Introduction
Gastric cancer is one of the leading causes of
cancer-related mortality worldwide (1). Progress in gastric cancer has been
slow, but steady (2). The
majority of gastric cancer patients have massive metastatic spread
at the time of initial diagnosis (3). Patients with advanced gastric cancer
have a poor prognosis (4), and
this results in approximately 800,000 deaths worldwide annually
(5). Therefore, it is necessary
to investigate the molecular mechanisms underlying the invasive
capacity of gastric cancer so as to enhance the curative effects
against gastric cancer.
Tetraspanins are cell-surface glycoproteins that are
characterized by the presence of 4 hydrophobic domains (6–8).
They form protein complexes with integrins and mediate signal
transduction events that play key roles in the regulation of cell
growth, activation, development and motility (9–13).
Tetraspanins have received attention as both suppressors and
promoters of metastasis (14–16).
CO-029 is a member of the tetraspanin family. CO-029
has been implicated to be a metastasis-promoting tetraspanin in
certain types of cancer, including colon carcinoma (17), esophageal carcinoma (18), pancreatic cancer (19) and hepatocellular carcinoma
(20). However, to the best of
our knowledge, studies on the functions of CO-029 in gastric cancer
cell proliferation and invasion are limited.
Epidermal growth factor (EGF) is a peptide of 53
amino acid residues, it binds to the epidermal growth factor
receptor (EGFR) and initiates the cascade of intracellular
signaling pathways. Therefore, EGF is involved in a variety of
physiological and pathologic processes, including cell
proliferation, apoptosis, migration, survival and angiogenesis
(21–23). However, the potential molecular
mechanisms underlying the effect of EGF on gastric cancer cell
growth have not yet been fully elucidated.
Hence, in the present study, we investigated the
expression of CO-029 in gastric cancer tissues in order to
determine whether CO-029 is involved in the effects of EGF on
gastric cancer cell proliferation and invasion.
Materials and methods
Tissues
Approval for the present study was obtained from the
Ethics Committee of The Second Affiliated Hospital of Zhejiang
University School of Medicine, Hangzhou, China and informed consent
was obtained from all patients prior to enrollment in this study.
Gastric cancer tissues, tumor-adjacent tissues and normal gastric
tissues were surgically resected from 32 patients with primary
gastric cancer. None of the 32 patients had receivedchemotherapy or
radiotherapy prior to surgery. The specimens were immediately used
for mRNA and protein extraction.
Cell culture
AGS cells purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s
modified Eagle’s medium with 10% fetal bovine serum (FBS) (both
from Invitrogen, Carlsbad, CA, USA) in the presence of 5%
CO2 at 37°C. The culture medium was replaced every 2–3
days. EGF was purchased from Sigma (St. Louis, MO, USA) and
dissolved in PBS at different concentrations (5, 10, 25, 50 and 100
ng/ml) for cell stimulation.
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen). Total RNA (2 μg) was used to perform reverse
transcription using the RevertAid First Strand cDNA Synthesis kit
(Fermentas, Vilnius, Lithuania). Real-time PCR was performed on a
7900 real-time PCR system using the SYBR-Green PCR kit (both from
Applied Biosystems, Foster City, CA, USA). All procedures were
carried out according to the manufacturer’s instructions. The Ct
value was calculated using the ΔΔCt method.
Western blot analysis
The protein samples from the tissues and cultured
cells were isolated using a total protein extraction kit (Sangon
Biotech, Shanghai, China) following the manufacturer’s
instructions. Protein concentrations were examined using the BCA
method with reagents from Pierce (Rockford, IL, USA). Protein
samples were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride membranes. Subsequently,
the membranes were incubated in blocking solution (Tris-buffered
saline containing 5% non-fat milk and 0.05% Tween-20) at 4°C
overnight. The blots were probed with the primary antibodies
[rabbit polyclonal to CO-029 (sc-292058), 1:400 dilution; rabbit
polyclonal to β-actin (sc-130656), 1:400 dilution] followed by
IgG-horse radish peroxidase (HRP)-conjugated secondary antibody
[goat anti-rabbit IgG-HRP (sc-2004), 1:2,000 dilution] (all from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The signal
was detected using an ECL western blotting kit (Pierce) and the
LabWork 4.0 software (UVP Inc., Upland, CA, USA) was used for
semi-quantitative analysis. β-actin was used as a loading
control.
Transfection
For siRNA transfection, the cells were seeded into
6-well plates and allowed to grow to approximately 70% confluence.
The cells were then transfected with 2 μM CO-029 siRNA or
scramble control siRNA using Lipofectamine 2000 (Invitrogen)
following the manufacturer’s instructions. Following incubation
with the transfection mixtures for 4 h, the medium was removed and
the cells were incubated with fresh Dulbecco’s modified Eagle’s
medium containing 10% FBS for 24 h. Scramble control siRNA
(Scr)-transfected cells were incubated with PBS or EGF; CO-029
siRNA (Si)-transfected cells were incubated with EGF.
MTT assay
Cell proliferation was determined by MTT assay. The
cells were seeded into 96-well plates and EGF was added to the
medium. Following treatment for 12, 24, 48 and 72 h, the cells were
incubated with 10 μl MTT (Sigma) at 37°C for 4 h. The medium
was then removed and the cells were incubated with 200 μl
dimethyl sulfoxide to solubilize the formazan crystals. Untreated
cells were used as the contrls. The absorbance at 570 nm was
measured using a microplate reader (Molecular Devices, Sunnyvale,
CA, USA).
