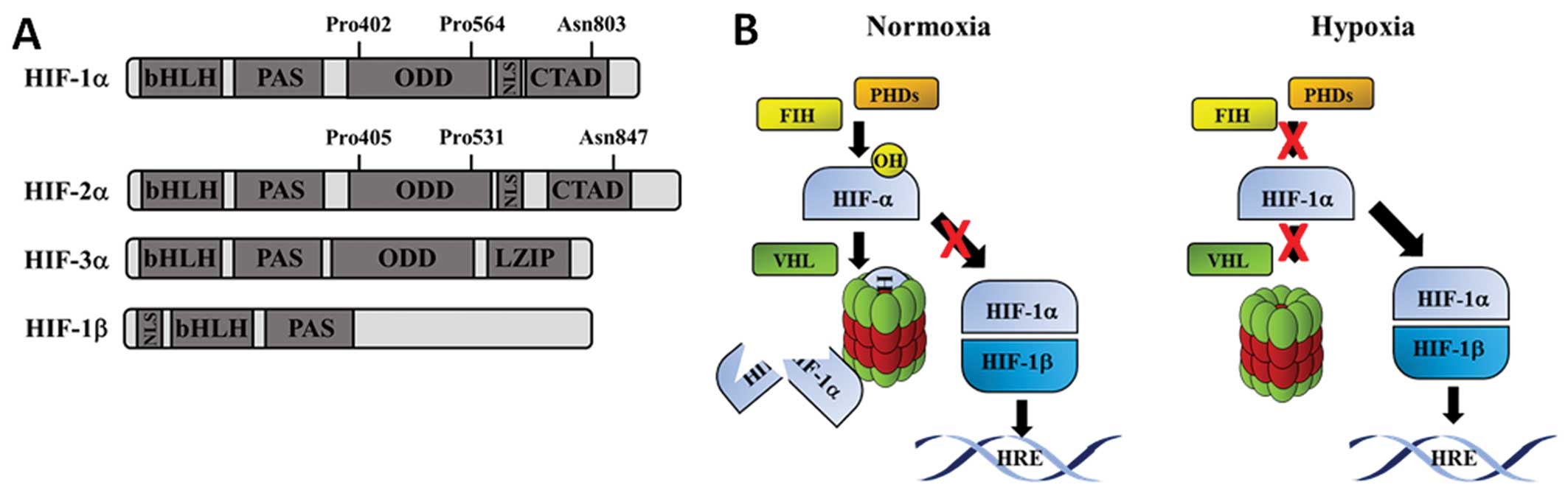
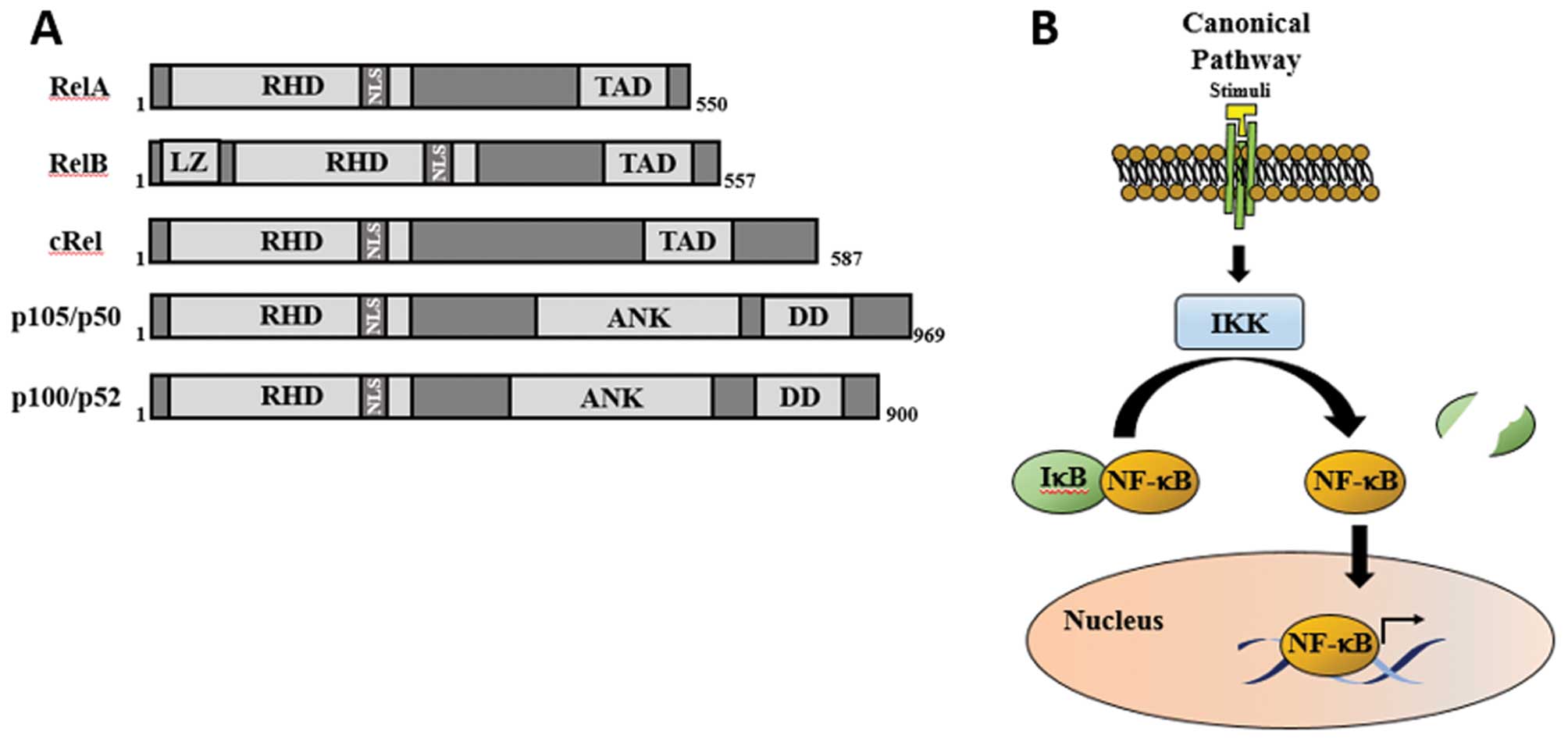
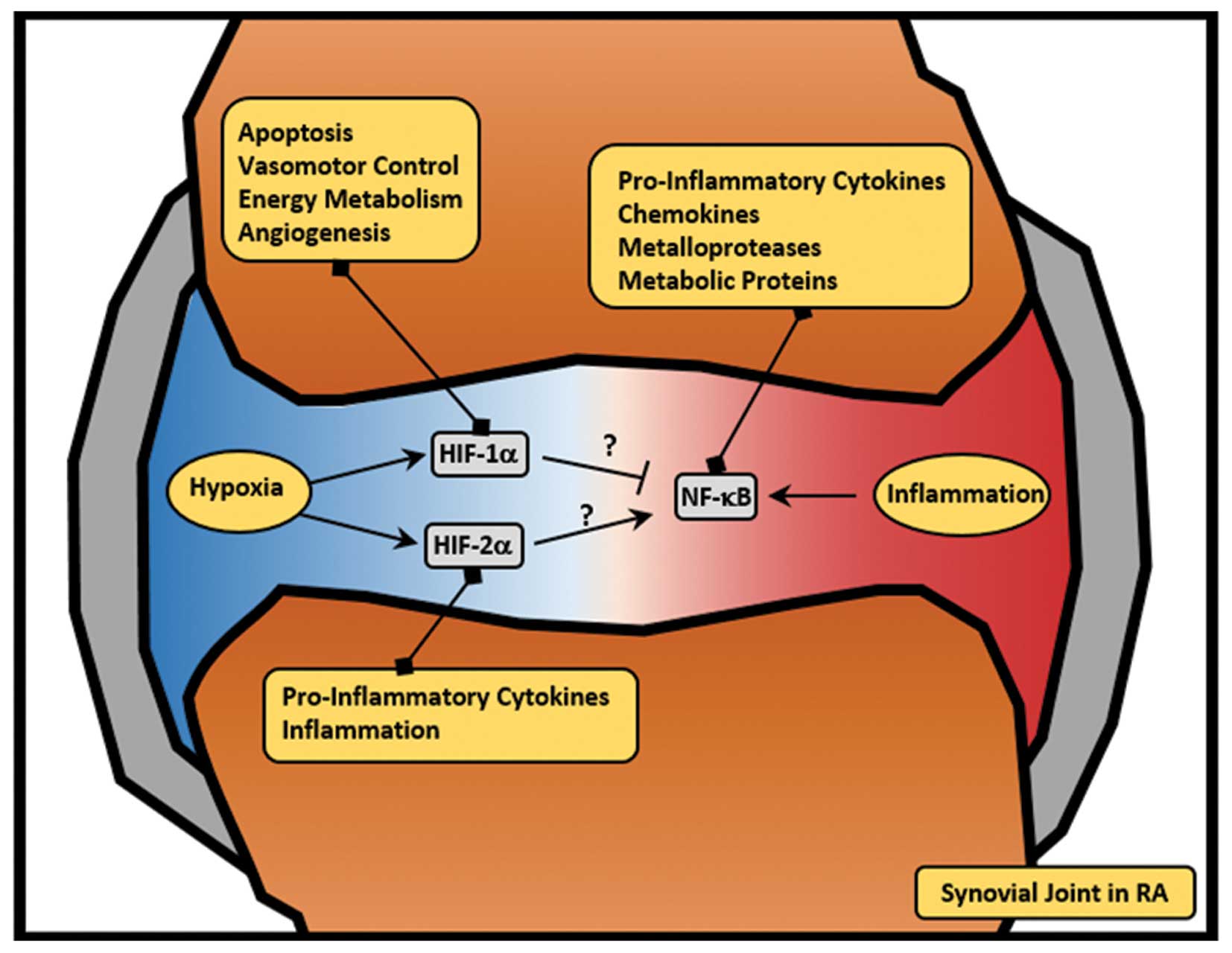
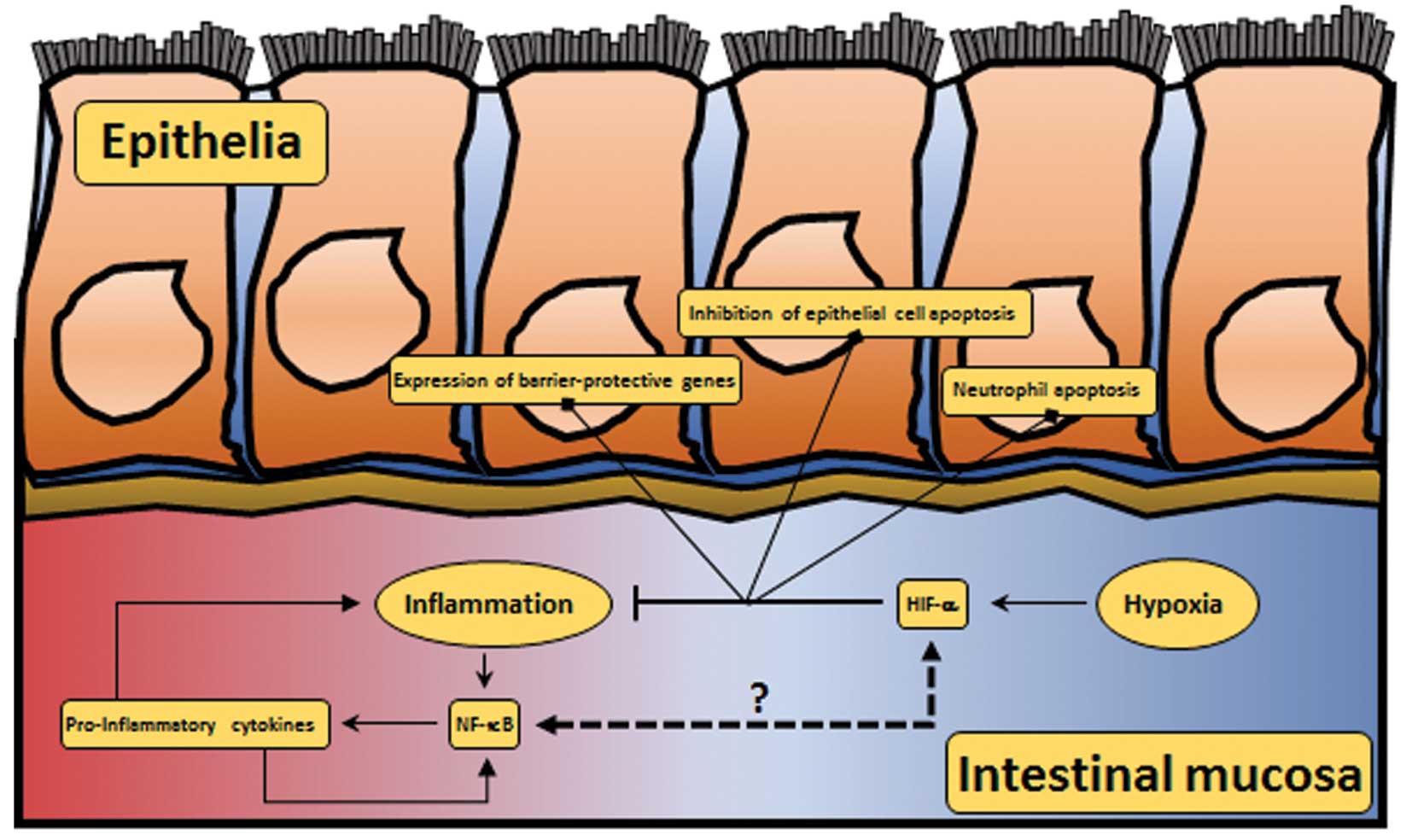
|
1
|
Semenza GL: Regulation of oxygen
homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda).
24:97–106. 2009. View Article : Google Scholar
|
|
2
|
Semenza GL: HIF-1 and human disease: One
highly involved factor. Genes Dev. 14:1983–1991. 2000.PubMed/NCBI
|
|
3
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI
|
|
4
|
Thornton RD, Lane P, Borghaei RC, Pease
EA, Caro J and Mochan E: Interleukin 1 induces hypoxia-inducible
factor 1 in human gingival and synovial fibroblasts. Biochem J.
350:307–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor CT: Interdependent roles for
hypoxia inducible factor and nuclear factor-kappaB in hypoxic
inflammation. J Physiol. 586:4055–4059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Näthke I and Rocha S: Antagonistic
