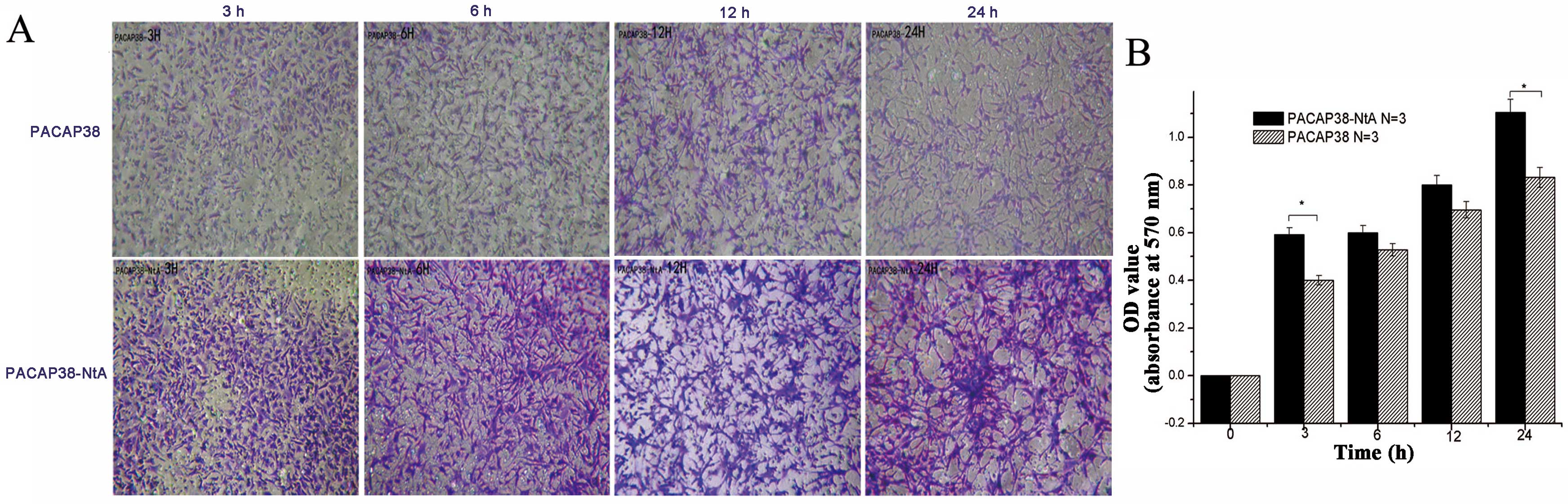 | Figure 3Effect of PACAP38-NtA and PACAP38 on
neurite outgrowth in PC12 cells by NEO. (A) Transwell at 0, 3, 6,
12 and 24 h (magnification, 4×5). (B) Absorbance value (OD570) of
PACAP38 and PACAP-NtA at 0, 3, 6, 12 and 24 h. PACAP, pituitary
adenylate cyclase-activating polypeptide; NtA, N-terminal agrin
domain; NEO, neurite outgrowth assay; OD, optical density. |
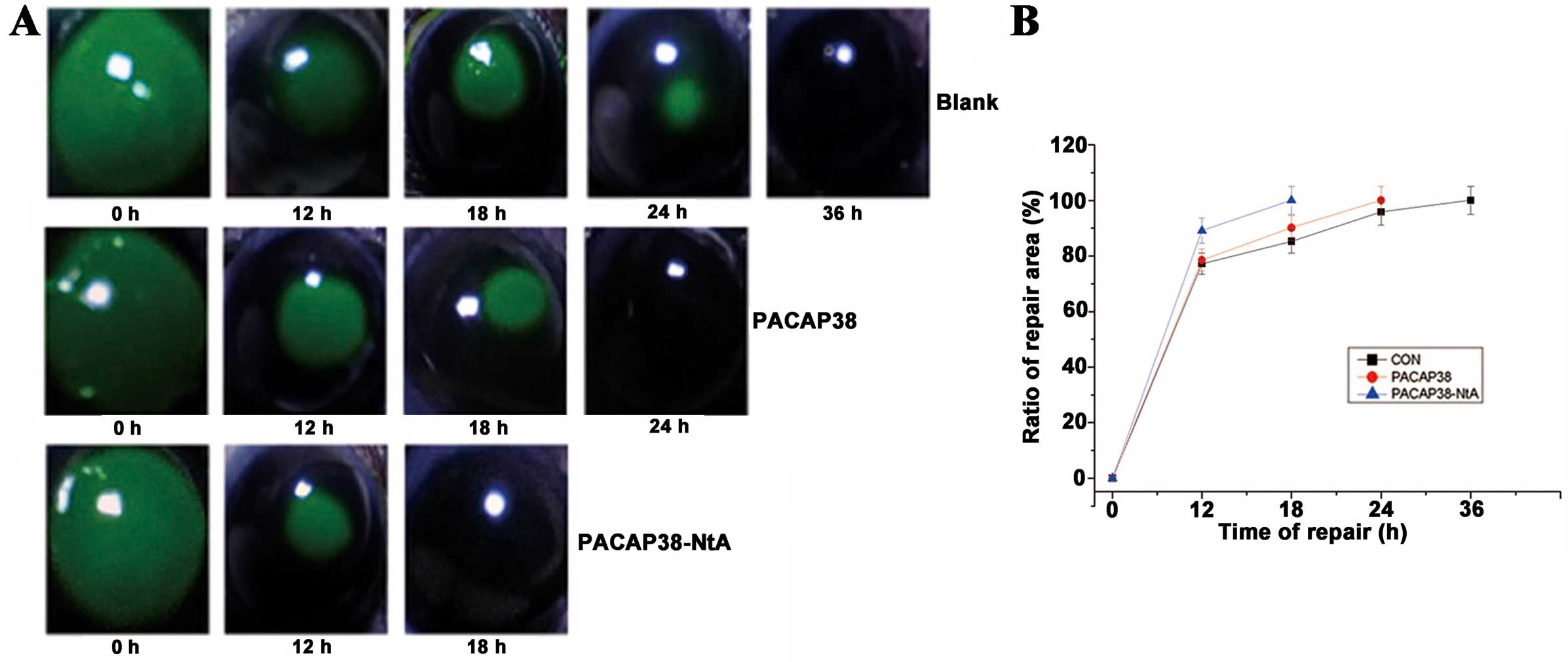
Recovery time following corneal
injury
The trephine injury method was applied for the
construction of the corneal injury model (35,36). Six C57 male mice were randomly
divided into three groups; normal saline, 100 nM PACAP38-NtA and
100 nM PACAP38 groups. Subsequent to injuring the corneal
epithelial cells in the left and right eyes of adult C57 mice with
mini-keratome, 2% sodium fluorescein was applied for coloration
(showing the injury area, Fig.
4A), and 5 μl normal saline, 5 μl 100 nM
PACAP38-NtA and 5 μl 100 nM PACAP38 were added,
respectively. The repair process of the cornea in each group is
shown in Fig. 4B. Recovery
occurred in 18 h in the PACAP38-NtA group, which was significantly
quicker compared to the control group with PACAP (24 h). The 100 nM
PACAP38 and control groups took less time compared to the blank
control group in repairing the cornea (36 h in the normal saline
group). PACAP38-NtA promotes the corneal epithelial cell repairing,
and its remediation effect is improved compared to wild-PACAP38 at
a concentration of 100 nM.
Discussion
PACAP has neurotrophy and a neural restoration