Cell invasion assay
Cells in the blank group were incubated with
Dulbecco’s modified Eagle’s medium containing 10% FBS. PBS was
added to the control group as the vehicle control. Cell invasion
ability was evaluated using Transwell inserts coated with Matrigel.
Transwell inserts (Corning, Inc., Corning, NY, USA) were coated
with Matrigel matrix (BD Biosciences, Franklin Lakes, NJ, USA) at a
final concentration of 200 μg/ml. The cell plates with the
coated inserts were incubated at 37°C for 2 h. Cell suspension was
prepared in serum-free medium containing 5×104 cells/ml
and added to the upper chambers. A total of 1 ml of cell medium
containing 10% FBS was added to the lower chambers. The cell
invasion chambers were incubated overnight in a 37°C, 5%
CO2 atmosphere. A cotton swab was used to gently remove
the non-invaded cells. The cells on the lower surface of the
membrane were fixed in 95% ethanol and then stained with
hematoxylin for 10 min. The number of invaded cells was evaluated
by counting the cells under an inverted microscope (Nikon, Tokyo,
Japan).
Statistical analysis
Descriptive data are presented as the means ±
standard deviation (SD). Differences between 2 groups were
evaluated using the Student’s t-test. A P-value ≤0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CO-029 in human gastric
cancer tissues
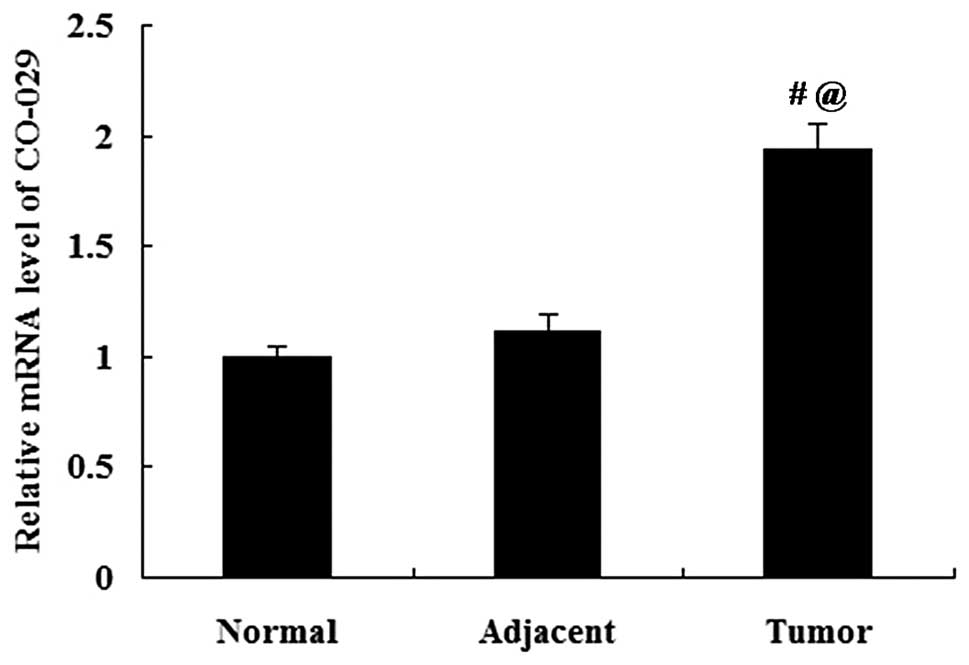
The expression of CO-029 in human normal gastric
tissues, gastric cancer tissues and tumor-adjacent tissues was
