Introduction
Preterm birth is an extremely common and problematic
complication in perinatal medicine and is one of the leading causes
of perinatal mortality (1,2).
Infants that survive preterm birth often suffer from health
problems, including cerebral palsy, cognitive dysfunction,
blindness, hearing loss and respiratory system damage (3,4),
which can place a significant responsibility on individuals and
families. Clinical studies have shown that 40% of preterm births
are caused by infection (5).
Accumulating evidence indicates that infection is not only an
important reason to induce preterm labor (6,7)
but also a potential target for the prevention of preterm birth
(8). Therefore, it is important
to further explore the pathogenesis of preterm birth caused by
infection to reduce the risk of preterm birth and improve the
perinatal survival rate.
One of the well-known main clinical manifestations
of preterm birth is early contractions of the uterus, and the
ultimate factor inducing uterine contraction is the changes of
calcium signals in uterine smooth muscle cells (9–11).
Voltage-gated calcium-channels (VGCCs) are heterologous multimeric
transmembrane proteins located in the cell membrane and they are
responsible for transporting extracellular calcium ions into the
cells. VGCCs play an important role in the regulation of
intracellular calcium signals. The VGCCs of uterine smooth muscles
are mainly L- and T-type (12).
Previous studies on the inducing factors of obstetric labor have
mainly focused on L-type calcium channels. L-type calcium channel
inhibitors, such as nifedipine, have been used as one of the
first-line drugs in the treatment of premature delivery (13–15). The roles of T-type calcium
channels are also extremely extensive, involving areas including
tumor development (16–19), and endocrine (20–22) and cardiovascular diseases
(23–26). However, there have been limited
studies on their role in uterine contractions and labor
induction.
In the present study, mouse uterine smooth muscle
cells were isolated. Lipopolysaccharides (LPS) were used for cell
treatment. Under infectious conditions, the expression of T-type
calcium channels and the changes in calcium concentration of
uterine smooth muscle cells were measured and the possible
mechanisms were explored. The results showed that infection induced
the upregulation of T-type calcium channel expression and further
increased the concentration of calcium in uterine smooth muscle
cells, in which the nuclear factor (NF)-κB/endothelin-1 (ET-1)
signaling pathway plays an important role.
Materials and methods
Establishing the mouse model of
pregnancy
Twelve-week-old female C57BL/6 mice weighing 22–25 g
were purchased from the Animal Center laboratory of the China
Medical University (Shenyang, China). The mice were fed and housed
in a standard laboratory environment for a week for adaptation
prior to the experiment. The maintenance and handling of
experimental animals was approved by the Committee on Animal
Research and Ethics of the China Medical University. On day 1, the
mice were housed together with a female to male ratio of 2:1 in the
cage. The vaginal plugs were checked on the morning of the day 2.
When positive for a vaginal plug, the mouse was listed as in
gestation day 0. Pregnancy was confirmed on gestation days 7 and
12. Healthy pregnant mice were selected for the experiments.
Isolation and culture of mouse uterine
smooth muscle cells
Mouse uterine smooth muscle cells were isolated
based on the methods used in previous studies (27,28). The uterine tissues of pregnant
mice were isolated under sterile conditions. Pre-chilled D-Han’s
solution (containing 100 U/ml of penicillin-streptomycin) was used
to remove impurities. The serosa and endometrium were removed. The
intermediate smooth muscle layers were cut into 2–3-mm3
sections. A digestive solution mixture of 0.25% trypsin (Beyotime
Institute of Biotechnology, Haimen, China) and 0.25% type I
collagenase (Invitrogen Life Technologies, Carlsbad, CA, USA) was
added, and digestion proceeded at 37°C for 50 min. Subsequently,
20% fetal bovine serum in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco Life Technologies, Grand Island, NY, USA) was added to
terminate the reaction. The cells were filtered using 200-mesh
filters. The supernatant was discarded following centrifugation.
After washing with phosphate-buffered saline (PBS), 10% fetal
bovine serum in DMEM was added to re-suspend the cells. The cells
were subsequently seeded into 6-well plates and placed in the
incubator. After 24 h, the cell adherence was observed and the
medium was changed. The medium was subsequently changed every 3
days. The cells from the third passage were selected to conduct the
experiments. In the LPS group, 2,000 ng/ml LPS was added to treat
the cells for 12 h, whereas in the saline group, an equal volume of
saline was added. The untreated uterine smooth muscle cells were
used as a control group. In the NF-κB inhibitor group, 100
μM BAY 11-7028 (Beyotime Institute of Biotechnology) was
added prior to treatment with LPS. In the ET-1 treatment group, 50
nM ET-1 (Sigma-Aldrich, St. Louis, MO, USA) was added. In the ET-1
antagonist group, 10 μM bosentan (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) was added.
Immunofluorescence
The third passage of uterine smooth muscle cells
were seeded in 6-well plates with cover slips. After the cells
became ~100% confluent, 4% paraformaldehyde was applied to fix the
cells for 15 min. Once the fixative was discarded, 0.1% Triton
X-100 was added until all the cells were covered. The cells were
incubated for 30 min at room temperature. Subsequent to blocking
the cells with goat serum, α-smooth muscle actin (SMA) antibody
(ab40863) with 1:100 dilution (Abcam, Cambridge, MA, USA) was
added, and the cells were incubated overnight at 4°C. The cells
were washed and fluorescein isothiocyanate-labeled secondary
antibody (A0562; Beyotime Institute of Biotechnology) with 1:200
dilution was added, after which the cells were incubated at room
temperature for 60 min. 4′,6-Diamidino-2-phenylindole (DAPI) was
added for nuclear staining. The fluorescence quencher was added
dropwise, and the slices were mounted. Images were obtained using a
laser scanning confocal microscope (FV1000S-SIM/IX81; Olympus,
Tokyo, Japan).
