Introduction
Toll-like receptors (TLRs) are a group of receptors
that recognize conserved pathogen-associated molecular patterns
(PAMPs) (1,2), playing a key role in the host
defense against infection. Thus far, 10 members of TLRs with
distinct PAMP ligands have been identified in humans (3). Triacyl lipopeptide and diacyl
lipopeptide are recognized by TLR1/2 and TLR2/6, respectively
(4–6). TLR3 recognizes double-stranded RNA
(7). The receptor for
lipopolysaccharide (LPS) is TLR4 (8). TLR5 recognizes bacterial flagellin
(9). Imidazoquinolines and
single-stranded RNA (ssRNA) are recognized by TLR7 and TLR8
(10). TLR9 recognizes bacterial
and viral CpG DNA motifs (11).
In addition to sensing exogenous ligands from microbial components,
TLRs can also recognize endogenous ligands, known as
danger-associated molecular patterns (12). For example, it is well-known that
heat-shock proteins (HSPs) can be locally released upon injury and
subsequently activate TLR2 or 4 (13). Certain self non-coding RNAs
damaged by ultraviolet (UV) radiation and released from necrotic
cells can be recognized by TLR3 to induce inflammatory cytokines
(14).
The TLR-mediated signaling pathways can be divided
into two major pathways: Myeloid differentiation primary response
protein 88 (MyD88)- and TIR-domain-containing adapter-inducing
interferon-β (TRIF)-dependent pathways. MyD88 is an adapter protein
used by almost all 10 TLRs (except TLR3) to initiate TLR signaling
(3). However, the signal
transduction pathway of TLR3 requires the other adaptor protein,
TRIF (3). TLR4 can utilize MyD88
and TRIF as signaling adaptors (3,15).
Following recognition of different ligands, TLRs trigger signaling
pathways and activate nuclear factor (NF)-κB and activator
protein-1 (AP-1), which results in the induction of inflammatory
cytokines and chemokines (2,3).
In skin, TLRs are expressed not only in professional
immune cells (such as Langerhans cells) but also in non-immune
cells (including keratinocytes and melanocytes) (16–18). Several studies have shown that
skin keratinocytes and melanocytes exhibit TLRs and respond to
corresponding PAMPs by producing pro-inflammatory cytokines
(17,18). Although recent studies have
reported that TLRs are expressed in skin fibroblasts (19,20), the expression levels and
functional responses of TLRs in skin fibroblasts have not been
thoroughly studied.
In the present study, the expression levels of all
the TLR family members (TLR1-10) were analyzed in skin fibroblasts.
Subsequently, using a specific ligand for each TLR, the functional
activation of each TLR was observed in fibroblasts. The signaling
pathways induced by TLR1/2, 3 and 4 were also examined.
Furthermore, the expression levels of TLRs were compared in skin
fibroblasts with those in skin keratinocytes. Finally, the mRNA
levels of interleukin-6 (IL-6) and IL-8 increased
upon exposure to a TLR1/2 ligand when compared between fibroblasts
and keratinocytes.
Materials and methods
Reagents
All the TLR ligands were purchased from InvivoGen,
Inc. (San Diego, CA, USA). For western blot analysis, antibodies to
phospho-inhibitor of nuclear factor κBα (IκBα) and phospho-and
total-extracellular-signal regulated kinase (ERK) 1/2 were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
The antibody to matrix metalloproteinase-1 (MMP-1) was manufactured
by Lab Frontier Co., Ltd. (Seoul, Korea). Antibodies to TLR2, 3, 4
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Cell culture
Human foreskin fibroblasts and keratinocytes were
established from biopsies obtained from four different healthy
donors. Skin fibroblasts were cultured in Dulbecco’s modified
Eagle’s media (DMEM) supplemented with glutamine (2 mM), penicillin
(400 U/ml), streptomycin (50 mg/ml) and 10% fetal bovine serum
(FBS) in a humidified 5% CO2 atmosphere at 37°C. Skin
fibroblasts were used for the experiments at passages 3-6. For
treatment, skin fibroblasts were serum-starved for 48 h in DMEM
containing 0.1% FBS.
Skin keratinocytes were cultured in keratinocyte
growth medium (Clonetics, Inc., San Diego, CA, USA) composed of
MCDB 153 medium supplemented with epidermal growth factor (10
ng/ml), bovine pituitary extract (70 μg/ml), hydrocortisone
(0.5 μg/ml), penicillin (100 μg/ml), streptomycin
(100 μg/ml) and fungizone (0.25 μg/ml). Skin
keratinocytes were used at passage 3-4 in the experiments. For
treatment, skin keratinocytes were maintained in keratinocyte basal
medium (MCDB 153 medium only) for 48 h.
Ligand treatment
To induce IL-6, IL-8 and MMP-1 production, skin
fibroblasts were plated at the concentration of 0.5×105
cells/500 μl in 24-well plates for 48 h in the presence of
different concentrations of Pam3CSK4 (TLR1/2 ligand; 0.01, 0.1 and
1 μg/ml), poly (I:C) (TLR3 ligand; 0.1, 1 and 10
μg/ml), LPS (TLR4 ligand; 0.001, 0.01 and 0.1 μg/ml),
flagellin (TLR5 ligand; 0.05, 0.5 and 5 μg/ml), FSL-1
(TLR2/6 ligand; 0.001, 0.01 and 0.1 μg/ml), imiquimod (TLR7
ligand; 0.1, 1 and 10 μg/ml), ssRNA40 (TLR8 ligand; 0.1, 1
and 10 μg/ml) and ODN2006 (TLR9 ligand, 0.05, 0.5 and 5
μM). The concentrations of each TLR ligand were screened and
determined using the information in previously published studies
(17,18).
RNA isolation, and semi-quantitative and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated using TRIzol (Invitrogen,
Carlsbad, CA, USA). Total RNA (1 μg) was used to generate
cDNA using a First Strand cDNA Synthesis kit (MBI Fermentas,
Vilnius, Lithuania) according to the manufacturer’s
instructions.
Semi-quantitative RT-PCR
Semi-quantitative RT-PCR was performed to detect
TLR1-10 and 36B4 mRNA using PCR Premix (Promega,
Madison, WI, USA). PCR conditions were as follows: 95°C for 2 min,
35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 1 min.
