Introduction
Germ cells are the biological route for the
transmission of genes from one generation to the next. With their
unique characteristics, germ cells constitute a cell population
which is very different to somatic cells, and display a haploid
chromosomal number following a delicate process of meiosis
(1). However, important questions
regarding how germ cells are specified remain unanswered in human
and mammalian developmental biology.
The generation of germ cells from somatic cells
in vitro may provide a valuable model for identifying
factors involved in germ cell formation and differentiation.
Accordingly, numerous attempts have been made over the past decade
to determine whether murine or human embryonic stem (ES) cells are
able to differentiate into primordial germ cells (PGCs) or
oocyte-like cells (OLCs) in vitro (2–7).
Moreover, it has been reported that germ cell-like cells can be
derived in vitro from multipotent stem cells derived from
newborn mice or porcine fetal skins (8–10),
mesenchymal stem cells (MSCs) derived from mouse bone marrow (BM)
(11), or human adult ovaries
(12).
Additionally, certain studies have reported that
human or murine ES cells can spontaneously differentiate into OLCs
in adherent cultures or through embryoid body formations (5–7).
Other studies have reported that human or mouse ES cells or
multipotent stem cells other than ES cells can form germ-like cells
and mature gametes by using various differentiation strategies,
such as the addition of exogenous factors (6,13)
or follicular fluid (8) to the
culture medium, or by co-culture with ovarian granulose cells
(3).
Previously, we isolated and characterized human
first-trimester umbilical cord (hFTUC)-derived stem cells and found
that the cells exhibited characteristics of pluripotent stem cells,
including the expression of pluripotent stem cell markers, such as
octamer-binding transcription factor 4 (OCT4), Nanog, (sex
determining region Y)-box 2 (SRY, also known as SOX2),
stage-specific embryonic antigen (SSEA)3, SSEA4, Tra-1-60 and
Tra-1-81, as well as formations of embryoid bodies (14). Furthermore, we found that
hFTUC-derived stem cells exhibited a significantly greater
proliferative potential, and were more efficient in their in
vitro differentiation toward selective mesenchymal cell types,
including chondrogenic and adipogenic lineages, as well as
neuronal- and hepatocyte-like lineages (15). Thus, we hypothesized that
hFTUC-derived stem cells may have an intrinsic ability to form germ
cells and differentiate into OLCs in vitro. In the present
study, we examined this hypothesis by first isolating and expanding
hFTUC-derived stems cells, and then inducing the differentiation of
cells using differentiation medium. Subsequently, we analyzed the
cells for their morphological appearance, the expression of markers
indicative of germ cell formation and oocyte development, as well
as estradiol production.
Materials and methods
Isolation and culture of hFTUC-derived
stem cells
The isolation and expansion of the hFTUC-derived
stem cells were based on a method employed in a recently published
study of ours (15). Briefly,
first-trimester umbilical cords were collected following
therapeutic pregnancy interruptions, which were carried out with
the written informed consent of the patients and approval from the
Second Medical College/Teaching Hospital Institutional Review
Board, Chengdu University of Traditional Chinese Medicine, Chengdu,
China. To isolate the stems cells, the cords were rinsed several
times with sterile saline. In order to completely avoid of any
contamination from maternal or fetal sources, the cells that were
not attached to/or within the umbilical cord tissue at the
beginning of the process were discarded during each rinsing.
Subsequently, the cords were rinsed several times with sterile
saline and cut into sections followed by an immersion in 1%
collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) solution for
1 h at 37°C. The denuded tissues were discarded following the
removal of the cells during digestion. The cells were then pelleted
by a low-speed centrifugation (250 × g for 5 min) and suspended in
fresh medium. The cells were then plated into a 25 cm culture flask
with an expansion medium (Minimum Essential Medium Eagle, Alpha
Modification; α-minimal essential medium (α-MEM) (Sigma-Aldrich),
which was supplemented with 10% fetal bovine serum (FBS), 2 mmol/l
L-glutamine, 50 IU/ml penicillin and 50 mg/ml streptomycin (both
from Gibco-BRL, Gaithersburg, MD, USA) in 5% CO2 at
37°C. The culture medium was replaced every 3 days. Subconfluent
(70–80%) cells were detached with 0.05% trypsin-0.01% EDTA
(Gibco-BRL) and plated at a density of 2.4×103
cells/cm2.
Karyotype analysis
Karyotype analysis of the hFTUC-derived stem cells
was performed by using an AneuVysion Multicolor DNA Probe kit
(Vysis CEP 18/X/Y-α-satellite/LSI 13/21) with DNA probes for
chromosomes X and Y (Abbott Molecular Diagnostics, Des Plaines, IL,
USA) in accordance with the manufacturer’s instructions.
Briefly, the cultured undifferentiated stem cells in
culture-ware (BD Biosciences, San Jose, CA, USA) were fixed with
3:1 methanol:acetic acid and exposed to denaturing solution (70%
formamide in 2X SSC pH 8.0) prior to hybridization. Subsequently,
the X and Y probes were applied to the slides, which were
immediately covered with coverslips and sealed. The slides were
subsequently incubated in hybridization chamber for 6 h at 37°C.
Following hybridization, the cover-slips were removed and the
slides were then washed with a solution of 2X SSC/0.1% NP-40
followed by a solution of 2X SSC/0.1% NP-40. The slides were
allowed to air-dry in the dark and DAPI II counterstain was
performed before covering the slides with a glass coverslip.
Finally, signal enumeration on the slides was visualized under a
fluorescence microscope (Leica M205 FA) (Leica Microsystems,
Wetzlar, Germany). In the present study, 6 hFTUC-derived stem cell
lines were isolated, with 3 cell lines with the XX karyotype and 3
cell lines with the XY karyotype.
In our preliminary experiments, we did not find any
significant difference in the efficiency of the induction of the
stem cells into germ cell-like cells between the female and male
umbilical cord cells. This result is similar to a recent report
regarding the effect of gender on the differentiation of MSCs into
germ cells (16). Thus, in order
to avoid the effects of possible contamination by the cells of the
mother, the XY karyotype cell line was used in the following
experiments.
Induction of differentiation
In order to induce differentiation into female germ
cells, the hFTUC-derived cells at the 3rd passage were trypsinized,
and 1–5×104 cells/well were plated into culturewares or
6-well culture plates (BD Biosciences) and cultured in α-MEM
containing 10% FBS, with or without 25% human follicular fluid
(hFF), 150 mIU follicle-stimulating hormone (FSH), 150 mIU
luteinizing hormone (LH) (Ferring Pharmaceuticals Inc., Parsippany,
NJ, USA) and 300 pg/ml of estradiol (Sigma-Aldrich) at 37°C in an
atmosphere of 5% CO2 air.
The culture medium was replaced every 3 days. The
morphology of the cells was examined and images were captured using
an inverted microscope (Leika DMI 3000B; Leica Microsystems) prior
to the medium changes. At 7 days after differentiation, a
subpopulation of cells showing a round shape became visible. The
cells were collected and analyzed either by RT-qPCR, western blot
analysis or immunofluorescence staining.
At 14 days after differentiation, cell aggregates
resembling follicle-like structures were appeared. The cell
aggregates were retained in this culture medium for a further 14
days by replacing the medium every 2–3 days. At 21–24 days after
differentiation, OLCs varying in size from 50 to 120 μm in
diameter were observed. The OLCs were collected and analyzed either
by RT-qPCR analysis, western blot analysis or immunofluorescence
staining.
The culture medium was collected at each medium
replacement and stored at −80°C until analysis to determine the
production of estradiol, vascular endothelial growth factor (VEGF)
and leukemia inhibitory factor (LIF).