analyzed by RT-qPCR and western blot analysis. The mRNA expression
of CO-029 did not differ significantly between the normal gastric
tissues and the tumor-adjacent tissues (P>0.05); however, the
CO-029 mRNA expression was significantly increased in the gastric
cancer tissues compared with both the normal gastric tissues and
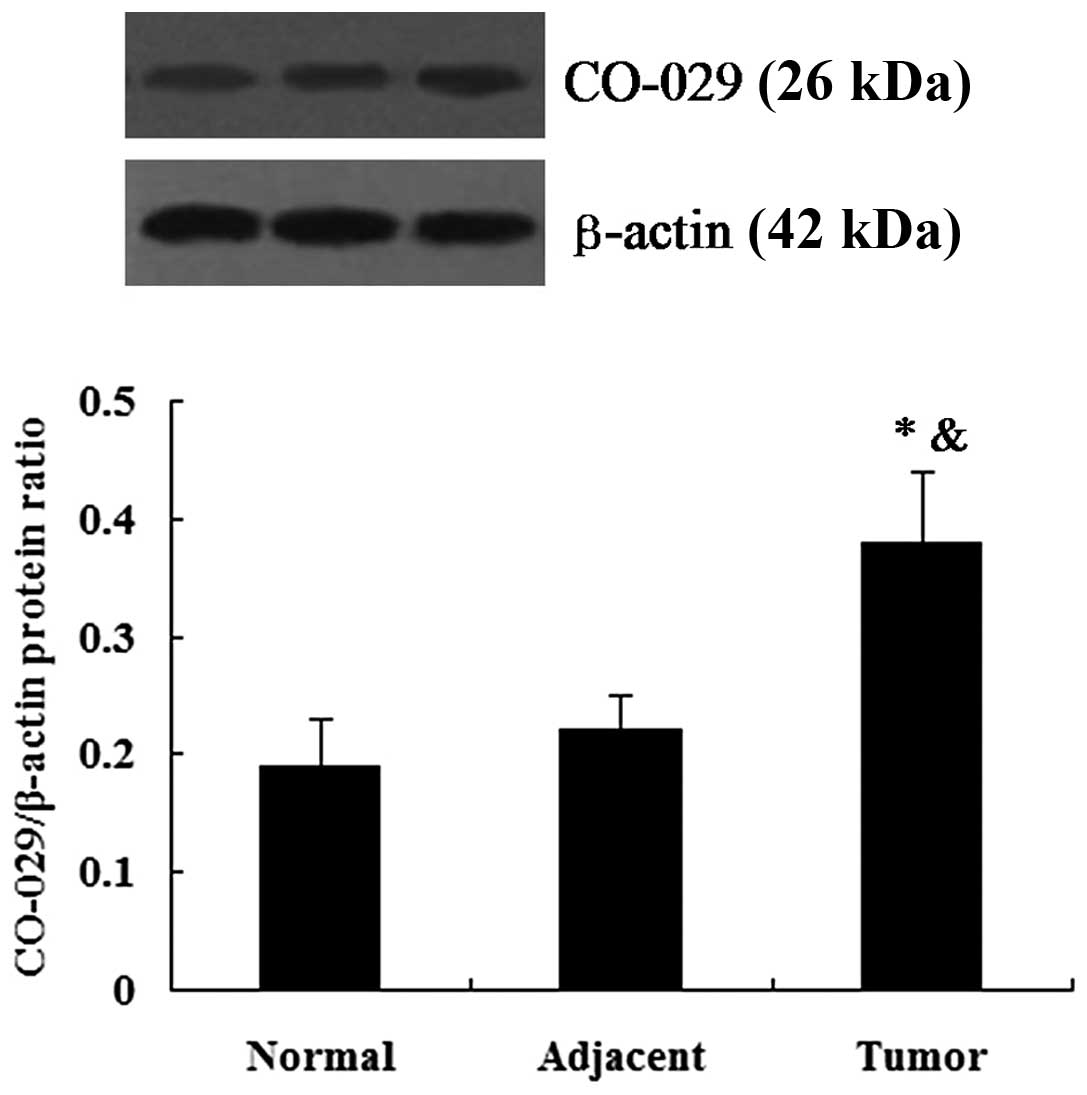
the tumor-adjacent tissues (P<0.01; Fig. 1). The results from western blot
analysis revealed that CO-029 protein expression was detectable in
the human normal gastric tissues, gastric cancer tissues and
tumor-adjacent tissues. Similar to the results obtained for the
mRNA expression, the gastric cancer tissues showed an upregulated
protein expression of CO-029 in comparison to the normal gastric
tissues and tumor-adjacent tissues (P<0.05l; Fig. 2).
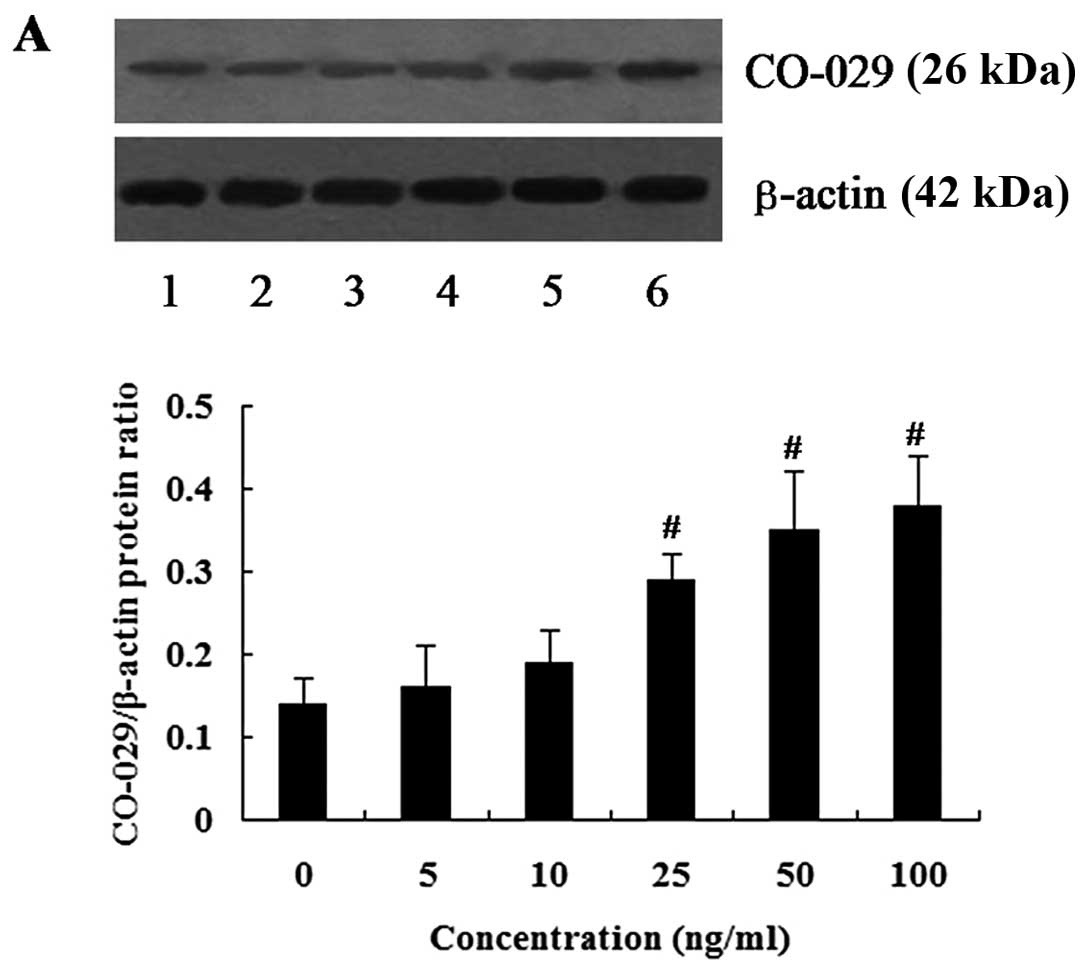
Effect of EGF on CO-029 protein
expression in AGS cells
The cells were treated with various concentrations
of EGF for 24 h, and western blot analysis was then performed to
examine the effect of EGF on CO-029 protein expression. We observed
that treatment with EGF increased the expression level of CO-029 in
a dose-dependent manner. EGF at the concentration of 5 and 10 ng/ml
did not seem to affect CO-029 protein expression. However, EGF at
the concentrations of 25 to 100 ng/ml increased the expression
level of CO-029 and its expression level reach a peak value at 100
ng/ml (P<0.01) (Fig. 3A).