function. PACAP and its special receptor, PAC1, are widely spread
in the peripheral nervous system, such as the cornea nerve
(29,37,38). According to these studies, PACAP
can promote the repair of corneal epithelium and regulation of the
inflammatory response of the ocular surface. Therefore, PACAP is a
candidate polypeptide drug with broad application. However,
according to clinical studies, PACAP through intravenous injection
is decomposed by various enzymes in the blood, such as dipeptidyl
peptidase (DPP)-IV and carboxypeptidase (CP), the half-life period
is 3–10 min (7,39,40). Consequently, PACAP as the neural
restoration drug for clinical treatment requires further study.
Improving the clinical effect of the biological activity of PACAP
and lengthening the action time of the biological activity would be
a significant research direction for PACAP polypeptide drug
development.
Using genetic engineering, two polypeptides with
different functions can be connected together (Fig. 1A), but the biological activity of
the two polypeptides mainly depends on whether the primary
structure of the polypeptide alters the space structure of the
active site region when folding into the space structure. In the
present study, the expression vector of PACAP38-NtA was constructed
with molecular cloning, and the active proteins were separated
using a two-step purification method, with the purity >90%
(Fig. 1B–D). This polypeptide was
identified as the amino acid sequence of PACAP38 through western
blotting analysis (Fig. 1E), and
it was preliminarily regarded as the polypeptide of interest.