Quantitative polymerase chain reaction
(qPCR)
TRIzol reagent (Invitrogen Life Technologies) was
used to extract the total RNA of the cells in each group. The RNA
was reverse transcribed to cDNA using a cDNA first strand synthesis
kit (Takara Bio, Inc., Dalian, China), according to the
manufacturer’s instructions. The upstream and downstream primer
sequences of Cav3.1 are 5′-ATAACAGTTCCAGCAATACCACC-3′ and
5′-GAATGAGCATCCATCACAAAGT-3′, respectively. The length of the
amplified fragment was 190 base pairs (bp). The upstream and
downstream primer sequences of Cav3.2 are
5′-AGAGCCGTTGGCGTAAGAAG-3′ and 5′-GCTGAAGTGGTAATGGTGGTGA-3′,
respectively. The length of the amplified fragment was 152 bp. The
upstream and downstream primer sequences of β-actin are
5′-CTGTGCCCATCTACGAGGGCTAT-3′ and 5′-TTTGATGTCACGCACGATTTCC-3′,
respectively. The length of the amplified fragment was 155 bp. qPCR
was conducted using an Exicycler™ 96 Quantitative fluorescence
Real-Time PCR System (Bioneer, Daejeon, Korea). The reaction volume
was 20 μl, including 1 μl cDNA, 0.5 μl of each
upstream and downstream primer, 10 μl SYBR GREEN mastermix
and 8 μl ddH2O. The PCR reaction program was 95°C
for 10 min followed by 40 cycles of 95°C for 10 sec, 58°C for 20
sec and 72°C for 30 sec; and subsequently 4°C for 5 min.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) was used for cell lysis. The
bicinchoninic acid method was used for protein quantification and
balancing. A total of 40 μg protein from each group was used
for SDS-PAGE. The sample was electronically transferred to a PVDF
membrane (Millipore, Bedford, MA, USA) following electrophoresis.
After 5% skimmed milk powder was added at room temperature and
blocked for 1 h, diluted primary antibody [Cav3.1 (sc-28617) and
Cav3.2 (sc-25691), 1:200 dilution; Santa Cruz Biotechnology, Inc.;
NF-κB p65 (WL00666), p-IκB (WL00020) and ET-1 (WL00138), 1:400
dilution; 1:400 dilution; Wanleibio, Shenyang, China] was added at
4°C and incubated overnight. Subsequently, 1:5,000 diluted
horseradish peroxidase-labeled secondary antibody (A0208; Beyotime
Institute of Biotechnology) was added and incubated at 37°C for 1
h. An electrochemiluminescence method was used for substrate
luminescence. Following exposure, the images were scanned into the
computer and grayscale analysis was performed by Image J software
(ImageJ Version 1.36b; National Institutes of Health, Bethesda, MD,
USA); β-actin was used as an internal reference to analyze the
expression level of each protein.
Detection of intracellular calcium ion
concentration