The cells were also treated with 100 ng/ml EGF for 6
to 48 h (Fig. 3B) and the results
demonstrated that EGF affected CO-029 protein expression in a
time-dependent manner. EGF at a concentration of 100 ng/ml
increased the expression level of CO-029 following 12 to 48 h of
treatment (P<0.01). However, there was no significant difference
observed between the 6-h treatment group and the control group
(P>0.05).
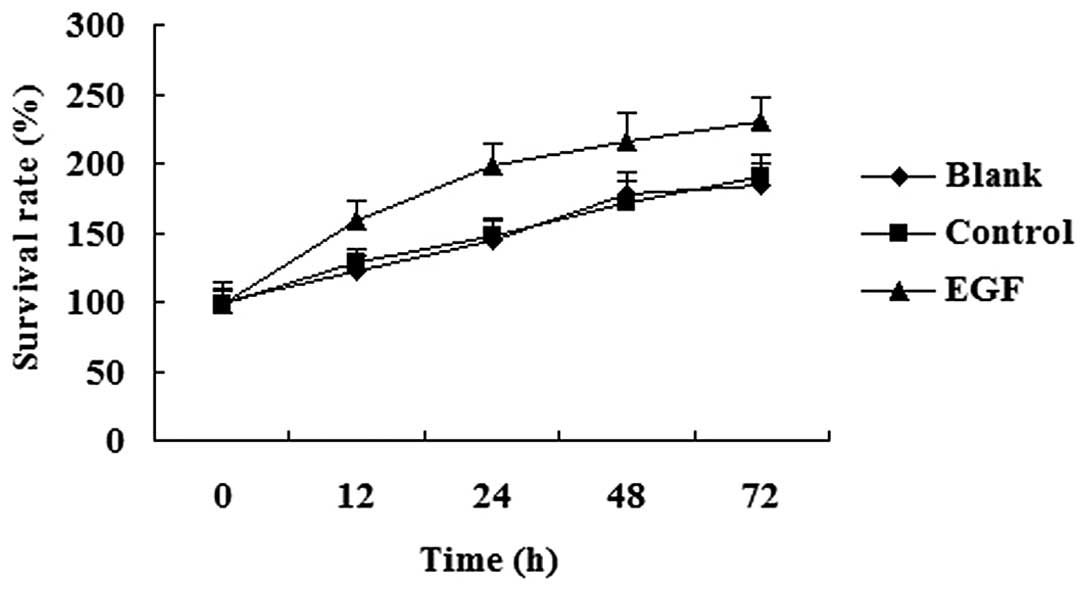
Effect of EGF on cell proliferation
The cells were treated with 100 ng/ml EGF for 12 to
72 h, and MTT assay was then performed to determine the effect of
EGF on cell proliferation. We found that EGF promoted cell growth,
and the cells treated with EGF proliferated at a higher rate
compared with the untreated controls (Fig. 4).