The model of PC12 injury can be generated with
numerous methods, such as H2O2,
MPP+, glutamic acid and serum-free injuries. In the
present study, the injury model of PC12 cell was created with
serum-free injury, which can decrease the influence of serum on the
experiment result, with a clear and stable effect. The
anti-apoptosis experiment of CCK8 shows that the recombinant
protein has the biological function of PACAP38, as it resists the
PC12 cellular damage and apoptosis. Under the same concentration,
the anti-apoptosis ability of the recombinant protein, PACAP38-NtA,
was shown to be more efficient compared to PACAP38 (Fig. 2B). The experiment of the PC12
serum-free injury model has shown that the recombinant polypeptide
also has the same in vitro biological activity of anti-nerve
cell apoptosis as PACAP38.
According to the experiment, PACAP, as a
neuropeptide, has the in vitro and in vivo biological
function of promoting the growth of nervous processes. In the
study, the biological activity of two types of polypeptide in
promoting the growth of nervous processes has been compared through
the in vitro experiments using the PC12 nerve regeneration
quantitative analysis. The nervous processes of PC12 cell were
detected according to the quantitative determination reported by
Smit et al (34), and
quantitative analysis was performed for 3, 6, 12 and 24 h after
PACAP38 and PACAP38-NtA were added. According to the results, the
recombinant polypeptide, PACAP38-NtA, promotes the growth of PC12
cellular processes, and the ability of the recombinant polypeptide
in promoting the growth of the nervous processes at different time
points is more efficient compared to the wild-polypeptide
PACAP38.
PACAP promotes the growth of trigeminal cells in the
corneal injury, as well as the restoration of the epithelial cell
and lacrimal gland in laser-assisted in situ keratomileusis
rabbit corneal surgery. The mechanism may be that it can promote
the trigeminal cell to secrete active neurotransmitters to enhance
the proliferation, differentiation and production of collagen VII.
In the present study, the mechanically-injured corneal epithelial
cells of C57 mice were adopted for the model, and 2% sodium
fluorescein staining directly exhibited the injured section. The
size of the stained section can distinguish the remediation effect
of the polypeptide at the same moment. The effect of repairing the
injured corneal epithelial cells with the recombinant polypeptide,
PACAP38-NtA, and wild-PACAP38 were studied with the mechanically
damaged cornea C57 mouse model. According to the experiment, under
the in vivo conditions, it takes 18 h for 100 nM of the
PACAP38-NtA polypeptide to repair the injured corneal epithelial
cell, while it takes 24 h for 100 nM of the PACAP38 polypeptide.
Evidently, it accelerates the process of repairing the injured
corneal epithelial cells of C57 mice.
According to previous studies, once the nerve is
damaged mechanically, Schwann cells will gather in the injured
section and secrete substantial laminin (16,22). The polypeptide PACAP-NtA
constructed in the present study has the biological function of
combining with laminin, and as a result, it can anchor near the
laminin of the injured nerve section, with a significant advantage
in repairing the injured nerve. The ELISA experiment of the
recombinant protein, PACAP38-NtA, and laminin has shown that the
recombinant protein can combine with laminin when it reaches the
nanomole and micromole concentration level, with an evident
comparative difference from the negative docking of PACAP38.
Consequently, it can be predicted that the recombinant protein
PACAP38-NtA has the biological activity of NtA, as it can combine
with laminin. The combination of the recombinant protein,
PACAP38-NtA, and laminin can gather in the injured section and
militate constantly, allowing the neural restoration function of
PACAP38.
In conclusion, the recombinant protein, PACAP-NtA,
has been constructed, expressed and purified with the genetic
engineering method, and according to the experiment, the
recombinant protein is equipped with such biological functions as
preventing the apoptosis of injured nerve and promoting the growth
of nervous processes of the PACAP38 polypeptide. In addition, the
polypeptide can anchor on the laminin of the injured section of the
nerve, and improve the utilization efficiency of the PACAP38
polypeptide by the injured section. The recombinant polypeptide may
become a novel type of candidate drug for promoting the restoration
of injured nerve, and further studies are required.
Acknowledgments
The present study was supported by the Science and
Technology Project of Guangzhou (grant no. 2011J4300107).
References
|
1
|
Miyata A, Arimura A, Dahl RR, et al:
Isolation of a novel 38 residue-hypothalamic polypeptide which
stimulates adenylate cyclase in pituitary cells. Biochem Biophys
Res Commun. 164:567–574. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wray V, Kakoschke C, Nokihara K and Naruse
S: Solution structure of pituitary adenylate cyclase activating
polypeptide by nuclear magnetic resonance spectroscopy.
