Introduction
Age-related macular degeneration (AMD) is the
leading cause of permanent blindness in older adults. The majority
of patients with AMD experience severe vision loss in the center of
the macula due to damage to the retina. There are two primary types
of AMD, ‘wet’ and ‘dry’. ‘Dry’ AMD is characterized by late-stage
geographic atrophy resulting from the gradual degeneration of
retinal cells, and ‘wet’ AMD is caused by choroidal
neovascularization (CNV) (1).
‘Wet’ AMD causes more severe visual loss, but is more treatable.
CNV is a common sign of the wet form of AMD, which is also referred
to as the exudative form of AMD (2). CNV sprouts from choroidal vessels
and extends through Bruch’s membrane and the retinal pigment
epithelium (RPE) to reach the subretinal space (3). The RPE, which lies between the
photoreceptor cell layer of the retina and Bruch’s membrane and
choroid, is a monolayer of pigmented cells (4). It plays a major role in retinal
physiology by supporting the function of the photoreceptors and
pathology in a variety of retinal diseases (5).
Retinal hypoxia (oxygen deficiency), which occurs
with capillary non-perfusion and leads to angiogenesis, is a major
pathological condition underlying a number of sight-threatening
diseases, including diabetic retinopathy (DR), retinopathy of
prematurity (ROP), AMD and neovascular glaucoma (6–8).
Hypoxia occurs secondary to a number of disease processes in the
human body (9). In response to
hypoxia, retinal pigment epithelial cells (RPE cells) rapidly
release various growth factors resulting in angiogenesis,
fibrovascular tissue formation and retinal ablation (10). Among the hypoxia-stimulated growth
factors, vascular endothelial growth factor (VEGF), a potent
vascular endothelial cell mitogen, is a pivotal regulator of
vasculogenesis and angiogenesis in CNV (11). It has been demonstrated that the
overproduction of VEGF by RPE cells induces CNV (12), and VEGF antagonists have been
shown to attenuate CNV in animal models (13,14). Therefore, therapeutic agents that
inhibit the overproduction of VEGF have shown promising inhibitory
effects on CNV in patients with AMD.
Caffeic acid phenethyl ester (CAPE), a potent
flavonoid antioxidant, is the active component of honeybee (Apis
mellifera) propolis. CAPE has profound antiviral, antitumoral,
anti-inflammatory, antioxidant, neuroprotective,
anti-atherosclerotic and immunomodulatory properties in diverse
systems (15). CAPE has also been
reported to have anti-angiogenic activity in cancer (16,17). However, to the best of our
knowledge, there is no study available to date evaluating the
effects of CAPE on VEGF expression in CNV-associated angiogenesis.
Previous studies have indicated that oxidative stress, inflammatory
responses and atherosclerotic properties are associated with the
pathogenesis of CNV in patients with AMD (18–20). Therefore, the antioxidant effects
of CAPE may be associated with a reduced incidence of CNV. However,
there is no experimental evidence to support this suggestion. In
the present study, we investigated whether treatment with CAPE
results in the inhibition of VEGF production and the possible
mechanisms involved in these effects in ARPE-19 cells under hypoxic
conditions.
Materials and methods
Reagents
CAPE, U0126 and diphenyleneiodonium (DPI) were
purchased from the Sigma Chemical Co. (St. Louis, MO, USA).
SB203580 was purchased from Enzo Life Sciences, Inc. (Farmingdale,
NY, USA). LY294002 was purchased from Calbiochem (San Diego, CA,
USA). YCG063 [an inhibitor of mitochondrial reactive oxygen species
(ROS)] was obtained from Millipore (Billerica, MA, USA). Dulbecco’s
modified Eagle’s medium/nutrient mixture F12 (DMEM/F12 medium),
fetal bovine serum (FBS) and trypsin-EDTA were obtained from
Invitrogen-Gibco (Carlsbad, CA, USA). Antibody against
hypoxia-inducible factor-1α (HIF-1α; NB100-105) was obtained from
Novus Biologicals (Littleton, CO, USA). Antibody against histone H3
(SC-10809) was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibodies against AKT (#9272), phosphorylated
(p)-AKT (ser473; #4058) and p-PI3K (#4255) were purchased from Cell
Signaling Technology (Beverly, MA, USA). Nitrocellulose membranes
and an enhanced chemiluminescence (ECL) kit were obtained from
Amersham Pharmacia Biotech (Uppsala, Sweden).
Cell culture
The human RPE cell line (ARPE-19) was obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
cultured in DMEM/F12 medium supplemented with 10% FBS plus 100
IU/ml penicillin and 100 μg/ml streptomycin mixture
(Gibco/BRL, Gaithersburg, MD, USA) in a 5% CO2
humidified atmosphere at 37°C. The ARPE-19 cells were trypsinized,
seeded in 10-cm diameter dishes, and incubated overnight until
attachment.
Induction of hypoxia
Following overnight incubation, the cells were moved
to a hypoxic chamber (Galaxy 14S; Eppendorf, Enfield, CT, USA).
Before the cells were exposed to hypoxia, the medium was replaced
with DMEM/F12 medium. The hypoxic chamber was equipped with an
oxygen sensor and gas regulator and was flushed with 1%
O2, 5% CO2 and 94% N2, sealed, and
placed at 37°C. After reaching ~80% confluency, the cells were
subjected to hypoxic conditions in the hypoxic chamber for 24 h.
The cells cultured under hypoxic conditions were processed in the
chamber itself to avoid any exposure to normoxic conditions.
Cell viability assay
The viability of the ARPE-19 cells was determined
using the cell counting kit-8 (CCK-8) according to the
manufacturer’s instructions (Dojindo Laboratories, Kumamoto,
Japan). Briefly, the cells were seeded in triplicate at a density
of 1×104 cells/well in 96-well culture plates and
allowed to attach overnight. The medium was then replaced with 100
μl DMEM/F12 medium containing 0, 10, 20 and 40 μM of
CAPE. The plates were incubated for 24 h under hypoxic conditions,
and 10 μl of CCK-8 reagent was added to each well. After
another 2 h incubation at 37°C, the plates were read at 450 nm
using a microplate reader (Model EL800; Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The VEGF levels in the cell culture medium were
assessed by ELISA. The cells were treated with various
concentrations of CAPE for 2 h before being exposed to hypoxic
conditions. Follwoing incubation for 24 h under hypoxic conditions,
the culture supernatant was collected and the VEGF levels were
measured using a VEGF DuoSet ELISA Development kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer’s
instructions. The absorbance at 450 nm was determined using a
microplate reader (Model EL800; Bio-Tek Instruments, Inc.).
