Introduction
Bone marrow stromal cells (e.g., fibroblasts,
osteoblasts, endothelial cells) support both normal and leukemic
hematopoiesis (1). This support
is mediated both via the local cytokine network and by direct
cell-cell contact, including gap junctions formed by connexin
molecules (2). This also seems to
be true in acute myeloid leukemia (AML), an aggressive myeloid
malignancy characterised by the accumulation of immature forms of
leukemic blasts in the bone marrow (3). Stromal cells seem to be important
both for leukemogenesis and chemosensitivity (1). This is supported by in vitro
studies on exogenous cytokines known to be released by stromal
cells (4–6) and by the in vitro co-culture
of primary human AML cells with human fibroblasts (7), endothelial cells (8) and osteoblasts (9). Murine stromal cells (10) also have growth-enhancing and
anti-apoptotic effects on primary human AML cells.
Gap junctions and connexins play an important role
in AML (11), and the
differentiation of the AML cell lines, HL-60 and PBL-985, is
inhibited by stromal cells; this effect is possibly mediated by gap
junctions (12). However, the
results from studies on the effects of gap junctions on AML cell
proliferation are conflicting. Firstly, gap junctions seem to exert
antiproliferative effects on the HL-60 and KG1 AML cell lines
(13). An antiproliferative
effect has also been observed in the U937 AML cell line when
increased Cx43 mRNA levels are induced by the expression of the
AML1-ETO fusion gene; however, it is not known whether this
antiproliferative effect is caused by the induction of Cx43 or by
another effect of the fusion protein (14). If this antiproliferative effect,
which is associated with increased Cx43 expression, is caused by
Cx43 itself and not by another effect of the AML-ETO fusion
protein, this may be associated with human AML, as increased Cx43
gap junction expression has also been detected in human AML bone
marrow biopsies (15). By
contrast, an in vitro study demonstrated a higher
proliferative capacity of the OCIM2 AML cells with increased Cx43
expression compared with cells showing a considerably lower Cx43
expression (16). Finally,
antileukemic chemotherapy has been shown to reduce Cx43 expression
in normal hematopoietic cells as bone marrow levels are reduced
following intensive chemotherapy compared with both AML marrow and
marrow from healthy controls; however, it remains unknown as to
whether chemotherapy has a similar effect on Cx43 expression in
primary AML cells and whether this is a determining factor for the
antileukemic effectiveness of the treatment (17). Taken together, these observations
suggest that gap junctions and connexins are involved in
leukemogenesis and are important for chemosensitivity. However,
many of these studies are mainly based on the use of AML cell
lines. Thus, in the present study, we investigated the expression
of connexins in well-characterised primary human AML cells derived
from unselected patients.
Materials and methods
Patients and cell preparation
This study was approved by the local ethics
committee (Regional Ethics Committee III, University of Bergen,
Bergen, Norway), and the samples were collected after obtaining
written informed consent from all participants. AML blasts were
derived from consecutive and thereby unselected patients with high
peripheral blood blast counts (18). This selection of patients together
with the analysis of fms-related tyrosine kinase 3 (FLT3)
and nucleophosmin (NPM)-1 mutations has been
described previously (18,19).
The expression of molecular markers for myeloid differentiation was
analysed by flow cytometry, as previously described (20). We investigated connexin protein
expression in 38 patients (referred to as cohort 1) and gene
expression profiling (GEP) in a second group of 48 patients (cohort
2). This cohort was comparable with cohort 1 and included
consecutive patients with relatively high peripheral blood blast
counts. The clinical and biological characteristics of the two
cohorts are presented in Table
I.
 | Table IClinical and biological
characteristics of AML patients analysed for connexin expression at
the protein (cohort 1) and mRNA level (cohort 2). |
Table I
Clinical and biological
characteristics of AML patients analysed for connexin expression at
the protein (cohort 1) and mRNA level (cohort 2).
| Patient
characteristics (consecutive patients in both cohorts) | Cohort 1 (cell
surface expression) | Cohort 2 (gene
expression profiles) |
|---|
| Demographic data and
disease history |
| Gender (no.) |
| Male/female | 17/21 | 23/25 |
| Age |
| Median (range) | 63.4 (29–88) | 60.5 (24–84) |
| History |
| De novo | 24 (63%) | 40 (83%) |
| Secondary | 8 (21%) | 4 (8%) |
| Relapse | 6 (16%) | 4 (8%) |
| AML cell
differentiation |
| FAB
classification |
| M0–1 | 9 (24%) | 16 (33%) |
| M2 | 9 (24%) | 10 (21%) |
| M3 | 1 (3%) | 0 (0%) |
| M4–5 | 19 (50%) | 22 (46%) |
| CD34
expression |
| 0–20% | 16 (42%) | 15 (36%) |
| 20–50% | 5 (13%) | 5 (12%) |
| 50–100% | 17 (45%) | 22 (52%) |
| Genetic
abnormalities |
| Cytogenetics |
| Favourable | 3 (9%) | 2 (4%) |
| Intermediate | 2 (6%) | 2 (4%) |
| Adverse | 8 (24%) | 10 (21%) |
| Normal | 10 (30%) | 25 (52%) |
| Not available | 10 (30%) | 9 (19%) |
| FLT3
mutations | 15 (44%) | 21 (44%) |
| NPM‑1
mutations | 12 (32%) | 17 (35%) |
Preparation of AML cells
The leukemic cells were isolated by density gradient
separation (Ficoll-Hypaque; specific density, 1.077; Nycomed
Pharma, Oslo, Norway), and were stored frozen in liquid nitrogen
(4,6,7,21).
Leukemia peripheral blood mononuclear cells were collected from the
AML patients with >95% of leukemic blasts among the mononuclear
cells.