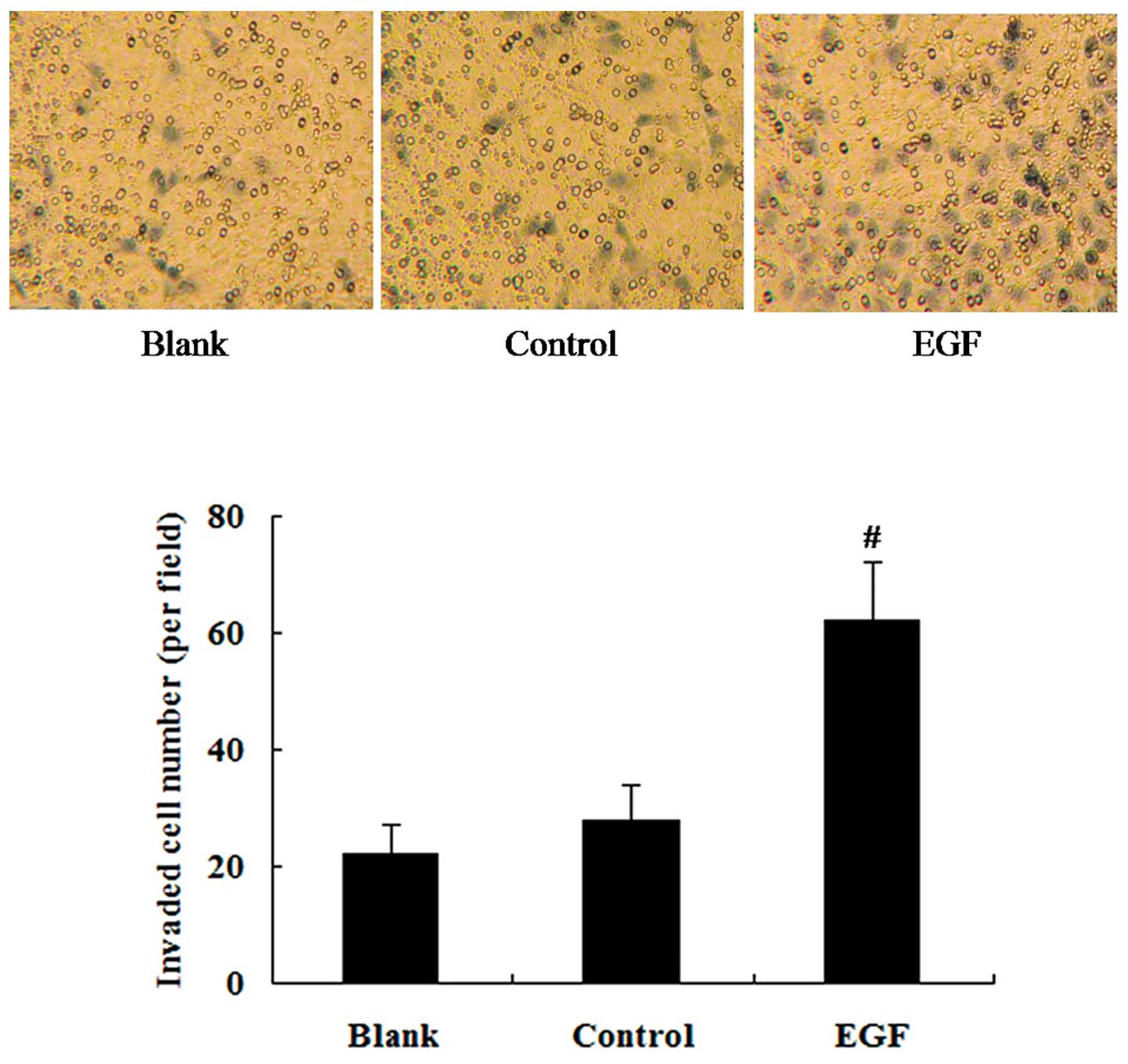
Effect of EGF on cell invasion
To determine the effect of EGF on the invasion
ability of the cells, the cells were treated with 100 ng/ml EGF for
24 h and Transwell-Matrigel invasion assay was then performed. Cell
invasion assay revealed that the cells treated with EGF showed an
enhanced invasion ability in comparison to the untreated controls
(P<0.01) (Fig. 5).
Suppression of CO-029 expression
attenuates the effects of EGF on cell proliferation and
invasion
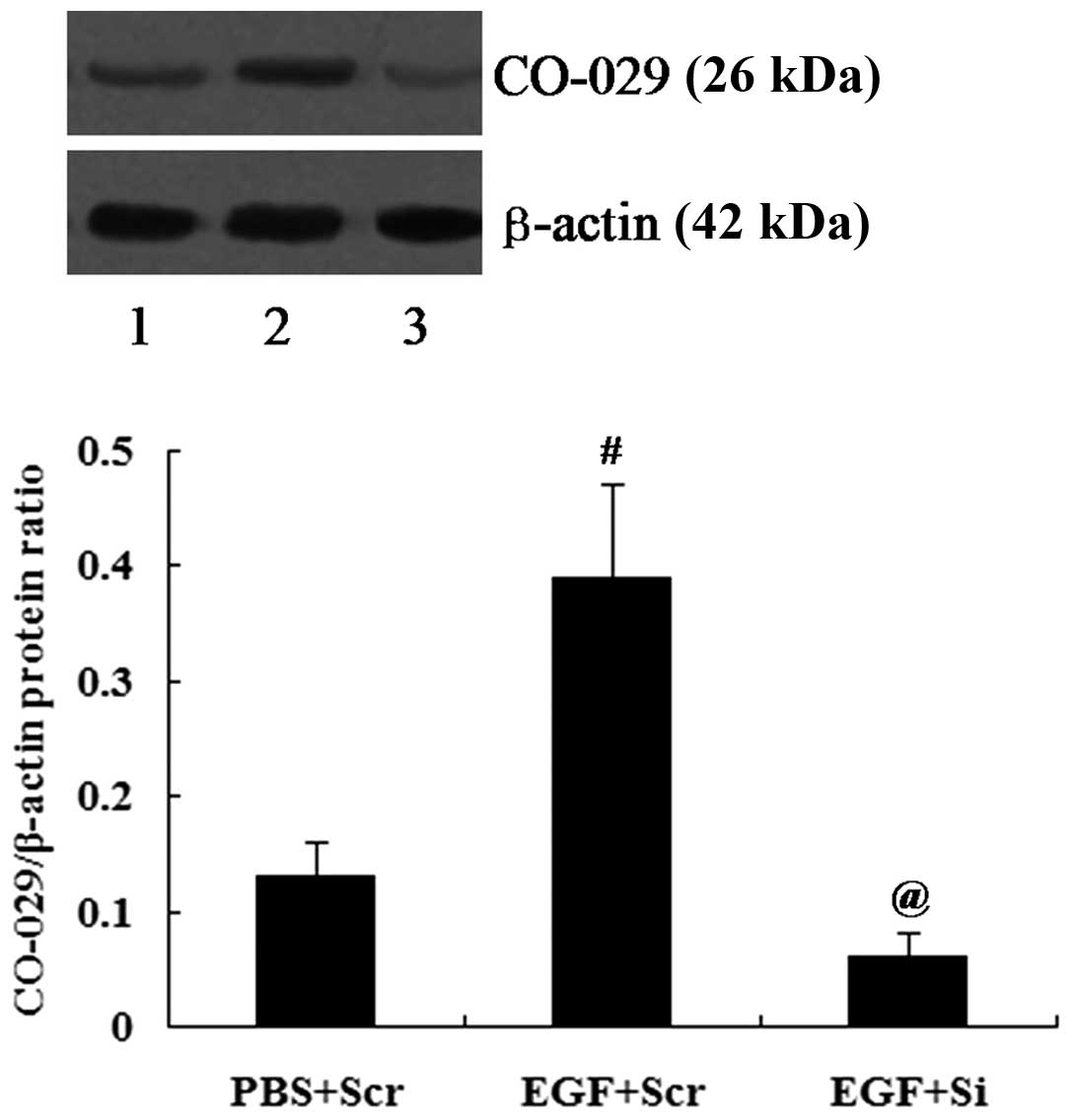
The cells transfected with CO-029 siRNA (Si) or
scramble control siRNA (Scr) were treated with 100 ng/ml EGF for 24
h followed by the determination of CO-029 protein expression levels
by western blot analysis. CO-029 expression was successfully
reduced by siRNA (P<0.01; Fig.