Immunofluorescence staining
The cells that were treated for 7 and 24 days with
or without the differentiation medium were washed with
phosphate-buffered saline (PBS) and fixed with 4% ice-cold
paraformaldehyde for 10 min. Following 3 washes with PBS, the fixed
cells were incubated for 1 h at room temperature with one of the
primary antibodies in an antibody dilution solution (Dako Denmark
A/S, Glostrup, Denmark). The source and dilution information of the
primary antibodies are presented in Table I.
 | Table ISource and dilution of primary
antibodies used in this study. |
Table I
Source and dilution of primary
antibodies used in this study.
| Antibodies | Clone | Catalog no. | Manufacturer | Dilution |
|---|
| OCT4 | Rabbit
polyclonal | Ab18976 | Abcam, Cambridge,
UK | 1:200 |
| VASA | Rabbit
polyclonal | SC-67185 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | 1:100 |
| STELLA | Mouse antibody | Ab74531 | Abcam, Cambridge,
UK | 1:200 |
| IFITM3 | Rabbit
polyclonal | SC-66827 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | 1:100 |
| DAZL | Rabbit
polyclonal | SC-36604 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | 1:200 |
| SCP3 | Rabbit
polyclonal | SC-33195 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | 1:200 |
| GDF9 | Rabbit
polyclonal | Ab38544 | Abcam, Cambridge,
UK | 1:100 |
| ZP1 | Rabbit
polyclonal | Ab171954 | Abcam, Cambridge,
UK | 1:200 |
| ZP3 | Rabbit
polyclonal | Ab48895 | Abcam, Cambridge,
UK | 1:200 |
Following incubation with the primary antibodies and
3 PBS washes, the cells were incubated with FITC-conjugated goat
anti-rabbit or rabbit anti-mouse antibodies (Sc2012; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) diluted at 1:1,000 in PBS-3%
(w/v) BSA for 1 h at room temperature. After washing another 3
times with PBS, the nuclei were stained with DAPI, and visualized
under a fluorescence microscope (Leica M205 FA; Leica
Microsystems).
Mouse oocytes, the maturation of which was promoted
by treatment with pregnant mare serum gonadotrophin (PMSG), were
used as the positive controls. Negative controls were prepared by
the omission of the primary antibodies.
RT-qPCR
Total cellular RNA was isolated using
TRIzol® reagent (Macherey-Nagel, Düren, Germany)
according to the manufacturer’s instructions. Subsequently, 0.5
μg of the total RNA was reverse transcribed into cDNA using
a Superscript II Reverse Transcriptase kit (Fermentas Life
Sciences, Schwerte, Germany) in accordance with the manufacturer’s
instructions.
Quantitative (real-time) PCR (qPCR) was performed
using the SYBR-Green mix kit on an ABI Prism 7900 sequence detector
(both from Applied Biosystems, Foster City, CA, USA). A total of
2.0 μl cDNA was added to 12.5 μl SYBR-Green mix with
0.3 μM each of the forward and reverse primers. Water was
added to produce a final volume of 25 μl. The reaction was
carried out for 40 cycles of 95°C for 15 sec, 56–62°C for 30 sec
(primer-dependent), 72°C for 30 sec, and a final cycle of 75°C for
30 sec. The primer sequences for OCT4, SSEA1, STELLA, VASA, B
lymphocyte-induced maturation protein-1 (BLIMP1), PR domain
containing 14 (PRDM14), transcription factor AP-2 gamma (TFAP2C),
synaptonemal complex protein 3 (SCP3), growth/differentiation
factor-9 (GDF9), ZP1, ZP2, ZP3 and β-actin are listed in Table II.
 | Table IIList of primers used in qPCR. |
Table II
List of primers used in qPCR.
| Genes | Primers | Amplified size
(bp) |
|---|
| OCT4 |
CCCACACTGCAGCAGATCAGTTGTGCATAGTCGCTGCTTGA | 110 |
| BLIMP1 |
TGGAGAACGGCCTTTCAAATCCTGGCATTCATGTGGCTTT | 110 |
| PRDM14 |
GAGTCAGGTTTGGGCCCTTTGTGGCTCAAATGACCATCTTCA | 110 |
| TFAP2C |
TATGTCTGTGAAGCCGAATTTCCGCCGCCAATAGCATGTTCTT | 110 |
| SSEA1 |
CACCAACTGAGCCAACATGTGGCCAGAGCTTCTCGGTGATATAA | 160 |
| STELLA |
GCGGAGTTCGTACGCATGACCATCCATTAGACACGCAGAAA | 110 |
| VASA |
TTTCCAAGAGAGGCGGCTATCAGTGCGCTGCATACATTCGT | 155 |
| SCP3 |
TGCAGTCATTGAGAAACGTAGGAGCAAGAAGAGCCTTGTTAATGTCA | 110 |
| GDF9 |
TCTCCAGTTCACACCATGGTACAATCGGGCTCAATGGTCAAAA | 110 |
| ZP1 |
CCGCTTCAAGGTGGTGGATCCTCTGTAATCGGCCGAGAA | 110 |
| ZP2 |
CAGAGGTGTCGGCTCATCTGAGCAGTCTTGTGCCCTTTGGT | 110 |
| ZP3 |
GACCCGGGCCAGATACACTCATCTGGGTCCTGCTCAGCTA | 110 |
| β-actin |
TGGCATTGCCGACAGGATGGACAGCGAGGCCAGGAT | 110 |
For each PCR product, the melting curve was
determined using the comparative threshold cycle number
(2−ΔΔCt) method, with the results being presented as the
fold change in the expression of the genes in the cells induced to
differentiate relative to the undifferentiated cells (controls), as
previously described (17). All
the experiments were performed in triplicate and were repeated at
least 3 times on different occasions.
Western blot analysis
To examine protein expression, the cells were
collected using 200 μl cell lysis buffer (50 mM Tris-HCl, pH
7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 1% Triton X-100 and 1%
SDS). An equal protein concentration of cell lysates/lane (10
μg/lane) was separated using 10% SDS-PAGE and electroblotted
onto polyvinylidene fluoride (PVFD) membranes (Invitrogen,
Carlsbad, CA, USA). The membranes were blocked in PBS containing
0.05% Tween-20 (PBS-T) and 5% skim milk for 2 h at room
temperature, and then incubated with polyclonal rabbit anti-human
OCT4, STELLA, VASA, GDF9, ZP1 (Table
I) and anti-β-actin antibodies (Abcam, Cambridge, UK) overnight
at 4°C. The membranes were washed 3 times (10 min/wash) with PBS-T,
and incubated with a horseradish peroxidase-conjugated goat
anti-rabbit antibody (1:2,000; Sigma-Aldrich) for 1 h at room
temperature. The membranes were washed 3 times again and
antigen-antibody complexes were visualized using
tetramethylbenzidine (TMB) (Sigma-Aldrich).
ELISA for estradiol, VEGF and LIF
expression in the culture supernatant
The concentrations of estradiol, VEGF and LIF in the
culture supernatant were determined using a specific estradiol
ELISA kit (Catalog no. 1920) (Alpha Diagnostic International, San
Antonio, TX, USA), a VEGF ELISA kit (Catalog no. DVE00) and a LIF
ELISA kit (Catalog no. DLF00) (both from R&D Systems,
Minneapolis, MN, USA). The assays were performed according to the
respective manufacturers’ instructions. The intra- and inter-assay
variations were 3.5–5.0 and 10.2–13.1%, respectively. ELISAs were
performed in a blinded manner.
Statistical analysis
Statistical analysis was performed using the SPSS
software package (SPSS Inc., Chicago, IL, USA). Differences in the
mRNA expression of the markers and the concentrations of estradiol,
VEGF and LIF in the culture supernatant between the cells induced
to differentiate and the undifferentiated cells were analyzed using
the one-way analysis of variance. If significance was found in the
analysis, the data underwent post-hoc comparisons. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differentiated cells with morphological
resemblance to PGCs and OLCs
As shown in our previous study, the hFTUC-derived
stem cells can be propagated in long-term culture for at least 15
passages without the induction of differentiation (15). Similarly, the cells in the present
study grew adherent, exhibited spindle-like shapes and resembled
the morphological characteristics of undifferentiated
fibroblast-like cells in α-MEM with 10% FBS during the 24 days of
experiments (Fig. 1A–E).