Flow cytometry
Flow cytometric analysis to assess cell surface
molecular expression was carried out as described in our previous
study (7). Briefly,
1×106 AML cells/ml were washed once in binding buffer
[phosphate buffered saline (PBS) with 0.2% bovine serum albumin
(BSA)] prior to incubation in the dark for 20 min at room
temperature with FITC-conjugated antibodies against Cx26 (sc-7261),
Cx32 (sc-7258), Cx37 (sc-27712), Cx43 (sc-13558) or Cx45
(sc-374354; Santa Cruz Biotechnology, Inc., CA, USA). The samples
were thereafter washed once before flow cytometric analysis. For
each measurement, 10,000 events were collected using a standard
FACSCalibur flow cytometer (Becton Dickinson Immunocytometry
Systems, San Jose, CA, USA) equipped with an Argon laser (488 nm)
and a red diode laser (635 nm). A cytogram based on the forward
angle light scatter (FSC) versus the right angle side scatter (SSC)
was used to eliminate aggregates, debris and dead cells before
fluorescence was detected. In the analysis of connexin membrane
expression, only green fluorescence (FL1) was detected through the
530/30 nm band-pass filter. The fluorescence measurements were
collected in the logarithmic mode. Both data from the positive cell
region diagrams and the mean fluorescence intensity (MFI) were
analysed using FCS Express version 3 (De Novo Software, Los
Angeles, CA, USA). The positive cell regions were defined for each
individual patient based on the FSC/SSC diagrams and contained
<2% and >1% of the cells of the unstained negative
controls.
Microarray analysis
Global gene expression analyses were performed for a
second patient cohort (Table I).
We then used the Illumina iScan Reader that is based on the
fluorescence detection of biotin-labelled cRNA. Total RNA (300 ng)
from each sample was reverse transcribed, amplified and
biotin-16-UTP-labelled, using the Illumina TotalPrep RNA
Amplification kit (Applied Biosystems/Ambion, Foster City, CA,
USA). The amount and quality of the biotin-labelled cRNA was
controlled both by NanoDrop spectrophotometer and Agilent 2100
Bioanalyzer. Biotin-labelled cRNA 750 ng was hybridised to the
HumanHT-12 v4 Expression BeadChip according to manufacturer’s
instructions. The HumanHT-12 v4 BeadChip targets 47,231 probes
derived primarily from genes in the NCBI RefSeq database (Release
38).
Bioinformatics and statistical
analyses
The AML cells were regarded as positive for cell
surface expression when >20% of the cells stained positive for
the marker by flow cytometry, as previously described (7). The statistical calculation for the
correlation and regression (R2) analyses was performed
using the Statistical Package for the Social Sciences (SPSS),