Western blot analysis
Western blot analysis was performed as previously
described (21). The ARPE-19
cells were washed 3 times with phosphate-buffered saline (PBS) and
lysed with lysis buffer (Mammalian Cell-PE LB; G-Biosciences, St.
Louis, MO, USA). Equal amounts of protein were separated on 10%
SDS-polyacrylamide minigels and transferred onto nitrocellulose
transfer membranes. Following incubation with the appropriate
primary antibody, the membranes were incubated for 1 h at room
temperature with a secondary antibody conjugated to horseradish
peroxidase. Following 3 washes in Tris-Buffered Saline and Tween-20
(TBST), the immunoreactive bands were visualized using the ECL
detection system (Pierce, Rockford, IL, USA).
Preparation of nuclear extracts and
electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using the NE-PER
nuclear extraction reagent (Pierce). For the gel retardation assay,
a typical double-stranded oligonucleotide for the HIF-1α binding
DNA sequence (5′-TCTGTACGTGA CCACACTCACCTC-3′ with the HIF-1α
binding site sequence underlined) was purchased from Santa Cruz
Biotechnology, Inc. (Cat. no. sc-2625). A non-radioactive method in
which the 3′ end of the probe was labeled with biotin was used in
these experiments (Pierce). The binding reactions contained 5
μg of nuclear extract protein, buffer (10 mM Tris, pH 7.5,
50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.05%
Nonidet P-40, and 2.5% glycerol), 50 ng of poly(dI-dC) and 20 fM of
the biotin-labeled DNA. The reactions were incubated for 20 min at
room temperature in a final volume of 20 μl. The competition
reactions were conducted by the addition of a 25-fold excess of
unlabeled HIF-1α to the reaction mixture. The mixture was then
separated by electrophoresis on a 5% polyacrylamide gel in 0.5X
Tris-borate buffer and transferred onto nylon membranes. The
biotin-labeled DNA was detected using a LightShift Chemiluminescent
EMSA kit (Pierce).
Assay of intracellular ROS levels
Intracellular ROS levels were measured using the
2′,7′-dichlorofluorescein diacetate (DCF-DA) assay as previously
described with some modifications (22). DCF-DA can be deacetylated in
cells, where it reacts quantitatively with intracellular radicals
to convert into its fluorescent product, DCF, which is retained
within the cells. Therefore, DCF-DA is used to evaluate the
generation of ROS under conditions of oxidative stress. The ARPE-19
cells (1×104 cells/well) were seeded in 96-well plates
in a humidified atmosphere containing 5% CO2 at 37°C for
16 h. Following 16 h of incubation, the cells were treated with
various concentrations (10 and 20 μM) of CAPE and further
incubated for 24 h under hypoxic conditions. Thereafter, the cells
were incubated with 10 μM DCF-DA for 30 min under hypoxia
conditions. Subsequently, the cells were fixed with an equal volume
of 4% formaldehyde and were analyzed immediately after that. The
intracellular ROS levels were measured using a fluorescent plate
reader (SpectraMax M2; Molecular Devices, Sunnyvale, CA, USA) at an
excitation wavelength of 492 nm and an emission wavelength of 515
nm.
Statistical analysis
Data values represent the means ± SD. To analyze the
data produced from the experiments with two independent variables,
a one-way analysis of variance (ANOVA) was performed using GraphPad
Prism software (GraphPad Software, La Jolla, CA, USA). A value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
Production of VEGF under hypoxic
conditions
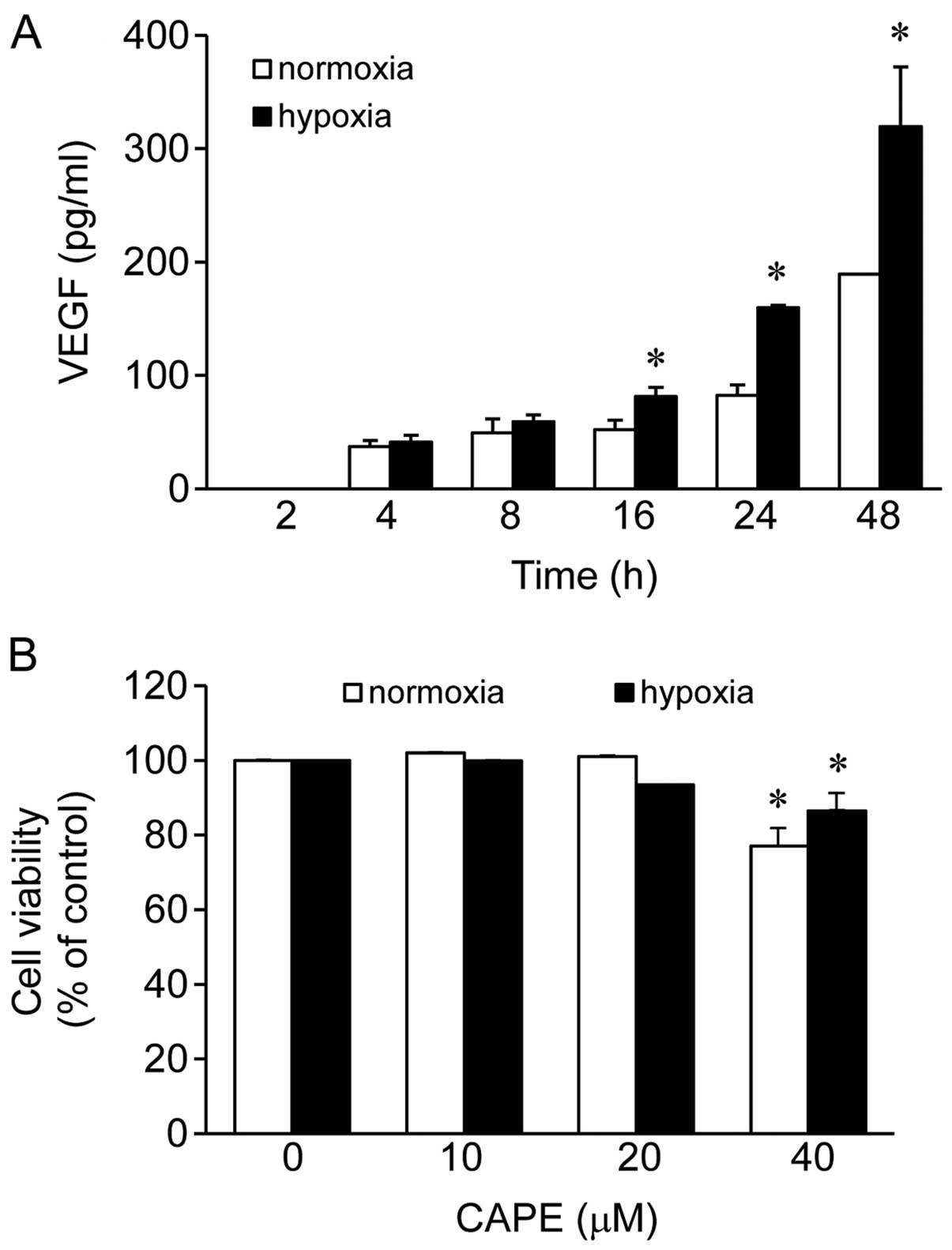
Initially, we wished to evaluate whether we could
use our system to induce the hypoxia signaling pathway by measuring
the production of VEGF. One of the main characteristics of CNV is
the formation of new blood vessels; this may be associated with the
VEGF levels as VEGF is the main inducer of neoangiogenesis
(23). Based on ELISA, the
ARPE-19 cells were exposed to normoxic and hypoxic conditions for
2, 4, 8, 16, 24 and 48 h (Fig.