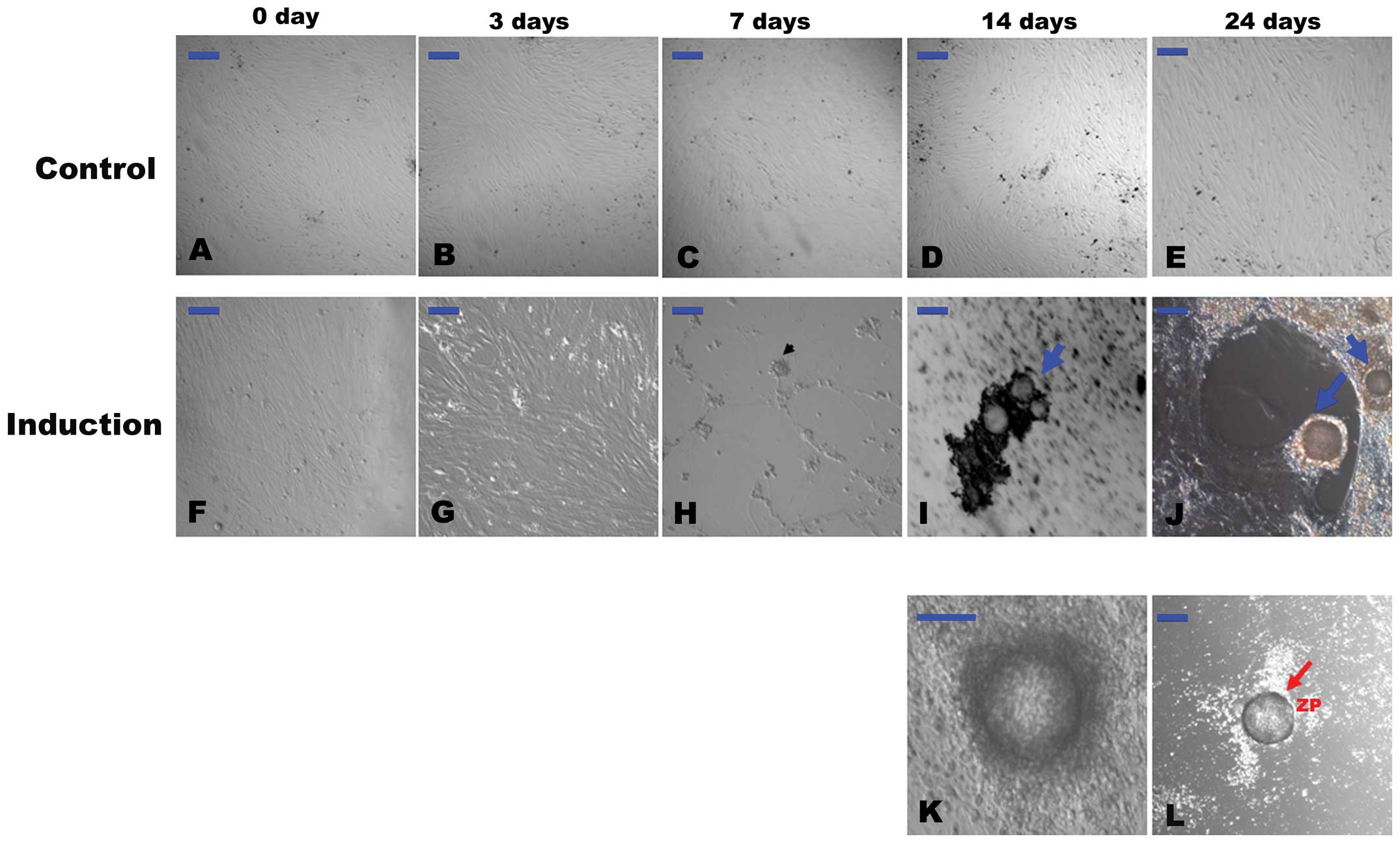 | Figure 1(A–E) Representative images of
untreated cells (controls) cultured in α-minimal essential medium
(α-MEM) containing 10% fetal bovine serum (FBS) from day 0–24,
during which no significant morphological changes were observed.
However, after 3 days of culture in the differentiation medium,
some subpopulations of the cells appeared morphologically distinct
from the starting culture (G). After 7 days of differentiation,
primordial germ cell (PGC)-like cells, which ranged from
approximately 15–20 μm in diameter, appeared (H, arrowhead).
After 14 days of differentiation, oocyte-like cells (OLCs)
developed and were surrounded by smaller cells (I and J, blue
arrows). Higher magnification images of OLCs are also presented to
show that they are in pachytene (K). Occasionally, some of the OLCs
were coated with a zona pellucida (ZP)-like structure (L, red
arrow). Scale bar is set to 100 μm. |
However, after 3 days of differentiation, some
subpopulations of the cells became morphologically distinct from
the starting cultures (Fig. 1G).
PGC-like cells, which ranged from approximately 15–20 μm in
diameter, appeared around at 7 days of differentiation (Fig. 1H). Aggregates were formed after 7
days of differentiation. These aggregates gradually became
morphological structures similar to a primordial follicle after 14
days (Fig. 1I). At 14–24 days of
differentiation, OLCs were developed much like those described in a
previous study (Fig. 1J)
(8). The OLCs became surrounded
by smaller cells over time that resembled cumulus-oocyte complex
(COC)-like cells (Fig. 1K).
Occasionally, some of the OLCs were observed to be coated with a
zona pellucida (ZP)-like structure (Fig. 1L), and some of the OLCs reached
>100 μm in diameter.
Immunofluerence staining for the
expression of markers related to germ cell formation and oocyte
development
In order to verify whether the PGC-like cells and
OLCs, that were identified based on morphological changes,
expressed specific gene markers related to germ cell formation and
oocyte development, we first performed immunofluerence staining of
the cells at 7 and 24 days of induction, respectively.
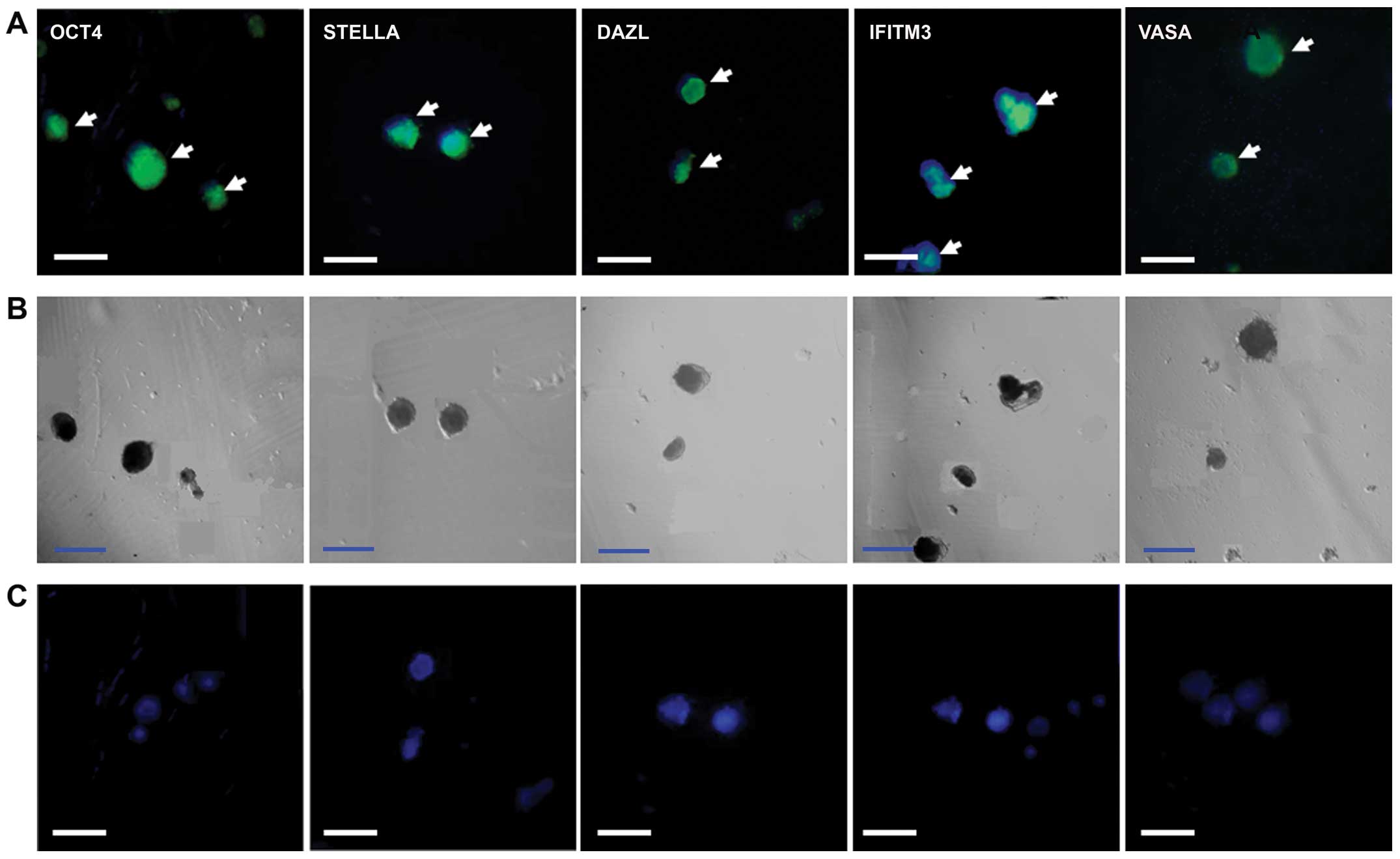
OCT4, VASA, STELLA, interferon-induced transmembrane
protein 3 (IFITM3) and DAZL were detected in the PGC-like cells on
day 7 of induction of differentiation, while no signal was detected
in the negative controls with only the anti-rabbit or anti-mouse
secondary antibody (Fig. 2).