version 15. Correlation analyses were performed using Spearman’s
rank correlation coefficient (ϱ), and differences were regarded as
statistically significant with a value of p<0.05. For
comparisons between different groups, the non-parametric
Mann-Whitney U test was used. Microarray mRNA levels are presented
as the relative expression (22).
Other bioinformatics analyses were performed using the J-Express
2012 analysis suite (MolMine AS, Hafrsfjord, Norway), as previously
described (23). Hierarchal
clustering analysis was performed using the Euclidean distance
formula with complete linkage. Data are presented as heatmaps and
genograms for each individual analysis. Gene set enrichment
analysis (GSEA) was used to search for related genes that follow
the same trends in the data set. A two-class unpaired data analysis
using 1,000 permutations was performed, and gene ontology (GO)
terms were ranked according to the enrichment score (ES).
Results
Primary human AML cells differ in their
cell surface connexin expression
The surface membrane expression of Cx32, Cx37, Cx43
and Cx45 was analysed for primary human AML blasts derived from 38
consecutive/unselected patients; Cx26 was only analysed for 16
unselected patients. The results from flow cytometry were analysed
both with regard to the percentages of positively stained cells and
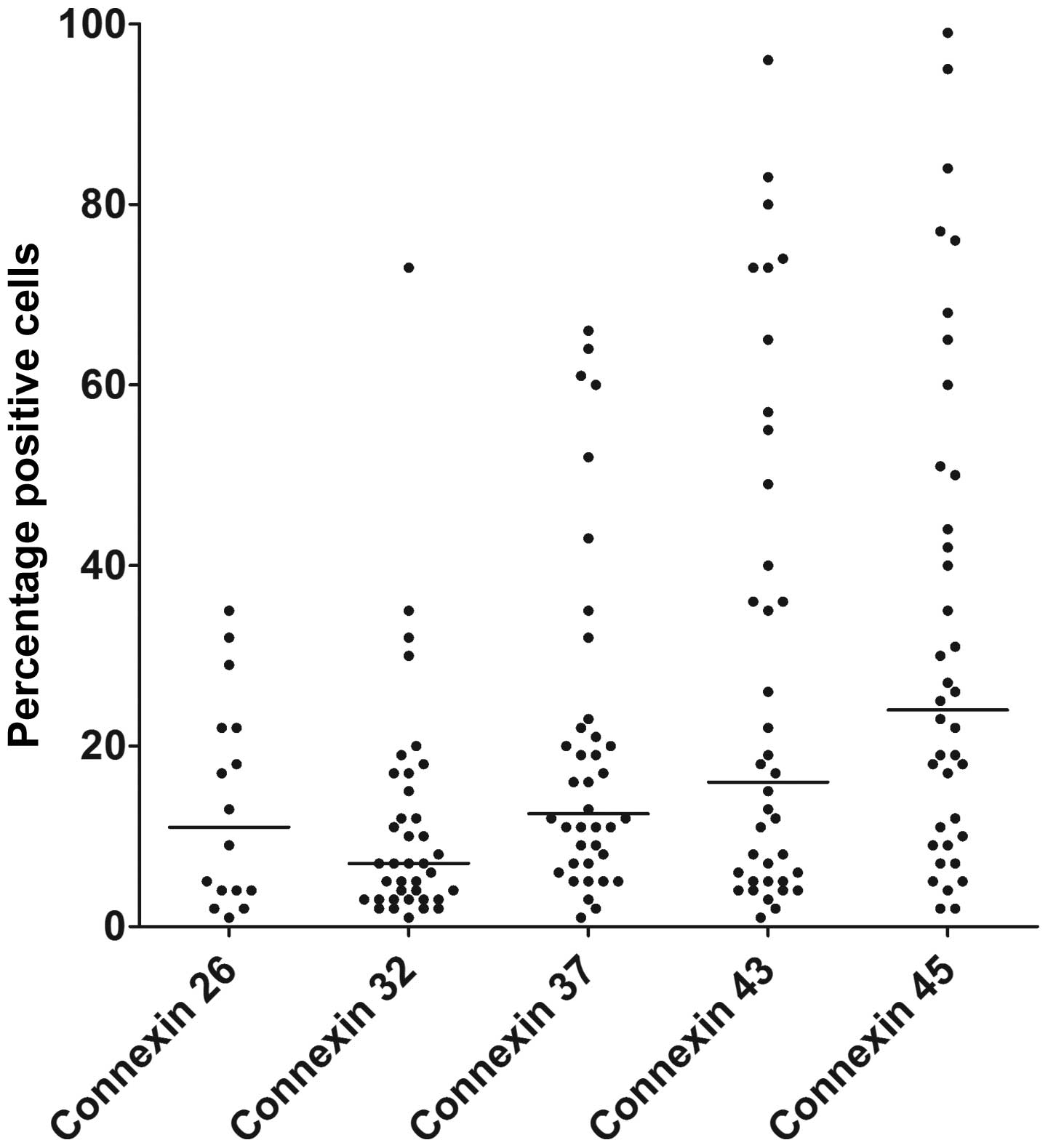
the MFI. The expression of all the connexins examined varied
between patients (Fig. 1). The
highest surface expression was observed for Cx45; 55% of the
patients (21/38) had at least 20% Cx45-positive cells, whereas the
fraction of patients showing at least 20% positive cells was lower
for Cx43 (16/38, 42%), Cx37 (13/38, 34%), Cx32 (5/38, 13%) and Cx26
(5/16, 31%). The MFI values for Cx32, Cx37, Cx43 and Cx45 showed
statistically significant correlations with each other (all
p<0.001, Table II). The
strongest correlation was observed between Cx43 and Cx45 expression
(Spearman’s correlation, ϱ=0.930), and a strong linear correlation
was also suggested by regression analysis
(R2=0.896).
 | Table IISignificant correlations between the
cell surface expression of different connexions in primary human
AML cells. |
Table II
Significant correlations between the
cell surface expression of different connexions in primary human
AML cells.
| Cx32 | Cx37 | Cx43 |
|---|
| Cx37 | <0.001
(0.674) | – | Not
significant |
| Cx43 | <0.001
(0.544) | <0.001
(0.722) | – |
| Cx45 | <0.001
(0.566) | <0.001
(0.745) | <0.001
(0.930) |
Connexin cell surface expression is
associated with the expression of myeloid differentiation markers
(CD11c, CD13, CD14, CD15), but not with the expression of the CD34