crosstalk between APC and HIF-1α. Cell Cycle. 10:1545–1547. 2011.
View Article : Google Scholar
|
|
7
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI
|
|
8
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
9
|
Carroll VA and Ashcroft M: Role of
hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the
regulation of HIF target genes in response to hypoxia, insulin-like
growth factor-I, or loss of von Hippel-Lindau function:
Implications for targeting the HIF pathway. Cancer Res.
66:6264–6270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
11
|
Patel SA and Simon MC: Biology of
hypoxia-inducible factor-2alpha in development and disease. Cell
Death Differ. 15:628–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino Y, Cao R, Svensson K, Bertilsson G,
Asman M, Tanaka H, Cao Y, Berkenstam A and Poellinger L: Inhibitory
PAS domain protein is a negative regulator of hypoxia-inducible
gene expression. Nature. 414:550–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita T, Ohneda O, Nagano M, et al:
Abnormal heart development and lung remodeling in mice lacking the
hypoxia-inducible factor-related basic helix-loop-helix PAS protein
NEPAS. Mol Cell Biol. 28:1285–1297. 2008. View Article : Google Scholar :
|
|
14
|
Zhang P, Yao Q, Lu L, Li Y, Chen PJ and
Duan C: Hypoxia-inducible factor 3 is an oxygen-dependent
transcription activator and regulates a distinct transcriptional
response to hypoxia. Cell Rep. 6:1110–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bárdos JI and Ashcroft M: Negative and
positive regulation of HIF-1: A complex network. Biochim Biophys
Acta. 1755:107–120. 2005.PubMed/NCBI
|
|
16
|
Rocha S: Gene regulation under low oxygen:
Holding your breath for transcription. Trends Biochem Sci.