Biochemistry. 32:5832–5841. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jüppner H, Schipani E, Bringhurst FR, et
al: The extracellular amino-terminal region of the parathyroid
hormone (PTH)/PTH-related peptide receptor determines the binding
affinity for carboxyl-terminal fragments of PTH (1-34).
Endocrinology. 134:879–884. 1994.
|
|
4
|
Wei Y and Mojsov S: Tissue specific
expression of different human receptor types for pituitary
adenylate cyclase activating polypeptide and vasoactive intestinal
polypeptide: implications for their role in human physiology. J
Neuroendocrinol. 8:811–817. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gou rlet P and Va nder me ers A:
Vasoactive intestinal peptide (VIP) and pituitary adenylate
cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation
by the neutral endopeptidase EC 3.4.24.11. Biochem Pharmacol.
54:509–515. 1997. View Article : Google Scholar
|
|
6
|
Ohkubo S, Kimura C, Ogi K, et al: Primary
structure and characterization of the precursor to human pituitary
adenylate cyclase activating polypeptide. DNA Cell Biol. 11:21–30.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaudry D, Gonzalez BJ, Basille M, Yon L,
Fournier A and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: from structure to functions.
Pharmacol Rev. 52:269–324. 2000.PubMed/NCBI
|
|
8
|
Inooka H, Ohtaki T, Kitahara O, et al:
Conformation of a peptide ligand bound to its G-protein coupled
receptor. Nat Struct Biol. 8:161–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimizu N, Guo J and Gardella TJ:
Parathyroid hormone (PTH)-(1-14) and -(1-11) analogs
conformationally constrained by alpha-aminoisobutyric acid mediate
full agonist responses via the juxtamembrane region of the PTH-1
receptor. J Biol Chem. 276:49003–49012. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tibaduiza EC, Chen C and Beinborn M: A
small molecule ligand of the glucagon-like peptide 1 receptor
targets its amino-terminal hormone binding domain. J Biol Chem.
276:37787–37793. 2001.PubMed/NCBI
|
|
11
|
Miyata A, Jiang L, Dahl RD, et al:
Isolation of a neuropeptide corresponding to the N-terminal 27
residues of the pituitary adenylate cyclase activating polypeptide
with 38 residues (PACAP38). Biochem Biophy Res Commun. 170:643–648.
1990. View Article : Google Scholar
|
|
12
|
Inooka H and Shirakawa M: Conformation of
a peptide ligand bound to its G-protein coupled receptor and its
implication for ligand transportation. Tanpakushitsu Kakusan Koso.
47:787–793. 2002.In Japanese. PubMed/NCBI
|
|
13
|
Laburthe M and Couvineau A: Molecular
pharmacology and structure of VPAC Receptors for VIP and PACAP.
Regul Pept. 108:165–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laburthe M, Couvineau A and Marie JC: VPAC
receptors for VIP and PACAP. Receptors Channels. 8:137–153. 2002.
View Article : Google Scholar
|
|
15
|
Runge S, Wulff BS, Madsen K,
Brauner-Osborne H and Knudsen LB: Different domains of the glucagon
and glucagon-like peptide-1 receptors provide the critical
determinants of ligand selectivity. Br J Pharmacol. 138:787–794.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shioda S, Shuto Y, Somogyvari-Vigh A, et
al: Localization and gene expression of the receptor for pituitary
adenylate cyclase-activating polypeptide in the rat brain. Neurosci
Res. 28:345–354. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nowak JZ and Zawilska JB: PACAP in avians:
origin, occurrence, and receptors-pharmacological and functional
considerations. Curr Pharm Des. 9:467–481. 2003. View Article : Google Scholar
|
|
18
|
Bourgault S, Vaudry D, Segalas-Milazzo I,
et al: Molecular and conformational determinants of pituitary
adenylate cyclase-activating polypeptide (PACAP) for activation of
the PAC1 receptor. J Med Chem. 52:3308–3316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linden A, Hansson L, Andersson A, et al:
Bronchodilation by an inhaled VPAC (2) receptor agonist in patients
with stable asthma. Thorax. 58:217–221. 2003. View Article : Google Scholar
|
|
20
|
Yuhara A, Nishio C, Abiru Y, Hatanaka H
and Takei N: PACAP has a neurotrophic effect on cultured basal
forebrain cholinergic neurons from adult rats. Brain Res Dev Brain
Res. 131:41–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laburthe M, Couvineau A and Tan V: Class
II G protein-coupled receptors for VIP and PACAP: structure, models
of activation and pharmacology. Peptides. 28:1631–1639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hautmann M, Friis UG, Desch M, et al:
Pituitary adenylate cyclase-activating polypeptide stimulates renin
secretion via activation of PAC1 receptors. J Am Soc Nephrol.