stem cell marker
The correlations between the cell surface expression
of connexins (Cx32, Cx37, Cx43, Cx45) and the myeloid
differentiation markers, CD11c, CD13, CD14, CD15 and CD33, as well
as the stem cell marker, CD34, were then analysed (Table III). All four connexins showed
inverse correlations with CD13 expression (p<0.05 for all), but
only Cx43 and Cx45 showed additional positive correlations with
CD14 and CD15 expression (p<0.05). Furthermore, Cx45 expression
positively correlated with CD11c expression (p=0.037), whereas none
of the connexins showed significant correlations with CD33 or CD34
expression (Table III).
 | Table IIICorrelations between the expression
of different connexions and molecular differentiation markers in
primary human AML cells. |
Table III
Correlations between the expression
of different connexions and molecular differentiation markers in
primary human AML cells.
| Cx32 | Cx37 | Cx43 | Cx45 |
|---|
| CD11c | 0.388 (−0.166) | 0.504 (−0.129) | 0.128 (0.290) | 0.037
(0.389) |
| CD13 | 0.046
(−0.326) | 0.024
(−0.366) | 0.033
(−0.347) | 0.020
(−0.376) |
| CD14 | 0.984 (0.003) | 0.103 (0.277) | 0.030
(0.362) | 0.005
(0.457) |
| CD15 | 0.938 (0.013) | 0.364 (0.154) | 0.003
(0.482) | 0.001
(0.542) |
| CD33 | 0.951 (0.010) | 0.431 (0.132) | 0.408 (0.138) | 0.130 (0.250) |
| CD34 | 0.258 (−0.188) | 0.498 (−0.133) | 0.437 (−0.130) | 0.180 (−0.222) |
Connexin expression correlates with the
morphological signs of differentiation, but not with cytogenetic
abnormalities, and FLT3 and NPM-1 mutations
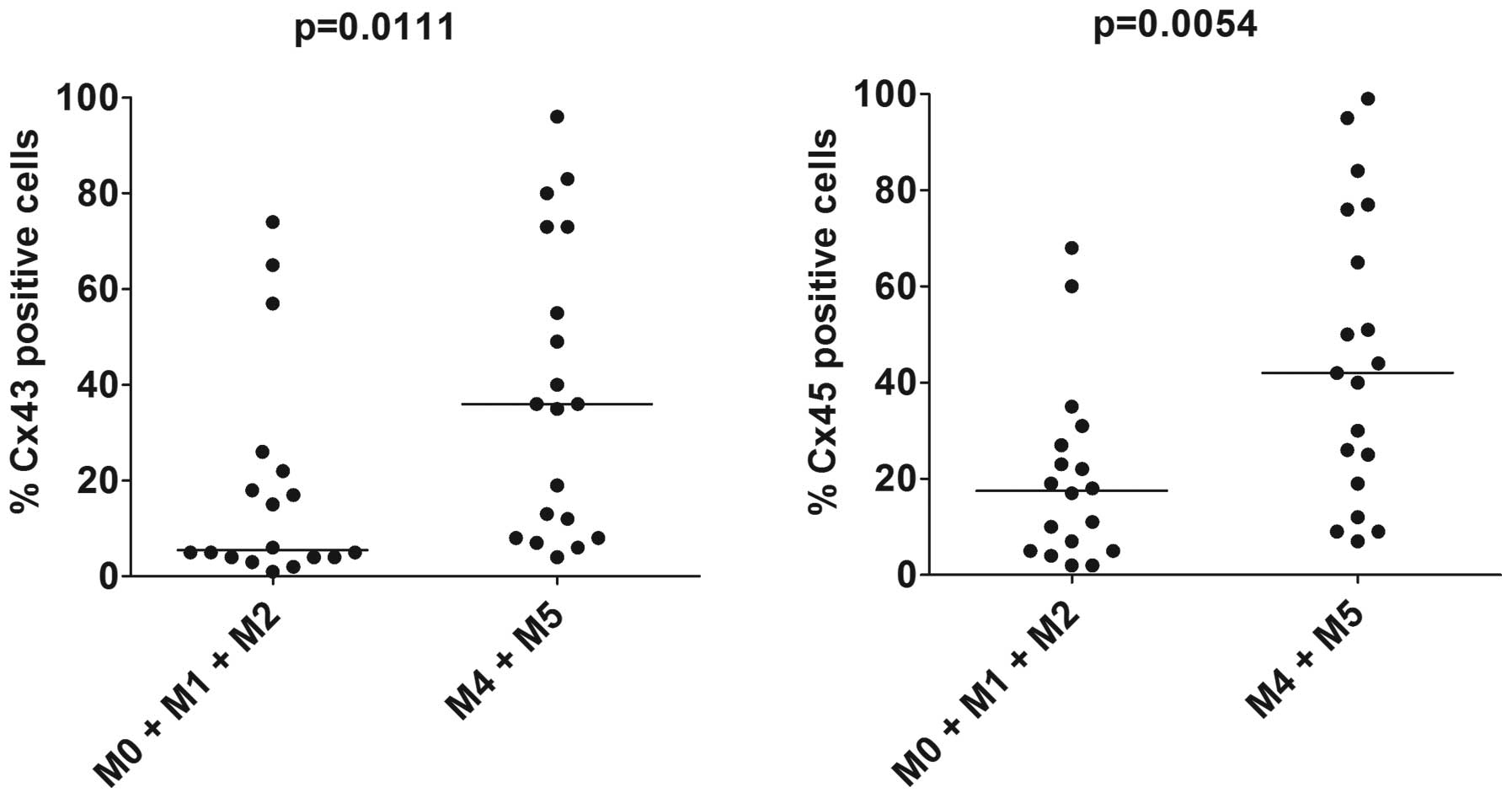
All patients analysed for connexin cell surface
expression were classified according to the FAB criteria and this
morphological/histochemical classification was used to group the
patients into two major subsets as follows: i) AML cells showing no
or minimal signs of differentiation (FAB classification M0, M1 and
M2); and ii) signs of monocytic differentiation (FAB M4 and M5)
(Fig. 2). The expression of Cx32
and Cx35 did not differ between the two subsets (data not shown),
whereas Cx43 and Cx45 expression was significantly increased in the
AML cells with monocytic differentiation (Fig. 2; p=0.0111 and p=0.0054,
respectively, Mann-Whitney U test). There were no differences
observed in connexin expression when comparing patients with
different cytogenetic abnormalities and patients with or without
FLT3/NPM‑1 mutations (data not shown).
Hierarchical clustering identifies a
subset of patients with a generally low cell surface connexin
expression
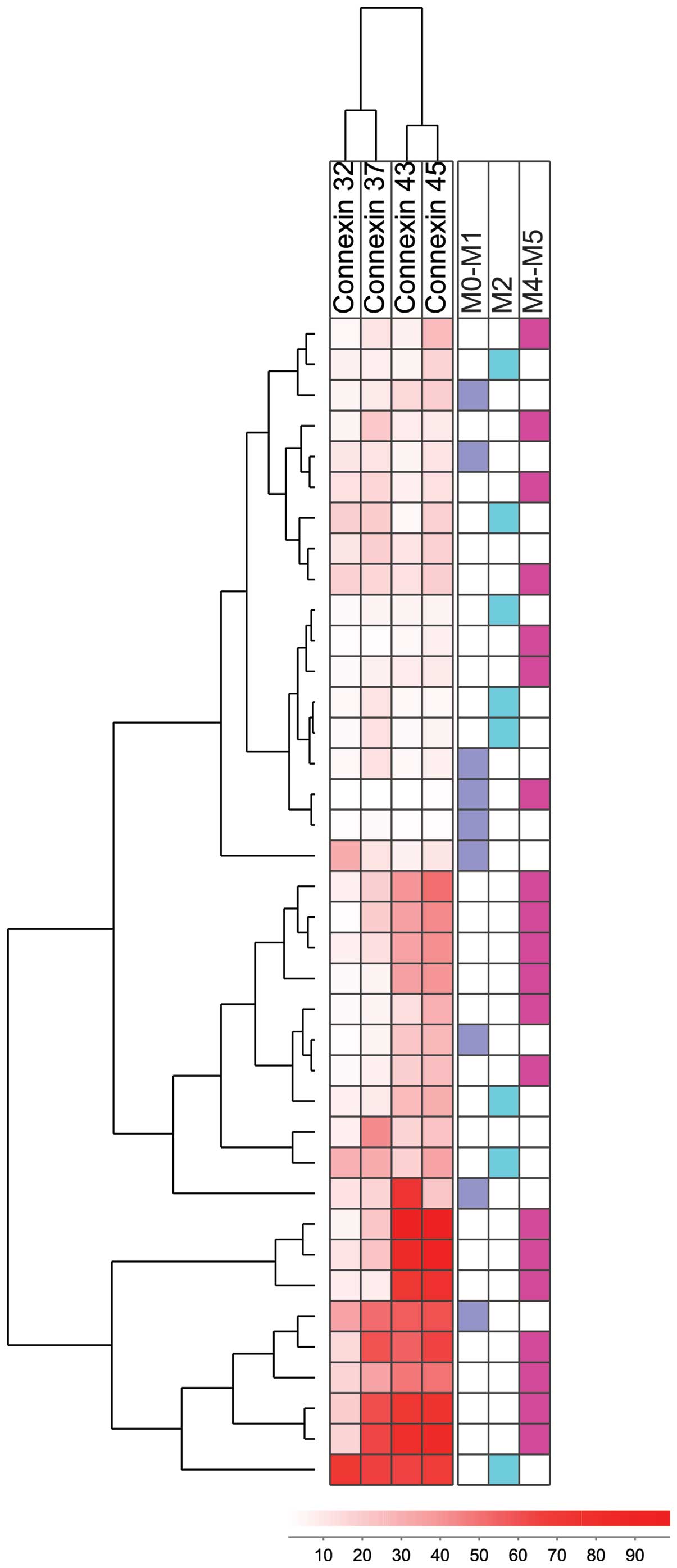
An unsupervised hierarchal clustering was performed
based on the expression of Cx32, Cx37, Cx43 and Cx45 in AML cells.