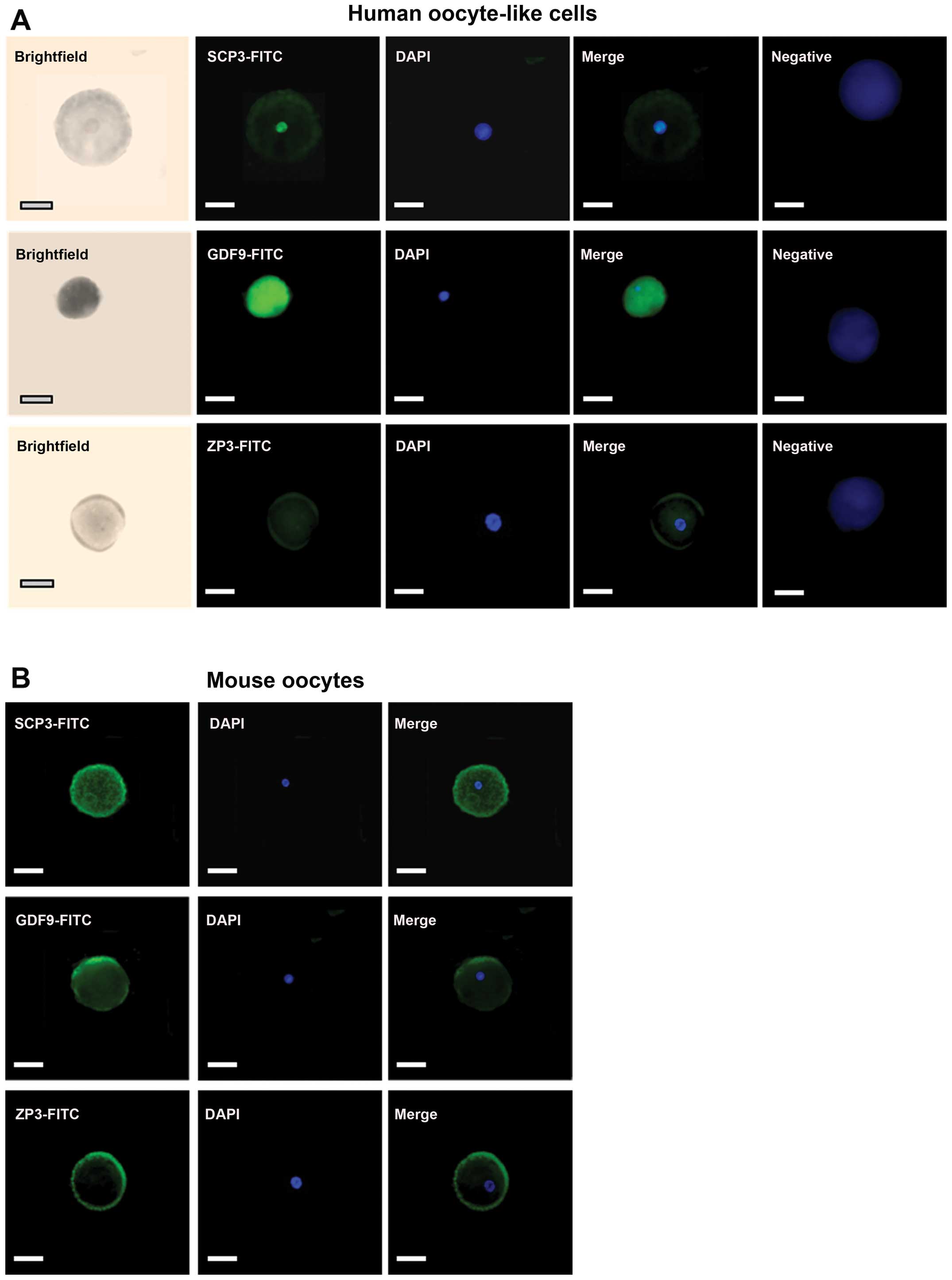
Furthermore, the OLCs at 24 days of induction were
positively stained with anti-SCP3, anti-GDF9 and anti-ZP3 primary
antibodies (Fig. 3A). The
specificity of the antibodies was identified using mouse oocytes as
positive controls as the primary antibodies used in this study all
reacted with humans and mice (Fig.
3B). More interestingly, DAPI staining revealed that the OLCs
contained a germinal vesicle. This demonstrated that the OLCs were
single cells rather than cell aggregations, which also were the
same as mouse oocytes (Fig. 3).
Notably, ZP-like structures surrounding OLCs could be detected in
some of the cells by using anti-ZP antibodies (Fig. 3A), which were very similar to
matured mouse oocytes (Fig.
3B).
RT-qPCR and western blot analysis of
specific markers of PGCs and oocytes
To further characterize the PGC-like cells and OLCs,
RT-qPCR was performed. In RT-qPCR, the specificity of each
amplified product in the controls and differentiated cells were
verified by a bi-directional sequence analysis. Once the
specificity of the products was established, temporal changes in
the relative mRNA levels of each of the markers were evaluated
using β-actin as an inner control. The Ct values for all the
products were <35, and the efficiencies of the targets and the
reference (β-actin) were approximately equal.
Transcripts of all the markers were detected in the
PGC-like cells and OLCs, as well as in the undifferentiated cell
population (Fig. 4). However,
fold changes in the differentiated compared to the undifferentiated
cells for all the markers showed certain dynamic changes during the
induction process. On day 7 of the induction of differentation, in
which the PGC-like cells appeared, the mRNA levels of OCT4, SSEA1,
STELLA, VASA, BLIMP1, PRDM14 and TFAP2C increased by approximately
1.02-fold (P=0.246), 11.6-fold (P=0.001), 9.8-fold (P=0.001),
13.3-fold (P=0.001), 1.4-fold (P=0.005), 3.3-fold (P=0.01) and
3.05-fold (P=0.0025), respectively, when compared to the levels
obtained on day 0 (Fig. 4). As
these genes are determinants in the transition of PGCs, their
increased expression may indicate initial PCG-like cells during the
induction process.