The concentration of intracellular calcium ions was
measured using the method of Wang et al (29). In the T-type calcium channel
inhibitor group, 5 μM NNC 55-0396 was added 10 min before
LPS treatment started (30).
After the cells in each group were collected and washed twice with
PBS, 1 ml 5 μM serum-free Fluo-3AM was added and incubated
at 37°C in the dark for 30 min. The cells were washed three times
and continued to incubate for another 20 min to allow the complete
conversion of Fluo-3AM to Fluo-3. Cells were collected and analyzed
by a flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The experimental data are presented as the means ±
standard deviation. One-way analysis of variance was used for
comparison between the groups. The Bonferroni post-hoc test was
used for multiple comparisons. GraphPad Prism 5.0 software (Graph
Pad, San Diego, CA, USA) was used for data processing. A value of
P<0.05 was considered to represent a statistically significant
difference.
Results
Isolation, culture and identification of
mouse uterine smooth muscle cells
The uterine tissues of pregnant mice were obtained.
Uterine smooth muscle cells were isolated using the
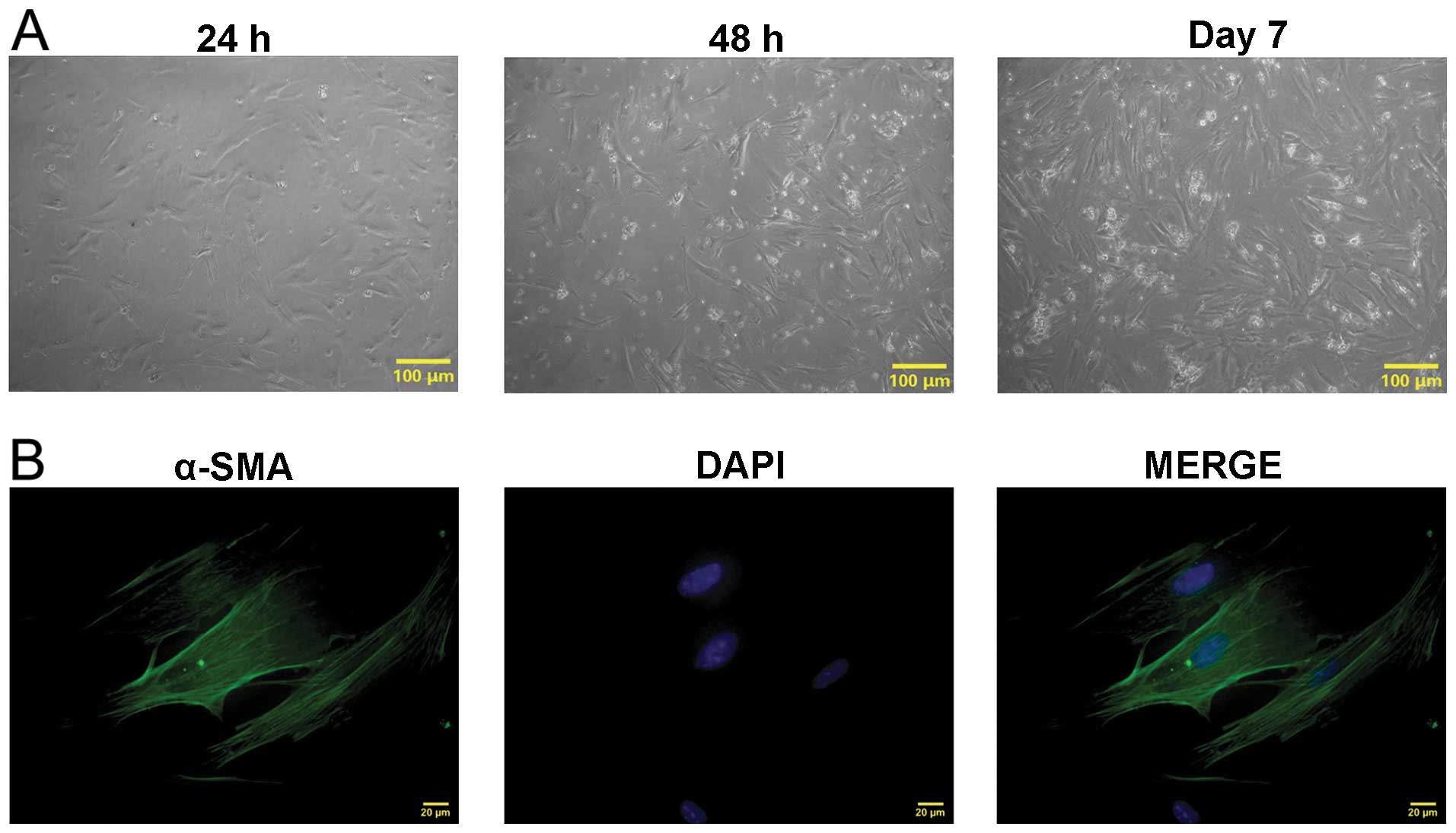
trypsin-collagenase I mixed enzyme digestion method (Fig. 1A). After 24 h, the cells had
already adhered to the wall of the wells and presented as fusiform
or polygonal shapes. The cell clones were visible after 48 h. Cells
were fused into pieces after 7 days. The cells from the third
passage were collected for α-SMA immunofluorescence staining
(Fig. 1B). The results showed
green filamentous actin in the cytoplasm, while the nuclei were
stained blue with DAPI indicating that mouse uterine smooth muscle
cells had been isolated.
LPS induces upregulation of T-type
calcium channel subtypes, Cav3.1 and Cav3.2, in mouse uterine
smooth muscle cells
Bacterial endotoxin LPS were used to treat uterine
smooth muscle cells to mimic an infectious uterine microenvironment
in vitro (31) to
investigate the effect of infection on T-type calcium channels
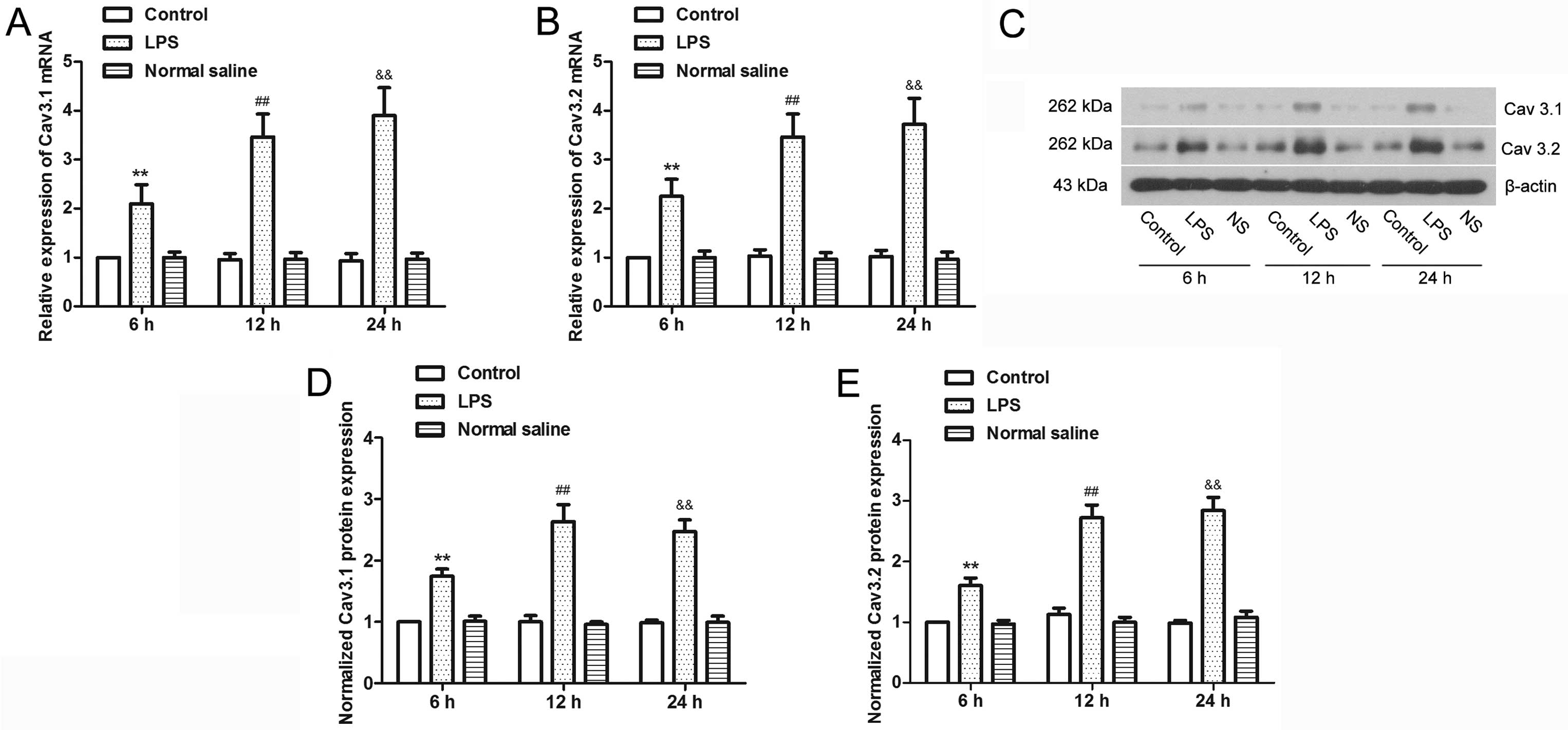
(Fig. 2). The results showed that
after 6 h of LPS treatment, there was a significant increase in
Cav3.1 and Cav3.2 mRNA (P<0.01), and the expression increased
with the increasing duration of LPS treatment. Western blot
analysis of the two proteins showed similar results to the mRNA
expression; LPS significantly increased the expression of Cav3.1
and Cav3.2 (P<0.01). Therefore, infection can cause the
upregulation of T-type calcium channel expression.