This analysis was based on the percentages of positively stained
cells, and the use of the complete linkage method and the Euclidean
distance (Fig. 3). Cx32 and Cx37
were clustered together, and Cx43 was clustered with Cx45. The
patients were divided into three main subsets based on their
connexin expression: i) the first cluster consisted of 18 patients
with a generally low expression of all connexins (upper section of
heatmap); ii) the second cluster (11 patients, middle section of
heatmap) showed intermediate levels of Cx43 and Cx45; and iii) the
third cluster (9 patients, lower section of heatmap) showed
generally higher connexin levels, particularly for Cx43 and Cx45.
The three clusters did not differ with regard to cytogenetic
abnormalities, FLT3 or NPM-1 mutations (data not
shown), and no significant differences were observed in FAB
classification between the clusters either (Fig. 3).
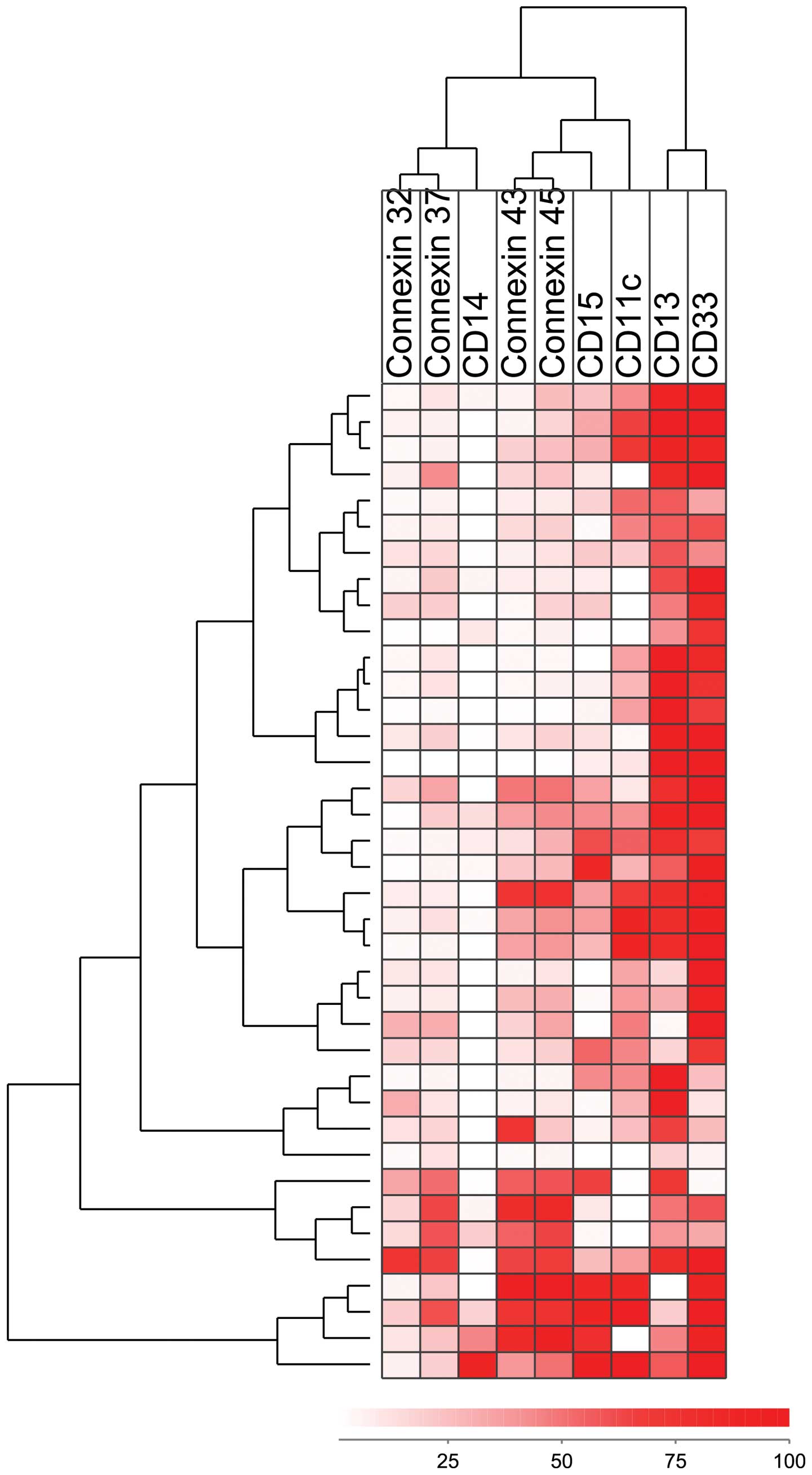
We performed an additional hierarchical clustering
analysis (complete linkage method and Euclidean distance) based on
the expression of connexins and the cell surface differentiation
markers, CD11c, CD13, CD14, CD15 and CD33 (Fig. 4). The two pairs of connexins
(Cx32/Cx37 and Cx43/Cx45) clustered closely together in this
analysis as well; CD14 clustered close to Cx32/Cx37, whereas CD15
clustered close to Cx43/Cx45. The majority of patients with a high
Cx43/Cx45 expression formed a separate cluster (8 patients, lower
section of heatmap).
