Introduction
Metal ions are required for a number of critical
functions in living organisms and are becoming increasingly
important as diagnostic and therapeutic tools in the study and and
treatment of a variety of human diseases (1,2).
Since the success of cisplatin, increasing attention has been paid
to metal complexes, which has been one of the most rapidly
developing areas of anticancer drugs (3-9).
It has been demonstrated that a number of bioinorganic complexes
contain metals, such as iron (Fe), ruthenium (Ru) and can trigger
reactive oxygen species (ROS)-mediated cell death (10).
Manganese (Mn) is a widely distributed metal which
is a required co-factor for many ubiquitous enzymes (11). It has been proven that Mn(II) ions
are mainly absorbed and transported by the transferrin
(Tf)-transferrin receptor (TfR) system (12,13). Studies have also demonstrated that
TfR is highly expressed in some types of tumor tissue (14–16). To date, simple Mn(II) salts have
been reported to exert anti-proliferate effects on several cancer
cell lines (17,18) and Mn(II) complexes containing
thiosemicarbazone or hydrazone groups have been reported as
antitumor agents (19), generally
by the induction of apoptotic cell death at rather high
concentrations (in the mM range). Based on these data, we designed
and synthesized Mn(II)-containing compounds, in an aim to develop
novel tumor-targeting lead chemotherapeutic agents. During our
research, a novel Mn(II) complex was synthesized and characterized,
and its anticancer activity was previously investigated in a
previous study of ours (20);
however, its mechanisms of action remain unclear.
Of note, the majority antitumor therapies, including
chemotherapy primarily act by inducing apoptosis (type I programmed
cell death) in cancer cells; thus, defects in the apoptotic
programs may cause resistance to the therapeutic agents (21,22). Thus, an alternative cell death
pathway termed autophagic cell death (type II programmed cell
death) has emerged as an important mechanism of cancer cell death
induced by chemotherapeutic agents (type II programmed cell death)
(10,23,24). Autophagy can act as a pro-death
mechanism which leads to the destruction of cancer cells, since
autophagy is a ‘self-cannibalistic’ process. There is evidence that
autophagy is required for the death of cancer cells with defects in
apoptosis (25,26). Moreover, new insights into the
molecular mechanisms of autophagy are now leading to the discovery
of exciting new potential drug targets (27) and researchers have pointed out
that apoptosis and autophagy are tightly connected and may be
regulated by the same trigger, such as ROS (28,29).
In the present study, we demonstrate that the novel
manganese (II) compound, Adpa-Mn {[(Adpa)Mn(Cl)(H2O)]
(Adpa=bis(2-pyridylmethyl)amino-2-propionic acid)}, is a promising
new anticancer agent which exerts potent selective activity against
a wide range of tumor cell lines in vitro and a carcinoma
xenograft model in vivo. Moreover, our results verify that
Adpa-Mn causes both apoptotic and autophagic cancer cell death
through the induction of ROS generation.
Materials and methods
Materials
The compound, Adpa-Mn, was synthesized by Professor
Chen Qiuyun. Cyclophosphamide (CTX) was produced by Jiangsu Hengrui
Medicine Co., Ltd. (Jiangsu, China).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) was purchased from Amresco LLC (Solon, OH, USA) and Annexin
V/PI kits for the detection of apoptosis were from Life
Technologies (Carlsbad, CA, USA). Culture medium (DMEM/1640),
trypsin and EDTA-2·Na were purchased from Thermo Fisher Scientific
(Waltham, MA, USA). Fetal bovine serum was obtained from Sijiqing
Biological Engineering Materials (Hangzhou, China). The 2',7'
dichlorofluorescin diacetate (DCFH-DA) kit was purchased from the
Beyotime Institute of Biotechnology (Nantong, China). Antibodies
against microtubule-associated protein 1 light chain 3 (LC3; 2775),
poly(ADP-ribose) polymerase (PARP; 9542), autophagy-related protein
(ATG)7 (2631) and ATG siRNA (6604) were obtained from Cell
Signaling Technology (Boston, MA, USA); β-actin (sc-8423), GAPDH
(sc-25778), cytochrome c (sc-13561), COX IV (sc-376731),
tubulin (sc-5546), TfR1 (sc-9099) and caspase-3 (sc-98785) were
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
3-Methyladenine (3-MA), N-acetyl cysteine (NAC), chloroquine (CQ),
cisplatin, ferric citrate and deferoxamine (DFO) were purchased
from Sigma-Aldrich. Wortmanin was purchased from Beyotime. All
other chemicals were of high purity and were from commercial
sources.
Cell culture
The human cancer cell lines, including HeLa
(cervical adenocarcinoma), HepG2 (hepatocellular carcinoma), A549
(lung adenocarcinoma), MCF-7 (breast cancer), U251 (glioblastoma),
LoVo (colon cancer), A875 (melanoma) and ECA-109 (human esophageal
squamous carcinoma) cells, as well as the human normal liver cell
line, WRL-68 (immortalized), were obtained from the Cancer Cell
Repository (Shanghai Cell Bank, Shanghai, China). The cells were
maintained in DMEM medium supplemented with 10% (v/v)
heat-inactivated fetal bovine serum, antibiotics (100 U/ml
penicillin and 100 U/ml streptomycin), at 37°C in a humidified
atmosphere of 5% CO2 (Thermo Fisher Scientific).
Animals
Female imprinting control region (ICR) mice (6–8
weeks old) were purchased from the Comparative Medicine Research
Center of Yangzhou University [Yangzhou, China, register no: SCXK
(JIANGSU) 2007-0001]. The mice were maintained on a standard diet
and water was made freely available.
Ethics statement
Animal welfare and experimental procedures were
carried out strictly in accordance with the Guide for the Care and
Use of Laboratory Animals (The Ministry of Science and Technology
of China, 2006) and the related ethical regulations of our
university. All efforts were made to minimize the suffering of the
animals and to reduce the number of animals used.
Histological analysis
For histological morphometry, tumor tissues were
fixed with 10% formalin and embedded in paraffin, and cut into
5-μm-thick sections and stained with hematoxylin and eosin
(H&E; Nanjing Jiancheng Technology Co., Nanjing, China).
Cell viability assay
The cells were plated at a density of approximately
4 × 103 viable cells per well in 96-well plates. Various
concentrations of the compound were used to treat the cells in
triplicate. Following incubation for the indicated periods of time,
MTT assay was performed to measure cell viability using a 96-well
plate reader (Spectra Max 190; Molecular Devices Corp., Sunnyvale,
CA, USA).
Cell morphological changes observed under
a Nikon TE2000 microscope
The morphological changes of the
H2B-GFP-labeled HeLa cells (stable cell line) were
observed under a Nikon TE2000 microscope (Nikon, Tokyo, Japan) with
a live cell system (LCS) which can provide CO2,
temperature control and position fixing. The
H2B-GFP-labeled HepG2 cells, which were incubated with
20 μM Adpa-Mn, were observed for 24 h. The bright and
fluorescence imaginations of the cells were recorded and
analyzed.
Cell apoptosis assay
The cells were stained with Annexin V/PI at room
temperature for 15 min in the dark and then analyzed using a
FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA). Annexin V+/PI− and Annexin
V+/PI+ cells were considered as apoptotic
cells in the early and late phase.