LPS upregulate calcium concentration
through T-type calcium channels in uterine smooth muscle cells
Increasing the intracellular calcium concentration
can directly cause uterine contractions (32). Therefore, the intracellular
calcium concentration of uterine smooth muscle cells was examined
to investigate the role of infection and T-type calcium channels in
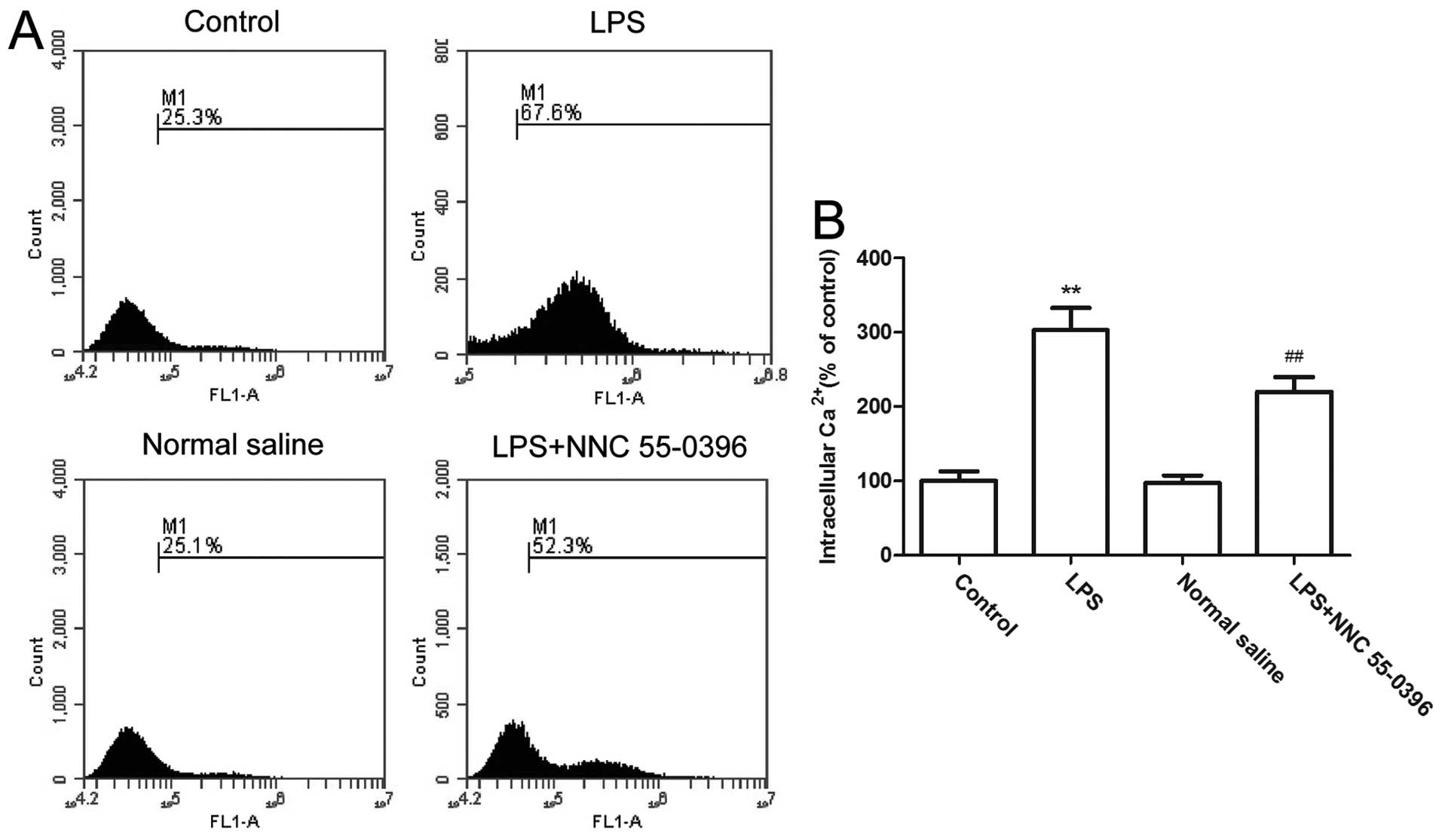
uterine contractions (Fig. 3).
The results showed that following LPS treatment, the intracellular
calcium concentration increased >3-fold (P<0.01). However,
subsequent to applying the T-type calcium channel inhibitors, the
calcium concentration was significantly reduced (P<0.01). As the
LPS-induced increases in calcium concentration can be reversed,
this indicates that the infection can lead to calcium influx in
uterine smooth muscle cells. In this process, T-type calcium
channels play an essential role.