Only Cx45 expression shows a wide
variation between patients at the mRNA level and differences in
Cx45 mRNA levels are associated with distinct gene expression
profiles
We used the microarray data obtained from a second
cohort of AML patients (cohort 2, Table I). This cohort was comparable with
cohort 1 and included consecutive patients with relatively high
peripheral blood blast counts (see Materials and methods) (Table I). Among the connexins
investigated, Cx45 (encoded by the GJC1 gene) was the only
one that showed a relatively high expression and a wide variation
in expression between patients, whereas the other connexins showed
relatively low mRNA levels and only minor variation in expression
between patients. Thus, the relatively wide variation in expression
between patients with regard to the connexin protein expression
profile is not reflected in the mRNA expression profile, an
observation suggesting that transcriptional regulation is important
for the expression of several connexins.
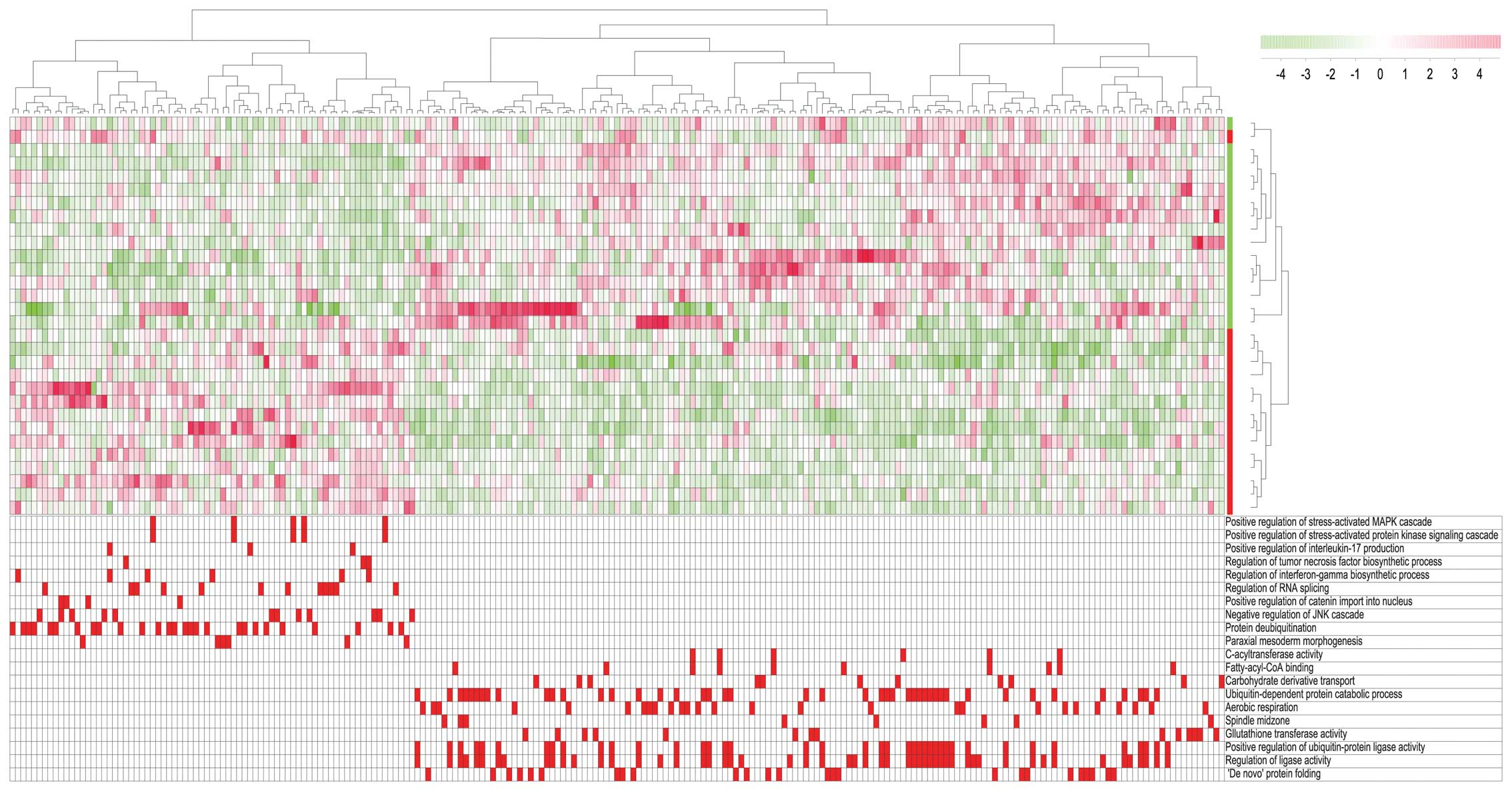
We then used gene set enrichment analysis (GSEA) to
compare the global gene expression profiles for two contrasting
patient subsets; the first subset included 15 AML patients with the
highest GJC1 expression (GJC1 high) and the second
included 15 patients with the lowest GJC1 expression
(GJC1 low). Using the leading genes belonging to the top ten
gene ontology (GO) terms enriched in both the low and the high
group, we identified 225 genes that were enriched in at least one
GO term (Fig. 5). We subsequently
performed a hierarchical clustering analysis based on these 225
genes; the 30 patients were then divided into two distinct subsets
corresponding to the original GJC1 high/low subsets except
for one outlier. The main GO terms enriched in the GJC1 high
group included annotations related to the mitogen-activated protein
kinase (MAPK) pathway and the synthesis of pro-inflammatory
cytokines, interleukin (IL)-17, tumor necrosis factor (TNF) and
interferon-γ. By contrast, the enriched GO terms for the
GJC1 low patients included terms related to the regulation
of ligase activity, protein folding and protein catabolism. Thus,
differences between AML patients with regard to Cx45 expression at
the mRNA level in leukemia cells are associated with additional
differences with regard to intra- and extracellular signaling, as
well as the regulation of protein function.
Combined data of mRNA and protein expression was
available for 19 overlapping patients between cohorts 1 and 2;
however, no significant correlations were detected between the mRNA
and protein levels for any connexin (data not shown). Thus, patient
subclassification based on connexin expression at the protein and
mRNA level identifies different subsets, and this is possibly due
to post-transcriptional regulation of the protein levels.