32:389–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin C, Wilson C, Blancher C, Taylor M,
Safe S and Harris AL: Association of ARNT splice variants with
estrogen receptor-negative breast cancer, poor induction of
vascular endothelial growth factor under hypoxia, and poor
prognosis. Clin Cancer Res. 7:818–823. 2001.PubMed/NCBI
|
|
18
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haase VH: Renal cancer: Oxygen meets
metabolism. Exp Cell Res. 318:1057–1067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berra E, Benizri E, Ginouvès A, Volmat V,
Roux D and Pouysségur J: HIF prolyl-hydroxylase 2 is the key oxygen
sensor setting low steady-state levels of HIF-1alpha in normoxia.
EMBO J. 22:4082–4090. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Appelhoff RJ, Tian YM, Raval RR, Turley H,
Harris AL, Pugh CW, Ratcliffe PJ and Gleadle JM: Differential
function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the
regulation of hypoxia-inducible factor. J Biol Chem.
279:38458–38465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Epstein AC, Gleadle JM, McNeill LA, et al:
C. elegans EGL-9 and mammalian homologs define a family of
dioxygenases that regulate HIF by prolyl hydroxylation. Cell.
107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fandrey J, Gorr TA and Gassmann M:
Regulating cellular oxygen sensing by hydroxylation. Cardiovasc
Res. 71:642–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruegge K, Jelkmann W and Metzen E:
Hydroxylation of hypoxia-inducible transcription factors and
chemical compounds targeting the HIF-alpha hydroxylases. Curr Med
Chem. 14:1853–1862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frede S, Stockmann C, Freitag P and
Fandrey J: Bacterial lipopolysaccharide induces HIF-1 activation in
human monocytes via p44/42 MAPK and NF-kappaB. Biochem J.
396:517–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jaakkola P, Mole DR, Tian YM, et al:
Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation
complex by O2-regulated prolyl hydroxylation. Science.
292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu F, White SB, Zhao Q and Lee FS:
HIF-1alpha binding to VHL is regulated by stimulus-sensitive
proline hydroxylation. Proc Natl Acad Sci USA. 98:9630–9635. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Durán RV, MacKenzie ED, Boulahbel H,
Frezza C, Heiserich L, Tardito S, Bussolati O, Rocha S, Hall MN and
Gottlieb E: HIF-independent role of prolyl hydroxylases in the
cellular response to amino acids. Oncogene. 32:4549–4556. 2013.
View Article : Google Scholar :
|
|
30
|
Moser SC, Bensaddek D, Ortmann B, Maure
JF, Mudie S, Blow JJ, Lamond AI, Swedlow JR and Rocha S: PHD1 links
cell-cycle progression to oxygen sensing through hydroxylation of
the centrosomal protein Cep192. Dev Cell. 26:381–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O’Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie L, Pi X, Mishra A, Fong G, Peng J and
Patterson C: PHD3-dependent hydroxylation of HCLK2 promotes the DNA
damage response. J Clin Invest. 122:2827–2836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pugh CW, Tan CC, Jones RW and Ratcliffe
PJ: Functional analysis of an oxygen-regulated transcriptional
enhancer lying 3′ to the mouse erythropoietin gene. Proc Natl Acad
Sci USA. 88:10553–10557. 1991. View Article : Google Scholar
|
|
34
|
Semenza GL, Jiang BH, Leung SW, Passantino
R, Concordet JP, Maire P and Giallongo A: Hypoxia response elements
in the aldolase A, enolase 1, and lactate dehydrogenase A gene
promoters contain essential binding sites for hypoxia-inducible
factor 1. J Biol Chem. 271:32529–32537. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schödel J, Oikonomopoulos S, Ragoussis J,
Pugh CW, Ratcliffe PJ and Mole DR: High-resolution genome-wide
mapping of HIF-binding sites by ChIP-seq. Blood. 117:e207–e217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Semenza GL: Regulation of cancer cell
metabolism by hypoxia-inducible factor 1. Semin Cancer Biol.
19:12–16. 2009. View Article : Google Scholar
|
|
37
|
Han YH, Xia L, Song LP, Zheng Y, Chen WL,
Zhang L, Huang Y, Chen GQ and Wang LS: Comparative proteomic
analysis of hypoxia-treated and untreated human leukemic U937
cells. Proteomics. 6:3262–3274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Djidja MC, Chang J, Hadjiprocopis A, et
al: Identification of hypoxia-regulated proteins using MALDI-mass
spectrometry imaging combined with quantitative proteomics. J
Proteome Res. 13:2297–2313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gustafsson MV, Zheng X, Pereira T, Gradin
K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U and
Bondesson M: Hypoxia requires notch signaling to maintain the
undifferentiated cell state. Dev Cell. 9:617–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gordan JD, Bertout JA, Hu CJ, Diehl JA and
Simon MC: HIF-2alpha promotes hypoxic cell proliferation by
enhancing c-myc transcriptional activity. Cancer Cell. 11:335–347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
An WG, Kanekal M, Simon MC, Maltepe E,
Blagosklonny MV and Neckers LM: Stabilization of wild-type p53 by
hypoxia-inducible factor 1alpha. Nature. 392:405–408. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View Article : Google Scholar
|
|
43
|
No authors listed. Celebrating 25 years of
NF-κB. Nat Immunol. 12:6812011. View Article : Google Scholar
|
|
44
|
Campbell KJ and Perkins ND: Regulation of
NF-kappaB function. Biochem Soc Symp. 73:165–180. 2006.PubMed/NCBI
|
|
45
|
Wong D, Teixeira A, Oikonomopoulos S, et
al: Extensive characterization of NF-κB binding uncovers
non-canonical motifs and advances the interpretation of genetic
functional traits. Genome Biol. 12:R702011. View Article : Google Scholar
|
|
46
|
Gilmore TD: The Rel/NF-kappaB signal
transduction pathway: Introduction. Oncogene. 18:6842–6844. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen F, Castranova V, Shi X and Demers LM:
New insights into the role of nuclear factor-kappaB, a ubiquitous
transcription factor in the initiation of diseases. Clin Chem.