Visualization of monodansylcadaverine
(MDC)-labeled vacuoles
Autophagic vacuoles were labeled with MDC by
incubating the HepG2 cells which were grown on coverslips with 0.05
mM MDC in PBS at 37°C for 10 min. The cellular fluorescent changes
were observed under a fluorescence microscope (Nikon; excitation,
380 to 420 nm; emission, 450 nm; Nikon).
GFP-LC3 plasmid transfection
The HepG2 cells transfected with green fluorescent
protein (GFP)-LC3-expressing plasmid were treated with Adpa-Mn, and
the fluorescence of GFP-LC3 was viewed under a fluorescence
microscope (Nikon).
Western blot analysis
Proteins were extracted in lysis buffer (30 mM Tris,
pH 7.5, 150 mM sodium chloride, 1 mM phenylmethanesulfonyl
fluoride, 1 mM sodium orthovanadate, 1% Nonidet P-40, 10% glycerol,
and phosphatase and protease inhibitors), separated by SDS-PAGE and
electrophcoretically transferred onto polyvinylidene fluoride
membranes. The membranes were probed with antibodies (LC3, PARP,
ATG7 and β-actin) overnight at 4°C, and then incubated with a horse
radish peroxidase-coupled secondary antibody. Detection was
performed using a LumiGLO chemiluminescent substrate system
[Kirkegaard & Perry Laboratories, Inc. (KPL), Gaithersburg, MD,
USA].
Mitochondrial membrane potential
assay
Changes in mitochondrial membrane potential were
measured using JC-1 staining. Briefly, following treatment, the
HepG2 cells were washed with PBS and incubated with 5 μg/ml
JC-1 at 37°C for 30 min. The cells were then washed twice with PBS
and immediately assessed by fluorescence spectrometry (Spectra
MaxGemini; Molecular Devices Corp.). A 488 nm filter was used for
the excitation of JC-1. Emission filters of 535 and 595 nm were
used to quantify the population of mitochondria with green (JC-1
monomers) and red (JC-1 aggregates) fluorescence. The ratio of
red/green was used to reflect the mitochondrial membrane
potential.
Measurement of intracellular ROS
production
The intracellular generation of ROS was analyzed
using the probe, DCFH-DA. Cells were incubated with 10 μM
DCFH-DA at 37°C for 15 min. The DCF fluorescence distribution of
1×104 cells was tehn measured by fluorescence
spectrometry (Spectra MaxGemini; Molecular Devices Corp.) at an
excitation wavelength of 488 nm and at an emission wavelength of
535 nm.
Evaluation of the antitumor effects of
Adpa-Mn in vivo
Mouse hepatocellular carcinoma (Hep-A;
1×107) cells (grown in donor mice) were transplanted
subcutaneously into the armpits of the ICR mice. One day following
transplantion, the mice were randomly allocated to either the
control (vehicle control, received PBS) or treatment groups, with
10 mice in each group. The drugs (Adpa-Mn and CTX) were
administered intraperitoneally on days 0–9. All efforts were made
to minimize the suffering of the animals and to reduce the number
of animals used and the mice were sacrificed by cervical
dislocation. After the mice were sacrificed, the solid tumors were
separated. Tumor weights were measured and the tumor growth
inhibition ratio was calculated. The toxic effects of Adpa-Mn on
the spleen were also observed.
Statistical analysis
Comparisons were made by one-way analysis of
variance (ANOVA). Differences were considered statistically
significant when p<0.05. All experiments were repeated at least
3 times. All graphs were created using GraphPad Prism 5
software.
Results
Adpa-Mn exerts inhibitory effects on
various types of cancer cells
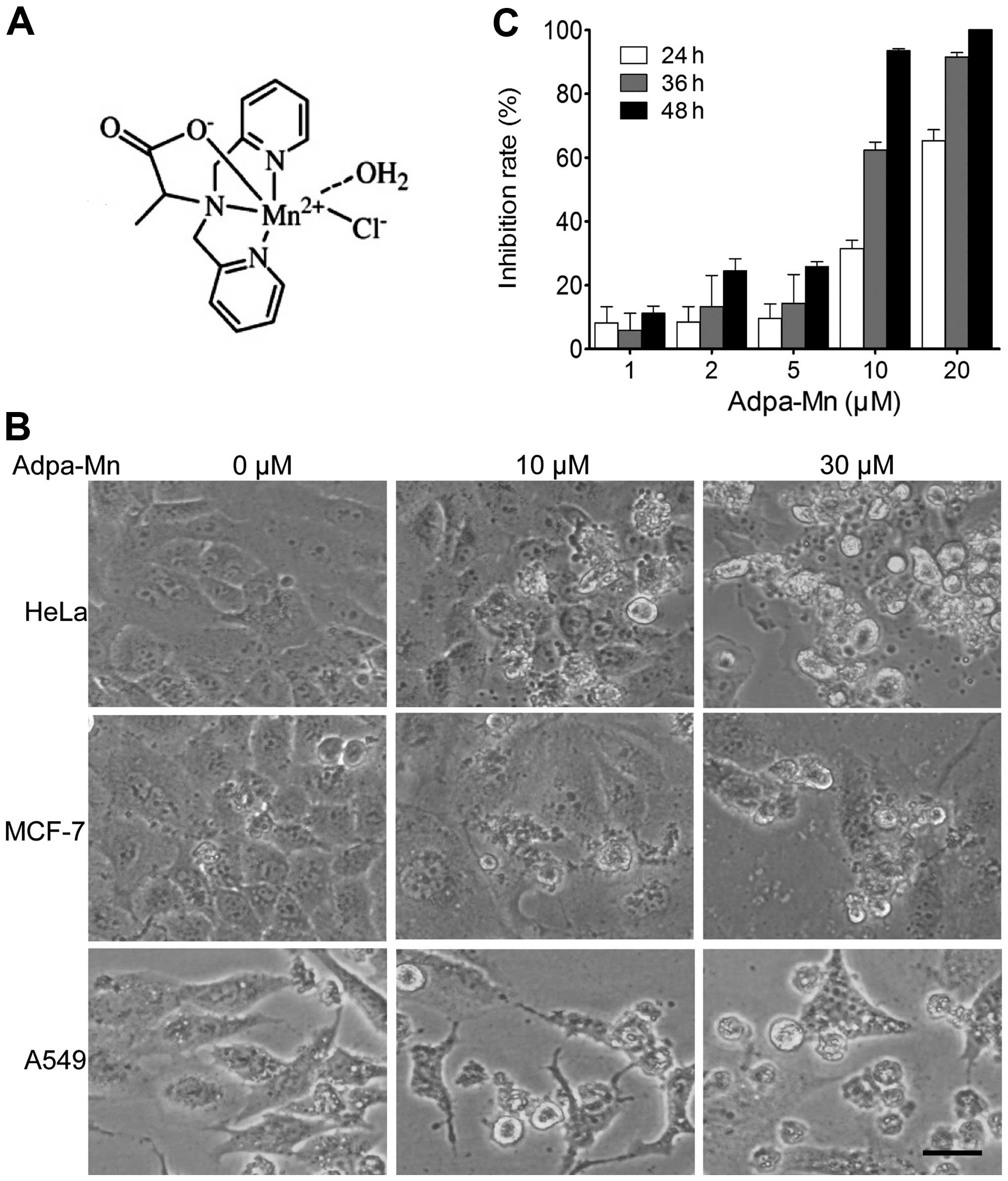
First we examined the cytotoxic effects of Adpa-Mn
(chemical structure shown in Fig.