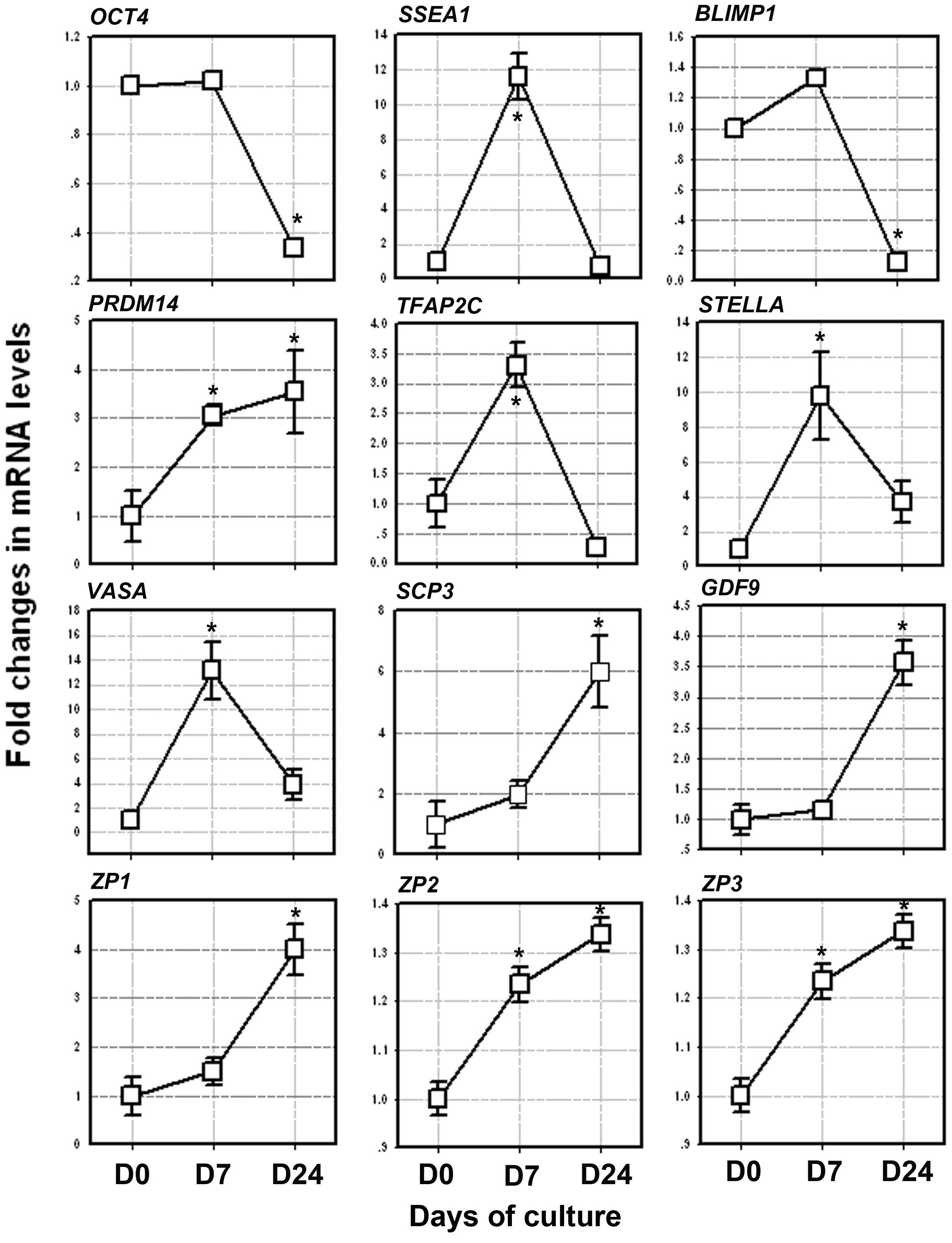 | Figure 4Expression of the germ cell markers,
octamer-binding transcription factor 4 (OCT4), stage-specific
embryonic antigen 1 (SSEA1), STELLA, VASA, B lymphocyte induced
maturation protein 1 (BLIMP1), PR domain containing 14 (PRDM14) and
transcription factor AP–2 gamma (TFAP2C), in the primordial germ
cell (PGC)-like cells after 7 days of differentiation and the
oocyte markers, synaptonemal complex protein 3 (SCP3),
growth/differentiation factor 9 (GDF9) and zona pellucida
glycoprotein (ZP)1, ZP2 and ZP3, after 24 days of differentiation
as determined by RT-qPCR. Relative mRNA levels are normalized for
the β-actin housekeeping gene. The results are presented relative
to the control cells (2−ΔΔCt). Data represent the means
± SEM of 3 independent experiments. *Statistical significance when
compared to day 0 of culture. |
From day 7–24, the mRNA levels of SCP3, GDF9, ZP1,
ZP2 and ZP3 gradually increased when compared to the levels
obtained on day 0. On day 24 of differentiation, in which the OLCs
were developed, the mRNA levels of SCP3, GDF9, ZP1, ZP2 and ZP3
increased by approxiamtely 6-fold (P=0.039), 3.5-fold (P=0.007),
4-fold (P=0.014), 1.33-fold (P=0.036), and 8-fold (P=0.008),
respectively, when compared to the levels obtained on day 0
(Fig. 4).
In order to further confirm the results of RT-qPCR,
western blot analysis was performed. The protein expression levels
of STELLA and VASA on day 7 and those of GDF9 and ZP1 on day 24
after the induction of differentiation were markedly increased,
while OCT4 protein expression showed a slight decrease from day
0–24 (Fig. 5). These changes in
the protein expression levels were similar to those of the mRNA
expression levels.