6).
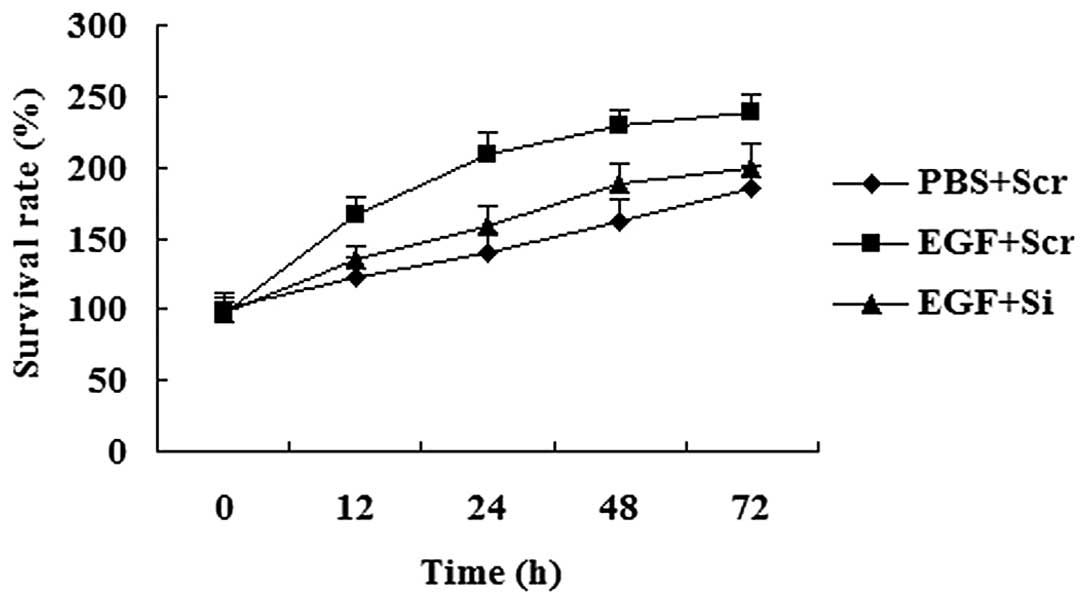
MTT assay revealed that EGF promoted cell growth;
however, the cells with a reduced expression of CO-029 proliferated
at a lower rate compared with the cells transfected with the
scramble control siRNA (Scr) in response to EGF treatment (Fig. 7).
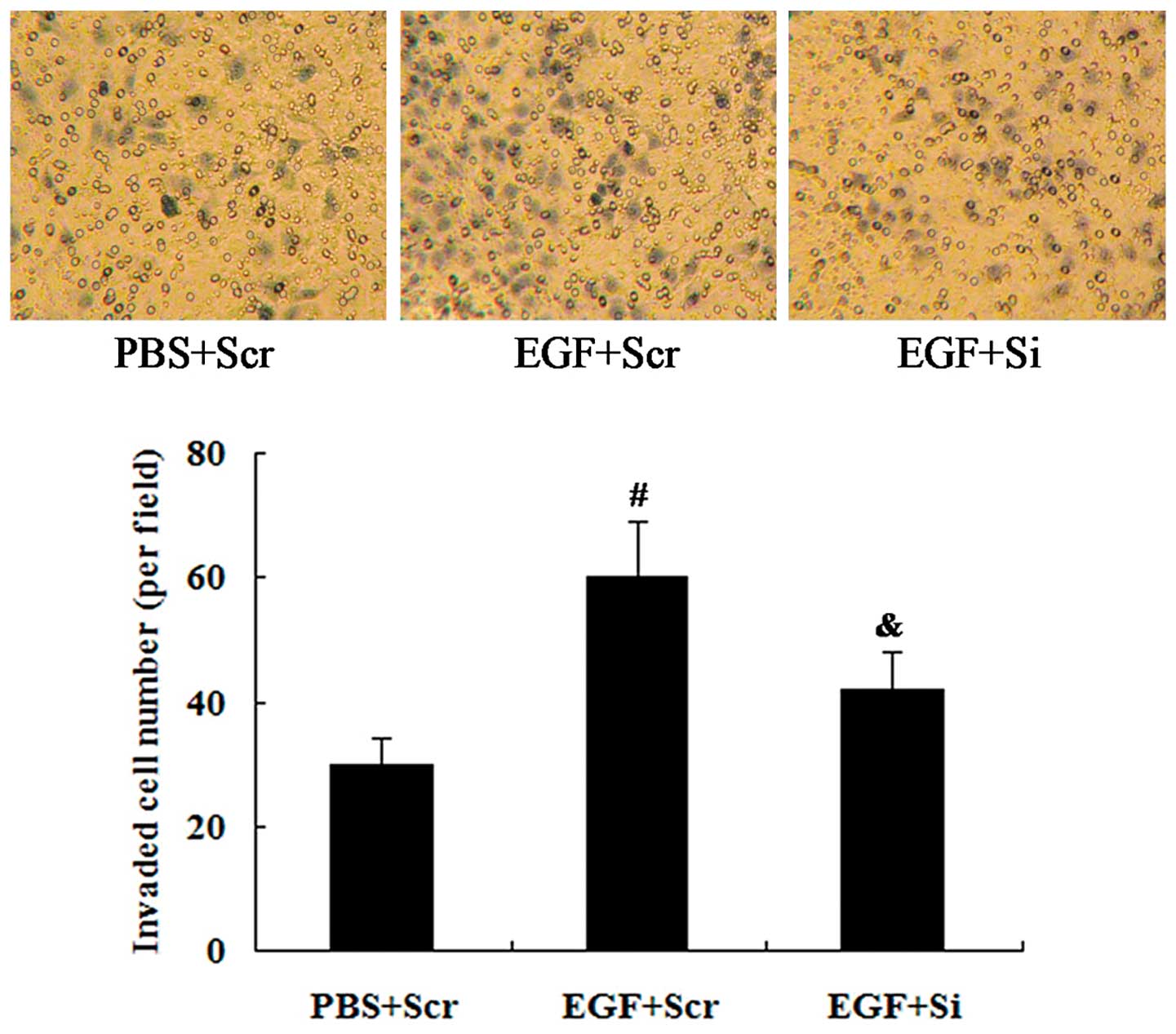
We also examined whether CO-029 is involved in the
effect of EGF on cell invasion. CO-029 siRNA was transfected into
the cells for 24 h, and the cells were subsequently treated with
100 ng/ml EGF for a further 24 h. The results from the
Transwell-Matrigel invasion assay revealed that the EGF-induced
cell invasion was attenuated by the knockdown of CO-029 expression
(P<0.05; Fig. 8).
Discussion
In the study by Matsumura et al, it was
reported that 53 genes were upregulated in advanced gastric cancer
by microarray analysis, and CO-029 was among these upregulated
genes (24). In the present
study, RT-qPCR and western blot analysis were used to assess the
expression of CO-029 in human normal gastric tissues, gastric
cancer and tumor-adjacent tissues. We observed that the expression
of CO-029 was increased both at the mRNA level and protein level in
gastric cancer tissues in comparison to normal gastric tissues and
tumor-adjacent tissues. These data indicate that CO-029 acts as an
oncogene in human gastric cancer.
Next, we performed in vitro experiments to
further investigate the role of CO-029 in gastric cancer cell
proliferation and invasion. Previous studies have suggested that
EGF acts as a mitogen in gastrointestinal tissue and stimulates
epithelial cell proliferation, differentiation and growth (25,26). It has been demonstrated that EGF
increases mucus synthesis in vitro, and during the
epithelial repair process, EGF aids the proliferating cells to
migrate into the superficial epithelium (27). The EGFR pathway appears to play a
crucial role in the progression of gastric cancer. The expression
of EGF and its receptor has been found to correlate with the
prognosis of patients with gastric cancer (28,29). In this study, to the best of our
knowledge, we investigated for the first time the regulatory effect
of EGF on CO-029 expression. The results from western blot analysis