The PCR products were visualized on 2% agarose gels using ethidium
bromide staining. The primer sequences for semi-quantitative RT-PCR
are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|
| TLR1 |
CTATACACCAAGTTGTCAGC |
GTCTCCAACTCAGTAAGGTG |
| TLR2 |
GCCAAAGTCTTGATTGATTG |
TTGAAGTTCTCCAGCTCCTG |
| TLR3 |
GATCTGTCTCATAATGGCTTG |
GACAGATTCCGAATGCTTGTG |
| TLR4 |
TGGATACGTTTCCTTATAAG |
GAAATGGAGGCACCCCTTC |
| TLR5 |
ATTGCCAATATCCAGGATGC |
CACCACCATGATGAGAGCAC |
| TLR6 |
TTGGGCTAACATTAGAGCCG |
AGCTCAGTTCCCCAGATGAA |
| TLR7 |
AATGTCACAGCCGTCCCTAC |
GCGCATCAAAAGCATTTACA |
| TLR8 |
TCAACAAATCCGCACTTGAA |
CAGGACTGGCACAAATGACA |
| TLR9 |
TACGATGCCTTCGTGGTCTT |
CTCAAAGAGGGTTTTGCCAG |
| TLR10 |
TCTCCCTGGATGCAGTCATT |
AACTTCCTGGCAGCTCTGAA |
| 36B4 |
TGGGCTCCAAGCAGATGC |
GGCTTCGCTGGCTCCCAC |
| β-actin |
AGAGATGGCCACGGCTGCTT |
ATTTGCGGTGGACGATGGAG |
| IL-6 |
CTCCTTCTCCACAAGCGCC |
GCCGAAGAGCCCTCAGGC |
| IL-8 |
CTCTTGGCAGCCTTCCTG |
TTGGGGTCCAGACAGAGC |
RT-qPCR
RT-qPCR was performed to measure the relative levels
of TLR1-10 mRNA using an ABI Prism 7500 (Applied Biosystems,
Darmstadt, Germany). Input cDNA was normalized according to
36B4 or actin as internal control genes. The primer
sequences for RT-qPCR were the same as for semi-quantitative
RT-PCR.
Enzyme-linked immunosorbent assay
(ELISA)
After stimulation for 48 h with TLR ligands, cell
culture supernatants were harvested and examined by ELISA for IL-8
and IL-6, following the manufacturer’s instructions (Endogen, Inc.,
Woburn, MA, USA).
Western blotting
For the MMP-1 measurement, an equal volume of cell
culture supernatants was prepared for western blot analysis.
β-actin was detected from an equal volume of cell lysate as a
loading control for MMP-1. For the detection of protein in cell
lysates, cells were washed twice with ice-cold phosphate-buffered
saline to remove all the serum proteins and were subsequently lysed
in cell lysis buffer. Separated proteins were transferred to
polyvinylidene fluoride membranes, which were subsequently blocked
with Tris-buffered saline containing 0.1% Tween-20 and 5% dried
skimmed milk. Membranes were incubated with the appropriate primary
antibody and horseradish peroxidase-conjugated secondary antibody.
Antibody-bound proteins were visualized by enhanced
chemiluminescence (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
Statistical analyses were performed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference. Results are presented as mean
values ± standard error of the mean.
Results
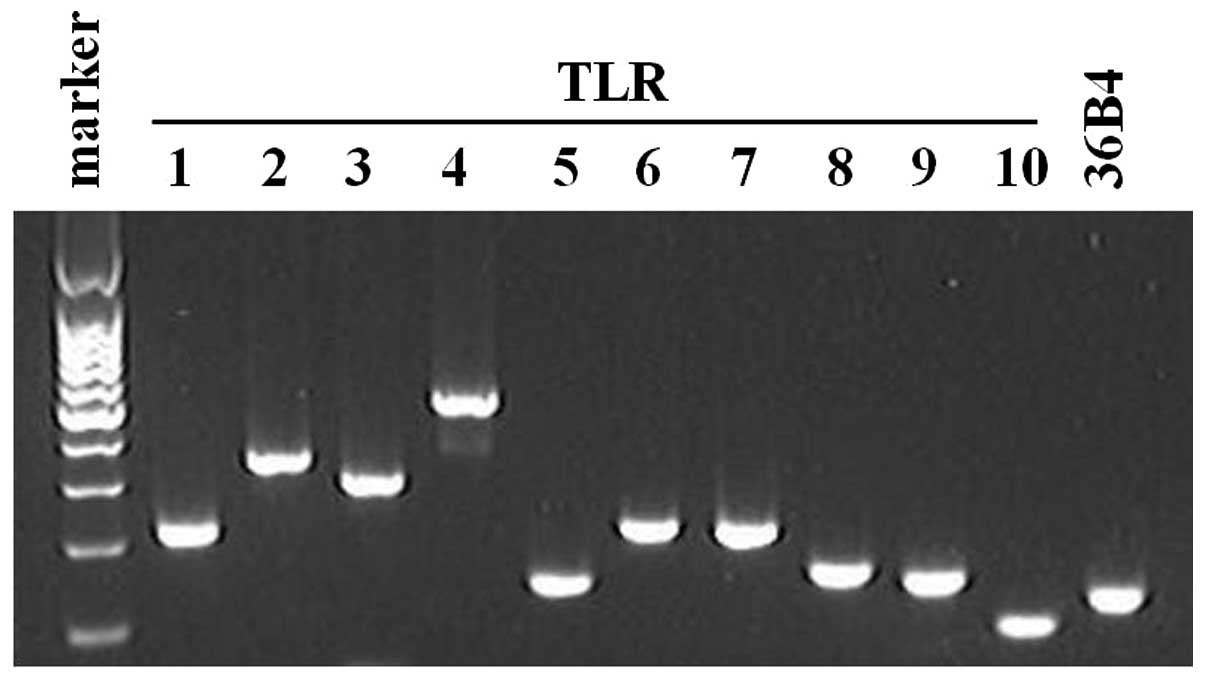
All 10 TLR family members are expressed
in human skin fibroblasts
In order to gain insight into the functions of TLRs
in skin fibroblasts, the expression levels of TLR family members
were assessed by RT-qPCR. Consistent with the previously published
studies (17,19,20), all the 10 TLR family members were
constitutively expressed in skin fibroblasts (Fig. 1).