45:7–17. 1999.PubMed/NCBI
|
|
48
|
Bandarra DR and Rocha S: A tale of two
transcription factors: NF-κB and HIF crosstalk. OA Mol Cell Biol.
1:62013. View Article : Google Scholar
|
|
49
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Perkins ND and Gilmore TD: Good cop, bad
cop: The different faces of NF-kappaB. Cell Death Differ.
13:759–772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aggarwal BB, Takada Y, Shishodia S,
Gutierrez AM, Oommen OV, Ichikawa H, Baba Y and Kumar A: Nuclear
transcription factor NF-kappa B: Role in biology and medicine.
Indian J Exp Biol. 42:341–353. 2004.PubMed/NCBI
|
|
52
|
Hackett PH and Roach RC: High-altitude
illness. N Engl J Med. 345:107–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hartmann G, Tschöp M, Fischer R,
Bidlingmaier C, Riepl R, Tschöp K, Hautmann H, Endres S and Toepfer
M: High altitude increases circulating interleukin-6, interleukin-1
receptor antagonist and C-reactive protein. Cytokine. 12:246–252.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim HL, Cho YS, Choi H, Chun YS, Lee ZH
and Park JW: Hypoxia-inducible factor 1alpha is deregulated by the
serum of rats with adjuvant-induced arthritis. Biochem Biophys Res
Commun. 378:123–128. 2009. View Article : Google Scholar
|
|
55
|
Boyd HK, Lappin TR and Bell AL: Evidence
for impaired erythropoietin response to anaemia in rheumatoid
disease. Br J Rheumatol. 30:255–259. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grenz A, Clambey E and Eltzschig HK:
Hypoxia signaling during intestinal ischemia and inflammation. Curr
Opin Crit Care. 18:178–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eltzschig HK, Sitkovsky MV and Robson SC:
Purinergic signaling during inflammation. N Engl J Med.
367:2322–2333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bandarra D, Biddlestone J, Mudie S, Muller
HA and Rocha S: Hypoxia activates IKK-NF-κB and the immune response
in Drosophila melanogaster. Biosci Rep. 34:342014. View Article : Google Scholar
|
|
59
|
Bandarra D, Biddlestone J, Mudie S, Muller
HA and Rocha S: HIF-1α restricts NF-κB dependent gene expression to
control innate immunity signals. Dis Model Mech. Dec 15–2014.Epub
ahead of print.
|
|
60
|
van Uden P, Kenneth NS, Webster R, Müller
HA, Mudie S and Rocha S: Evolutionary conserved regulation of
HIF-1β by NF-κB. PLoS Genet. 7:e10012852011. View Article : Google Scholar
|
|
61
|
van Uden P, Kenneth NS and Rocha S:
Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem
J. 412:477–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xue X, Ramakrishnan S, Anderson E, Taylor
M, Zimmermann EM, Spence JR, Huang S, Greenson JK and Shah YM:
Endothelial PAS domain protein 1 activates the inflammatory
response in the intestinal epithelium to promote colitis in mice.
Gastroenterology. 145:831–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Karhausen J, Furuta GT, Tomaszewski JE,
Johnson RS, Colgan SP and Haase VH: Epithelial hypoxia-inducible
factor-1 is protective in murine experimental colitis. J Clin
Invest. 114:1098–1106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sewell KL and Trentham DE: Pathogenesis of
rheumatoid arthritis. Lancet. 341:283–286. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Al-Shukaili AK and Al-Jabri AA: Rheumatoid
arthritis, cytokines and hypoxia. What is the link. Saudi Med J.
27:1642–1649. 2006.PubMed/NCBI
|
|
66
|
Gaber T, Dziurla R, Tripmacher R,
Burmester GR and Buttgereit F: Hypoxia inducible factor (HIF) in
rheumatology: Low O2! See what HIF can do. Ann Rheum
Dis. 64:971–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hueber W, Kidd BA, Tomooka BH, et al:
Antigen microarray profiling of autoantibodies in rheumatoid
arthritis. Arthritis Rheum. 52:2645–2655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
van Baarsen LG, Wijbrandts CA, Rustenburg
F, Cantaert T, van der Pouw Kraan TC, Baeten DL, Dijkmans BA, Tak
PP and Verweij CL: Regulation of IFN response gene activity during
infliximab treatment in rheumatoid arthritis is associated with
clinical response to treatment. Arthritis Res Ther. 12:R112010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
van Wietmarschen HA, Dai W, van der Kooij
AJ, et al: Characterization of rheumatoid arthritis subtypes using
symptom profiles, clinical chemistry and metabolomics measurements.
PLoS One. 7:e443312012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sweeney SE and Firestein GS: Signal
transduction in rheumatoid arthritis. Curr Opin Rheumatol.
16:231–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Morel J and Berenbaum F: Signal
transduction pathways: new targets for treating rheumatoid
arthritis. Joint Bone Spine. 71:503–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Firestein GS and Manning AM: Signal
transduction and transcription factors in rheumatic disease.