1A) on various types of human cancer cells, including human
cervical cancer cells (HeLa), human hepatocellular carcinoma cells
(HepG2), human lung cancer cells (A549), human breast cancer cells
(MCF-7), human glioblastoma cells (U251), human colon cancer cells
(LoVo), human melanoma cells (A875) and human esophageal squamous
carcinoma cells (ECA-109). The morphological changes (cell body
shrinkage and cell number reduction) of the cells were photographed
(Fig. 1B) and the 50% inhibitory
concentration (IC50) was calculated (Table I). Our results revealed that
Adpa-Mn exerted a significant cytotoxic effect with an
IC50 between 5 and 20 μM. Furthermore, we
demonstrated that Adpa-Mn inhibited HepG2 cell proliferation not
only in a dose-dependent manner, but also in a time-dependent
manner (Fig. 1C).
 | Table IHalf maximal inhibitory concentration
(IC50) of Adpa-Mn in various cancer cell lines. |
Table I
Half maximal inhibitory concentration
(IC50) of Adpa-Mn in various cancer cell lines.
| Cell line | IC50
(M) |
|---|
| HeLa | 12.3±1.5 |
| HepG2 | 10.8±0.9 |
| A549 | 14.2±0.5 |
| MCF-7 | 6.5±0.7 |
| U251 | 9.1±0.6 |
| LoVo | 10.2±0.4 |
| A875 | 18.6±0.3 |
| ECA-109 | 16.2±0.3 |
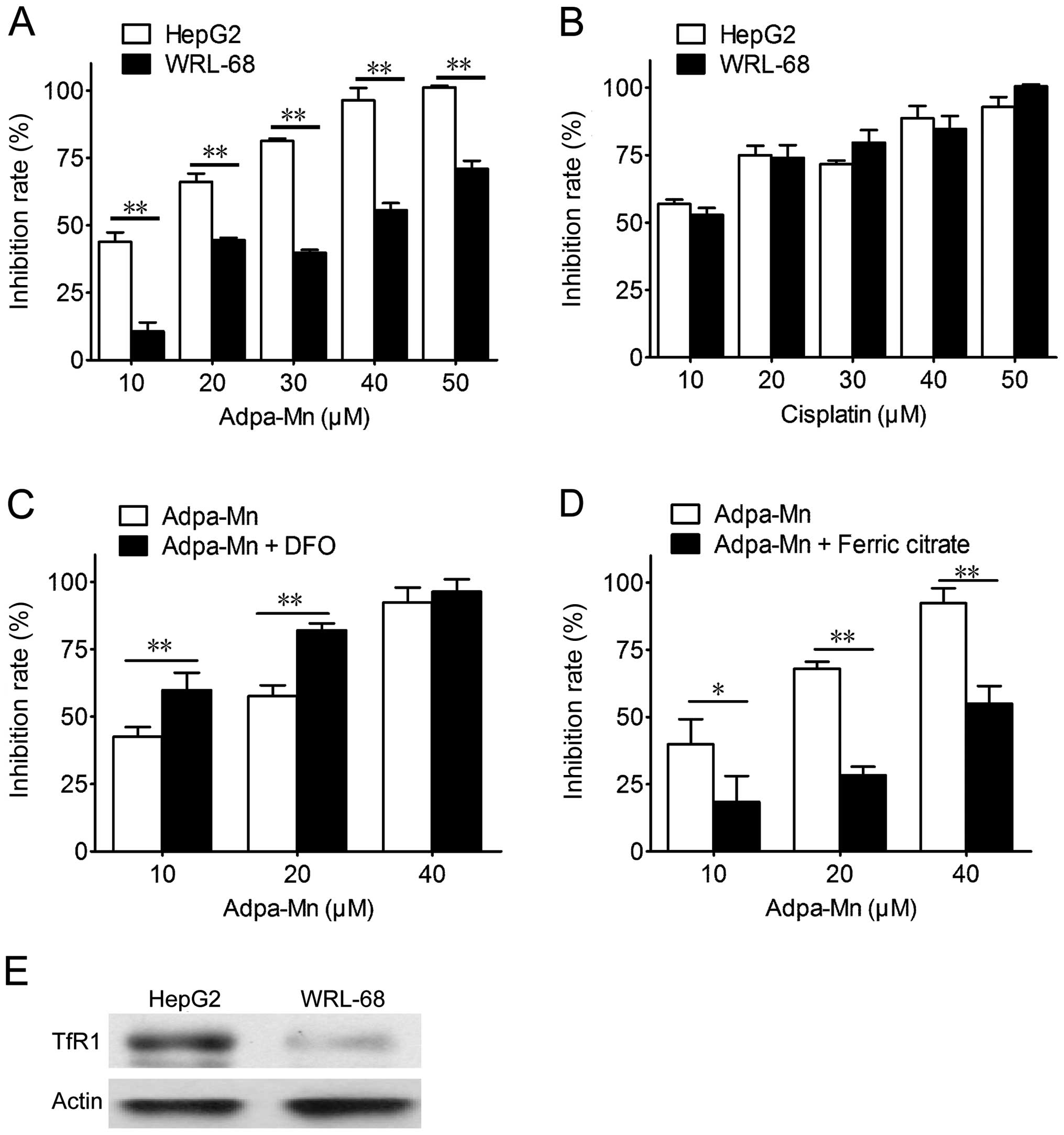
Adpa-Mn selectively kills cancer cells
through the Tf-TfR system
The selectivity of Adpa-Mn on cancer cells was
examined. Adpa-Mn demonstrated significant selectivity towards the
liver cancer cells (HepG2) compared with the non-malignant liver
epithelial cells (WRL-68) (Fig.
2A), and compared to treatment with cisplatin (Fig. 2B). The preferential toxicities
toward the cancer cells compared to the non-cancer cells suggest
the possibile use of this compound as an antitumor agent. Due to
the high expression level of TfR in the tumor cells, the
selectivity of Adpa-Mn may be due to its transport mechanisms. As
shown in Fig. 2E, the expression
of TfR1 in the HepG2 cells was higher than that in the WRL-68
cells. As shown in Fig. 2C and D,
pre-treatment with ferric citrate reduced the inhibitory effects of
Adpa-Mn on the growth of HepG2 cells, while pre-treatment with
deferoxamine (DFO) promoted them.