1A). The production of VEGF was elevated following the exposure
of the cells to both hypoxic and normoxic conditions as the
exposure time increased (Fig.
1A). The production of VEGF was not significantly altered
following the exposure of the cells to hypoxic conditions for 4 and
8 h as indicated by the 1.1- and 1.2-fold-change in its levels,
respectively, compared to exposure to normoxic conditions (Fig. 1A). At the 2-h time point, VEGF was
not produced under either normoxic or hypoxic conditions. However,
the levels of VEGF were significantly elevated following the
exposure of the cells to hypoxic conditions for 16, 24 and 48 h as
indicated by the 1.6- (change from 52.2 to 81.34), 1.9- (change
from 82.45 to 159.6) and 1.7- (change from 189.2 to 319.52) fold
change, respectively, compared to exposure to normoxic conditions
(Fig. 1A). Among these 3 time
points, compared to the levels observed following exposure to
normoxic conditions for 24 h, exposure to hypoxic conditions for 24
h demonstrated the maximum elevation in the VEGF levels (Fig. 1A). Therefore, we selected this
time point for all the subsequent experiments.
Effects of CAPE on the viability of human
ARPE-19 cells
We then examined the viability of human ARPE-19
cells treated with CAPE (10, 20 and 40 μM) using the CCK-8
assay under normoxic or hypoxic conditions. No cytotoxic effect on
the human ARPE-19 cells was observed at doses of up to 20
μM, but cell viability was reduced by 23 and 13.5% with the
40 μM dose of CAPE (Fig.
1B) under normoxic and hypoxic conditions, respectively. Based
on these results, a concentration of 10–20 μM of CAPE was
selected for the subsequent experiments.
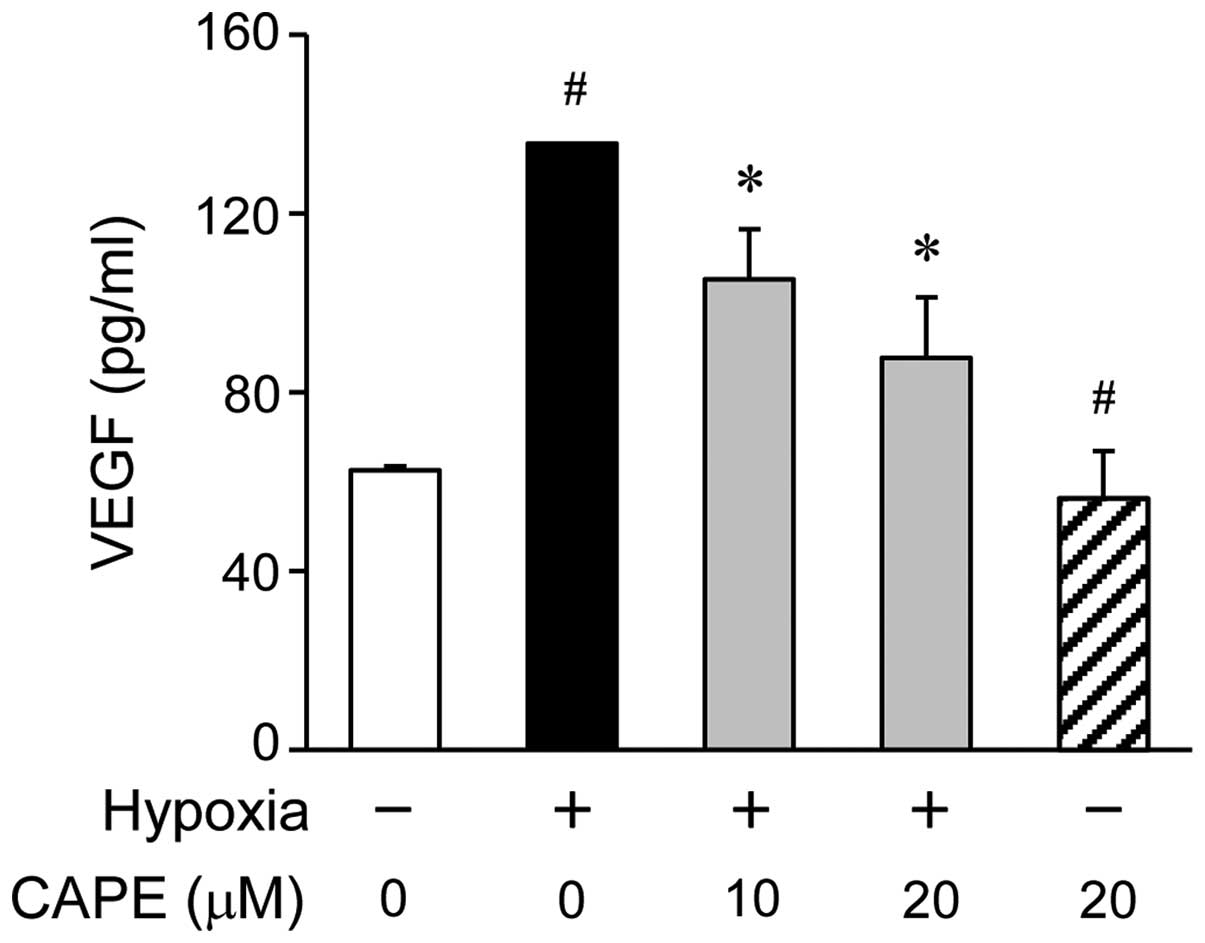
Effect of CAPE on VEGF production under
hypoxic conditions
To examine the inhibitory effects of CAPE on the
hypoxia-induced production of VEGF in the ARPE-19 cells, we
measured the amount of VEGF secretion into the culture mediaum by
ELISA. The ARPE-19 cells were treated with various concentrations
of CAPE (0, 10 or 20 μM) for 2 h prior to exposure to
hypoxic conditions. Pre-treatment with various doses of CAPE led to
a significant decrease in the production of VEGF, as measured in
the cell supernatants 24 h following exposure to hypoxia (Fig. 2). As shown by the ELISA results,
the VEGF levels were significantly increased in the ARPE-19 cells
after 24 h of exposure to hypoxic conditions compared to normoxic
conditions, and this increase was reversed by treatment with CAPE
in a dose-dependent manner (Fig.