LPS upregulates the expression of T-type
calcium channels via the NF-κB signaling pathway
The activation of the NF-κB signaling pathway was
determined to investigate the mechanism of T-type calcium channel
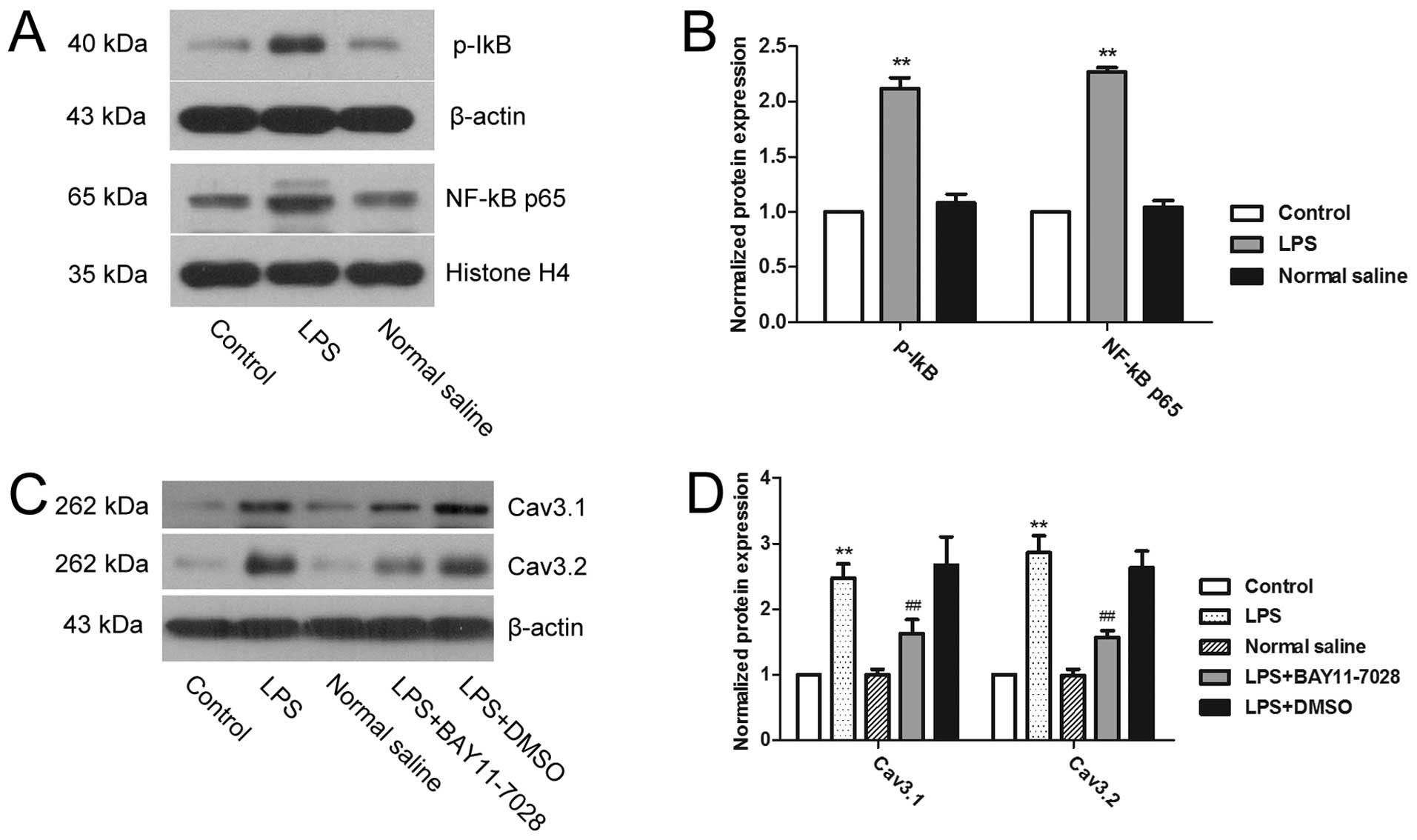
upregulation by LPS (Fig. 4). The
results showed that compared to the control group, the expression
of p-IκB in the cytoplasm and NF-κB p65 in the nucleus was
significantly higher in the LPS treatment group (P<0.01),
indicating that LPS can activate the NF-κB signaling pathway of
mouse uterine smooth muscle cells. Subsequent to applying the NF-κB
inhibitors, the LPS-induced upregulation of Cav3.1 and Cav3.2 was
significantly reversed (P<0.01). Therefore, NF-κB played a role
in the LPS-induced upregulation of T-type calcium channel
expression.
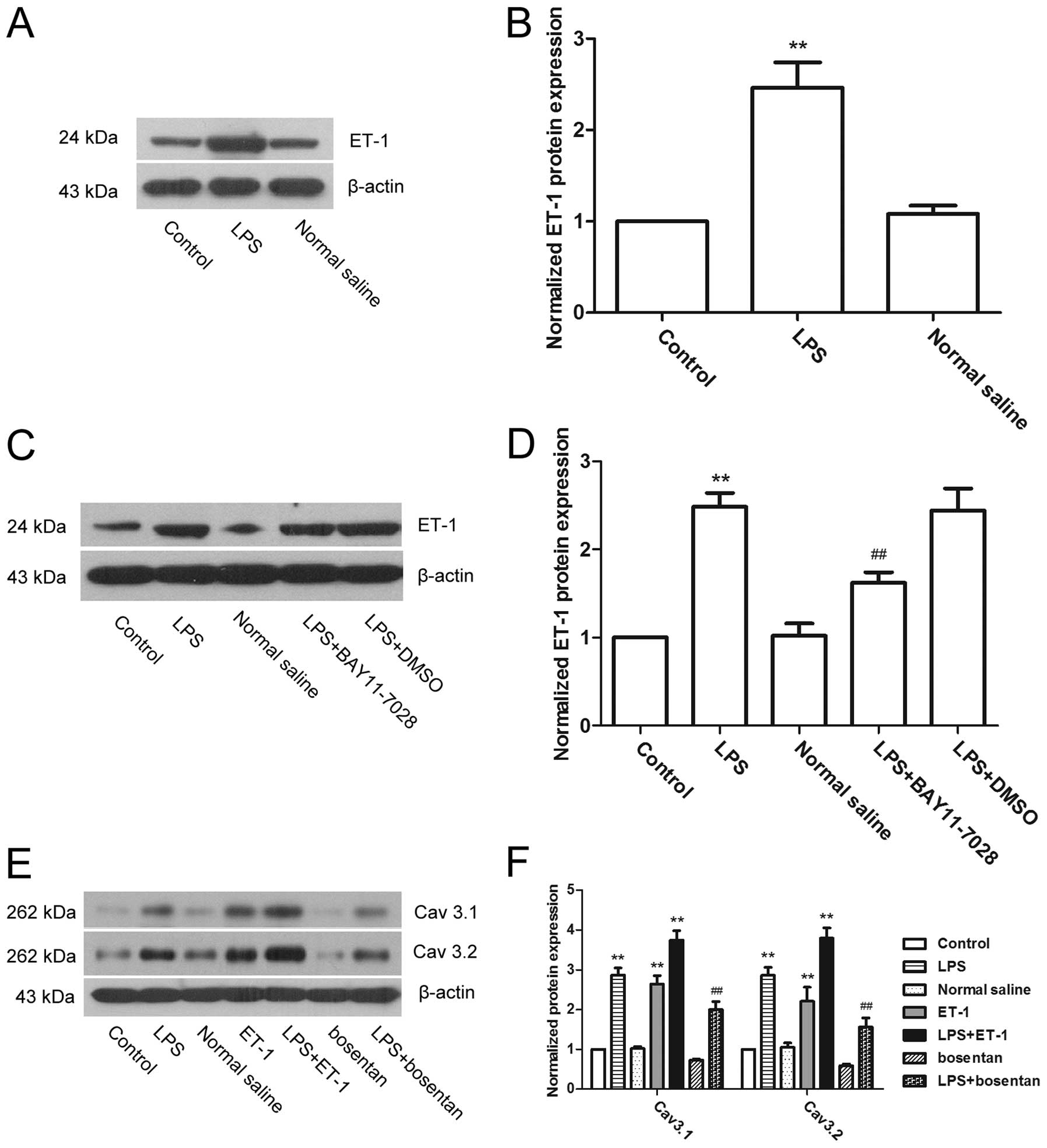
ET-1-mediated NF-κB signaling is involved
in the regulation of T-type calcium channels by LPS
ET-1 is an important factor that can induce calcium
influx, as well as induce cell contraction (33,34). Therefore, the expression of ET-1
was measured to investigate whether it was involved in the
regulation of T-type calcium channels by LPS (Fig. 5). The results showed that the
expression of ET-1 was significantly increased subsequent to
treatment with LPS (P<0.01), indicating that LPS can induce the
expression of ET-1 in uterine smooth muscle cells. Further
experiments showed that NF-κB inhibitors reduced the LPS-induced
upregulation of ET-1 expression (Fig.
5C and D). Therefore, ET-1 was regulated by NF-κB. Subsequent
to direct treatment of the uterine smooth muscle cells with ET-1,
the expression of Cav3.1 and Cav3.2 was upregulated, while the ET-1
antagonist, bosentan, reversed the effect of LPS on the above two
factors (Fig. 5E and F). These
results indicate that ET-1 mediates NF-κB signaling and
participates in the regulation of T-type calcium channels by
LPS.
Discussion
In the present study, mouse uterine smooth muscle
cells were isolated. LPS were applied to treat the cells to mimic
the microenvironment of uterine infection in vitro. The
mechanisms through which an infection affects the T-type calcium
channels and intracellular calcium concentration were also
explored. The results indicated that LPS significantly upregulated
the expression of T-type calcium channel subtypes Cav3.1 and Cav3.2
and increased the concentration of calcium in the uterine smooth
muscle cells. This process depends on the activation of the
NF-κB/ET-1 signaling pathways. The above results show that T-type
calcium channels play an important role in infection-induced
calcium influx. T-type calcium channels are expected to become a
novel target for the prevention of preterm birth.
In the signal transduction process during uterine
smooth muscle cell contraction in pregnant females, the ultimate
step controlling contraction is the changes in cytosolic calcium
signaling (32). T-type calcium
channels are a class of low activation potential, small
single-channel conductance voltage-gated calcium channels that can
directly regulate intracellular calcium ion concentrations. T-type