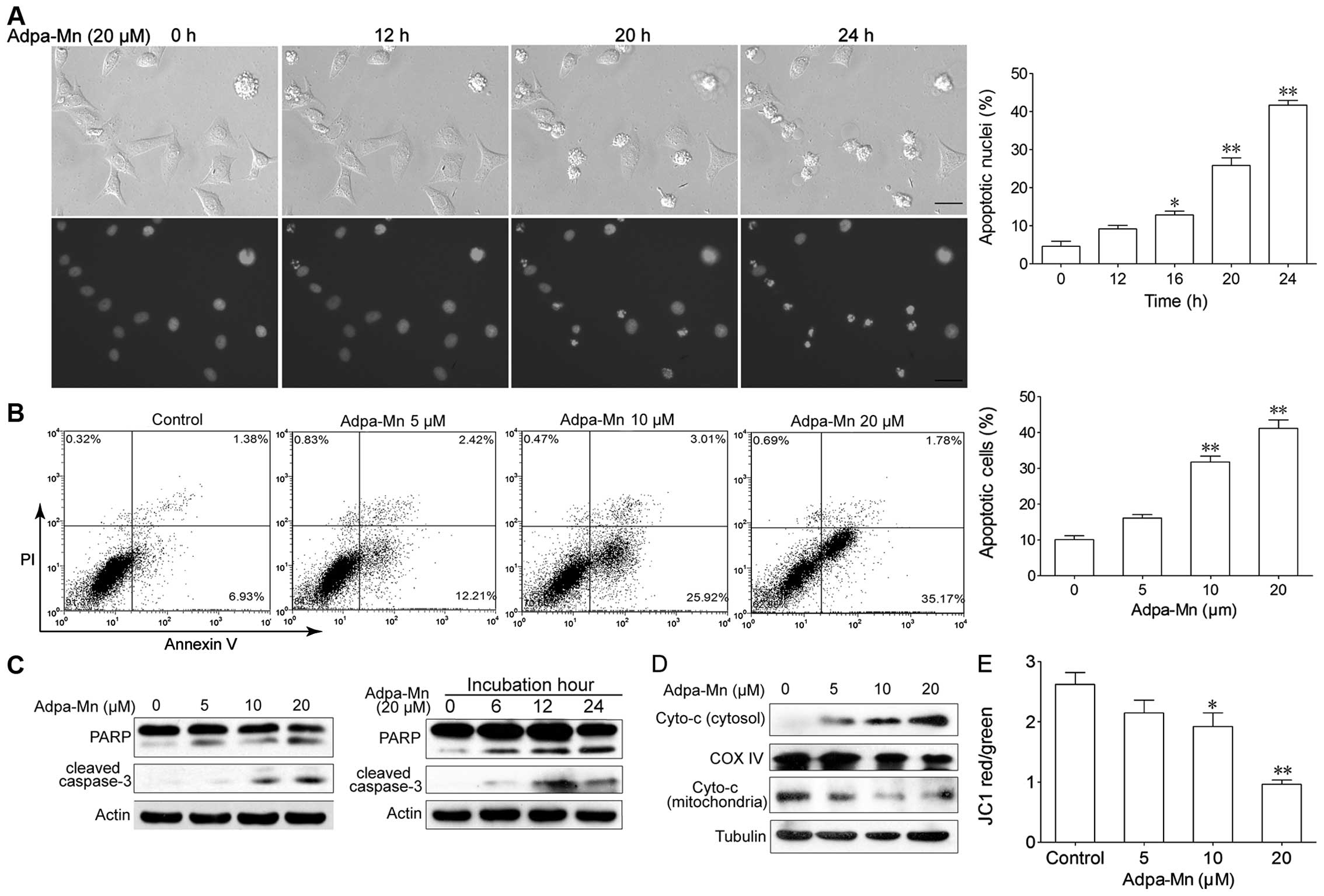
Adpa-Mn induces apoptotic cell death
through the mitochondrial pathway
We then wished to determine which cell death pathway
was employed when the cells were treated with Adpa-Mn. The
possibility of apoptosis was investigated. As shown in Fig. 3A, following incubation with
Adpa-Mn for 12 h, cell shrinkage and chromatin condensation were
observed. As the inubation time increased, the nuclei became
condensed and had divided into several parts; apoptotic bodies had
emerged, and an increasing number of cells began to exhibit these
characteristics. We calculated the percentage of cells which showed
these characteristics and found that this percentage increased in a
time-dependent manner (Fig. 3A).
FACS analyses revealed that the number of Annexin
V+/PI− cells had increased from 6.9 to 25.9%
(10 μM) and from 35.2% (20 μM) following treatment
with Adpa-Mn (Fig. 3B). Western
blot analysis revealed that Adpa-Mn triggered the activation of
PARP and the cleavage of caspase-3 (Fig. 3C) in a dose- and time-dependent
manner.
To further examine the pathway of apoptosis, we
monitored the changes in apoptotic molecules related to the
mitochondrial pathway in the HepG2 cells. As shown in Fig. 3D and E, treatment with Adpa-Mn
disrupted the mitochondrial trans-membrane potential and with the
collapse of the mitochondrial transmembrane potential, the release
of cytochrome c from the mitochondrion to the cytosol was
greatly increased in a dose-dependent manner (Fig. 3C). These results indicate that the
mitochondrial apoptotic pathway is involved in the Adpa-Mn-induced
apoptosis of cancer cells.
Adpa-Mn induces autophagic cell
death
We also wished to determine whether autophagic cell
death contributes to the cytotoxic effects of Adpa-Mn. The
possibility of the induction of autophagy was analyzed by
autophagic vacuole organelle (AVO) formation, the formation of
GFP-LC3 vacuoles and LC3 conversion. AVO formation was detected and
measured by staining with MDC, as previously described (30). The Adpa-Mn-treated HepG2 cells
showed a greater fluorescence intensity and a greater number of
MDC-labeled particles compared with the control (untreated) group
(Fig. 4A), indicating that
Adpa-Mn increased MDC recruitment to autophagosomes in the
cytoplasm which was suppressed by the autophagy inhibitor, 3-MA
(Fig. 4A).
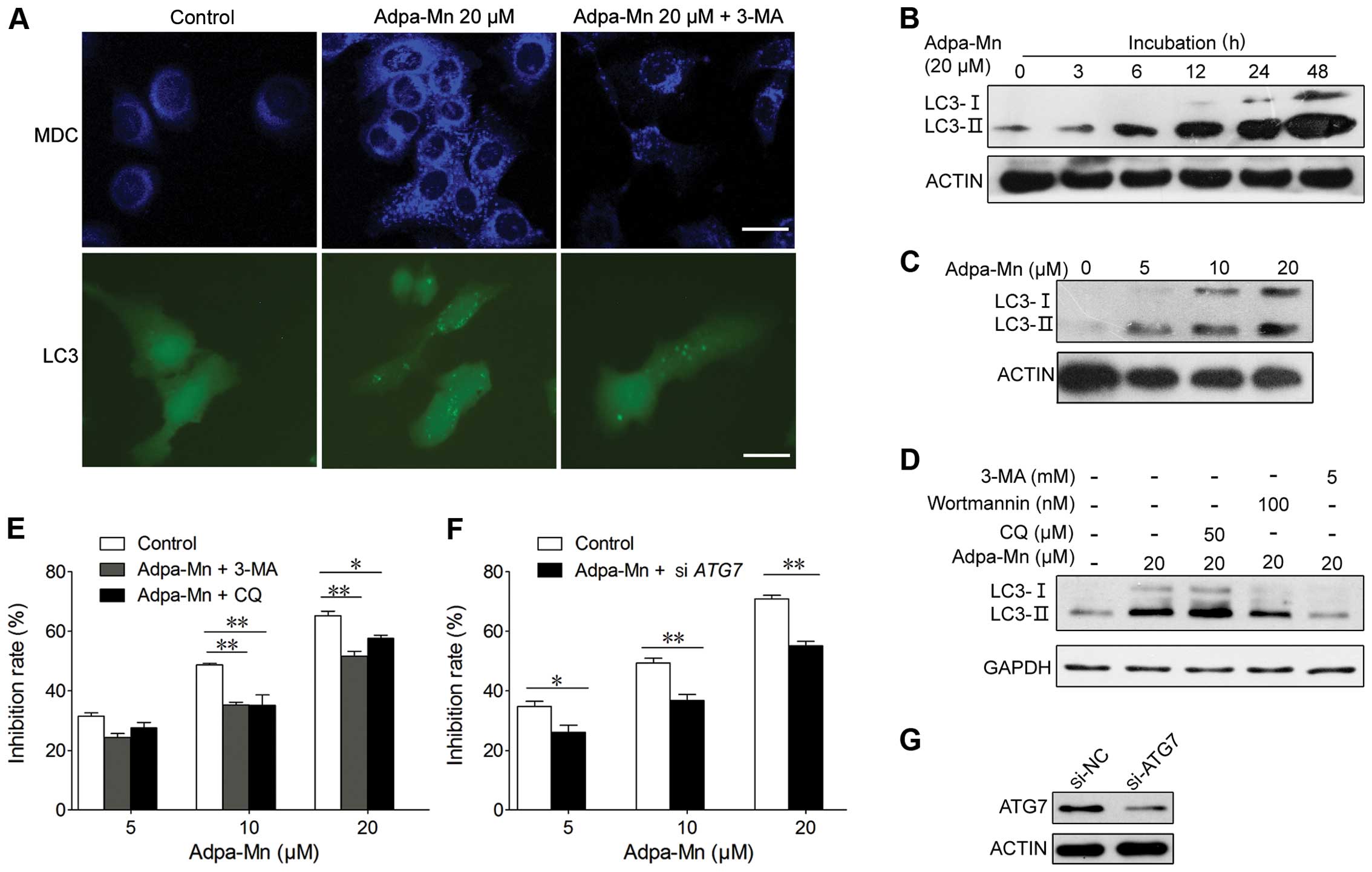 | Figure 4Adpa-Mn induces autophagic cell
death. (A) HepG2 cells transfected with GFP-LC3 cDNA were treated
with 20 μM Adpa-Mn for 12 h with or without pre-treatment
with 5 mM 3-methyladenine (3-MA) for 2 h. The formation of vacuoles
containing GFP-LC3 (dots) was examined by fluorescence microscopy.