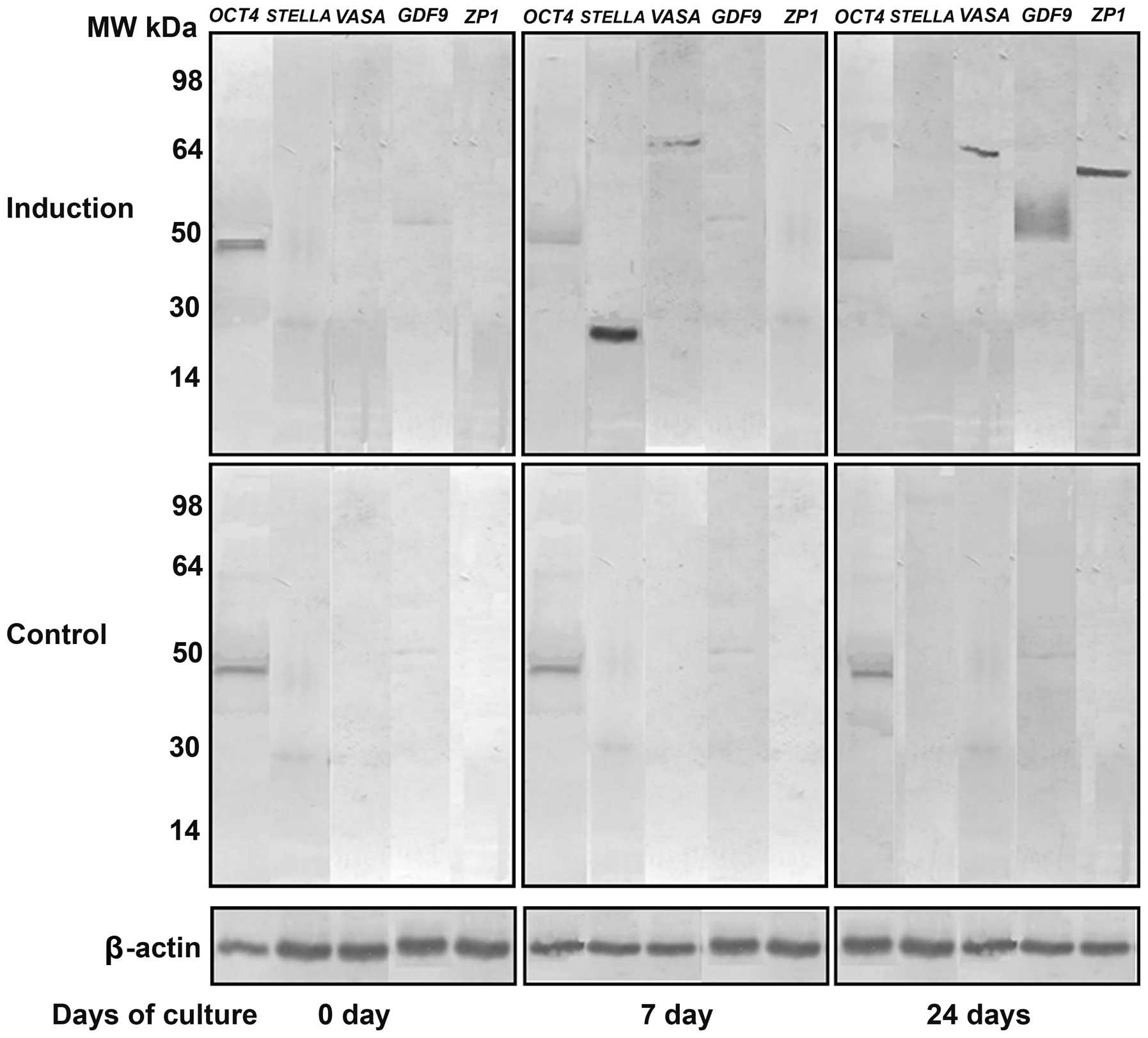 | Figure 5Western blot analysis of the protein
expression of octamer-binding transcription factor 4 (OCT4; MW, 45
kDa), STELLA (MW, 23 kDa), VASA (MW, 80 kDa),
growth/differentiation factor 9 (GDF9; MW, 51 kDa) and zona
pellucida glycoprotein 1 (ZP1; MW, 70 kDa) on day 0, 7 and 24 after
the induction of differentiation. Protein expression of STELLA,
VASA, GDF9 and ZP1 was not detected in the untreated stems cells
except for OCT4. β-actin (MW, 42 kDa) was used as an internal
control. |
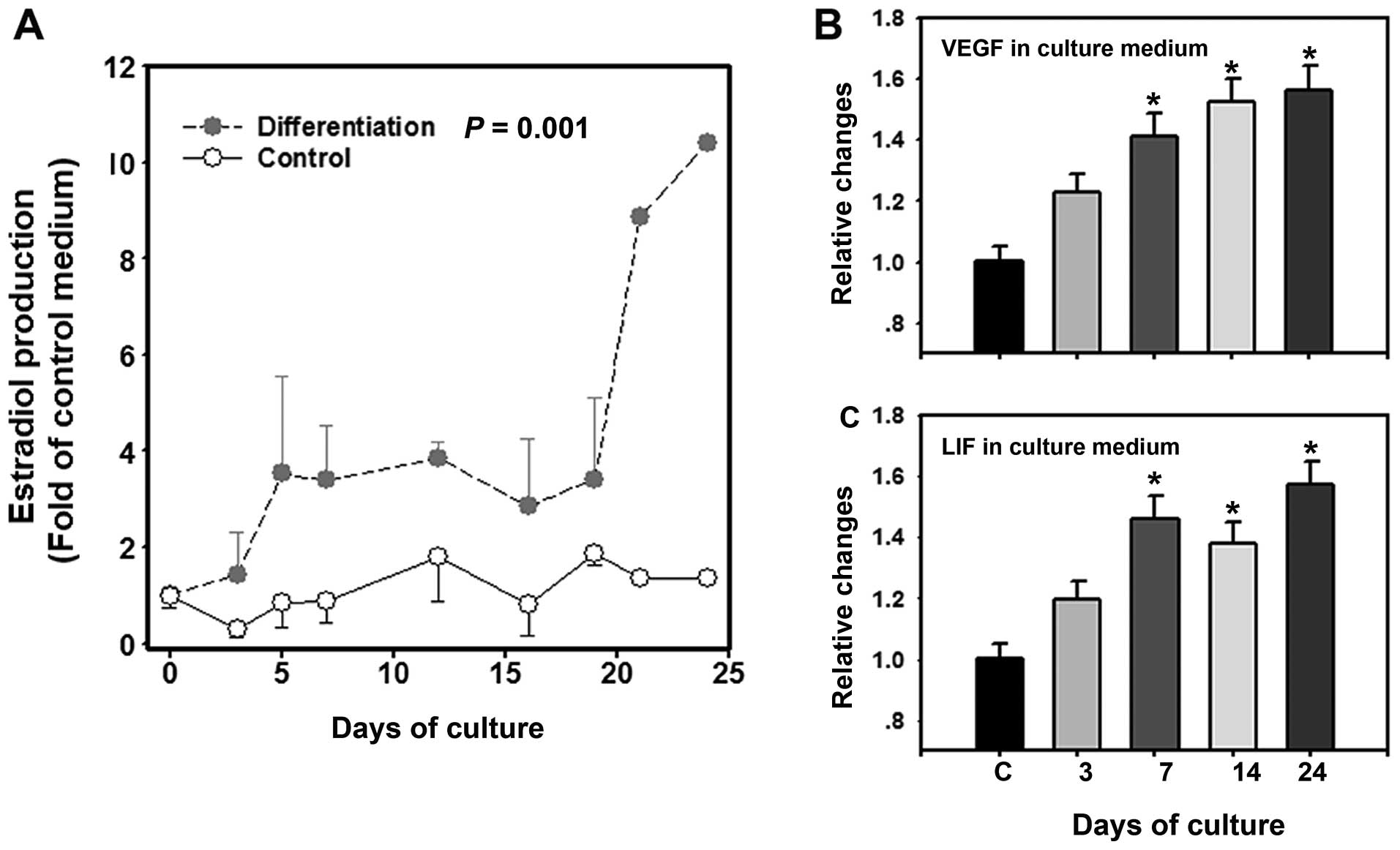
Production of estradiol, VEGF and LIF by
COC-like cells
Before day 18, there were low levels (ranging from
0.24–2.79 ng/ml) of estradiol in the culture medium of the
undifferentiated cells (controls), while the estradiol levels in
the culture medium of the cells induced to differentiate were
1-4-fold (ranging from 1.42–8.72 ng/ml) higher than the controls.
The estradiol concentrations increased consistently after 18 days,
with the levels peaking (22.3–27.9 ng/ml; 8-10-fold increase) on
day 21–24 (Fig. 6A). In addition,
the concentrations of LIF and VEGF in the culture medium also
gradually increased from day 0–24 (Fig. 6B and C). The concentration of LIF
in the human follicular fluid (control, C) was 0.91±0.04 ng/l,
while the concentrations of LIF in the culture medium were
1.07±0.05 ng/l (day 3), 1.33±0.04 ng/l (day 7), 1.25±0.03 ng/l (day
14) and 1.43±0.03 ng/l (day 24). The concentration of VEGF in the
human follicular fluid (control, C) was 410.6±20.5 pg/ml, while the
concentrations of VEGF in the culture medium were 503.5±25.1 pg/ml
(day 3), 572.4±26.8 pg/ml (day 7), 625.1±30.2 pg/ml (day 14) and
641.2±23.8 pg/ml (day 24) (Fig. 6B
and C).
Discussion
A number of previous studies have demonstrated that
PGC-like cells and OLCs can be generated from embryonic,
differentiated pluripotent and adult stem cells in vitro
(2–7,18).
In these studies, the induction of embryonic or somatic stem cells
into OLCs was generally performed by culturing the cells with
growth factors (3,6), estrogenic stimuli (12), conditional medium from testicular
cell cultures (19), follicular
fluid and gonadotrophins (8), or
with ovarian granulose cells (3).
In the present study, we demonstrated that stem cells derived from
hFTUC also differentiate into PGC-like cells and OLCs by the
addition of human follicular fluid, gonadotrophins and estradiol to
the culture medium.