Activation of TLRs induces expression of
IL-6, IL-8 and MMP-1 in human skin fibroblasts
Activation of TLRs in skin keratinocytes and
melanocytes are known to produce various cytokines and chemokines
(17,18,21). As all 10 TLRs are expressed in
skin fibroblasts, whether these TLRs are functional or not was
questioned. Therefore, skin fibroblasts were stimulated with each
available TLR ligand (TLR10 ligand was unavailable for purchasing)
for 48 h and the protein expression of IL-6 and IL-8 induced by
each TLR ligand was analyzed. The concentrations of each TLR ligand
were screened and determined using the information in previously
published studies (17,18). IL-6 and IL-8 were selected as
representatives of cytokines and chemokines, respectively. All the
TLR ligands induced the production of IL-6 (Fig. 2A) and IL-8 (Fig. 2B) in a dose-dependent manner,
except IL-8 and IL-6 in the activation of TLR8 and 9, respectively.
Activation of TLR8 and 9 induced IL-6 and IL-8 compared to the
activation of the other TLRs.
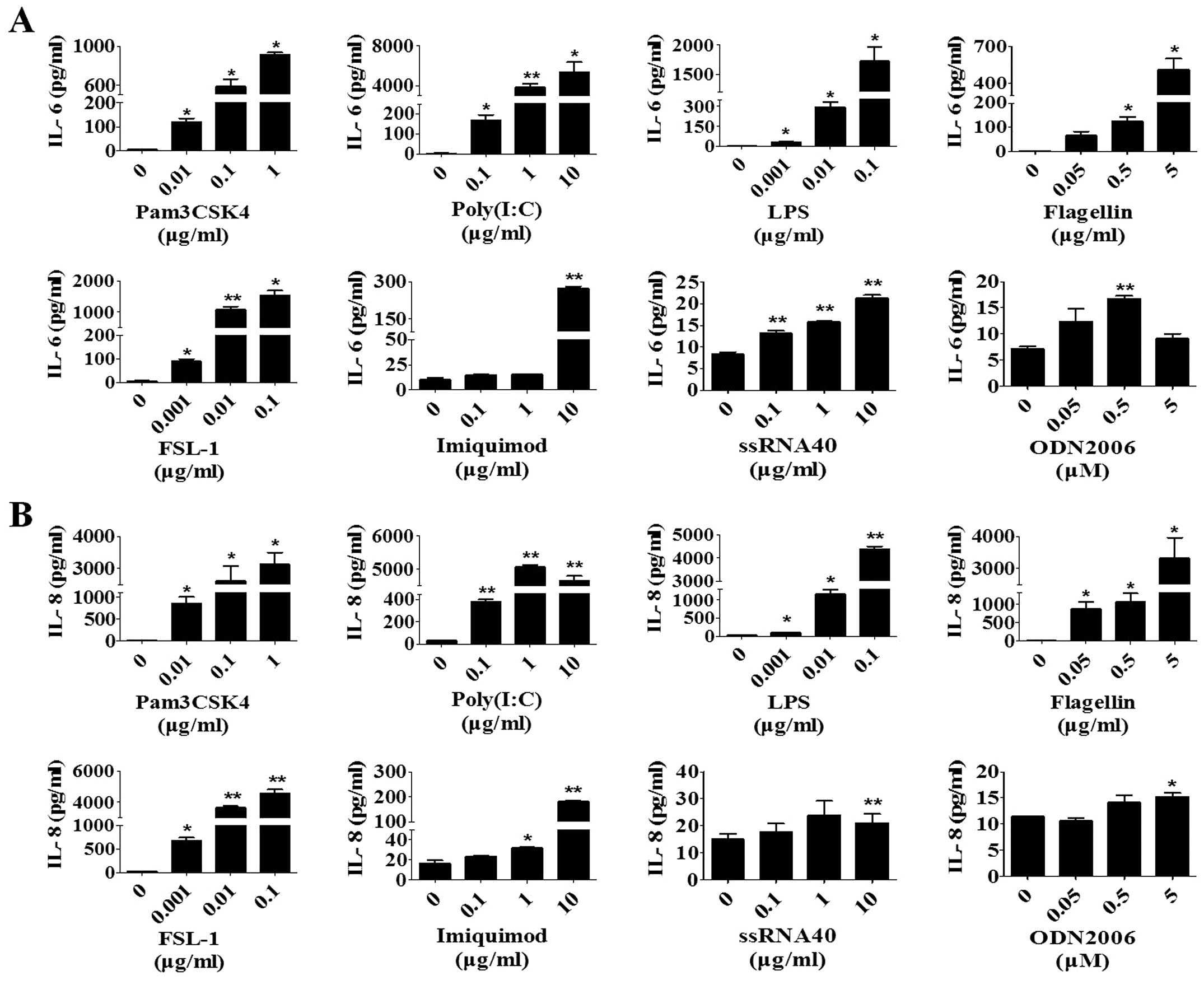 | Figure 2Activation of TLRs induces the
expression of IL-6 and IL-8 in human skin fibroblasts. After
culture in basal medium for 48 h, skin fibroblasts were incubated
with different concentrations of Pam3CSK4 (TLR1/2 ligand), poly
(I:C) (TLR3 ligand), LPS (TLR4 ligand), flagellin (TLR5 ligand),
FSL-1 (TLR2/6 ligand), imiquimod (TLR7 ligand), ssRNA40 (TLR8
ligand) and ODN2006 (TLR9 ligand). At 48 h after treatment, the
concentrations of (A) IL-6 and (B) IL-8 in the cell culture medium
were measured with specific ELISA. The results expressed as mean ±
standard error of the mean of triplicate cultures are from one
experiment representative of three with different donors.