Discussion
Leukemic hematopoiesis is supported by various
stromal cells (1). In human AML,
this support seems to be caused both by communication through gap
junctions formed by connexins (2), as well as adhesion molecule binding
and the stromal release of leukemia-supporting cytokines (7–10).
This support may be important both for AML development and
chemosensitivity (11). The
supportive role of gap junctions/connexins has mainly been studied
in AML cell lines. In the present study, we therefore investigated
connexin expression in primary human AML cells. Cx43 and Cx45 in
particular showed a relatively high cell-surface expression
compared with the other connexins examined. These differences
between connexins may be caused by several mechanisms, including
differences in mRNA expression, post-transcriptional regulation or
protein turnover/degradation (24).
Animal studies have indicated that the knockout of
Cx32 in bone marrow facilitates leukemogenesis (25). The low mRNA levels of Cx32
detected in the present study suggest that low Cx32 expression
levels contribute to leukemogenesis in human AML, even though 13%
of the AML patients showed detectable expression levles of Cx32 on
the cell surface. The role of Cx32 in leukemogenesis may therefore
differ and contribute to the heterogeneity in leukemogenesis
between patients (26).
This study demonstrated that all investigated
connexins (i.e., Cx26, Cx32, Cx37, Cx43 and Cx45) were expressed in
AML cells, but detectable expression for >40% of the patients
was observed only for Cx43 (42%) and Cx45 (55%), even though these
two connexins showed the lowest mRNA expression. Furthermore,
studies on both normal and leukemic bone marrow cells have
demonstrated the formation of Cx43 gap junctions between stromal
and hematopoietic cells, as well as the upregulation of Cx43 gap
junctions in AML marrow (15).
Cx43 gap junctions also mediate the communication between normal
CD34+ hematopoietic stem cells and stromal cells
(27); animal models with the
loss of a single Cx43 allele have shown decreased normal
hematopoiesis (28), and gene
deletion of Cx43 in mice has been shown to decrease hematological
recovery following treatment with 5-fluorouracil (29). The present study suggests that
Cx43 is also important in leukemic hematopoiesis at least for a
subset ofpatients.
The protein expression of several connexins showed a
significant inverse (CD13) or positive correlation (CD11c, CD14,
CD15) with molecular differentiation markers, and increased levels
were also associated with morphological signs of differentiation.
By contrast, connexin protein expression showed no association with
cytogenetic abnormalities or mutations of the FLT3 or
NPM-1 genes. Taken together, these observations suggest that
differentiation is important for connexin expresssion, whereas
NPM-1 or FLT3 mutations only have a minor impact on
connexin expression.
Both Cx45 and Cx43 have been linked to stem cell
functions and are expressed by human, as well as mouse embryonic
stem cells (30–32). In murine cells, they can form
heterodimeric gap junction channels (32). Our experiments revealed that Cx43
and Cx45 expression was closely correlated (Fig. 3); however, based on our data, it
cannot be concluded whether this correlation represents a molecular
basis for the formation of heterodimers.
We observed several significant correlations between
the expression of various connexins (Fig. 2), even though the protein
expression of Cx26 and Cx32 was very low or absent in the majority
of patients, and several patients were negative for Cx37
expression. It is not certain whether Cx37 plays a role in normal
or neoplastic hematopoiesis, although its expression has been
detected in bone marrow endothelial cells (15), where it is important for monocyte
adhesion (33). Cx37 expression
may indirectly affect leukemic hematopoiesis through its
involvement in AML-associated angiogenesis or interactions with AML
cells in the endothelial stem cell niches.
The present study demonstrates that several
connexins, Cx43 and Cx45 in particular, are expressed on the
surface of primary human AML cells in a large subset of patients.
The profile of connexin protein expression can be used for the
subclassification of patients. This variation in protein expression
cannot be detected at the mRNA level, where only Cx45 expression
shows a considerable variation between patients. This Cx45 mRNA
variation is also associated with differences in intracellular and
extracellular cytokine signaling, as well as the regulation of
protein function. These observations suggest that different subsets
of patients are identified when the subclassification is based on
the protein and mRNA expression of connexins.
References
|
1
|
Shiozawa Y, Havens AM, Pienta KJ and
Taichman RS: The bone marrow niche: habitat to hematopoietic and
mesenchymal stem cells, and unwitting host to molecular parasites.
Leukemia. 22:941–950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foss B, Hervig T and Bruserud Ø: Connexins
are active participants of hematopoietic stem cell regulation. Stem
Cells Dev. 18:807–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shipley JL and Butera JN: Acute
myelogenous leukemia. Exper Hematol. 37:649–658. 2009. View Article : Google Scholar
|
|
4
|
Foss B, Ulvestad E and Bruserud Ø:
Platelet-derived growth factor (PDGF) in human acute myelogenous
leukemia: PDGF receptor expression, endogenous PDGF release and
responsiveness to exogenous PDGF isoforms by in vitro cultured
acute myelogenous leukemia blasts. Eur J Haematol. 66:365–376.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruserud Ø: IL-4, IL-10 and IL-13 in acute
myelogenous leukemia. Cytokines Cell Mol Ther. 4:187–198.
1998.PubMed/NCBI
|
|
6
|
Foss B, Mentzoni L and Bruserud Ø: Effects
of vascular endothelial growth factor on acute myelogenous leukemia
blasts. J Hematother Stem Cell Res. 10:81–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryningen A, Wergeland L, Glenjen N,
Gjertsen BT and Bruserud Ø: In vitro crosstalk between fibroblasts
and native human acute myelogenous leukemia (AML) blasts via local
cytokine networks results in increased proliferation and decreased
apoptosis of AML cells as well as increased levels of proangiogenic
Interleukin 8. Leuk Res. 29:185–196. 2005. View Article : Google Scholar
|
|
8
|
Hatfield K, Ryningen A, Corbascio M and
Bruserud Ø: Microvascular endothelial cells increase proliferation
and inhibit apoptosis of native human acute myelogenous leukemia
blasts. Int J Cancer. 119:2313–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruserud Ø, Ryningen A, Wergeland L,
Glenjen NI and Gjertsen BT: Osteoblasts increase proliferation and
release of proangiogenic interleukin 8 by native human acute
myelogenous leukemia blasts. Haematologica. 89:391–402.