18:1150–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cazillis M, Gonzalez BJ, Billardon C, et
al: VIP and PACAP induce selective neuronal differentiation of
mouse embryonic stem cells. Eur J Neurosci. 19:798–808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Yu R, Liu X, Guo X and Zeng Z:
The expression of PAC1 increases in the degenerative thymus and low
dose PACAP protects female mice from cyclophosphamide induced
thymus atrophy. Peptides. 38:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harmar AJ, Fahrenkrug J, Gozes I, et al:
Pharmacology and functions of receptors for vasoactive intestinal
peptide and pituitary adenylate cyclase-activating polypeptide:
IUPHAR review 1. B J Pharmacol. 166:4–17. 2012. View Article : Google Scholar
|
|
26
|
Racz B, Gasz B, Borsiczky B, et al:
Protective effects of pituitary adenylate cyclase activating
polypeptide in endothelial cells against oxidative stress-induced
apoptosis. Gen Comp Endocrinol. 153:115–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horvath G, Brubel R, Kovacs K, et al:
Effects of PACAP on oxidative stress-induced cell death in rat
kidney and human hepatocyte cells. J Mol Neurosci. 43:67–75. 2011.
View Article : Google Scholar
|
|
28
|
Horvath G, Reglodi D, Opper B, et al:
Effects of PACAP on the oxidative stress-induced cell death in
chicken pinealocytes is influenced by the phase of the circadian
clock. Neurosci Lett. 484:148–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukiage C, Nakajima T, Takayama Y,
Minagawa Y, Shearer TR and Azuma M: PACAP induces neurite outgrowth
in cultured trigeminal ganglion cells and recovery of corneal
sensitivity after flap surgery in rabbits. Am J Ophthalmol.
143:255–262. 2007. View Article : Google Scholar
|
|
30
|
Wang ZY, Alm P and Hakanson R:
Distribution and effects of pituitary adenylate cyclase-activating
peptide in the rabbit eye. Neuroscience. 69:297–308. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu SY and Gordon T: The cellular and
molecular basis of peripheral nerve regeneration. Mol Neurobiol.
14:67–116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daggett DF, Cohen MW, Stone D, Nikolics K,
Rauvala H and Peng HB: The role of an agrin-growth factor
interaction in ACh receptor clustering. Mol Cell Neurosci.
8:272–285. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun W, Sun C, Zhao H, et al: Improvement
of sciatic nerve regeneration using laminin-binding human NGF-beta.
PloS One. 4:e61802009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smit M, Leng J and Klemke RL: Assay for
neurite outgrowth quantification. Biotechniques. 35:254–256.
2003.PubMed/NCBI
|
|
35
|
Li Z, Burns AR, Han L, Rumbaut RE and
Smith CW: IL-17 and VEGF are necessary for efficient corneal nerve
regeneration. Am J Pathol. 178:1106–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SJ, Kim JK, Seo KY, Kim EK and Lee HK:
Comparison of corneal nerve regeneration and sensitivity between
LASIK and laser epithelial keratomileusis (LASEK). Am J Ophthalmol.
141:1009–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waschek JA: Multiple actions of pituitary
adenylyl cyclase activating peptide in nervous system development
and regeneration. Dev Neurosci. 24:14–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Somogyvári-Vigh A and Reglodi D: Pituitary
adenylate cyclase activating polypeptide: a potential
neuroprotective peptide. Curr Pharma Des. 10:2861–2889. 2004.
View Article : Google Scholar
|
|
39
|
Li M, Maderdrut JL, Lertora JJ and Batuman
V: Intravenous infusion of pituitary adenylate cyclase-activating
polypeptide (PACAP) in a patient with multiple myeloma and myeloma
kidney: a case study. Peptides. 28:1891–1895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mentlein R: Dipeptidyl-peptidase IV (CD26)
- role in the inactivation of regulatory peptides. Regul Pept.
85:9–24. 1999. View Article : Google Scholar : PubMed/NCBI
|