*P<0.05, **P<0.01 vs. corresponding
control. TLR, Toll-like receptor; IL, interleukin; LPS,
lipopolysaccharide; ssRNA, single-stranded RNA; ELISA,
enzyme-linked immunosorbent assay. |
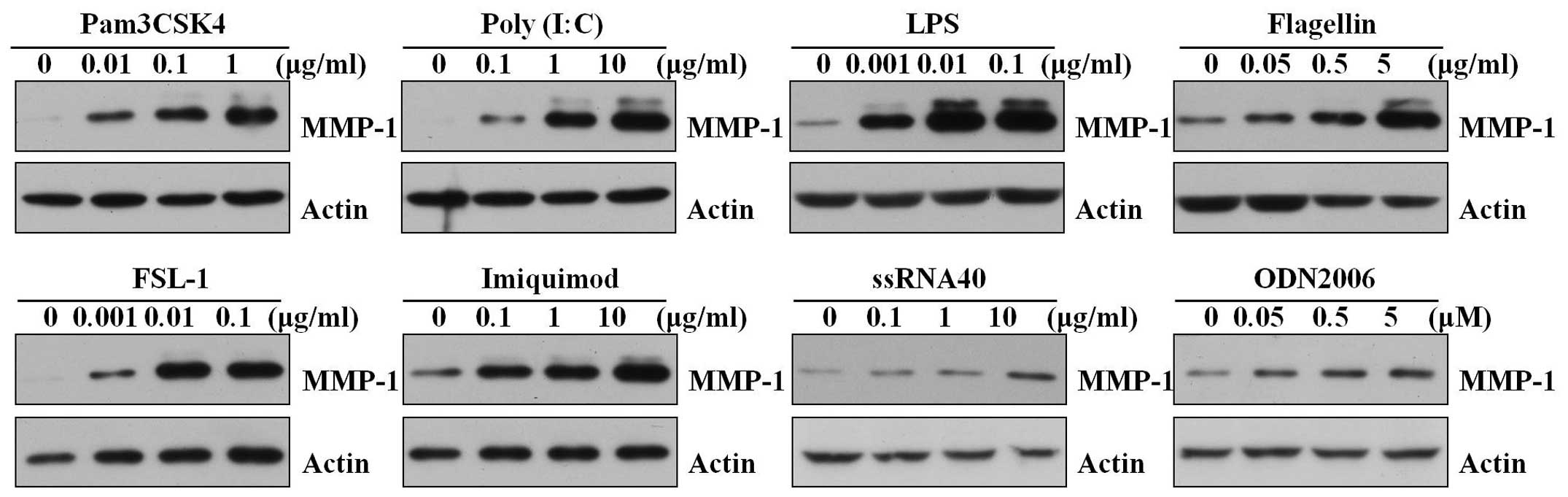
The expression of members of the MMP family is
important during inflammation, cell migration and during the
remodeling of damaged tissues (22,23). In addition to IL-6 and IL-8,
whether the activation of TLRs in skin fibroblasts induced MMPs,
such as MMP-1, was investigated. All the TLR ligands induced the
production of MMP-1 in a dose-dependent manner (Fig. 3). Activation of TLR8 and 9 induced
MMP-1 compared to the activation of the other TLRs.
Taken together, these results suggest that TLR1-9 in
human skin fibroblasts are functional and that activation of these
TLRs can induce the expression of IL-6, IL-8 and MMP-1.
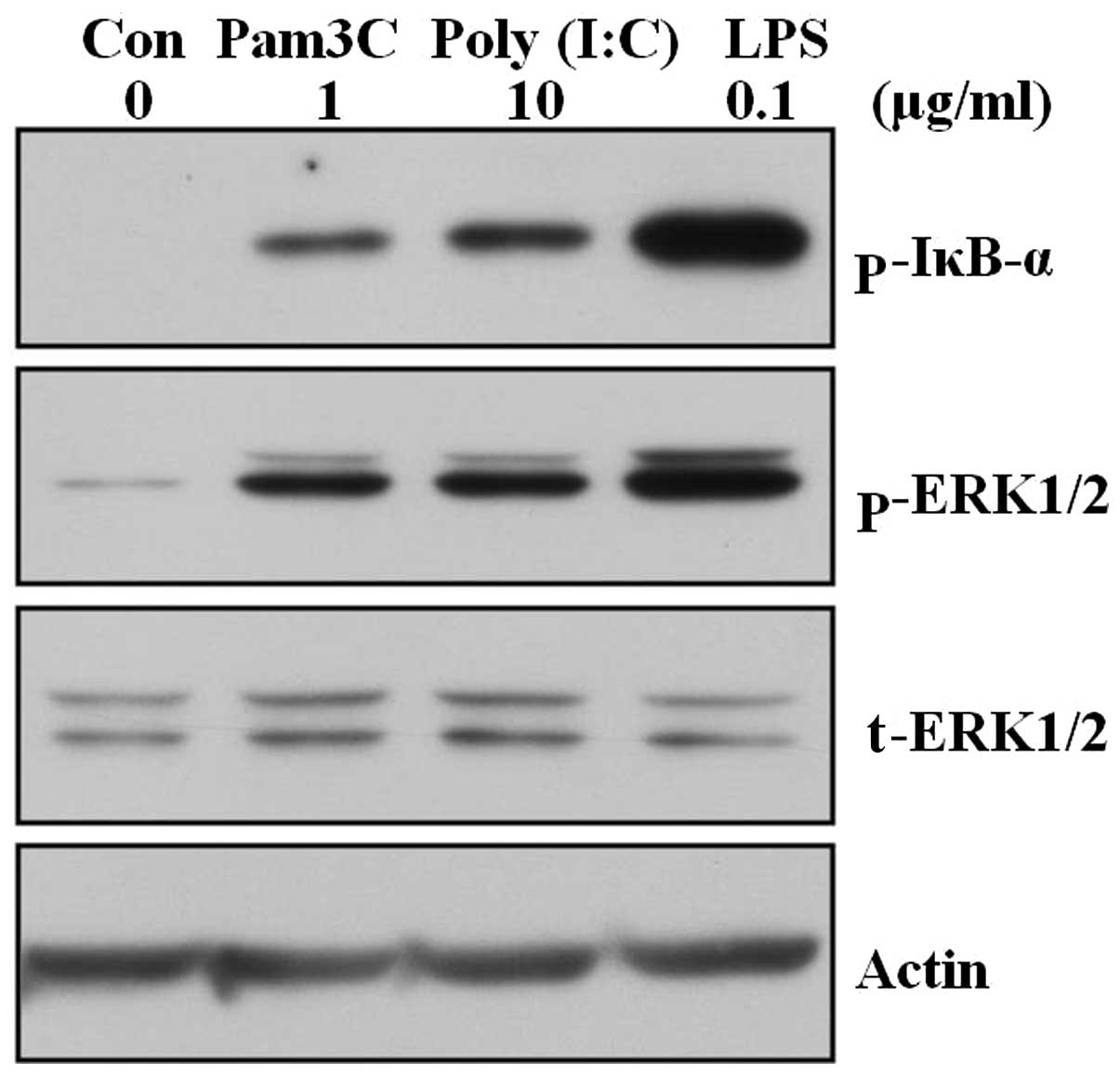
Triggering TLR1/2, 3 and 4 by each ligand
induces phosphorylation of IκBα and activation of ERK in human skin
fibroblasts
Activation of NF-κB and mitogen-activated protein
(MAP) kinases has been shown to play a central role in TLR-mediated
cellular activation and gene expression in a variety of cell types
(17,18,21). Therefore, whether triggering TLRs
with each ligand can induce the activation of NF-κB and MAP kinase
signaling pathways in skin fibroblasts was tested. Skin fibroblasts
were stimulated with TLR1/2, 3 and 4 ligands and harvested at 3 h
after treatment. The samples were assessed by western blot analysis
for the phosphorylation of IκBα, which is frequently used as one of
the markers for activation of the NF-κB pathway, and active
phosphorylation of ERK, which is representative of MAP kinases.