2).
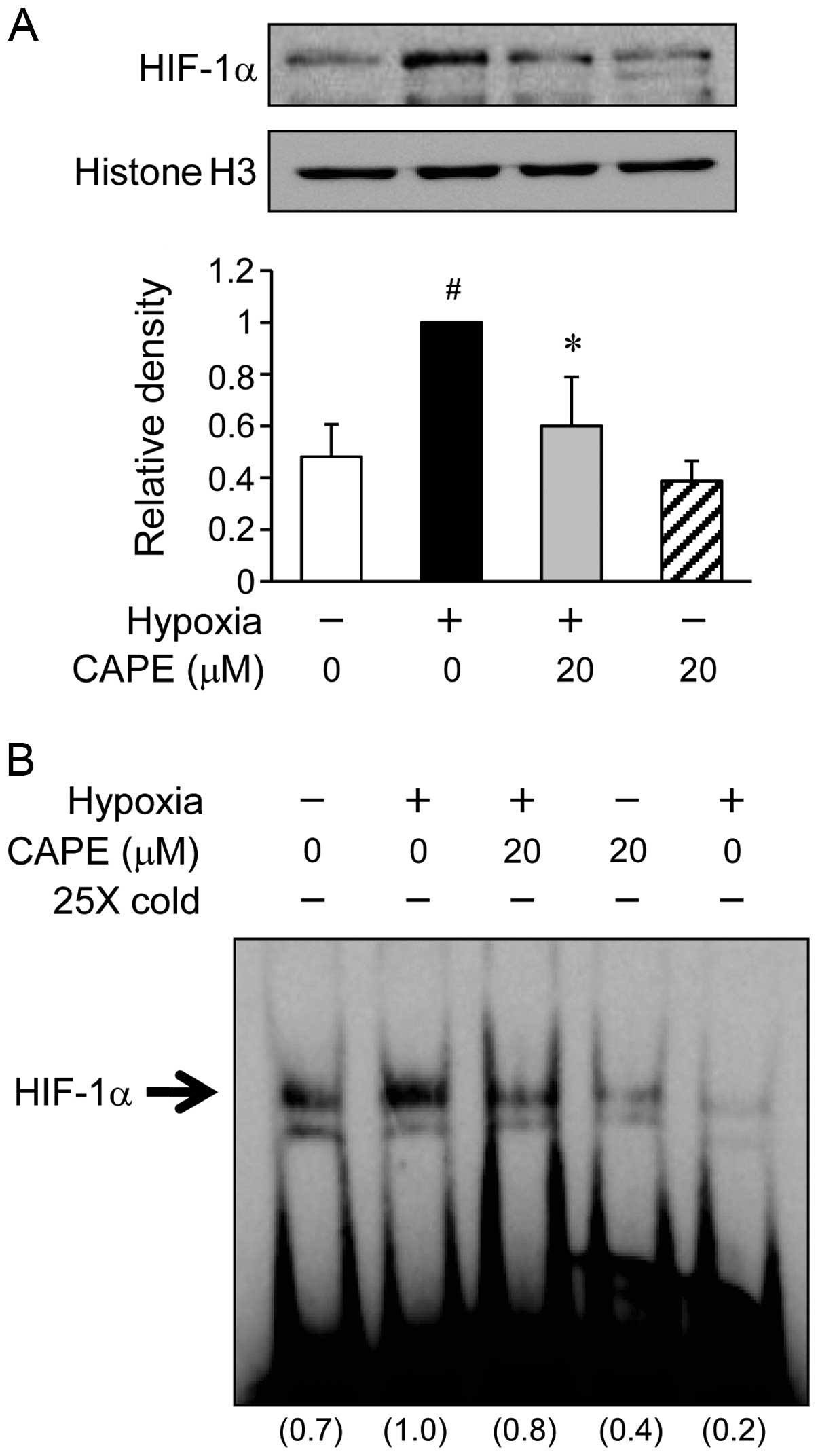
Effects of CAPE on HIF-1α activation
under hypoxic conditions
Initially, in order to assess whether CAPE inhibits
the HIF-1α translocation, the ARPE-19 cells were incubated with 20
μM CAPE under hypoxic conditions for 6 h (Fig. 3A). In the hypoxia-exposed ARPE-19
cells, the translocation of HIF-1α to the nucleus was increased by
almost 2-fold compared to the cells exposed to normoxic conditions.
By contrast, pre-treatment of the cells with 20 μM CAPE
significantly inhibited the trans-location of HIF-1α under hypoxic
conditions (Fig. 3A). Further
experiments were carried out to determine whether the activation of
HIF-1α in the ARPE-19 cells is altered under hypoxic conditions.
When nuclear extract proteins from the cells were probed with
oligonucleotides within the VEGF promoter, subsequent gel shift
analysis revealed a marked increase in HIF-1α transcriptional
activity in the ARPE-19 cells exposed to hypoxic conditions
(Fig. 3B). However, the induction
of specific HIF-1α DNA binding activity by exposure to hypoxic
conditions was inhibited by CAPE (20 μM) (Fig. 3B). These results indicate that
CAPE inhibits HIF-1α activity by preventing the translocation of
this transcription factor into the nucleus during hypoxia.