revealed that EGF increased the expression of CO-029 in a
concentration- and a time-dependent manner. In addition, AGS cells
treated with EGF proliferated at a higher rate and showed an
enhanced invasion ability.
To determine whether CO-029 is involved in the
effects of EGF on gastric cancer cell proliferation and invasion,
CO-029 was knocked down by siRNA in the AGS cells. The cells were
then treated with EGF. We found that the effects of EGF on gastric
cancer cell proliferation and invasion were attenuated by the
knockdown of CO-029. These results indicate that CO-029 promotes
gastric cancer cell proliferation and invasion mediated by EGF.
Taken together, our study strongly suggests that
CO-029 is an oncogene in human gastric cancer. The results from our
in vitro experiment demonstrate a specific upregulation of
CO-029 in AGS cells treated with EGF, and CO-029 at least partially
mediates the effects of EGF on gastric cancer cell proliferation
and invasion. It can thus be hypothesized that CO-029 plays an
important role in the progression of cancer and may thus emerge as
a novel target for therapeutic intervention in gastric cancer.
References
|
1
|
Thun MJ, DeLancey JO, Center MM, Jemal A
and Ward EM: The global burden of cancer: priorities for
prevention. Carcinogenesis. 31:100–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer–slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rohatgi PR, Yao JC, Hess K, et al: Outcome
of gastric cancer patients after successful gastrectomy: influence
of the type of recurrence and histology on survival. Cancer.
107:2576–2580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ott K, Lordick F, Blank S and Büchler M:
Gastric cancer: surgery in 2011. Langenbecks Arch Surg.
396:743–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tetzlaff ED, Cheng JD and Ajani JA: Review
of docetaxel in the treatment of gastric cancer. Ther Clin Risk
Manag. 4:999–1007. 2008.
|
|
6
|
Claas C, Stipp CS and Hemler ME:
Evaluation of prototype transmembrane 4 superfamily protein
complexes and their relation to lipid rafts. J Biol Chem.
276:7974–7984. 2001. View Article : Google Scholar
|
|
7
|
Maecker HT, Todd SC and Levy S: The
tetraspanin superfamily: molecular facilitators. FASEB J.