Treatment of TLR1/2, 3 and 4 ligands induced phosphorylation of
IκBα and active phosphorylation of ERK (Fig. 4).
In conclusion, the data suggest that activation of
TLR can induce activation of the NF-κB and ERK pathways in skin
fibroblasts.
Expression levels of TLR family members
are higher in skin fibroblasts compared to skin keratinocytes
Skin keratinocytes are the major cells expressed in
the epidermis, which is the first defense line of the skin against
pathogens. The expression levels of TLRs were well-studied in skin
keratinocytes in several research studies (17,24). To improve the understanding of the
TLR expression levels in skin fibroblasts, the expression levels of
TLRs in skin keratinocytes were compared with fibroblasts.
To eliminate the possibility that the expression
level of TLRs could be affected by different cell culture
conditions, growth culture (Fig.
5A) and basal culture conditions (Fig. 5B) were used. Consistent with the
previously published studies (17), all the TLRs were constitutively
expressed in skin keratinocytes, except TLR7 and 8 (Fig. 5A and B). Regardless of culture
conditions, each TLR was expressed much higher in skin fibroblasts
compared to skin keratinocytes (Fig.
5A and B). Furthermore, these results were not dependent on
different endogenous controls, as the same results were almost
obtained when β-actin was used as an endogenous control (data not
shown). Subsequently, the protein expression levels of certain TLRs
between fibroblasts and keratinocytes were confirmed. The protein
expression levels of TLR2, 3 and 4 are much higher in skin
fibroblasts compared to keratinocytes (Fig. 5C).
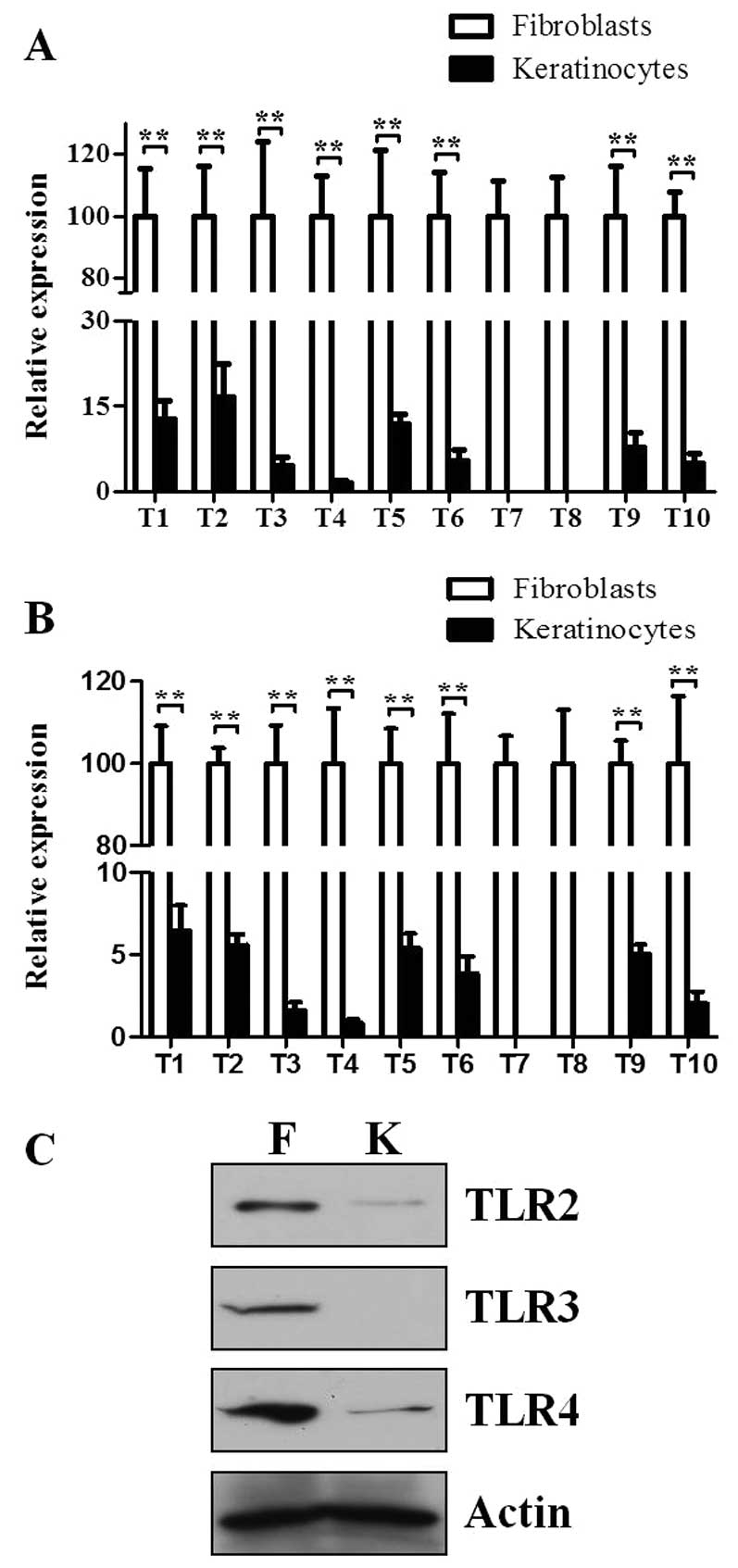 | Figure 5Expression levels of the TLR family
members are higher in skin fibroblasts compared to skin
keratinocytes. RNA was extracted from skin fibroblasts and
keratinocytes obtained from four different donors. An equal amount
of cDNA was used to assess mRNA expression of TLRs by RT-qPCR.
36B4 was used as an endogenous control. (A) Cells were
cultured in growth medium (DMEM containing 10% FBS for fibroblasts
and keratinocyte growth medium for keratinocytes, respectively).