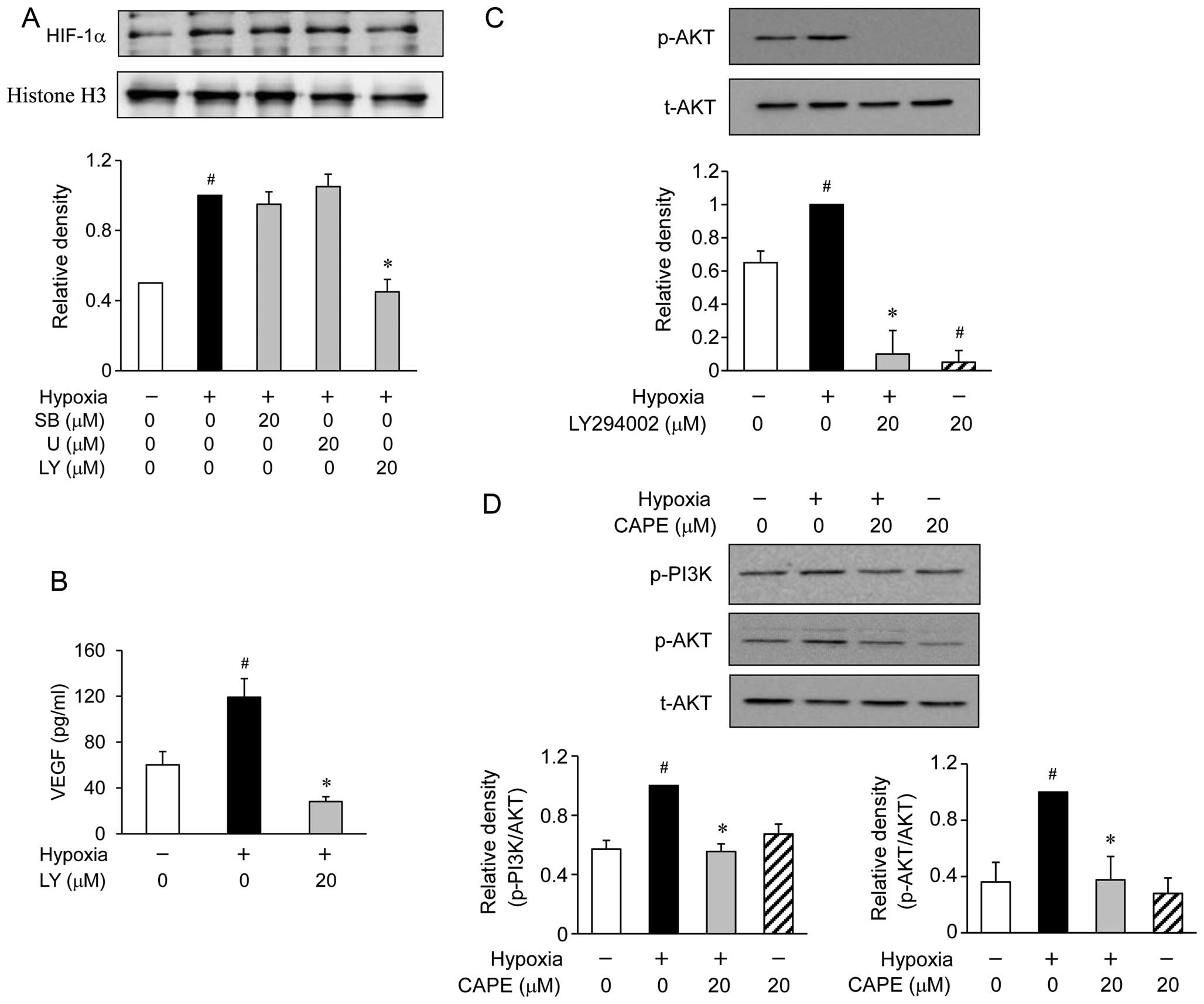
Effects of CAPE on the phosphorylation of
phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated protein
(MAP) kinases in hypoxia-exposed ARPE-19 cells
It is well known that the PI3K/AKT and MAP kinase
signaling molecules are able to regulate HIF-1α activation
(24,25). Therefore, we examined the effects
of CAPE on the hypoxia-induced PI3K/AKT and MAP kinase activation.
Western blot analysis revealed the accumulation of HIF-1α at 6 h
following exposure to hypoxic conditions (Fig. 4A). When the ARPE-19 cells were
treated with various inhibitors of signaling transduction pathways,
such as SB203580 for p38 MAP kinase and U0126 for extracellular
signal-regulated kinase (ERK), these inhibitors were not found to
affect the accumulation of HIF-1α during hypoxia (Fig. 4A). However, the ARPE-19 cells
treated with LY294002 (an inhibitor of PI3K/AKT) showed a reduced
accumulation of HIF-1α induced by hypoxia (Fig. 4A). To determine whether the
hypoxia-induced secretion of VEGF is associated with PI3K/AKT, the
ARPE-19 cells were exposed to hypoxia for 24 h in the presence or
absence of 20 μM LY294002. We found that pre-treatment with
LY294002 substantially reduced the hypoxia-induced production of
VEGF (Fig. 4B). In addition,
pre-treatment with LY294002 also reduced the phosphorylation of AKT
(Fig. 4C). Based on these
results, we investigated the effects of CAPE on the hypoxia-induced
activation of PI3K/AKT. The phosphorylation of AKT showed a marked
increase within 2 h following exposure to hypoxic conditions.
However, pre-treatment with CAPE resulted in a significant
inhibition of the hypoxia-induced AKT phosphorylation (Fig. 4D). These results demonstrated that
the inhibition of VEGF production by CAPE in the hypoxia-exposed
ARPE-19 cells was associated with the downregulation of PI3K/AKT
phosphorylation.
Effect of CAPE on hypoxia-induced
intracellular ROS generation in ARPE-19 cells
In a previous study, ROS stimulated CNV by fostering
a pro-angiogenic environment in the retina and choroid, and
antioxidants were shown to reduce CNV (26). Therefore, in this study, we
examined the inhibitory effects of CAPE on the hypoxia-induced
generation of ROS (Fig. 5).