calcium channels also play a role as a secondary messenger in a
variety of physiological and pathological processes (23,35–37). T-type calcium channels expressed
in uterine smooth muscle cells are mainly Cav3.1 and Cav3.2
subtypes (38). LPS is the main
toxic component of Gram-negative bacteria (39). Levels of LPS were increased in the
amniotic cavity of premature patients with bacterial infection
(40). In the present study, the
use of LPS for the treatment of uterine smooth muscle cells to
mimic an in vitro bacterial infection microenvironment found
that the expression of Cav3.1 and Cav3.2 was significantly
upregulated in the infectious condition, and the intracellular
calcium concentration also increased. However, T-type calcium
channel antagonists can effectively inhibit the infection-induced
increase of calcium concentration, indicating that T-type calcium
channels play an indispensable role in the LPS signal transmission
and the regulation of intracellular calcium concentration
processes. Increased calcium concentration by excitable cells is
known to lead to cell shrinkage (41). T-type calcium channels in the
infectious condition are speculated to trigger uterine
contractions, while T-type calcium channel antagonist agents can
prevent preterm birth caused by infection.
NF-κB is an important transcription factor.
Activation can cause uterine smooth muscle cells to produce a
variety of inflammatory cytokines and active substances, promoting
intracellular calcium influx and inducing uterine contractions that
lead to preterm birth (42). In
the present study, under the treatment of LPS, there was a
significant increase in the expression of cytosolic p-IκB and NF-κB
p65 in the nucleus, suggesting that infection conditions can
activate the NF-κB signaling pathway, which has been confirmed in
numerous previous studies (43,44). NF-κB antagonists were further
demonstrated to reverse the LPS-induced increase in Cav3.1 and
Cav3.2 expression. Therefore, the increased expression of T-type
calcium channels by infection partially depends on the activation
of the NF-κB signaling pathway.
ET-1 is one of the small proteins with strong
vasoconstriction activities (45,46). Under stimulation, the uterus can
produce ET-1 and release it in the paracrine form to induce
contraction (47). The process of
ET-1-induced uterine contractions ultimately depends on the
increase in intracellular calcium concentration (48). In the present study, it was found
that following LPS treatment the expression of ET-1 was elevated,
whereas NF-κB inhibitors reversed the effect of LPS on ET-1.
Therefore, infection induces the release of ET-1 through the NF-κB
signaling pathway, which is consistent with previous studies
(49,50). The present study also found that
ET-1 can directly increase the expression of Cav3.1 and Cav3.2. In
addition, ET-1 antagonists inhibited the LPS-induced upregulation
of Cav3.1 and Cav3.2, indicating an important role of ET-1 in the
upregulation of T-type calcium channels and calcium influx in
uterine smooth muscle cells. Similar results have also been
demonstrated in myocardial cells (51,52).