(B) Cells were first cultured in growth medium for 24 h and were
maintained in basal medium (DMEM containing 0.1% FBS for
fibroblasts and keratinocyte basal medium for keratinocytes,
respectively) for 48 h. Data are presented as mean ± standard error
of the mean (n=4; **P<0.01). (C) Cells were cultured
in growth medium for 24 h and were maintained in basal medium,
after which lysates were prepared and the protein levels of TLR2, 3
and 4 were analyzed by western blotting. A single representative
experiment is shown from three different experiments. TLR,
Toll-like receptor; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; DMEM, Dulbecco’s modified Eagle’s media;
FBS, fetal bovine serum; F, fibroblasts; K, keratinocytes. |
Taken together, the data indicate that the
expression level of each TLR was higher in skin fibroblasts
compared to skin keratinocytes.
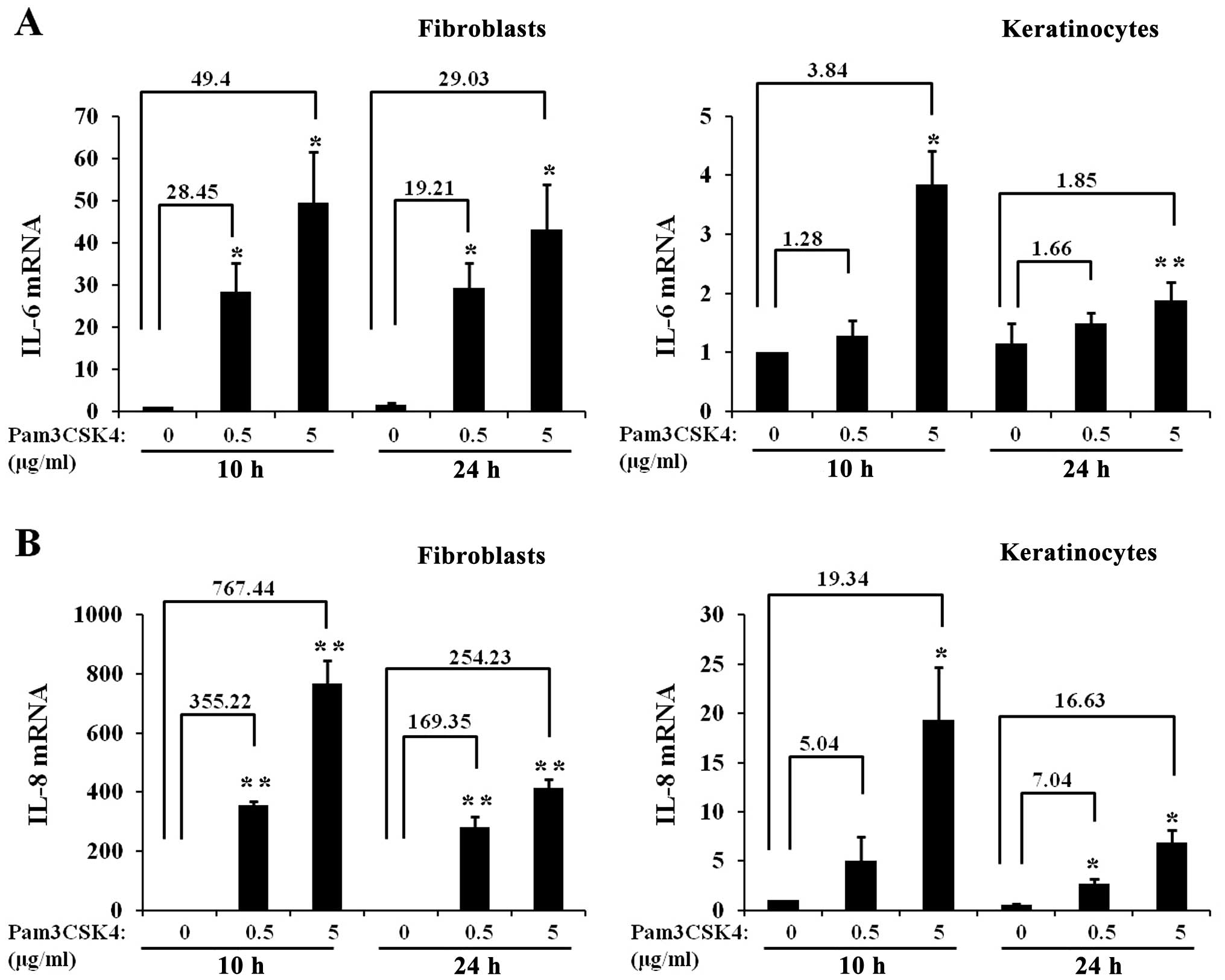
Fold increases in IL-6 and IL-8 mRNA
expression level upon exposure to a TLR1/2 ligand are much higher
in skin fibroblasts compared to skin keratinocytes
To confirm the competence of TLR signaling in skin
fibroblasts, the activation of TLR1/2 signaling pathways in skin
fibroblasts was compared with those in skin keratinocytes.
Fibroblasts and keratinocytes were treated with Pam3CSK4 and the
cells were harvested at 10 and 24 h after treatment. Subsequently,
the mRNA levels of IL-6 and IL-8 were analyzed by
RT-qPCR, and the fold-increase was calculated in fibroblasts and
keratinocytes. Pam3CSK4 treatment increased mRNA levels of
IL-6 (Fig. 6A) and
IL-8 (Fig. 6B) in
fibroblasts and keratinocytes. However, at the two time points and
concentrations, the fold-increase in IL-6 and IL-8
mRNA expression levels induced by Pam3CSK4 treatment was much
higher in fibroblasts compared to keratinocytes.
Therefore, these results indicate that TLR signaling
pathways in skin fibroblasts are functional and extremely active,
and suggest that TLRs in skin fibroblasts may provide strong
responses to pathogens and/or danger signals.
Discussion
In the present study, all 10 TLR family members were
constitutively expressed in skin fibroblasts (Fig. 1), which is in agreement with the
previously published results (19,20). To understand whether the TLRs in
skin fibroblasts are functional, skin fibroblasts were treated with
each TLR ligand. Treatment with TLR1/2, 3, 4, 5, 2/6, 7, 8 and 9
ligands induced protein expression levels of IL-6, IL-8 and MMP-1,
which are representatives of cytokines, chemokines and MMPs,
respectively (Figs. 2 and
3). Since the effects of TLR1-9
activation by each ligand on the protein expression levels of IL-6,
IL-8 and MMP-1 were not systemically examined in skin fibroblasts
previously, the present data may extend the knowledge to understand
expression and activation of TLRs in skin fibroblasts.
UV can directly activate growth factor or cytokine
receptors on the irradiated skin cells, leading to AP-1 activation
and MMP-1 upregulation, which degrades extracellular matrix
proteins, including collagen (25). Decreased collagen in skin is
responsible for the characteristic aged appearance of photo-damaged
skin (25,26). UV irradiation on keratinocytes can
also induce the expression of HSP60 (27) and damage non-coding RNA (14). HSP60 is recognized in murine and
human cells via TLR2 and 4 (28).