Arthritis Rheum. 42:609–621. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Benito MJ, Murphy E, Murphy EP, van den
Berg WB, FitzGerald O and Bresnihan B: Increased synovial tissue
NF-kappa B1 expression at sites adjacent to the cartilage-pannus
junction in rheumatoid arthritis. Arthritis Rheum. 50:1781–1787.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Handel ML, McMorrow LB and Gravallese EM:
Nuclear factor-kappa B in rheumatoid synovium. Localization of p50
and p65. Arthritis Rheum. 38:1762–1770. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Müller-Ladner U, Pap T, Gay RE, Neidhart M
and Gay S: Mechanisms of disease: The molecular and cellular basis
of joint destruction in rheumatoid arthritis. Nat Clin Pract
Rheumatol. 1:102–110. 2005. View Article : Google Scholar
|
|
76
|
Simmonds RE and Foxwell BM: Signalling,
inflammation and arthritis: NF-kappaB and its relevance to
arthritis and inflammation. Rheumatology (Oxford). 47:584–590.
2008. View Article : Google Scholar
|
|
77
|
Westra J, Molema G and Kallenberg CG:
Hypoxia-inducible factor-1 as regulator of angiogenesis in
rheumatoid arthritis -therapeutic implications. Curr Med Chem.
17:254–263. 2010. View Article : Google Scholar
|
|
78
|
Ryu JH, Chae CS, Kwak JS, et al:
Hypoxia-inducible factor-2α is an essential catabolic regulator of
inflammatory rheumatoid arthritis. PLoS Biol. 12:e10018812014.
View Article : Google Scholar
|
|
79
|
Hu F, Shi L, Mu R, et al:
Hypoxia-inducible factor-1α and interleukin 33 form a regulatory
circuit to perpetuate the inflammation in rheumatoid arthritis.
PLoS One. 8:e726502013. View Article : Google Scholar
|
|
80
|
Brouwer E, Gouw AS, Posthumus MD, van
Leeuwen MA, Boerboom AL, Bijzet J, Bos R, Limburg PC, Kallenberg CG
and Westra J: Hypoxia inducible factor-1-alpha (HIF-1alpha) is
related to both angiogenesis and inflammation in rheumatoid
arthritis. Clin Exp Rheumatol. 27:945–951. 2009.
|
|
81
|
Muz B, Khan MN, Kiriakidis S and Paleolog
EM: Hypoxia. The role of hypoxia and HIF-dependent signalling
events in rheumatoid arthritis. Arthritis Res Ther. 11:2012009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Moniz S, Biddlestone J and Rocha S:
Grow2: The HIF system, energy homeostasis and the cell
cycle. Histol Histopathol. 29:589–600. 2014.PubMed/NCBI
|
|
83
|
Kenneth NS and Rocha S: Regulation of gene
expression by hypoxia. Biochem J. 414:19–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Poonam P: The biology of oral tolerance
and issues related to oral vaccine design. Curr Pharm Des.
13:2001–2007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cummins EP, Doherty GA and Taylor CT:
Hydroxylases as therapeutic targets in inflammatory bowel disease.
Lab Invest. 93:378–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Abraham C and Cho JH: Inflammatory bowel
disease. N Engl J Med. 361:2066–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Giatromanolaki A, Sivridis E, Maltezos E,
Papazoglou D, Simopoulos C, Gatter KC, Harris AL and Koukourakis
MI: Hypoxia inducible factor 1alpha and 2alpha overexpression in
inflammatory bowel disease. J Clin Pathol. 56:209–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Danese S, Dejana E and Fiocchi C: Immune
regulation by microvascular endothelial cells: Directing innate and
adaptive immunity, coagulation, and inflammation. J Immunol.
178:6017–6022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Werth N, Beerlage C, Rosenberger C, et al:
Activation of hypoxia inducible factor 1 is a general phenomenon in
infections with human pathogens. PLoS One. 5:e115762010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cummins EP, Seeballuck F, Keely SJ, Mangan
NE, Callanan JJ, Fallon PG and Taylor CT: The hydroxylase inhibitor
dimethyloxalylglycine is protective in a murine model of colitis.
Gastroenterology. 134:156–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tambuwala MM, Cummins EP, Lenihan CR, et
al: Loss of prolyl hydroxylase-1 protects against colitis through
reduced epithelial cell apoptosis and increased barrier function.
Gastroenterology. 139:2093–2101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Louis NA, Hamilton KE, Kong T and Colgan
SP: HIF-dependent induction of apical CD55 coordinates epithelial
clearance of neutrophils. FASEB J. 19:950–959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Synnestvedt K, Furuta GT, Comerford KM,
Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF and
Colgan SP: Ecto-5′-nucleotidase (CD73) regulation by
hypoxia-inducible factor-1 mediates permeability changes in
intestinal epithelia. J Clin Invest. 110:993–1002. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kong T, Westerman KA, Faigle M, Eltzschig
HK and Colgan SP: HIF-dependent induction of adenosine A2B receptor
in hypoxia. FASEB J. 20:2242–2250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Louis NA, Hamilton KE, Canny G, Shekels
LL, Ho SB and Colgan SP: Selective induction of mucin-3 by hypoxia
in intestinal epithelia. J Cell Biochem. 99:1616–1627. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Furuta GT, Turner JR, Taylor CT, Hershberg
RM, Comerford K, Narravula S, Podolsky DK and Colgan SP:
Hypoxia-inducible factor 1-dependent induction of intestinal
trefoil factor protects barrier function during hypoxia. J Exp Med.