We demonstrated that our germ cell precursors
closely resembled PGCs or oocytes based on the following factors:
i) morphologic changes; ii) marker expression profiles at the mRNA
and/or protein level; and iii) the production of estradiol from
COC-like structures.
As has been previously demonstrated, germ cell
development requires a series of multiple well-orchestrated steps,
which involve the concurrent up- and downregulation of the
expression of specific genes (20). In the present study, on day 7 of
differentiation, the PGC-like cells expressed the proteins OCT4,
IFITM3, VASA, STELLA and DAZL (Figs.
2 and 5), which are markers
indicative of germ cell formation. In particular, OCT4 has been
suggested to be required for PGC survival (20). IFITM3 is believed to initiate the
repression of homeobox genes in early germ cell precursors
(20), while STELLA plays a role
in facilitating germline and endodermal differentiation of human ES
cells (21). A lack of STELLA
expression at the earlier stage can reflect a transition of cells
committing to the germ lineage (19). DAZL is considered essential for
PGC development, as knockout mice lack a germ cell population
(22,23). VASA is expressed in post-migratory
PGCs until the post-meiotic stage of oocytes (24,25).
Moreover, in this study, the mRNA levels of BLIMP1,
PRDM14, TFAP2C, SSEA1, STELLA and VASA first increased and then
decreased at later stages of the induction of differentiation
(Fig. 4). BLIMP1, PRDM14 and
TFAP2C are key germ cell determinants for regulating PGC
specification (26,27). It has been reported that BLIMP1
binds directly to suppress the epxression of somatic and cell
proliferation-related genes, and directly induces TFAP2C
expression, which together with PRDM14, initiates the PGC-specific
fate (28). Furthermore, it has
been reported that human germ cells express SSEA1 (29). These results together with
morphologic similarities indicate that some subpopulations of stem
cells are able to differentiate into PGC-like cells.
In this study, indeed, after day 7 of
differentiation, the PGC-like cells continued to form structures
that morphologically resembled primordial follicles, and COC-like
cells/OLCs finally appeared in the culture during day 4–24 of the
induction of differentiation. The COC-like cells/OLCs express
proteins, such as SCP3, GDF9, ZP1 and ZP3 (Figs. 3 and 5) and the mRNA levels of oocyte-specific
markers, such as SCP3, GDF9, ZP1, ZP2 and ZP3 increased during the
differentiation process (Fig. 4).
SCP3 is a meiosis-specific protein (30), while GDF9 is required for normal
folliculogenesis (31), and ZP
glycoproteins are expressed only in oocytes (32). Taken together, these results
indicate that the some of the PGC-like cells are able to further
develop into OLCs.
Furthermore, the detection of estradiol production
provides evidence of the functional activity of somatic cells in
the COCs. Although we added a small amount of estradiol into the
culture medium during the induction process, the 8-10-fold increase
in the estradiol concentration over the controls on days 21 and 23
was probably due to the COC-like cells rather than the accumulation
of exogenous estradiol as the culture medium was changed every 3
days. This is supported by the fact that the protein concentrations
of LIF and VEGF in the culture medium increased consistently during
the differentiation process even though the follicular fluid
contained a certain amount of the two factors. It is known that
granulosa cells express a number of growth factors and cytokines,
including LIF (33) and VEGF
(34), which have been reported
to have beneficial effects on mouse (35) or porcine (36) oocyte maturation. Our findings in
this study further suggest that differentiated COC-like cells may
include functional granulose cells.
It is interesting to determine the reason that
hFTUC-derived stem cells are capable of differentiating into germ
cell-like cells, COC-like cells and OLCs. In our previous study,
the stem cells highly expressed BMP1 and BMP4 (15). As mentioned earlier, BLIMP1
together with PRDM14 and TFAP2C are key germ cell determinants for
regulating PGC specification (26,27). Thus, this suggests that
hFTUC-derived stem cells may be an ideal cell resource for an in
vitro model for the investigation of the occurrence of germ
cell formation and differentiation.
Moreover, our previous study indicated that stem
cells derived from hFTUC exhibited a significantly greater
proliferative potential and were more efficient in their in
vitro differentiation when compared to stem cells derived from
term umbilical cords (15). This
may be due to the fact that younger sources of adult stem cell
populations have a greater proliferative potential and greater
plasticity than their older counterparts (37,38). Therefore, it can be assumed that
hFTUC-derived stem cells may be more efficient when differentiating
into OLCs. ES cells are the primary stem cells that are capable of
developing into any type of cells. It has been reported that
ES-derived OLCs are produced after an induction of 26 days in a
monolayer culture without feeder cells and without the addition of
growth factors (2,10). At the same time, skin stem
cell-derived OLCs were generated by the addition of 5% porcine
follicular fluid, growth factor EGF and hormones, such as ITS, FSH
and LH, and by extending the induction from 30 to 50 days (2,10).
Compared to ES-derived and skin stem cell-derived OLCs, the
hFTUC-derived OLCs in the present study were generated within a
shorter time frame (14–24 days); these results are similar to those
of a recent study in which human amniotic fluid stem cells
generated OLCs within 3 weeks (39).
Overall, our findings support the idea that the
hFTUC-derived stem cells have an intrinsic ability to differentiate
into PGC-like cells and OLCs. However, whether the OLCs contain
synapsed homologous chromosomes or whether the OLCs become embryos
after subsequent in vitro fertilization are questions that
require further investigation in future studies.
Currently, factors regarding on how to induce the
up- or downregulation of markers of PGCs and oocytes remain
unknown. Human follicular fluid contains a variety of biochemical
substances both transferred from the blood plasma and secreted from
granulosa and theca cells (40).
As oocytes secrete soluble paracrine growth factors that can
regulate granulose cell development, and granulosa cells in turn
regulate oocyte growth during follicle formation (41), it is apparent that the biochemical
substances from the granulosa cells may play a key role in the
initiation of germ cell formation and oocyte development.
Nonetheless, in order to answer the question of whether these
factors, alone or in combination, are responsible for initiating
the differentiation of stem cells into germ cell lineages, further
studies are required in the future.
In conclusion, in this study, we demonstrated that
stem cells derived from hFTUC have an intrinsic ability to
differentiate into OLCs. This provides a novel in vitro
model for the investigation of the mechanisms through which germ
cell formation and differentiation occurs. Nevertheless, further
studies are warranted in order to fully elucidate the accurate
functionality of these stem cells, and which factors are
responsible for the initiation of these stem cells into germ cell
lineages.
Acknowledgments
This study was supported by grants from the National
Basic Research Program of China (973 Plan, grant nos. 2010CB530403
and 2010CB530400).
References
|
1
|
Marques-Mari AI, Lacham-Kaplan O, Medrano
JV, Pellicer A and Simón C: Differentiation of germ cells and
gametes from stem cells. Hum Reprod Update. 15:379–390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hübner K, Fuhrmann G, Christenson LK,
Kehler J, Reinbold R, De La, Fuente R, Wood J, Strauss JF III,
Boiani M and Schöler HR: Derivation of oocytes from mouse embryonic
stem cells. Science. 300:1251–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qing T, Shi Y, Qin H, Ye X, Wei W, Liu H,
Ding M and Deng H: Induction of oocyte-like cells from mouse
embryonic stem cells by co-culture with ovarian granulosa cells.
Differentiation. 75:902–911. 2007.PubMed/NCBI
|
|
4
|
Wei W, Qing T, Ye X, Liu H, Zhang D, Yang
W and Deng H: Primordial germ cell specification from embryonic
stem cells. PLoS One. 3:e40132008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark AT, Bodnar MS, Fox M, Rodriquez RT,
Abeyta MJ, Firpo MT and Pera RA: Spontaneous differentiation of
germ cells from human embryonic stem cells in vitro. Hum Mol Genet.
13:727–739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kee K, Gonsalves JM, Clark AT and Pera RA:
Bone morphogenetic proteins induce germ cell differentiation from
human embryonic stem cells. Stem Cells Dev. 15:831–837. 2006.