11:428–442. 1997.PubMed/NCBI
|
|
8
|
Todres E, Nardi JB and Robertson HM: The
tetraspanin superfamily in insects. Insect Mol Biol. 9:581–590.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fitter S, Sincock PM, Joliffe CN and
Ashman LK: Transmembrane 4 superfamily protein CD151 (PETA-3)
associates with beta1 and alpha IIb beta3 integrins in
haematopoietic cell lines and modulates cell-cell adhesion. Biochem
J. 338:61–70. 1999. View Article : Google Scholar
|
|
10
|
Horváth G, Serru V, Clay D, Billard M,
Boucheix C and Rubinstein E: CD19 is linked to the
integrin-associated tetraspans CD9, CD81, and CD82. J Biol Chem.
273:30537–30543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Indig FE, Diaz-Gonzales F and Ginsberg MH:
Analysis of the tetraspanin CD9-integrin alphaIIbbeta3 (GPIIb-IIIa)
complex in platelet membranes and transfected cells. Biochem J.
327:291–298. 1997.PubMed/NCBI
|
|
12
|
Lozahic S, Christiansen D, Manié S,
Gerlier D, Billard M, Boucheix C and Rubinstein E: CD46 (membrane
cofactor protein) associates with multiple beta1 integrins and
tetraspans. Eur J Immunol. 30:900–907. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tiwari-Woodruff SK, Buznikov AG, Vu TQ,
Micevych PE, Chen K, Kornblum HI and Bronstein JM: OSP/claudin-11
forms a complex with a novel member of the tetraspanin super family
and beta1 integrin and regulates proliferation and migration of
oligodendrocytes. J Cell Biol. 153:295–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Claas C, Seiter S, Claas A, Savelyeva L,
Schwab M and Zöller M: Association between the rat homologue of
CO-029, a metastasis-associated tetraspanin molecule and
consumption coagulopathy. J Cell Biol. 141:267–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Odintsova E, Sugiura T and Berditchevski
F: Attenuation of EGF receptor signaling by a metastasis
suppressor, the tetraspanin CD82/KAI-1. Curr Biol. 10:1009–1012.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Testa JE, Brooks PC, Lin JM and Quigley
JP: Eukaryotic expression cloning with an antimetastatic monoclonal
antibody identifies a tetraspanin (PETA-3/CD151) as an effector of
human tumor cell migration and metastasis. Cancer Res.
59:3812–3820. 1999.PubMed/NCBI
|
|
17
|
Greco C, Bralet MP, Ailane N,
Dubart-Kupperschmitt A, Rubinstein E, Le Naour F and Boucheix C:
E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell
motility control in human colon carcinoma. Cancer Res.
170:7674–7683. 2010. View Article : Google Scholar
|
|
18
|
Zhou Z, Ran YL, Hu H, et al: TM4SF3
promotes esophageal carcinoma metastasis via upregulating ADAM12m
expression. Clin Exp Metastasis. 25:537–548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herlevsen M, Schmidt DS, Miyazaki K and
Zöller M: The association of the tetraspanin D6.1A with the
alpha6beta4 integrin supports cell motility and liver metastasis
formation. J Cell Sci. 116:4373–4390. 2006. View Article : Google Scholar
|
|
20
|
Kanetaka K, Sakamoto M, Yamamoto Y,
Yamasaki S, Lanza F, Kanematsu T and Hirohashi S: Overexpression of
tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol.
35:637–642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sartore-Bianchi A, Bencardino K, Di
Nicolantonio F, et al: Integrated molecular dissection of the
epidermal growth factor receptor (EFGR) oncogenic pathway to
predict response to EGFR-targeted monoclonal antibodies in
metastatic colorectal cancer. Target Oncol. 5:19–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lafky JM, Wilken JA, Baron AT and Maihle
NJ: Clinical implications of the ErbB/epidermal growth factor (EGF)
receptor family and its ligands in ovarian cancer. Biochim Biophys
Acta. 1785:232–265. 2008.PubMed/NCBI
|
|
23
|
Lin JX, Jia YD and Zhang CQ: Effect of
epidermal growth factor on follicle-stimulating hormone-induced
proliferation of granulosa cells from chicken prehierarchical
follicles. J Zhejiang Univ Sci B. 12:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumura N, Zembutsu H, Yamaguchi K, et
al: Identification of novel molecular markers for detection of
gastric cancer cells in the peripheral blood circulation using
genome-wide microarray analysis. Exp Ther Med. 2:705–713.
2011.PubMed/NCBI
|
|
25
|
Ross JS and McKenna BJ: The HER-2/neu
oncogene in tumors of the gastrointestinal tract. Cancer Invest.
19:554–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Wilson EJ, Osburn J and Delvalle
J: Epidermal growth factor inhibits carbachol-stimulated canine
parietal cell function via protein kinase C. Gastroenterology.
110:469–477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L, Liu ES, Chow JY, Wang JY and Cho CH:
Interactions of EGF and ornithine decarboxylase activity in the
regulation of gastric mucus synthesis in cigarette smoke exposed
rats. Chin J Physiol. 42:137–143. 1999.
|
|
28
|
Yasui W, Hata J, Yokozaki H, Nakatani H,
Ochiai A, Ito H and Tahara E: Interaction between epidermal growth
factor and its receptor in progression of human gastric carcinoma.
Int J Cancer. 41:211–217. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jonjić N1, Kovac K, Krasević M, Valković
T, Ernjak N, Sasso F and Melato M: Epidermal growth factor-receptor
expression correlates with tumour cell proliferation and prognosis
in gastric cancer. Anticancer Res. 17:3883–3888. 1997.
|