193:1027–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Comerford KM, Wallace TJ, Karhausen J,
Louis NA, Montalto MC and Colgan SP: Hypoxia-inducible
factor-1-dependent regulation of the multidrug resistance (MDR1)
gene. Cancer Res. 62:3387–3394. 2002.PubMed/NCBI
|
|
99
|
Neurath MF, Pettersson S, Meyer zum
Büschenfelde KH and Strober W: Local administration of antisense
phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B
abrogates established experimental colitis in mice. Nat Med.
2:998–1004. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Holtmann MH and Neurath MF: Differential
TNF-signaling in chronic inflammatory disorders. Curr Mol Med.
4:439–444. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, Egan LJ, Kagnoff MF and Karin M: IKKbeta links inflammation
and tumorigenesis in a mouse model of colitis-associated cancer.
Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pasparakis M: IKK/NF-kappaB signaling in
intestinal epithelial cells controls immune homeostasis in the gut.
Mucosal Immunol. 1(Suppl 1): S54–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zaph C, Troy AE, Taylor BC, et al:
Epithelial-cell-intrinsic IKK-beta expression regulates intestinal
immune homeostasis. Nature. 446:552–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hauser CJ, Locke RR, Kao HW, Patterson J
and Zipser RD: Visceral surface oxygen tension in experimental
colitis in the rabbit. J Lab Clin Med. 112:68–71. 1988.PubMed/NCBI
|
|
105
|
Shah YM, Ito S, Morimura K, et al:
Hypoxia-inducible factor augments experimental colitis through an
MIF-dependent inflammatory signaling cascade. Gastroenterology.
134:2036–2048. 2048 e2031–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hara H and Saito T: CARD9 versus CARMA1 in
innate and adaptive immunity. Trends Immunol. 30:234–242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang H, Minamishima YA, Yan Q, Schlisio S,
Ebert BL, Zhang X, Zhang L, Kim WY, Olumi AF and Kaelin WG Jr: pVHL
acts as an adaptor to promote the inhibitory phosphorylation of the
NF-kappaB agonist Card9 by CK2. Mol Cell. 28:15–27. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rius J, Guma M, Schachtrup C, Akassoglou
K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG and Karin M:
NF-kappaB links innate immunity to the hypoxic response through
transcriptional regulation of HIF-1alpha. Nature. 453:807–811.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bracken CP, Whitelaw ML and Peet DJ:
Activity of hypoxia-inducible factor 2alpha is regulated by
association with the NF-kappaB essential modulator. J Biol Chem.
280:14240–14251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
O’Connell RM, Rao DS, Chaudhuri AA, Boldin
MP, Taganov KD, Nicoll J, Paquette RL and Baltimore D: Sustained
expression of microRNA-155 in hematopoietic stem cells causes a
myeloproliferative disorder. J Exp Med. 205:585–594. 2008.
View Article : Google Scholar
|
|
111
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tsuzuki Y, Fukumura D, Oosthuyse B, Koike
C, Carmeliet P and Jain RK: Vascular endothelial growth factor
(VEGF) modulation by targeting hypoxia-inducible
factor-1alpha--> hypoxia response element--> VEGF cascade
differentially regulates vascular response and growth rate in
tumors. Cancer Res. 60:6248–6252. 2000.PubMed/NCBI
|
|
114
|
Shay JE, Imtiyaz HZ, Sivanand S, et al:
Inhibition of hypoxia-inducible factors limits tumor progression in
a mouse model of colorectal cancer. Carcinogenesis. 35:1067–1077.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rawluszko-Wieczorek AA, Horbacka K,
Krokowicz P, Misztal M and Jagodzinski PP: Prognostic potential of
DNA methylation and transcript levels of HIF1A and EPAS1 in
colorectal cancer. Mol Cancer Res. 12:1112–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Koshiji M, Kageyama Y, Pete EA, Horikawa
I, Barrett JC and Huang LE: HIF-1alpha induces cell cycle arrest by
functionally counteracting Myc. EMBO J. 23:1949–1956. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sánchez-Puig N, Veprintsev DB and Fersht
AR: Binding of natively unfolded HIF-1alpha ODD domain to p53. Mol
Cell. 17:11–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter
CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL and Bedi A:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
119
|
Moeller BJ, Dreher MR, Rabbani ZN,
Schroeder T, Cao Y, Li CY and Dewhirst MW: Pleiotropic effects of
HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 8:99–110.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bertout JA, Majmundar AJ, Gordan JD, Lam
JC, Ditsworth D, Keith B, Brown EJ, Nathanson KL and Simon MC:
HIF2alpha inhibition promotes p53 pathway activity, tumor cell
death, and radiation responses. Proc Natl Acad Sci USA.
106:14391–14396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Volm M and Koomägi R: Hypoxia-inducible
factor (HIF-1) and its relationship to apoptosis and proliferation
in lung cancer. Anticancer Res. 20:1527–1533. 2000.PubMed/NCBI
|
|
122
|
Evans AJ, Russell RC, Roche O, et al: VHL
promotes E2 box-dependent E-cadherin transcription by HIF-mediated
regulation of SIP1 and snail. Mol Cell Biol. 27:157–169. 2007.
View Article : Google Scholar :
|
|
123
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gort EH, van Haaften G, Verlaan I, et al:
The TWIST1 oncogene is a direct target of hypoxia-inducible
factor-2alpha. Oncogene. 27:1501–1510. 2008. View Article : Google Scholar
|
|
125
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lakatos PL and Lakatos L: Risk for
colorectal cancer in ulcerative colitis: Changes, causes and
management strategies. World J Gastroenterol. 14:3937–3947. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Munkholm P: Review article: The incidence
and prevalence of colorectal cancer in inflammatory bowel disease.