In conclusion, the results demonstrated that
infection can induce the upregulation of T-type calcium channels
and facilitate calcium influx in uterine smooth muscle cells. This
process is closely associated with the activation of the NF-κB/ET-1
signaling pathway. The study illustrated the role of T-type calcium
channels in premature birth caused by infection. T-type calcium
channels are expected to become a novel target for the prevention
of infection-induced preterm birth.
Reference
|
1
|
Goldenberg RL, Hauth JC and Andrews WW:
Intrauterine infection and preterm delivery. N Engl J Med.
342:1500–1507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawn JE, Cousens S and Zupan J; Lancet
Neonatal Survival Steering Team: 4 million neonatal deaths: when?
where? why? Lancet. 365:891–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trachtenbarg DE and Golemon TB: Care of
the premature infant: Part I. Monitoring growth and development. Am
Fam Physician. 57:2123–2130. 1998.PubMed/NCBI
|
|
4
|
Howson CP, Kinney MV, McDougall L and Lawn
JE; Born Too Soon Preterm Birth Action Group: Born too soon:
preterm birth matters. Reprod Health. 10(Suppl 1): S12013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamont RF: Infection in the prediction and
antibiotics in the prevention of spontaneous preterm labour and
preterm birth. BJOG. 110(Suppl 20): 71–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shim SS, Romero R, Hong JS, et al:
Clinical significance of intra-amniotic inflammation in patients
with preterm premature rupture of membranes. Am J Obstet Gynecol.
191:1339–1345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christiaens I, Zaragoza DB, Guilbert L,
Robertson SA, Mitchell BF and Olson DM: Inflammatory processes in
preterm and term parturition. J Reprod Immunol. 79:50–57. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shynlova O, Dorogin A, Li Y and Lye S:
Inhibition of infection-mediated preterm birth by administration of
broad spectrum chemokine inhibitor in mice. J Cell Mol Med.
18:1816–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shmygol A and Wray S: Modulation of
agonist-induced Ca2+ release by SR Ca2+ load:
direct SR and cytosolic Ca2+ measurements in rat uterine
myocytes. Cell Calcium. 37:215–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Floyd R and Wray S: Calcium transporters
and signalling in smooth muscles. Cell Calcium. 42:467–476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young RC: Myocytes, myometrium, and
uterine contractions. Ann N Y Acad Sci. 1101:72–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamkin AG, Kiseleva IS, Kirishchuk SI and
Lozinskiĭ IT: Voltage-gated calcium channels. Usp Fiziol Nauk.
37:3–33. 2006.(In Russian). PubMed/NCBI
|
|
13
|
Flenady V, Wojcieszek AM, Papatsonis DN,
et al: Calcium channel blockers for inhibiting preterm labour and
birth. Cochrane Database Syst Rev. 6:CD0022552014.PubMed/NCBI
|
|
14
|
King JF, Flenady VJ, Papatsonis DN, Dekker
GA and Carbonne B: Calcium channel blockers for inhibiting preterm
labour. Cochrane Database Syst Rev. CD0022552003.PubMed/NCBI
|
|
15
|
Tranquilli AL and Giannubilo SR: Use and
safety of calcium channel blockers in obstetrics. Curr Med Chem.
16:3330–3340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang SJ, Choi HW, Choi DL, et al: In vitro
cytotoxicity on human ovarian cancer cells by T-type calcium
channel blockers. Bioorg Med Chem Lett. 23:6656–6662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santoni G, Santoni M and Nabissi M:
Functional role of T-type calcium channels in tumour growth and
progression: prospective in cancer therapy. Br J Pharmacol.
166:1244–1246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi DL, Jang SJ, Cho S, et al: Inhibition
of cellular proliferation and induction of apoptosis in human lung
adenocarcinoma A549 cells by T-type calcium channel antagonist.
Bioorg Med Chem Lett. 24:1565–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohkubo T and Yamazaki J: T-type
voltage-activated calcium channel Cav3.1, but not Cav3.2, is
involved in the inhibition of proliferation and apoptosis in MCF-7
human breast cancer cells. Int J Oncol. 41:267–275. 2012.PubMed/NCBI
|
|
20
|
Bhattacharjee A, Whitehurst RM Jr, Zhang
M, Wang L and Li M: T-type calcium channels facilitate insulin
secretion by enhancing general excitability in the
insulin-secreting beta-cell line, INS-1. Endocrinology.
138:3735–3740. 1997.PubMed/NCBI
|
|
21
|
Scholze A, Plant TD, Dolphin AC and
Nürnberg B: Functional expression and characterization of a
voltage-gated CaV1.3 (alpha1D) calcium channel subunit from an
insulin-secreting cell line. Mol Endocrinol. 15:1211–1221.
2001.PubMed/NCBI
|
|
22
|
Young RC and Zhang P: Inhibition of in
vitro contractions of human myometrium by mibefradil, a T-type
calcium channel blocker: support for a model using
excitation-contraction coupling, and autocrine and paracrine
signaling mechanisms. J Soc Gynecol Investig. 12:e7–e12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lalevée N, Rebsamen MC, Barrère-Lemaire S,
et al: Aldosterone increases T-type calcium channel expression and
in vitro beating frequency in neonatal rat cardiomyocytes.
Cardiovasc Res. 67:216–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cribbs LL: T-type Ca2+ channels
in vascular smooth muscle: multiple functions. Cell Calcium.
40:221–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pluteanu F and Cribbs LL: Regulation and
function of Cav3.1 T-type calcium channels in IGF-i-stimulated
pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol.
300:C517–C525. 2011. View Article : Google Scholar :
|
|
26
|
Tzeng BH, Chen YH, Huang CH, Lin SS, Lee
KR and Chen CC: The Ca(v)3.1 T-type calcium channel is required for
neointimal formation in response to vascular injury in mice.