In another set of experiments, HepG2 cells were treated with 20
μM Adpa-Mn for 12 h with or without pre-treatment with 5 mM
3-MA for 2 h and then incubated with 0.05 mM monodansylcadaverine
(MDC) for 10 min. The cells were then analyzed by fluorescence
microscopy. Scale bar, 5 μm. Protein expression of LC3 in
(B-D) was determined by western blot analysis. (B) HepG2 cells were
cultured with 20 μM Adpa-Mn for 3, 6, 12, 24 and 48 h. (C)
HepG2 cells were cultured with the indicated concentrations of
Adpa-Mn for 24 h. (D) HepG2 cells were treated with 20 μM
Adpa-Mn for 24 h with or without 3-MA, wartmanin and chloroquine
(CQ) pre-treatment for 2 h. (E) HepG2 cells were treated with 20
μM Adpa-Mn for 24 h with or without 3-MA, and CQ
pre-treatment for 2 h. MTT assay was used to evaluate the cell
death rate. (F) HepG2 cells were transfected with control siRNA or
siRNA targeting autophagy-related gene (ATG7). After 48 h, the
cells were treated with 0, 5, 10 or 20 μM Adpa-Mn for 24 h,
and cell death was measured by MTT assay. (G) The knockdown of ATG7
was confirmed by western blot analysis. Data represent the means ±
SEM of 3 different experiments. *p<0.05 and
**p<0.01 vs. respective control. |
Conversion of LC3-I (19 kDa) to the
pre-autophagosomal and autophagosomal membrane-bound form of LC3-II
(17 kDa) is another specific marker of autophagosome formation
(31). GFP-fused LC3 was
transfected into the cells to detect autophagy. As shown in
Fig. 4B, the formation of
GFP-LC3-labeled vacuoles in the HepG2 cells was markedly increased
12 h following treatment with 20 μM Adpa-Mn. The formation
of these vacuoles was inhibited by treatment with 3-MA, a specific
inhibitor of the autophagic process during the early stages
(Fig. 4A). Consistent with the
above results, the LC3-I to LC3-II conversion markedly increased
with the increasing incubation time or the increasing dose of
Adpa-Mn in the HepG2 cells, as shown by western blot analysis
(Fig. 4B and C); this was also
inhibited by treatment with the autophagy inhibitors, wortmanin and
3-MA (Fig. 4D).
To determine whether autophagy is associated with
the cell death induced by Adpa-Mn in the HepG2 cells, we examined
whether the inhibition of autophagy using autophagy inhibitors or
by silencing autophagy-related genes affects cell death in the
HepG2 cells. First, using MTT assay, we found that pre-treatment
with the autophagy inhibitor, 3-MA, or the autophagolysosome fusion
inhibitor, CQ, significantly inhibited Adpa-Mn-induced cell death
in a dose-dependent manner (Fig.
4E). Second, we also found that silencing autophagy-related
genes (ATG7) using siRNA significantly reduced the cell death
induced by Adpa-Mn (Fig. 4F and