Initially, in order to determine whether the hypoxia-induced
production of VEGF is associated with ROS, the ARPE-19 cells were
exposed to hypoxic conditions for 24 h in the presence or absence
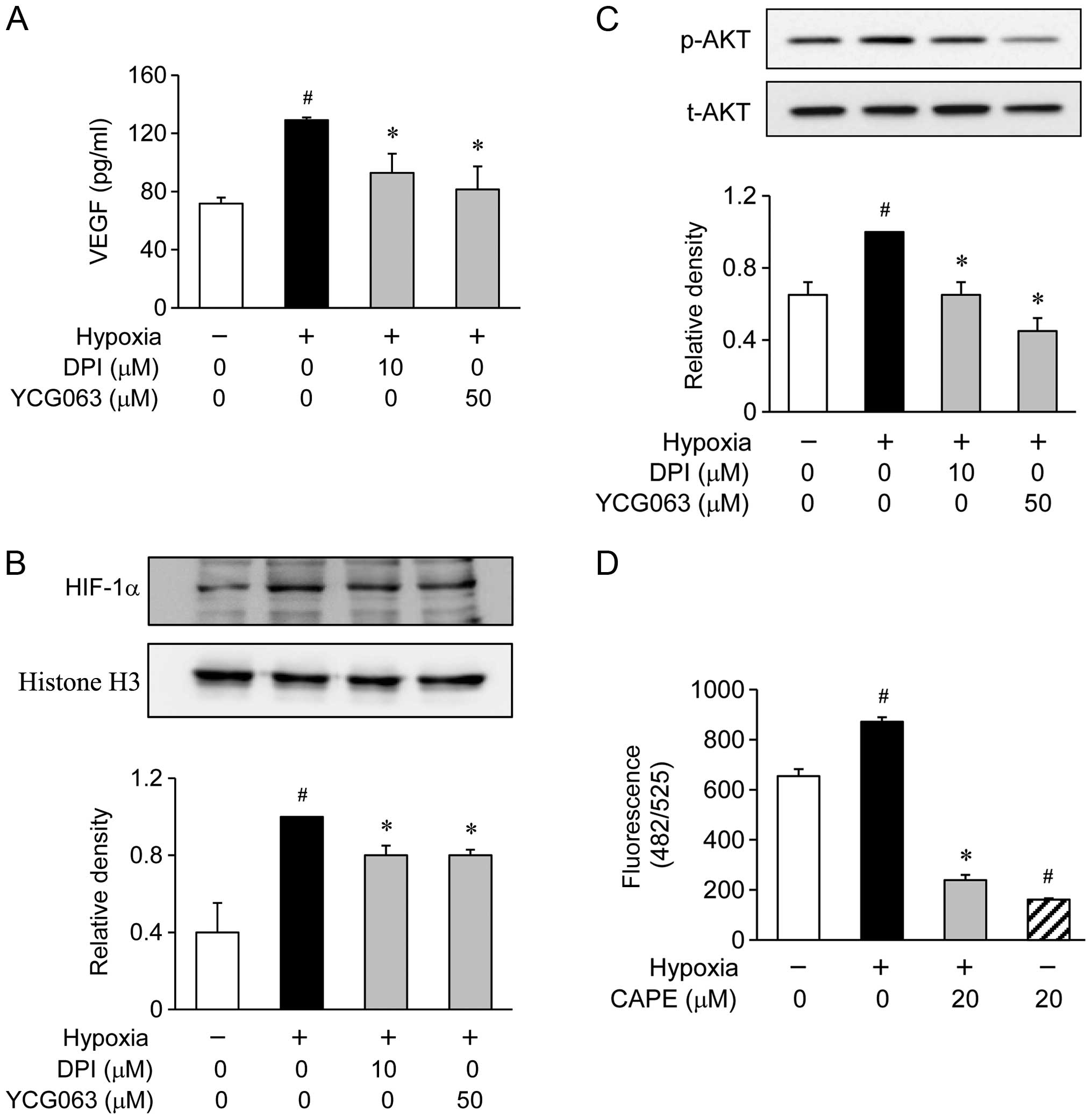
of ROS inhibitors, such as 10 μM DPI for NADPH oxidase or 50
μM YCG063 for mitochondrial ROS (Fig. 5A). These inhibitors significantly
inhibited the hypoxia-induced production of VEGF (Fig. 5A). Furthermore, when the ARPE-19
cells were treated with DPI and YCG063, these inhibitors reduced
the accumulation of HIF-1α in the nuclei during hypoxia (Fig. 5B). Since the production of VEGF in
the hypoxia-exposed ARPE-19 cells was associated with the
downregulation of AKT phosphorylation levels, we investigated
whether AKT phosphorylation is associated with the generation of
ROS. The ARPE-19 cells were exposed to hypoxic conditions for 2 h
in the presence or absence of DPI and YCG063. The phosphorylation
of AKT showed an increase within 2 h following exposure to hypoxic
conditions. However, pre-treatment with DPI and YCG063 resulted in
the significant attenuation of the hypoxia-induced AKT
phosphorylation (Fig. 5C). These
results demonstrate that the generation of ROS by exposure to
hypoxic conditions serves as an upstream signal for the induction
of VEGF production by PI3K/AKT activation. Therefore, we
investigated whether pre-treatment with CAPE inhibits the
generation of ROS. We found that pre-treatment with CAPE markedly
reduced the generation of ROS under hypoxic conditions (Fig. 5D).
Discussion
VEGF is known to be the most important modulator of
both normal and pathological angiogenesis. The inhibition of VEGF
function has been shown to lead to reduced pathological vessel
formation in a murine model of ocular disease (27). RPE cells are one of the major cell
constituents and secretors of VEGF in the retina (28). In the present study, in order to
examine the association between hypoxia and angiogenesis, we
measured the levels of VEGF production in human ARPE-19 cells using
ELISA. The levels of VEGF were increased in a time-dependent
manner. However, the maximum level of VEGF production was observed
at the 24 h time point, and the levels subsequently decreased under
hypoxic conditions compared to normoxic conditions in human ARPE-19
cells (Fig. 1). Treatment with
concentrations of 10 and 20 μM CAPE inhibited VEGF
production under both normoxic and hypoxic conditions without
inducing cytotoxicity.
It has been demonstrated that hypoxia induces the
production of VEGF through the HIF transcriptional complex
(29,30). HIF comprises the α- and
β-subunits, which heterodimerize to form a competent transcription
factor (31). There are three
isoforms of the α-subunit, HIF-1α, HIF-2α and HIF-3α, of which
HIF-1α is the most well characterized (32). HIF-1α is constitutively expressed
in the cytoplasm under normoxic conditions and is continually
degraded. Under hypoxic conditions, HIF-1α accumulates and is
translocated to the nucleus, where it binds to the hypoxia response
element of the VEGF promoter and induces transcriptional activity
(31). HIF-1α plays a pivotal
role in angiogenesis. This suggests that hypoxia mediates CNV
through HIF-1α-regulated VEGF production. Therefore, in this study,
we investigated whether CAPE attenuates the translocation of HIF-1α
to the nucleus and its binding to hypoxia response element of the
VEGF promoter. Our results demonstrated that CAPE reduces HIF-1α
accumulation (Fig. 3) and
HIF-1α-dependent transcriptional activity (Fig. 3B). In previous studies, CAPE was
shown to be associated with HIF stabilization under normoxic
conditions (33,34). In this study, however, CAPE
reduced HIF-1α accumulation. The reason for this discrepancy may
lie in the different experimental conditions used (e.g., cell type,
stimulus, species, etc.). In this regard, the inhibition of VEGF
production by pre-treatment with CAPE may prove to be an effective
therapeutic approach to relieve the progression of ocular
angiogenesis through the regulation of HIF-1α.