UV-damaged RNA can be recognized by TLR3 in healthy keratinocytes
(14). Taken together with the
present finding that treatment of TLR1/2, 3 and 4 ligands can
strongly induce MMP-1 expression in skin fibroblasts (Fig. 3), UV can possibly indirectly
increase MMP-1 expression in skin fibroblasts through activation of
TLR pathways by an increased in the generation of endogenous TLR
ligands, which may eventually contribute to photoaging.
Activation of NF-κB and MAP kinases plays a central
role in TLR-mediated cellular activation and gene expression in a
variety of cell types (17,18,21). NF-κB activation is essential for
the upregulation of several cytokines and chemokines, including
IL-6 and IL-8 (29,30). Activation of MAP kinases (ERK1/2,
c-Jun N-terminal kinase and p38) is known to increase the
expression of MMPs in skin cells (25). Therefore, whether triggering TLRs
can give rise to activation of NF-κB and MAP kinases in skin
fibroblasts was investigated. TLR signaling pathways can be divided
into MyD88 and TRIF pathways (3,15).
TLR2, 3 and 4 are known to initiate signaling by MyD88, TRIF or the
two pathways, respectively (3,15).
Thus, the ligands of TLR1/2, 3 and 4 were used to study NF-κB and
MAP kinases signaling in fibroblasts. Pam3CSK4, poly (I:C) and LPS
induced the phosphorylation of IκBα (Fig. 4). Phosphorylation of IκBα is known
to induce the degradation of IκBα and the translocation of NF-κB
into the nucleus, and subsequently leads to transcription of
NF-κB-sensitive genes, such as IL-6 and IL-8. The
present finding also supports the notion that IκBα is an important
cellular factor involved in the regulation of the host innate
antimicrobial response (31). In
addition, ERK1/2 was phosphorylated at 3 h after Pam3CSK4, poly
(I:C) and LPS treatment (Fig. 4).
The data suggest that MAP kinases can be activated by TLR ligands,
and that the activated MAP kinases may contribute to the
upregulation of MMP-1 expression by TLR ligands in skin
fibroblasts.
The epidermis is generally considered as the first
defense line of skin against pathogens and skin keratinocytes are
major cells in the epidermis. To this point, the expression level
of certain (if not all) TLR family members may be higher in skin
keratinocytes compared to skin fibroblasts. Thus, the expression
levels of TLRs in skin keratinocytes with fibroblasts were
compared. Of note, the relative expression levels of each TLR
family member were much higher in fibroblasts compared to
keratinocytes, regardless of cell culture conditions. The protein
expression levels of TLR2, 3 and 4 were also checked and compared
between fibroblasts and keratinocytes using each specific antibody.
Consistent with the mRNA levels, protein expression levels of TLR2,
3 and 4 are higher in skin fibroblasts compared to keratinocytes
(Fig. 5). These results were
noteworthy, considering that the epidermis is the first defense
line of skin against pathogens and that skin keratinocytes are
major cells in the epidermis. However, numerous studies also show
that in addition to exogenous ligands derived from pathogens,
various endogenous ligands can activate TLRs and initiate responses
to danger signals (13–14,32).
In addition, a recent study has shown that certain
TLR4 endogenous ligands, such as tenascin C and hyaluronic acid,
are elevated in scleroderma skin lesions, and has suggested that
TLR4 signaling activated by endogenous ligands in skin fibroblasts
may be involved in pathogenesis of scleroderma (33). Thus, high expression levels of
TLRs found in skin fibroblasts may play important roles when they
are activated by exogenous and/or endogenous ligands. In addition
to the cell culture system, it may also be necessary to compare the
expression levels of TLRs in fibroblasts with those in
keratinocytes in skin tissue. Since differentiated skin
keratinocytes may have different expression levels of TLRs in skin
tissue, the expression patterns of TLRs in skin tissue will be
investigated in our future study. Taken together, these results
show that TLRs are highly expressed in skin fibroblasts in
vitro and indicate that TLRs in skin fibroblasts may play an
important role on numerous physiological or pathological
conditions.
Finally, to check the competence of TLR signaling
pathways in skin fibroblasts, the activities of TLR signaling
pathways were compared in fibroblasts with those in keratinocytes.
Since the TLR1/2 signaling pathway has been well-studied in skin
keratinocytes in several research studies (34,35), the activities of TLR1/2 signaling
pathways in fibroblasts were compared with those in keratinocytes.
Fibroblasts and keratinocytes were treated with TLR1/2 ligand
Pam3CSK4 for 10 and 24 h, and the expression level of IL-6 and IL-8
were analyzed. Since fibroblasts and keratinocytes are different
types of cells, and IL-6 and IL-8 are secreted cytokines, it may be
difficult to normalize and compare the expression levels of these
proteins in cell culture media between fibroblasts and
keratinocytes. Therefore, the mRNA levels of IL-6 and
IL-8 were analyzed by RT-qPCR, in which expression levels
can be easily normalized by the same housekeeping gene. As shown in
Fig. 6, Pam3CSK4 treatment
increased the mRNA levels of IL-6 and IL-8 in
fibroblasts and keratinocytes and the Pam3CSK4-induced expression
folds of IL-6 and IL-8 were much higher in
fibroblasts compared to keratinocytes at every time point and
concentration. For example, after treatment with 0.5 μg/ml
of Pam3CSK4 for 10 h, the IL-6 mRNA level was increased by
~28.45-fold in skin fibroblasts but only ~1.28-fold in skin
keratinocytes, as compared with their non-treated controls. These
data may result, at least partially, from the fact that expression
levels of TLR1 and 2 are higher in fibroblasts compared to
keratinocytes (Fig. 5). Thus, the
data indicate that activation of the TLR signaling pathway induced
by Pam3CSK4 appears to be much stronger in fibroblasts compared to
keratinocytes and suggest that TLR signaling pathways in
fibroblasts may play important roles in the host defense against
infection and/or in response to danger signals.
In conclusion, the present findings provide evidence
for the constitutive expression levels of TLR family members and
their functional responses to each TLR ligand in skin fibroblasts,
and suggest that TLRs in skin fibroblasts may play an important
role in the detection of and response to different classes of
pathogens and/or danger signals.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea grant funded by the Korea Government
(Ministry of Science, ICT and Future Planning) (no.
2009-0092835).