G). These results suggest that autophagy contributes to the
death of HepG2 cells treated with Adpa-Mn.
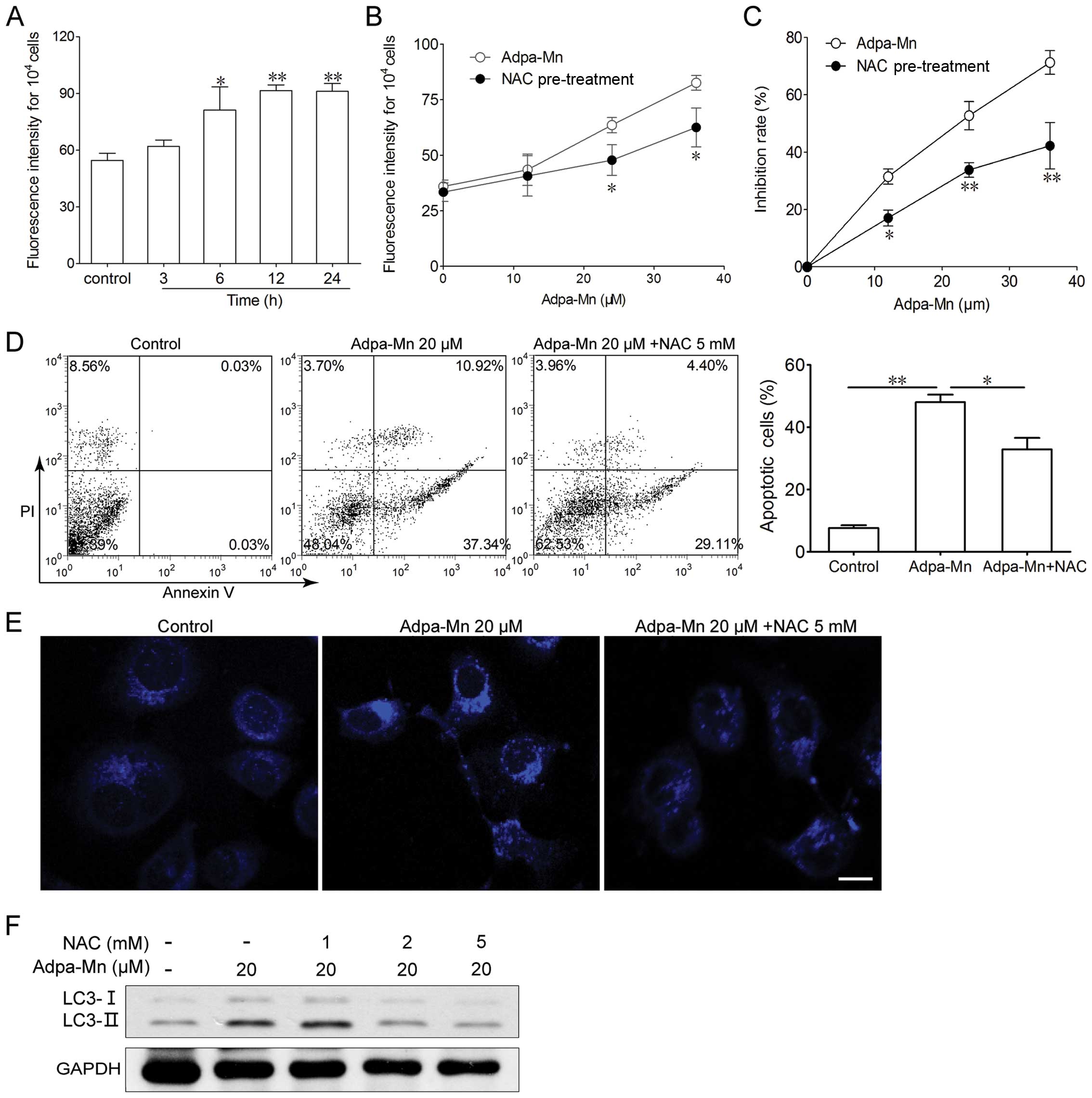
ROS generation triggered by Adpa-Mn is
indispensable for the induction of apoptosis and autophagy
To determine whether ROS play an important role in
the cell death induced by Adpa-Mn, the intracellular ROS levels
were measured by fluorescence spectrometry after the cells were
labeled with DCFH-DA. As shown in Fig. 5A, treatment with 20 μM of
Adpa-Mn for 6 h led to an increase in ROS generation in the HepG2
cells. The generation of ROS significantly increased, as detected
by the higher fluorescence intensity compared to the control
(untreated) group. The generation of ROS increased in a dose- and
time-dependent manner, suggesting that the continuous generation of
ROS is involved in the whole process of Adpa-Mn-induced cell death
(Fig. 5A). To determine the role
of ROS in the Adpa-Mn-induced cell death, we examined whether the
inhibition of ROS by NAC affects apoptosis or autophagy in HepG2
cells. As shown in Fig. 5B,
pre-treatment with NAC effectively suppressed the generation of
ROS. MTT assay revealed that the cell death induced by Adpa-Mn was
markedly reduced by NAC (Fig.
5C). Annexin V/PI staining also revealed that pre-treatment
with NAC inhibited the apoptosis induced by Adpa-Mn (Fig. 5D). At the same time, the
Adpa-Mn-induced MDC-labeled particle formation and LC3 conversion
were inhibited by NAC (Fig. 5E and
F). These results demonstrate that ROS are necessary for the
Adpa-Mn-induced apoptotic and autophagic death of HepG2 cells.
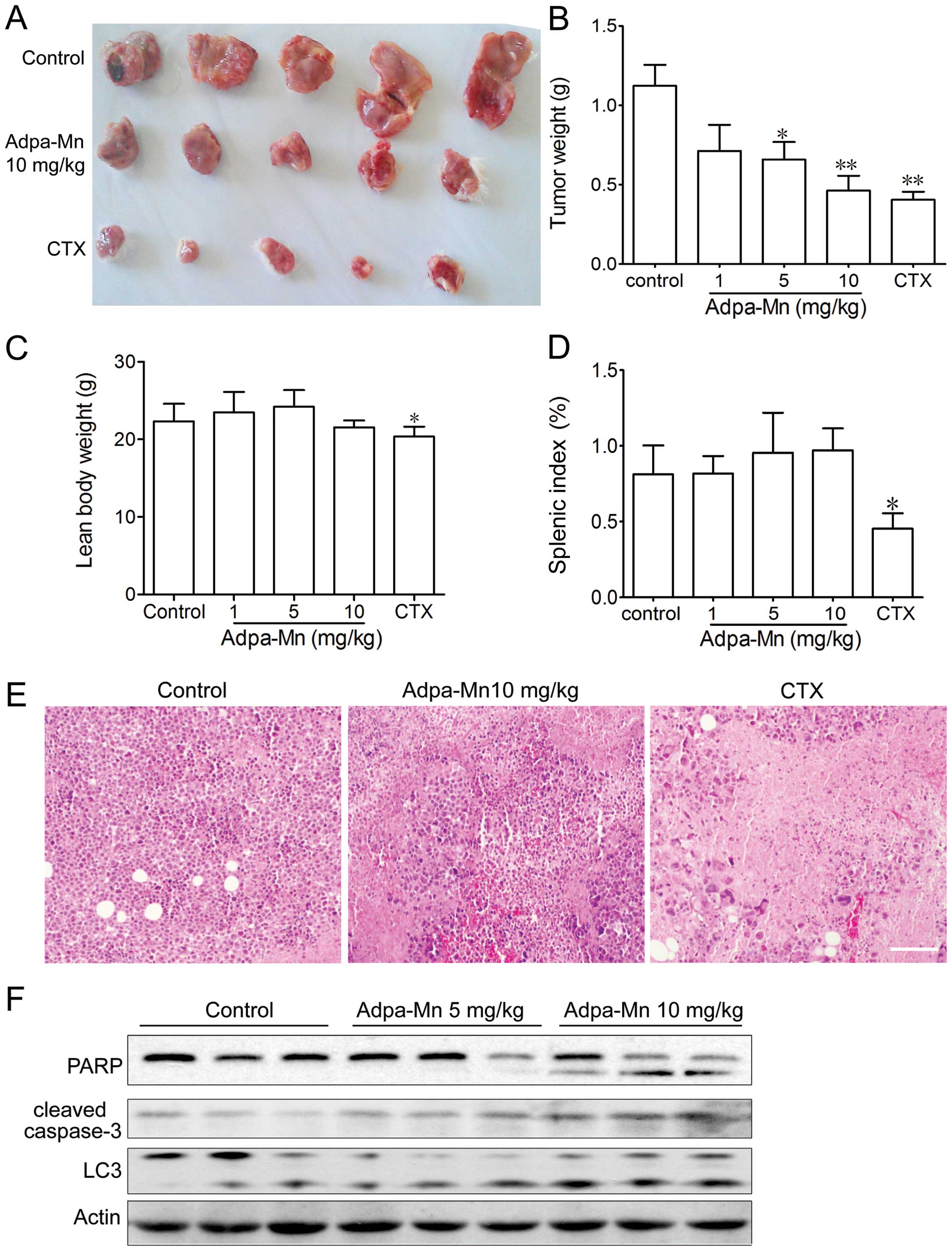
In vivo anticancer activity of Adpa-Mn
against a mouse hepatocellular carcinoma xenograft
To examine the antitumor activity of Adpa-Mn in
vivo, we developed a mouse xenograft model of Hep-A cells in
ICR mice. The administration of Adpa-Mn (1, 5, 10 mg/kg, once a
day) inhibited tumor growth in the mice in a dose-dependent manner
(Fig. 6A and B). The tumor
inhibition rate at the dose of 10 mg/kg was 60%, which is
comparable to that of 20 mg kg CTX, and H&E staining revealed
evident cell death in the tumor tissue (Fig. 6E). Treatment with CTX
significantly reduced the body weight and splenic index in the
mice, whereas no significant changes were observed in the
Adpa-Mn-treated mice (Fig. 6C and
D). According to the results in vitro, both PARP and
caspase-3 activation and LC3 conversion in the tumor tissue from
mice were significantly enhanced following treatment with Adpa-Mn,
which proved that apoptosis and autophagy were induced (Fig. 6F). These results indicated that
Adpa-Mn effectively suppressed tumor growth in vivo, while
no significant side-effects were observed.