Cardiovasc Res. 96:533–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oldenhof AD, Shynlova OP, Liu M, Langille
BL and Lye SJ: Mitogen-activated protein kinases mediate
stretch-induced c-fos mRNA expression in myometrial smooth muscle
cells. Am J Physiol Cell Physiol. 283:C1530–C1539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitchell JA, Shynlova O, Langille BL and
Lye SJ: Mechanical stretch and progesterone differentially regulate
activator protein-1 transcription factors in primary rat myometrial
smooth muscle cells. Am J Physiol Endocrinol Metab. 287:E439–E445.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Ruan Y, Chen Q, Li S, Wang Q and
Cai J: Curcumin induced HepG2 cell apoptosis-associated
mitochondrial membrane potential and intracellular free Ca(2+)
concentration. Eur J Pharmacol. 650:41–47. 2011. View Article : Google Scholar
|
|
30
|
Okubo K, Takahashi T, Sekiguchi F, et al:
Inhibition of T-type calcium channels and hydrogen sulfide-forming
enzyme reverses paclitaxel-evoked neuropathic hyperalgesia in rats.
Neuroscience. 188:148–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thota C, Farmer T, Garfield RE, Menon R
and Al-Hendy A: Vitamin D elicits anti-inflammatory response,
inhibits contractile-associated proteins, and modulates toll-like
receptors in human myometrial cells. Reprod Sci. 20:463–475. 2013.
View Article : Google Scholar :
|
|
32
|
Wray S, Jones K, Kupittayanant S, et al:
Calcium signaling and uterine contractility. J Soc Gynecol
Investig. 10:252–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bouallegue A, Daou GB and Srivastava AK:
Endothelin-1-induced signaling pathways in vascular smooth muscle
cells. Curr Vasc Pharmacol. 5:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masamune A, Satoh M, Kikuta K, Suzuki N
and Shimosegawa T: Endothelin-1 stimulates contraction and
migration of rat pancreatic stellate cells. World J Gastroenterol.
11:6144–6151. 2005.PubMed/NCBI
|
|
35
|
Chemin J, Monteil A, Perez-Reyes E,
Bourinet E, Nargeot J and Lory P: Specific contribution of human
T-type calcium channel isotypes (alpha(1 G), alpha(1 H) and
alpha(1I)) to neuronal excitability. J Physiol. 540:3–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu W, Wang P, Ma H, et al: Suppression of
T-type Ca2+ channels inhibited human laryngeal squamous
cell carcinoma cell proliferation running title: roles of T-type
Ca2+ channels in LSCC cell proliferation. Clin Lab.
60:621–628. 2014.
|
|
37
|
Chen Y, Parker WD and Wang K: The role of
T-type calcium channel genes in absence seizures. Front Neurol.
5:452014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cens T, Rousset M, Kajava A and Charnet P:
Molecular determinant for specific ca/ba selectivity profiles of
low and high threshold Ca2+ channels. J Gen Physiol.
130:415–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sperandeo P, Deho G and Polissi A: The
lipopolysaccharide transport system of gram-negative bacteria.
Biochim Biophys Acta. 1791:594–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Romero R, Espinoza J, Goncalves LF,
Kusanovic JP, Friel LA and Nien JK: Inflammation in preterm and
term labour and delivery. Semin Fetal Neonatal Med. 11:317–326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karaki H, Ozaki H, Hori M, et al: Calcium
movements, distribution, and functions in smooth muscle. Pharmacol
Rev. 49:157–230. 1997.PubMed/NCBI
|
|
42
|
Hua R, Pease JE, Sooranna SR, et al:
Stretch and inflammatory cytokines drive myometrial chemokine
expression via NF-κB activation. Endocrinology. 153:481–491. 2012.
View Article : Google Scholar
|
|
43
|
Ikebe M, Kitaura Y, Nakamura M, et al:
Lipopolysaccharide (lPS) increases the invasive ability of
pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J
Surg Oncol. 100:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamamoto Y and Gaynor RB: Role of the
NF-kappaB pathway in the pathogenesis of human disease states. Curr
Mol Med. 1:287–296. 2001. View Article : Google Scholar
|
|
45
|
Mitchell MD, Branch DW, Lamarche S and
Dudley DJ: The regulation of endothelin production in human
umbilical vein endothelial cells: unique inhibitory action of
calcium ionophores. J Clin Endocrinol Metab. 75:665–668.
1992.PubMed/NCBI
|
|
46
|
Wolff K, Nisell H, Modin A, Lundberg JM,
Lunell NO and Lindblom B: Contractile effects of endothelin 1 and
endothelin 3 on myometrium and small intramyometrial arteries of
pregnant women at term. Gynecol Obstet Invest. 36:166–171. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rae GA, Calixto JB and D’Orléans-Juste P:
Effects and mechanisms of action of endothelins on non-vascular
smooth muscle of the respiratory, gastrointestinal and urogenital
tracts. Regul Pept. 55:1–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Robin P, Boulven I, Desmyter C, Harbon S
and Leiber D: ET-1 stimulates ERK signaling pathway through
sequential activation of PKC and src in rat myometrial cells. Am J
Physiol Cell Physiol. 283:C251–C260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ohkita M, Takaoka M, Shiota Y, Nojiri R,
Sugii M and Matsumura Y: A nuclear factor-kappaB inhibitor BAY
11-7082 suppresses endothelin-1 production in cultured vascular
endothelial cells. Jpn J Pharmacol. 89:81–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sugii M, Ohkita M, Taniguchi M, et al:
Xanthoangelol D isolated from the roots of Angelica keiskei
inhibits endothelin-1 production through the suppression of nuclear
factor-kappaB. Biol Pharm Bull. 28:607–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Furukawa T, Ito H, Nitta J, et al:
Endothelin-1 enhances calcium entry through T-type calcium channels
in cultured neonatal rat ventricular myocytes. Circ Res.
71:1242–1253. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Izumi T, Kihara Y, Sarai N, et al:
Reinduction of T-type calcium channels by endothelin-1 in failing
hearts in vivo and in adult rat ventricular myocytes in vitro.
Circulation. 108:2530–2535. 2003. View Article : Google Scholar : PubMed/NCBI
|