View Article : Google Scholar
|
|
7
|
Chen HF, Kuo HC, Chien CL, Shun CT, Yao
YL, Ip PL, Chuang CY, Wang CC, Yang YS and Ho HN: Derivation,
characterization and differentiation of human embryonic stem cells:
Comparing serum-containing versus serum-free media and evidence of
germ cell differentiation. Hum Reprod. 22:567–577. 2007. View Article : Google Scholar
|
|
8
|
Dyce PW, Wen L and Li J: In vitro germline
potential of stem cells derived from fetal porcine skin. Nat Cell
Biol. 8:384–390. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dyce PW, Liu J, Tayade C, Kidder GM, Betts
DH and Li J: In vitro and in vivo germ line potential of stem cells
derived from newborn mouse skin. PLoS One. 6:e203392011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dyce PW, Shen W, Huynh E, Shao H,
Villagómez DA, Kidder GM, King WA and Li J: Analysis of oocyte-like
cells differentiated from porcine fetal skin-derived stem cells.
Stem Cells Dev. 20:809–819. 2011. View Article : Google Scholar
|
|
11
|
Johnson J, Bagley J, Skaznik-Wikiel M, et
al: Oocyte generation in adult mammalian ovaries by putative germ
cells in bone marrow and peripheral blood. Cell. 122:303–315. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bukovsky A, Svetlikova M and Caudle MR:
Oogenesis in cultures derived from adult human ovaries. Reprod Biol
Endocrinol. 3:172005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toyooka Y, Tsunekawa N, Akasu R and Noce
T: Embryonic stem cells can form germ cells in vitro. Proc Natl
Acad Sci USA. 100:11457–11462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Librach CL, Yie SM and Xiao R: Method of
isolation and use of cells derived from first trimester umbilical
cord tissue. US Patent 20090074731 A1. Filed May 2, 2008; issued
March 19, 2009.
|
|
15
|
Hong SH, Maghen L, Kenigsberg S, et al:
Ontogeny of human umbilical cord perivascular cells: Molecular and
fate potential changes during gestation. Stem Cells Dev.
22:2425–2439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu P, Bai Y, Pan S, Li W, Liu W and Hua
J: Gender depended potentiality of differentiation of human
umbilical cord mesenchymal stem cells into oocyte-Like cells in
vitro. Cell Biochem Funct. 31:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Panula S, Medrano JV, Kee K, et al: Human
germ cell differentiation from fetal- and adult-derived induced
pluripotent stem cells. Hum Mol Genet. 20:752–762. 2011. View Article : Google Scholar :
|
|
19
|
Lacham-Kaplan O, Chy H and Trounson A:
Testicular cell conditioned medium supports differentiation of
embryonic stem cells into ovarian structures containing oocytes.
Stem Cells. 24:266–273. 2006. View Article : Google Scholar
|
|
20
|
Lange UC, Saitou M, Western PS, Barton SC
and Surani MA: The fragilis interferon-inducible gene family of
transmembrane proteins is associated with germ cell specification
in mice. BMC Dev Biol. 3:12003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wongtrakoongate P, Jones M, Gokhale PJ and
Andrews PW: STELLA facilitates differentiation of germ cell and
endodermal lineages of human embryonic stem cells. PLoS One.
8:e568932013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruggiu M, Speed R, Taggart M, McKay SJ,
Kilanowski F, Saunders P, Dorin J and Cooke HJ: The mouse Dazla
gene encodes a cytoplasmic protein essential for gametogenesis.
Nature. 389:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kee K, Angeles VT, Flores M, Nguyen HN and
Reijo Pera RA: Human DAZL, DAZ and BOULE genes modulate primordial
germ-cell and haploid gamete formation. Nature. 462:222–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toyooka Y, Tsunekawa N, Takahashi Y,
Matsui Y, Satoh M and Noce T: Expression and intracellular
localization of mouse Vasa-homologue protein during germ cell
development. Mech Dev. 93:139–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castrillon DH, Quade BJ, Wang TY, Quigley
C and Crum CP: The human VASA gene is specifically expressed in the
germ cell lineage. Proc Natl Acad Sci USA. 97:9585–9590. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kehler J, Tolkunova E, Koschorz B, et al:
Oct4 is required for primordial germ cell survival. EMBO Rep.
5:1078–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magnúsdóttir E, Dietmann S, Murakami K,
Günesdogan U, Tang F, Bao S, Diamanti E, Lao K, Gottgens B and Azim
Surani M: A tripartite transcription factor network regulates
primordial germ cell specification in mice. Nat Cell Biol.
15:905–915. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu LF, Surani MA, Jaenisch R and Zwaka
TP: Blimp1 expression predicts embryonic stem cell development in
vitro. Curr Biol. 21:1759–1765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kerr CL, Hill CM, Blumenthal PD and
Gearhart JD: Expression of pluripotent stem cell markers in the
human fetal testis. Stem Cells. 26:412–421. 2008. View Article : Google Scholar
|
|
30
|
Yuan L, Liu JG, Hoja MR, Wilbertz J,
Nordqvist K and Höög C: Female germ cell aneuploidy and embryo
death in mice lacking the meiosis-specific protein SCP3. Science.
296:1115–1118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aaltonen J, Laitinen MP, Vuojolainen K, et
al: Human growth differentiation factor 9 (GDF-9) and its novel
homolog GDF-9B are expressed in oocytes during early
folliculogenesis. J Clin Endocrinol Metab. 84:2744–2750.
1999.PubMed/NCBI
|
|
32
|
Lefièvre L, Conner SJ, Salpekar A, et al:
Four zona pellucida glycoproteins are expressed in the human. Hum
Reprod. 19:1580–1586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abir R, Fisch B, Jin S, Barnnet M,
Freimann S, Van den Hurk R, Feldberg D, Nitke S, Krissi H and Ao A:
Immunocytochemical detection and RT-PCR expression of leukaemia
inhibitory factor and its receptor in human fetal and adult
ovaries. Mol Hum Reprod. 10:313–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee A, Christenson LK, Patton PE, Burry KA
and Stouffer RL: Vascular endothelial growth factor production by
human luteinized granulosa cells in vitro. Hum Reprod.
12:2756–2761. 1997. View Article : Google Scholar
|
|
35
|
De Matos DG, Miller K, Scott R, Tran CA,
Kagan D, Nataraja SG, Clark A and Palmer S: Leukemia inhibitory
factor induces cumulus expansion in immature human and mouse
oocytes and improves mouse two-cell rate and delivery rates when it
is present during mouse in vitro oocyte maturation. Fertil Steril.
90:2367–2375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biswas D and Hyun SH: Supplementation with
vascular endothelial growth factor during in vitro maturation of
porcine cumulus oocyte complexes and subsequent developmental
competence after in vitro fertilization. Theriogenology.
76:153–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mareschi K, Ferrero I, Rustichelli D,
Aschero S, Gammaitoni L, Aglietta M, Madon E and Fagioli F:
Expansion of mesenchymal stem cells isolated from pediatric and
adult donor bone marrow. J Cell Biochem. 97:744–754. 2006.
View Article : Google Scholar
|
|
38
|
Choumerianou DM, Martimianaki G, Stiakaki
E, Kalmanti L, Kalmanti M and Dimitriou H: Comparative study of
stemness characteristics of mesenchymal cells from bone marrow of
children and adults. Cytotherapy. 12:881–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu X, Wang N, Qiang R, Wan Q, Qin M, Chen
S and Wang H: Human amniotic fluid stem cells possess the potential
to differentiate into primordial follicle oocytes in vitro. Biol
Reprod. 90:732014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fortune JE: Ovarian follicular growth and
development in mammals. Biol Reprod. 50:225–232. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gilchrist RB, Ritter LJ and Armstrong DT:
Oocyte-somatic cell interactions during follicle development in
mammals. Anim Reprod Sci. 82–83:431–446. 2004. View Article : Google Scholar
|