Disscusion
The success of cisplatin in the treatment of cancer
patients suggests that other metal complexes may also be potential
drugs in future chemotherapy regimens. In this study, experiments
were performed to verify whether the designed manganese compound,
Adpa-Mn, can be used as an anticancer lead compound. In
vitro, Adpa-Mn was demonstrated to be active against various
types of tumor cells in a dose- and time-dependent manner. In
vivo, the growth of mouse hepatocellular carcinoma tumor
xeno-grafts was significantly attenuated by Adpa-Mn, which was
comparable to the effect of CTX.
The anticancer activity of Mn(II) has, however, been
distinctly enhanced by combination with diverse ligands, including
chrysin (32,33). For most of these compounds,
interactions with DNA involving intercalation or coordinative
binding have been demonstrated. However, the knowledge of the
precise molecular mechanisms underlying their increased cytotoxic
activity against cancer cells remains limited. N-allyl
di(picolyl)amine (Adpa) has been shown to be active against the
proliferation of cancer cells (34), capable of complexing Cu(II) and
inducing cell death by DNA interaction and ROS-mediated autophagy
(35,36).
The majority of metal complexes, such as platinum or
copper complexes have shown activity for DNA binding and cleavage
and the ability to induce cell cycle arrest and apoptosis (36–39). In a previous study of ours, we
found that the Adpa-Mn complex exhibited high toxicity against
cancer cell lines, but showed weak DNA binding and cleavage
activity (34). In agreement with
the study that manganese can induce apoptosis in neuronal cells
(40), in this study, treatment
with Adpa-Mn induced apoptosis, as indicated by nuclei condensation
and the appearance of apoptotic bodies, which occurred through the
mitochondrial pathway (Fig.
3).
Usually, apoptosis is the major mechanism which
destroys cancer cells. A number of chemotherapeutic agents have
been designed to kill cancer cells through the induction of
apoptosis (41). For example,
cisplatin has been reported to induce apoptosis in various types of
human tumor cells (42). However,
as is known, the decreased effects of anticancer drugs or
resistance to apoptosis are becoming a major concern with the
long-term use of chemotherapeutic agents. Thus, an alternative form
of programmed cell death known as autophagy is becoming
increasingly important in cancer therapy (24,43,44). Previously, autophagy was referred
to as a physiological process that plays a protective role as cells
encounter environmental stresses, such as starvation and pathogen
infection (45). It was also
classified as type II programmed cell death or autophagic cell
death (46). Excessive autophagy
can also act as a pro-death mechanism. Rapamycin and its analogs,
which induce autophagic cell death by inhibiting mTOR, have been
demonstrated to be a potent therapeutic strategy for many tumor
types in preclinical clinical studies (47). In this study, we demonstrated that
Adpa-Mn induced autophagy, which indeed contributed to its cell
death-inducing mechanisms (Fig.
4). The following characteristics of autophagy were observed in
the present study: the formation of AVO and the punctate
distribution of LC3 and the elevated ratio of LC3-II to LC3-I.
Furthermore, we confirmed that the Adpa-Mn-induced cell death was
mediated through autophagy: cell death was significantly suppressed
by the inhibition of autophagy, by pre-treatment of the cells with
various autophagy inhibitors or the transfection of siRNA targeting
ATG7 (Fig. 4).
It is well established that mitochondria and ROS
play a central role in the process of cell death, including
apoptosis and autophagy (48–53). It has been suggested that the
mitochondria can regulate the release of proteins inducing
apoptosis and autophagy through the excessive generation of ROS and
the self-directed induction of mitochondrial permeability
transition (MPT), while ROS play several roles in cellular
processes, including DNA damage, mitochondrial dysfunction, the
activation of signaling pathways and the activation of
transcription factors, leading to the upregulation of genes
(54). Consistent with these
observations, in this study, Adpa-Mn induced mitochondrial
dysfunction, including the collpase of mitochondrial membrane
potential following the accumulation of ROS. When ROS were
scavenged, both the Adpa-Mn-induced autophagy and apoptosis were
hampered (Fig. 5) which proved
that ROS generation triggered by Adpa-Mn was responsible for the
apoptotic and autophagic cell death. However, the association
between the apoptosis and autophagy induced by Adpa-Mn warrant
further investigations, which may provide some strategies for the
regulation of apoptosis/autophagy and drug design.
Taken together, our findings suggest that Adpa-Mn
exhibits potent and stable anti-proliferative and cytotoxic
activity against diverse tumor types in vitro, as well as
against tumor xenografts mediated by the ROS-dependent apoptotic
and autophagic cell death. Our study thus provides useful insight
into the investigation of apoptosis and autophagy in cancer cells
and offers a rationale for the development of complexes as
effective chemotherapeutic agents against human cancer in clinical
settings.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 21271090), the Natural
Science Foundation of Jiangsu Province (no. BK2012710), Jiangsu
University (no. 13JDG064) and the Graduate Research and Innovation
Projects in Jiangsu Province (no. 1293000504). We would also like
ot thank Professor Qin Zhenghong for providing the GFP-LC3
expression vector and Professor Li Chaojun for providing the
H2B-GFP-labeled HeLa cell line.
References
|
1
|
Guo Z and Sadler PJ: Metals in Medicine.
Angew Chem Int Ed. 38:1512–1531. 1999. View Article : Google Scholar
|
|
2
|
Orvig C and Abrams MJ: Medicinal inorganic
chemistry: introduction. Chem Rev. 99:2201–2204. 1999. View Article : Google Scholar
|
|
3
|
Hartinger CG, Nazarov AA, Ashraf SM, Dyson
PJ and Keppler BK: Carbohydrate-metal complexes and their potential
as anticancer agents. Curr Med Chem. 15:2574–2591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen D, Milacic V, Frezza M and Dou QP:
Metal complexes, their cellular targets and potential for cancer
therapy. Curr Pharm Des. 15:777–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marzano C, Pellei M, Tisato F and Santini
C: Copper complexes as anticancer agents. Anticancer Agents Med
Chem. 9:185–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ott I: On the medicinal chemistry of gold
complexes as anti cancer drugs. Coord Chem Rev. 253:1670–1681.
2009. View Article : Google Scholar
|
|
7
|
Timerbaev AR: Advances in developing
tris(8-quinolinolato) gallium(iii) as an anticancer drug: critical
appraisal and prospects. Metallomics. 1:193–198. 2009. View Article : Google Scholar
|
|
8
|
Kostova I: Titanium and vanadium complexes
as anticancer agents. Anticancer Agents in Med Chem. 9:827–842.
2009. View Article : Google Scholar
|
|
9
|
Zhang CX and Lippard SJ: New metal
complexes as potential therapeutics. Curr Opin Chem Biol.
7:481–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rafique S, Idrees M, Nasim A, Akbar H and
Athar A: Transition metal complexes as potential therapeutic
agents. Biotech Mol Biol Rev. 5:38–45. 2010.
|
|
11
|
Wedler FC: Biological significance of
manganese in mammalian systems. Progress in Medicinal Chemistry.