References
|
1
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar
|
|
3
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
4
|
Takeuchi O, Kaufmann A, Grote K, et al:
Cutting edge: preferentially the R-stereoisomer of the mycoplasmal
lipopeptide macrophage-activating lipopeptide-2 activates immune
cells through a toll-like receptor 2- and MyD88-dependent signaling
pathway. J Immunol. 164:554–557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeuchi O, Sato S, Horiuchi T, et al:
Cutting edge: role of Toll-like receptor 1 in mediating immune
response to microbial lipoproteins. J Immunol. 169:10–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeuchi O, Kawai T, Muhlradt PF, et al:
Discrimination of bacterial lipoproteins by Toll-like receptor 6.
Int Immunol. 13:933–940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexopoulou L, Holt AC, Medzhitov R and
Flavell RA: Recognition of double-stranded RNA and activation of
NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tapping RI, Akashi S, Miyake K, Godowski
PJ and Tobias PS: Toll-like receptor 4, but not toll-like receptor
2, is a signaling receptor for Escherichia and Salmonella
lipopolysaccharides. J Immunol. 165:5780–5787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi F, Smith KD, Ozinsky A, et al: The
innate immune response to bacterial flagellin is mediated by
Toll-like receptor 5. Nature. 410:1099–1103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heil F, Hemmi H, Hochrein H, et al:
Species-specific recognition of single-stranded RNA via toll-like
receptor 7 and 8. Science. 303:1526–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hemmi H, Takeuchi O, Kawai T, et al: A
Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sloane JA, Blitz D, Margolin Z and
Vartanian T: A clear and present danger: endogenous ligands of
Toll-like receptors. Neuromolecular Med. 12:149–163. 2010.
View Article : Google Scholar :
|
|
13
|
Calderwood SK, Mambula SS and Gray PJ Jr:
Extracellular heat shock proteins in cell signaling and immunity.
Ann NY Acad Sci. 1113:28–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernard JJ, Cowing–Zitron C, Nakatsuji T,
et al: Ultraviolet radiation damages self noncoding RNA and is
detected by TLR3. Nat Med. 18:1286–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasai M and Yamamoto M: Pathogen
recognition receptors: ligands and signaling pathways by Toll-like
receptors. Int Rev Immunol. 32:116–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flacher V, Bouschbacher M, Verronese E, et
al: Human Langerhans cells express a specific TLR profile and
differentially respond to viruses and Gram-positive bacteria. J
Immunol. 177:7959–7967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lebre MC, van der Aar AM, van Baarsen L,
et al: Human keratinocytes express functional Toll-like receptor 3,
4, 5, and 9. J Invest Dermatol. 127:331–341. 2007. View Article : Google Scholar
|
|
18
|
Yu N, Zhang S, Zuo F, Kang K, Guan M and
Xiang L: Cultured human melanocytes express functional toll-like
receptors 2–4, 7 and 9. J Dermatol Sci. 56:113–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang S, Park JS, Won YH, Yun SJ and Kim
SJ: The expression of toll-like receptors (TLRs) in cultured human
skin fibroblast is modulated by histamine. Chonnam Med J. 48:7–14.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Hori K, Ding J, et al: Toll-like
receptors expressed by dermal fibroblasts contribute to
hypertrophic scarring. J Cell Physiol. 226:1265–1273. 2011.
View Article : Google Scholar
|
|
21
|
Lee Y, Kim H, Kim S, Kim KH and Chung JH:
Activation of toll-like receptors 2, 3 or 5 induces matrix
metalloproteinase-1 and -9 expression with the involvement of MAPKs
and NF-kappaB in human epidermal keratinocytes. Exp Dermatol.
19:e44–e49. 2010. View Article : Google Scholar
|
|
22
|
McMillan SJ, Kearley J, Campbell JD, et
al: Matrix metallopro-teinase-9 deficiency results in enhanced
allergen-induced airway inflammation. J Immunol. 172:2586–2594.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YM, Li WH, Kim YK, Kim KH and Chung
JH: Heat-induced MMP-1 expression is mediated by TRPV1 through
PKCalpha signaling in HaCaT cells. Exp Dermatol. 17:864–870. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kollisch G, Kalali BN, Voelcker V, et al:
Various members of the Toll-like receptor family contribute to the
innate immune response of human epidermal keratinocytes.
Immunology. 114:531–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XY, Tao CJ, Wu QY and Yuan CD:
Protein extract of ultraviolet-irradiated human skin keratinocytes
promote the expression of mitogen-activated protein kinases,
nuclear factor-kappaB and interferon regulatory factor-3 in
Langerhans cells via Toll-like receptor 2 and 4. Photodermatol
Photoimmunol Photomed. 29:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vabulas RM, Ahmad-Nejad P, da Costa C, et
al: Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to
activate the toll/interleukin-1 receptor signaling pathway in
innate immune cells. J Biol Chem. 276:31332–31339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Libermann TA and Baltimore D: Activation
of interleukin-6 gene expression through the NF-kappa B
transcription factor. Mol Cell Biol. 10:2327–2334. 1990.PubMed/NCBI
|
|
30
|
Elliott CL, Allport VC, Loudon JA, Wu GD
and Bennett PR: Nuclear factor-kappa B is essential for
up-regulation of interleukin-8 expression in human amnion and
cervical epithelial cells. Mol Hum Reprod. 7:787–790. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siebenlist U, Franzoso G and Brown K:
Structure, regulation and function of NF-kappa B. Annu Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu-Bryan R, Scott P, Sydlaske A, Rose DM
and Terkeltaub R: Innate immunity conferred by Toll-like receptors
2 and 4 and myeloid differentiation factor 88 expression is pivotal
to monosodium urate monohydrate crystal-induced inflammation.
Arthritis Rheum. 52:2936–2946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhattacharyya S, Kelley K, Melichian DS,
et al: Toll-like receptor 4 signaling augments transforming growth
factor-beta responses: a novel mechanism for maintaining and
amplifying fibrosis in scleroderma. Am J Pathol. 182:192–205. 2013.
View Article : Google Scholar :
|
|
34
|
Niebuhr M, Baumert K and Werfel T:
TLR-2-mediated cytokine and chemokine secretion in human
keratinocytes. Exp Dermatol. 19:873–877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meisgen F, Xu Landen N, Wang A, et al:
MiR-146a negatively regulates TLR2-induced inflammatory responses
in keratinocytes. J Invest Dermatol. 134:1931–1940. 2014.
View Article : Google Scholar : PubMed/NCBI
|