2004.PubMed/NCBI
|
|
10
|
Konopleva M, Konoplev S, Hu W, Zaritskey
AY, Afanasiev BV and Andreeff M: Stromal cells prevent apoptosis of
AML cells by up-regulation of anti-apoptotic proteins. Leukemia.
16:1713–1724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Foss B, Tronstad KJ and Bruserud Ø:
Connexin-based signaling in acute myelogenous leukemia (AML).
Biochim Biophys Acta. 1798:1–8. 2010. View Article : Google Scholar
|
|
12
|
Weber MC and Tykocinski ML: Bone marrow
stromal cell blockade of human leukemic cell differentiation.
Blood. 83:2221–2229. 1994.PubMed/NCBI
|
|
13
|
Paraguassú-Braga FH, Borojevic R, Bouzas
LF, Barcinski MA and Bonomo A: Bone marrow stroma inhibits
proliferation and apoptosis in leukemic cells through gap
junction-mediated cell communication. Cell Death Differ.
10:1101–1108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Xu Y-B, Wang Q, et al: Leukemogenic
AML1-ETO fusion protein upregulates expression of connexin 43: the
role in AML1-ETO-induced growth arrest in leukemic cells. J Cell
Physiol. 208:594–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krenacs T and Rosendaal M: Connexin43 gap
junctions in normal, regenerating, and cultured mouse bone marrow
and in human leukemias: their possible involvement in blood
formation. Am J Pathol. 152:993–1004. 1998.PubMed/NCBI
|
|
16
|
Yi S, Chen Y, Wen L, Yang L and Cui G:
Expression of connexin 32 and connexin 43 in acute myeloid leukemia
and their roles in proliferation. Oncol Lett. 4:1003–1007.
2012.PubMed/NCBI
|
|
17
|
Liu Y, Zhang X, Li ZJ and Chen XH:
Up-regulation of Cx43 expression and GJIC function in acute
leukemia bone marrow stromal cells post-chemotherapy. Leuk Res.
34:631–640. 2010. View Article : Google Scholar
|
|
18
|
Bruserud Ø, Hovland R, Wergeland L, Huang
TS and Gjertsen BT: Flt3-mediated signaling in human acute
myelogenous leukemia (AML) blasts: a functional characterization of
Flt3-ligand effects in AML cell populations with and without
genetic Flt3 abnormalities. Haematologica. 88:416–428.
2003.PubMed/NCBI
|
|
19
|
Hatfield KJ, Hovland R, Øyan AM, et al:
Release of angio-poietin-1 by primary human acute myelogenous
leukemia cells is associated with mutations of nucleophosmin,
increased by bone marrow stromal cells and possibly antagonized by
high systemic angiopoietin-2 levels. Leukemia. 22:287–293. 2008.
View Article : Google Scholar
|
|
20
|
Tsykunova G, Reikvam H, Hovland R and
Bruserud Ø: The surface molecule signature of primary human acute
myeloid leukemia (AML) cells is highly associated with NPM1
mutation status. Leukemia. 26:557–559. 2012. View Article : Google Scholar
|
|
21
|
Bruserud Ø: Effect of dipyridamole,
theophyllamine and verapamil on spontaneous in vitro proliferation
of myelogenous leukaemia cells. Acta Oncol. 31:53–58. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dysvik B and Jonassen I: J-Express:
exploring gene expression data using Java. Bioinformatics.
17:369–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stavrum AK, Petersen K, Jonassen I and
Dysvik B: Analysis of gene-expression data using J-Express. Curr
Protoc Bioinformatics. Chapter 7: Unit 7.3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
VanSlyke JK and Musil LS: Cytosolic stress
reduces degradation of connexin43 internalized from the cell
surface and enhances gap junction formation and function. Mol Biol
Cell. 16:5247–5257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirabayashi Y, Yoon BI, Tsuboi I, et al:
Protective role of connexin 32 in steady-state hematopoiesis,
regeneration state, and leukemogenesis. Exp Biol Med. 232:700–712.
2007.
|
|
26
|
Döhner H, Estey EH, Amadori S, et al:
Diagnosis and management of acute myeloid leukemia in adults:
recommendations from an international expert panel, on behalf of
the European LeukemiaNet. Blood. 115:453–474. 2010. View Article : Google Scholar
|
|
27
|
Dürig J, Rosenthal C, Halfmeyer K, et al:
Intercellular communication between bone marrow stromal cells and
CD34+ haematopoietic progenitor cells is mediated by
connexin 43-type gap junctions. Br J Haematol. 111:416–425. 2000.
View Article : Google Scholar
|
|
28
|
Montecino-Rodriguez E, Leathers H and
Dorshkind K: Expression of connexin 43 (Cx43) is critical for
normal hematopoiesis. Blood. 96:917–924. 2000.PubMed/NCBI
|
|
29
|
Presley CA, Lee AW, Kastl B, et al: Bone
marrow connexin-43 expression is critical for hematopoietic
regeneration after chemotherapy. Cell Commun Adhes. 12:307–317.
2005. View Article : Google Scholar
|
|
30
|
Wong RC, Pébay A, Nguyen LT, Koh KL and
Pera MF: Presence of functional gap junctions in human embryonic
stem cells. Stem Cells. 22:883–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huettner JE, Lu A, Qu Y, Wu Y, Kim M and
McDonald JW: Gap junctions and connexon hemichannels in human
embryonic stem cells. Stem Cells. 24:1654–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wörsdörfer P, Maxeiner S, Markopoulos C,
et al: Connexin expression and functional analysis of gap
junctional communication in mouse embryonic stem cells. Stem Cells.
26:431–439. 2008. View Article : Google Scholar
|
|
33
|
Wong CW, Christen T, Roth I, et al:
Connexin37 protects against atherosclerosis by regulating monocyte
adhesion. Nat Med. 12:950–954. 2006. View
Article : Google Scholar : PubMed/NCBI
|