Ellis GP and Luscombe DK: Elsevier; Cardiff: pp. 89–133. 1993,
View Article : Google Scholar
|
|
12
|
Aschner M, Guilarte TR, Schneider JS and
Zheng W: Manganese: recent advances in understanding its transport
and neurotoxicity. Toxicol and Appl Pharmacol. 221:131–147. 2007.
View Article : Google Scholar
|
|
13
|
Au C, Benedetto A and Aschner M: Manganese
transport in eukaryotes: the role of DMT1. Neurotoxicology.
29:569–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calzolari A, Oliviero I, Deaglio S, et al:
Transferrin receptor 2 is frequently expressed in human cancer cell
lines. Blood Cells Mol Dis. 39:82–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sciot R, Paterson AC, van Eyken P, Callea
F, Kew MC and Desmet VJ: Transferrin receptor expression in human
hepato-cellular carcinoma: an immunohistochemical study of 34
cases. Histopathology. 12:53–63. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sciot R, Van Eyken P and Desmet VJ:
Transferrin receptor expression in benign tumours and in
hepatoblastoma of the liver. Histopathology. 16:59–62. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Mchichi B, Hadji A, Vazquez A and Leca
G: p38 MAPK and MSK1 mediate caspase-8 activation in
manganese-induced mitochondria-dependent cell death. Cell Death and
Differ. 14:1826–1836. 2007. View Article : Google Scholar
|
|
18
|
Oubrahim H, Stadtman ER and Chock PB:
Mitochondria play no roles in Mn(II)-induced apoptosis in HeLa
cells. Proc Natl Acad Sci USA. 98:9505–9510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kovala-Demertzi D, Hadjipavlou-Litina D,
Staninska M, Primikiri A, Kotoglou C and Demertzis MA:
Anti-oxidant, in vitro, in vivo anti-inflammatory activity and
antiproliferative activity of mefenamic acid and its metal
complexes with manganese(II), cobalt(II), nickel(II), copper(II)
and zinc(II). J Enzym Inhib Med Chem. 24:742–752. 2009. View Article : Google Scholar
|
|
20
|
Qiu-Yun C, Dong-Fang Z, Juan H, Wen-Jie G
and Jing G: Synthesis, anticancer activities, interaction with DNA
and mitochondria of manganese complexes. J Inorg Biochem.
11:1141–1417. 2010. View Article : Google Scholar
|
|
21
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, et al: Principles and current strategies for targeting
autophagy for cancer treatment. Clin Cancer Res. 17:654–666. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu EY and Ryan KM: Autophagy and cancer -
issues we need to digest. J Cell Sci. 125:2349–2358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen HM and Codogno P: Autophagic cell
death: Loch Ness monster or endangered species? Autophagy.
7:457–465. 2011. View Article : Google Scholar
|
|
24
|
Kondo Y and Kondo S: Autophagy and cancer
therapy. Autophagy. 2:85–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mujumdar N and Saluja AK: Autophagy in
pancreatic cancer: an emerging mechanism of cell death. Autophagy.
6:997–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu L, Alva A, Su H, et al: Regulation of
an ATG7-beclin 1 program of autophagic cell death by caspase-8.
Science. 304:1500–1502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Cheng Y, Zhang B, Bian HJ and Bao
JK: Polygonatum cyrtonema lectin induces apoptosis and autophagy in
human melanoma A375 cells through a mitochondria-mediated
ROS-p38-p53 pathway. Cancer letters. 275:54–60. 2009. View Article : Google Scholar
|
|
29
|
Ghavami S, Eshragi M, Ande SR, et al:
S100A8/A9 induces autophagy and apoptosis via ROS-mediated
cross-talk between mitochondria and lysosomes that involves BNIP3.
Cell Res. 20:314–331. 2010. View Article : Google Scholar
|
|
30
|
Biederbick A, Kern HF and Elsässer HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
31
|
Klionsky DJ, Abeliovich H, Agostinis P, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy in higher eukaryotes. Autophagy. 4:151–175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ansari KI, Grant JD, Kasiri S, Woldemariam
G, Shrestha B and Mandal SS: Manganese(III)-salens induce tumor
selective apoptosis in human cells. J Inorg Biochem. 103:818–826.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hille A, Ott I, Kitanovic A, et al:
N,N’-Bis(salicylidene)-1,2-phenylenediamine]metal complexes with
cell death promoting properties. J Biol Inorg Chem. 14:711–725.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen QY, Huang J, Li JF and Gao J:
Synthesis, interaction with mitochondrial and cancer cells of a
dinuclear manganese(II) complex:
Mn2(Adpa)2Cl4. Chinese J Inorg
Chem. 24:1789–1793. 2008.(In Chinese).
|
|
35
|
Guo WJ, Ye SS, Cao N, Huang J, Gao J and
Chen QY: ROS-mediated autophagy was involved in cancer cell death
induced by novel copper(II) complex. Exp Toxicol Pathol.
62:577–582. 2010. View Article : Google Scholar
|
|
36
|
Chen QY, Huang J, Guo WJ and Gao J:
Synthesis, characterization, DNA interaction and cytotoxic
activities of copper complexes with ethyl
2-[bis(2-pyridylmethyl)amino]propionate. Spectrochim Acta A Mol
Biomol Spectrosc. 72:648–653. 2009. View Article : Google Scholar
|
|
37
|
Rajendiran V, Karthik R, Palaniandavar M,
et al: Mixed-ligand copper(II)-phenolate complexes: effect of
coligand on enhanced DNA and protein binding, DNA cleavage, and
anticancer activity. Inorg Chem. 46:8208–8221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Selvakumar B, Rajendiran V, Uma Maheswari
P, Stoeckli-Evans H and Palaniandavar M: Structures, spectra, and
DNA-binding properties of mixed ligand copper(II) complexes of
iminodiacetic acid: The novel role of diimine co-ligands on DNA
conformation and hydrolytic and oxidative double strand DNA
cleavage. J Inorg Biochem. 100:316–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dhar S, Nethaji M and Chakravarty AR: DNA
cleavage on photoexposure at the d-d band in ternary copper(II)
complexes using red-light laser. Inorg Chem. 45:11043–11050. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shibata S, Maeda M, Furuta K, et al:
Neuroprotective effects of (arylthio)cyclopentenone derivatives on
manganese-induced apoptosis in PC12 cells. Brain Res. 1294:218–225.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qin LF and Ng IO: Induction of apoptosis
by cisplatin and its effect on cell cycle-related proteins and cell
cycle changes in hepatoma cells. Cancer Lett. 175:27–38. 2002.
View Article : Google Scholar
|
|
43
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Galluzzi L, Maiuri MC, Vitale I, et al:
Cell death modalities: classification and pathophysiological
implications. Cell Death Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iwamaru A, Kondo Y, Iwado E, et al:
Silencing mammalian target of rapamycin signaling by small
interfering RNA enhances rapamycin-induced autophagy in malignant
glioma cells. Oncogene. 26:1840–1851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zamzami N, Susin SA, Marchetti P, et al:
Mitochondrial control of nuclear apoptosis. J Exp Med.
183:1533–1544. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Y, McMillan-Ward E, Kong J, Israels
SJ and Gibson SB: Oxidative stress induces autophagic cell death
independent of apoptosis in transformed and cancer cells. Cell
Death Differ. 15:171–182. 2008. View Article : Google Scholar
|
|
51
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
26:1749–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
54
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|