In a previous study, the MAP kinase and PI3K/AKT
signaling pathways were shown to be involved in regulating the
expression of HIF-1α and VEGF in laser-induced CNV in rats
(35). Hypoxia activates the MAP
kinases (36). The ERK and
PI3K/AKT pathways have been known to be of critical importance for
neovascularization, ischemia and angiogenesis (37,38). It is possible that
anti-neovascularization mechanisms are associated with the PI3K/AKT
or MAP kinase pathways. To further elucidate the regulatory
mechanisms of CAPE in these processes, we investigated whether the
PI3K/AKT and MAP kinase signaling pathways are involved in
regulating HIF-1α/VEGF. Of note, p38 MAP kinase- and ERK-specific
inhibitors did not suppress the expression of HIF-1α. However, the
PI3K-specific inhibitor completely inhibited the expression of
HIF-1α. In addition, the PI3K-specific inhibitor significantly
suppressed the expression of VEGF. Therefore, this finding strongly
suggests that the PI3K/AKT signaling pathway, but not MAP kinases,
plays a critical role in the expression of HIF-1α/VEGF. Based on
these results, we demonstrated a marked inhibition of the
hypoxia-induced phosphorylation of AKT by CAPE in the ARPE-19
cells. Our findings suggest that the decrease in the
hypoxia-induced production of VEGF by CAPE is due to the inhibition
of HIF-1α through the inactivation of the PI3K/AKT signaling
pathway.
It has been reported that oxidative stress, which
refers to the cellular damage caused by reactive oxygen
intermediates (ROI), plays a causative role in both the initiation
and progression of CNV and a contributing factor in AMD (18,39). ROS also play a role in HIF-1α
induction (40). ROS are commonly
produced during inflammatory processes, are involved in signal
transduction and gene activation, and contribute to host cell and
organ damage (41). ROS,
including superoxide anion, hydroxyl radical and hydrogen peroxide,
may play multiple roles in a number of diseases, such as
atherosclerosis, angiogenesis, cancer, diabetes mellitus,
neurological degeneration and asthma (42). N-acetylcysteine (NAC), a potent
antioxidant, has been shown to inhibit the development of CNV in
mice (43). It is well known that
CAPE possesses significant antioxidant properties (44,45). However, the effects of CAPE on
hypoxia-induced ocular neovascularization and its molecular
mechanisms have not yet been elucidated. In the present study, we
demonstrated that ROS inhibitors significantly inhibited the
activation of PI3K/AKT, as well as the expression of HIF-1α and
VEGF. Furthermore, we demonstrated that CAPE has intracellular ROS
scavenging activity in ARPE-19 cells. Thus, the potential
inhibition of ROS generation by CAPE is consistent with the
inhibition of PI3K/AKT activation, HIF-1α and VEGF expression and,
thus, reduced ocular neovascularization.
In conclusion, the results obtained in the present
study indicate that treatment of the ARPE-19 cells with CAPE
decreases VEGF production following exposure to hypoxic conditions.
CAPE significantly inhibited the accumulation of HIF-1α under
hypoxic conditions. The inhibitory effects of CAPE are mediated by
the downregulation of PI3K/AKT activation and the inhibition of ROS
signaling in ARPE-19 cells. These findings indicate that CAPE has
the potential to target ROS, PI3K/AKT and HIF-1α and inhibit VEGF
production in ARPE-19 cells under hypoxic conditions. Such
inhibitory effects may contribute to the treatment of various
intraocular angiogenic diseases, such as AMD complicated by CNV,
and may thus provide novel therapeutic efficacy.
Acknowledgments
This study was supported by a grant of the Korea
Healthcare Technology R&D Project, Ministry of Health and
Welfare and Family Affairs, Republic of Korea (HI12C0005).
Abbreviations:
|
AMD
|
age-related macular degeneration
|
|
CNV
|
choroidal neovascularization
|
|
VEGF
|
vascular endothelial growth factor
|
|
CAPE
|
caffeic acid phenethyl ester
|
|
RPE
|
retinal pigment epithelium
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Oh JH, Oh J, Togloom A, Kim SW and Huh K:
Effects of Ginkgo biloba extract on cultured human retinal pigment
epithelial cells under chemical hypoxia. Curr Eye Res.
38:1072–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao J, Zhao L, Li Y, et al: A subretinal
matrigel rat choroidal neovascularization (CNV) model and
inhibition of CNV and associated inflammation and fibrosis by VEGF
trap. Invest Ophthalmol Vis Sci. 51:6009–6017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campochiaro PA: Ocular neovascularization.
J Mol Med Berl. 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
da Cruz L, Chen FK, Ahmado A, Greenwood J
and Coffey P: RPE transplantation and its role in retinal disease.
Prog Retin Eye Res. 26:598–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin J, Zhou KK, Park K, Hu Y, Xu X, Zheng
Z, Tyagi P, Kompella UB and Ma JX: Anti-inflammatory and
antiangiogenic effects of nanoparticle-mediated delivery of a
natural angiogenic inhibitor. Invest Ophthalmol Vis Sci.
52:6230–6237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vadlapatla RK, Vadlapudi AD and Mitra AK:
Hypoxia-inducible factor-1 (HIF-1): A potential target for
intervention in ocular neovascular diseases. Curr Drug Targets.
14:919–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caprara C and Grimm C: From oxygen to
erythropoietin: Relevance of hypoxia for retinal development,
health and disease. Prog Retin Eye Res. 31:89–119. 2012. View Article : Google Scholar
|
|
8
|
Lutty G, Grunwald J, Majji AB, Uyama M and
Yoneya S: Changes in choriocapillaris and retinal pigment
epithelium in age-related macular degeneration. Mol Vis. 5:35–38.
1999.PubMed/NCBI
|
|
9
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vadlapatla RK, Vadlapudi AD, Pal D,
Mukherji M and Mitra AK: Ritonavir inhibits HIF-1α-mediated VEGF
expression in retinal pigment epithelial cells in vitro. Eye
(Lond). 28:93–101. 2014. View Article : Google Scholar
|
|
11
|
Moreira EF, Larrayoz IM, Lee JW and
Rodríguez IR: 7-Ketocholesterol is present in lipid deposits in the
primate retina: potential implication in the induction of VEGF and
CNV formation. Invest Ophthalmol Vis Sci. 50:523–532. 2009.
View Article : Google Scholar
|
|
12
|
Spilsbury K, Garrett KL, Shen WY,
Constable IJ and Rakoczy PE: Overexpression of vascular endothelial
growth factor (VEGF) in the retinal pigment epithelium leads to the
development of choroidal neovascularization. Am J Pathol.
157:135–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak N, Okamoto N, Wood JM and Campochiaro
PA: VEGF is major stimulator in model of choroidal
neovascularization. Invest Ophthalmol Vis Sci. 41:3158–3164.
2000.PubMed/NCBI
|
|
14
|
Krzystolik MG, Afshari MA, Adamis AP,
Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O’Neill
CA and Miller JW: Prevention of experimental choroidal
neovascularization with intravitreal anti-vascular endothelial
growth factor antibody fragment. Arch Ophthalmol. 120:338–346.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SG, Lee DY, Seo SK, et al: Evaluation
of anti-allergic properties of caffeic acid phenethyl ester in a
murine model of systemic anaphylaxis. Toxicol Appl Pharmacol.