Aliment Pharmacol Ther. 18(Suppl 2): 1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fernández-Majada V, Aguilera C, Villanueva
A, et al: Nuclear IKK activity leads to dysregulated
notch-dependent gene expression in colorectal cancer. Proc Natl
Acad Sci USA. 104:276–281. 2007. View Article : Google Scholar :
|
|
130
|
Seril DN, Liao J, Yang GY and Yang CS:
Oxidative stress and ulcerative colitis-associated carcinogenesis:
Studies in humans and animal models. Carcinogenesis. 24:353–362.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sangha S, Yao M and Wolfe MM:
Non-steroidal anti-inflammatory drugs and colorectal cancer
prevention. Postgrad Med J. 81:223–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hoffmeister M, Chang-Claude J and Brenner
H: Do older adults using NSAIDs have a reduced risk of colorectal
cancer. Drugs Aging. 23:513–523. 2006. View Article : Google Scholar
|
|
133
|
Becker C, Fantini MC, Schramm C, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 21:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Greten FR, Arkan MC, Bollrath J, et al:
NF-kappaB is a negative regulator of IL-1beta secretion as revealed
by genetic and pharmacological inhibition of IKKbeta. Cell.
130:918–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
136
|
Richmond A: Nf-kappa B, chemokine gene
transcription and tumour growth. Nat Rev Immunol. 2:664–674. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schulze-Bergkamen H and Krammer PH:
Apoptosis in cancer-implications for therapy. Semin Oncol.
31:90–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Kucharczak J, Simmons MJ, Fan Y and
Gélinas C: To be, or not to be: NF-kappaB is the answer - role of
Rel/NF-kappaB in the regulation of apoptosis. Oncogene.
22:8961–8982. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Luo JL, Kamata H and Karin M:
IKK/NF-kappaB signaling: Balancing life and death - a new approach
to cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cornejo MG, Boggon TJ and Mercher T: JAK3:
A two-faced player in hematological disorders. Int J Biochem Cell
Biol. 41:2376–2379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lin Q, Lai R, Chirieac LR, et al:
Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and
cell lines: Inhibition of JAK3/STAT3 signaling induces apoptosis
and cell cycle arrest of colon carcinoma cells. Am J Pathol.
167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tsareva SA, Moriggl R, Corvinus FM,
Wiederanders B, Schütz A, Kovacic B and Friedrich K: Signal
transducer and activator of transcription 3 activation promotes
invasive growth of colon carcinomas through matrix
metalloproteinase induction. Neoplasia. 9:279–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Guttridge DC, Albanese C, Reuther JY,
Pestell RG and Baldwin AS Jr: NF-kappaB controls cell growth and
differentiation through transcriptional regulation of cyclin D1.
Mol Cell Biol. 19:5785–5799. 1999.PubMed/NCBI
|
|
145
|
Chen C, Edelstein LC and Gélinas C: The
Rel/NF-kappaB family directly activates expression of the apoptosis
inhibitor Bcl-x(L). Mol Cell Biol. 20:2687–2695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Choo MK, Sakurai H, Kim DH and Saiki I: A
ginseng saponin metabolite suppresses tumor necrosis
factor-α-promoted metastasis by suppressing nuclear factor-κB
signaling in murine colon cancer cells. Oncol Rep. 19:595–600.
2008.PubMed/NCBI
|
|
148
|
Thomasova D, Mulay SR, Bruns H and Anders
HJ: p53-independent roles of MDM2 in NF-κB signaling: Implications
for cancer therapy, wound healing, and autoimmune diseases.
Neoplasia. 14:1097–1101. 2012.
|
|
149
|
Puvvada SD, Funkhouser WK, Greene K, Deal
A, Chu H, Baldwin AS, Tepper JE and O’Neil BH: NF-κB and Bcl-3
activation are prognostic in metastatic colorectal cancer.
Oncology. 78:181–188. 2010. View Article : Google Scholar :
|
|
150
|
Kwon HC, Kim SH, Oh SY, et al:
Clinicopathological significance of nuclear factor-kappa B, HIF-1
alpha, and vascular endothelial growth factor expression in stage
III colorectal cancer. Cancer Sci. 101:1557–1561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Schwitalla S, Ziegler PK, Horst D, et al:
Loss of p53 in enterocytes generates an inflammatory
microenvironment enabling invasion and lymph node metastasis of
carcinogen-induced colorectal tumors. Cancer Cell. 23:93–106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Terzic J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114.
e21052010. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Newton IP, Kenneth NS, Appleton PL, Näthke
I and Rocha S: Adenomatous polyposis coli and hypoxia-inducible
factor-1{alpha} have an antagonistic connection. Mol Biol Cell.
21:3630–3638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bienz M and Clevers H: Linking colorectal
cancer to Wnt signaling. Cell. 103:311–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
McCartney BM and Näthke IS: Cell
regulation by the Apc protein Apc as master regulator of epithelia.
Curr Opin Cell Biol. 20:186–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wen D, Zhang N, Shan B and Wang S:
Helicobacter pylori infection may be implicated in the topography
and geographic variation of upper gastrointestinal cancers in the
Taihang Mountain high-risk region in northern China. Helicobacter.
15:416–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Krüger B, Krick S, Dhillon N, et al: Donor
Toll-like receptor 4 contributes to ischemia and reperfusion injury
following human kidney transplantation. Proc Natl Acad Sci USA.
106:3390–3395. 2009. View Article : Google Scholar : PubMed/NCBI
|