226:22–29. 2008. View Article : Google Scholar
|
|
16
|
El-Refaei MF and El-Naa MM: Inhibitory
effect of caffeic acid phenethyl ester on mice bearing tumor
involving angiostatic and apoptotic activities. Chem Biol Interact.
186:152–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin UH, Song KH, Motomura M, Suzuki I, Gu
YH, Kang YJ, Moon TC and Kim CH: Caffeic acid phenethyl ester
induces mitochondria-mediated apoptosis in human myeloid leukemia
U937 cells. Mol Cell Biochem. 310:43–48. 2008. View Article : Google Scholar
|
|
18
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Izumi-Nagai K, Nagai N, Ohgami K, Satofuka
S, Ozawa Y, Tsubota K, Ohno S, Oike Y and Ishida S: Inhibition of
choroidal neovascularization with an anti-inflammatory carotenoid
astaxanthin. Invest Ophthalmol Vis Sci. 49:1679–1685. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Apte RS, Richter J, Herndon J and Ferguson
TA: Macrophages inhibit neovascularization in a murine model of
age-related macular degeneration. PLoS Med. 3:e3102006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu BC, Lee DS, Bae SM, et al: The effect
of cilostazol on the expression of matrix metalloproteinase-1 and
type I procollagen in ultraviolet-irradiated human dermal
fibroblasts. Life Sci. 92:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H and Joseph JA: Quantifying cellular
oxidative stress by dichlorofluorescein assay using microplate
reader. Free Radic Biol Med. 27:612–616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uehara H, Luo L, Simonis J, Singh N,
Taylor EW and Ambati BK: Anti-SPARC oligopeptide inhibits
laser-induced CNV in mice. Vision Res. 50:674–679. 2010. View Article : Google Scholar :
|
|
24
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minet E, Michel G, Mottet D, Raes M and
Michiels C: Transduction pathways involved in hypoxia-inducible
factor-1 phosphorylation and activation. Free Radic Biol Med.
31:847–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HS, Jun JH, Jung EH, Koo BA and Kim
YS: Epigalloccatechin-3-gallate inhibits ocular neovascularization
and vascular permeability in human retinal pigment epithelial and
human retinal microvascular endothelial cells via suppression of
MMP-9 and VEGF activation. Molecules. 19:12150–12172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adamis AP and Shima DT: The role of
vascular endothelial growth factor in ocular health and disease.
Retina. 25:111–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forooghian F, Razavi R and Timms L:
Hypoxia-inducible factor expression in human RPE cells. Br J
Ophthalmol. 91:1406–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ratcliffe PJ, O’Rourke JF, Maxwell PH and
Pugh CW: Oxygen sensing, hypoxia-inducible factor-1 and the
regulation of mammalian gene expression. J Exp Biol. 201:1153–1162.
1998.PubMed/NCBI
|
|
30
|
Ratcliffe PJ, Pugh CW and Maxwell PH:
Targeting tumors through the HIF system. Nat Med. 6:1315–1316.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000.
|
|
32
|
Sparkenbaugh EM, Ganey PE and Roth RA:
Hypoxia sensitization of hepatocytes to neutrophil
elastase-mediated cell death depends on MAPKs and HIF-1α. Am J
Physiol Gastrointest Liver Physiol. 302:G748–G757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Zhang B, Zhang J, Wang S, Yao H, He
L, Chen L, Yue W, Li Y and Pei X: CAPE promotes the expansion of
human umbilical cord blood-derived hematopoietic stem and
progenitor cells in vitro. Sci China Life Sci. 57:188–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roos TU, Heiss EH, Schwaiberger AV,
Schachner D, Sroka IM, Oberan T, Vollmar AM and Dirsch VM: Caffeic
acid phenethyl ester inhibits PDGF-induced proliferation of
vascular smooth muscle cells via activation of p38 MAPK, HIF-1α,
and heme oxygenase-1. J Nat Prod. 74:352–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar
|
|
36
|
Seta KA, Spicer Z, Yuan Y, Lu G and
Millhorn DE: Responding to hypoxia: Lessons from a model cell line.
Sci STKE. 2002:re112002.PubMed/NCBI
|
|
37
|
Bullard LE, Qi X and Penn JS: Role for
extracellular signal-responsive kinase-1 and -2 in retinal
angiogenesis. Invest Ophthalmol Vis Sci. 44:1722–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ackah E, Yu J, Zoellner S, et al:
Akt1/protein kinase Balpha is critical for ischemic and
VEGF-mediated angiogenesis. J Clin Invest. 115:2119–2127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Cai Y, Wang YS, Shi YY, Hou W, Xu
CS, Wang HY, Ye Z, Yao LB and Zhang J: Hyperglycaemia exacerbates
choroidal neovascularisation in mice via the oxidative
stress-induced activation of STAT3 signalling in RPE cells. PLoS
One. 7:e476002012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandel NS, McClintock DS, Feliciano CE,
Wood TM, Melendez JA, Rodriguez AM and Schumacker PT: Reactive
oxygen species generated at mitochondrial complex III stabilize
hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2
sensing. J Biol Chem. 275:25130–25138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM,
Byun HG, Choi YH, Park SG and Choi IW: Inhibitory effects and
molecular mechanism of dieckol isolated from marine brown alga on
COX-2 and iNOS in microglial cells. J Agric Food Chem.
57:4439–4446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang D, Elner SG, Bian ZM, Till GO, Petty
HR and Elner VM: Pro-inflammatory cytokines increase reactive
oxygen species through mitochondria and NADPH oxidase in cultured
RPE cells. Exp Eye Res. 85:462–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hara R, Inomata Y, Kawaji T, Sagara N,
Inatani M, Fukushima M and Tanihara H: Suppression of choroidal
neovascularization by N-acetyl-cysteine in mice. Curr Eye Res.
35:1012–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bai H, Liu R, Chen HL, Zhang W, Wang X,
Zhang XD, Li WL and Hai CX: Enhanced antioxidant effect of caffeic
acid phenethyl ester and Trolox in combination against radiation
induced-oxidative stress. Chem Biol Interact. 207:7–15. 2014.
View Article : Google Scholar
|
|
45
|
Ozguner F, Altinbas A, Ozaydin M, Dogan A,
Vural H, Kisioglu AN, Cesur G and Yildirim NG: Mobile phone-induced
myocardial oxidative stress: Protection by a novel antioxidant
agent caffeic acid phenethyl ester. Toxicol Ind Health. 21:223–230.
2005. View Article : Google Scholar : PubMed/NCBI
|