Introduction
Inflammatory bowel disease (IBD), including
ulcerative colitis (UC) and Crohn's disease (CD), is a group of
chronic inflammatory disorders of the gastrointestinal tract. The
incidence of IBD is more frequent in Western countries, but it is
rapidly increasing in Asian populations (1). Although the pathogenesis of IBD
remains unknown, genetic and environmental factors resulting in an
aberrant immune response to commensal bacteria seem to play a
pivotal role in the development of IBD (2). One of the key histological
characteristics of IBD, particularly in UC, is the accumulation of
neutrophils in crypt lumens. Neutrophils provide the first line of
cellular immune defense against foreign microbes. However,
uncontrolled neutrophil trafficking has been implicated in the
pathogenesis of IBD (3).
Human neutrophils generate four α-defensins, human
neutrophil peptides (HNPs) 1 to 4. We have previously reported that
the plasma concentrations of HNP 1–3 in patients with active UC are
higher than in healthy subjects or in those with inactive UC, CD or
infectious enterocolitis (4).
Thus, HNP 1–3 are considered to be useful biomarkers that may be
used to diagnose and predict treatment outcomes in patients with
UC. Moreover, we demonstrated that high concentrations of HNP-1
aggravated dextran sodium sulfate (DSS)-induced colitis by
elevating the levels of inflammatory cytokines, suggesting a
potential pro-inflammatory role for HNP-1 in colitis (5). In addition to their direct
antimicrobial abilities, HNPs have a broad range of immune
activation functions. HNPs are chemotactic in vitro for
human monocytes, T-cells and immature dendritic cells (6–8).
HNPs induce the production of interleukin-8 (IL-8, also known as
CXCL8) by epithelial cells of the lungs and bronchus (9–14),
monocytes (13), lung fibroblasts
(14), conjunctival epithelial
cells (15) and rheumatoid
fibroblast-like synoviocytes (16). IL-8 primarily mediates the
activation and migration of neutrophils into tissue from peripheral
blood. In addition to this pro-inflammatory function, IL-8 is also
known to be a potent promoter of angiogenesis (17). The increased expression of IL-8 in
the colonic tissues of patients with UC has been demonstrated and
may contribute to the pathogenesis of UC (18,19). The serum concentrations of IL-8
have also been shown to be related to the endoscopic and
histological severity of UC (20). In lung epithelial cells and
monocytes, the HNP-induced production of IL-8 is regulated by the
P2Y6 receptor (10,13). P2 receptors are activated by
extracellular nucleotides. These receptors are divided into two
subfamilies: ligand-gated ion channels (P2X) and G-protein-coupled
receptors (P2Y). Both P2X and P2Y are expressed widely throughout
the intestinal tract and participate in the regulation of a variety
of physiological functions (21).
However, the association among HNPs, IL-8 and P2 receptors in the
intestinal mucosa has not yet been investigated. In the present
study, we sought to determine whether HNP-1 induces IL-8 in
intestinal epithelial cells, and if so, to elucidate the mechanisms
that underlie this activity.
Materials and methods
Chemicals
The synthetic products of HNP-1 were purchased from
Peptide Institute, Inc. (Osaka, Japan). MEM and McCoy's 5A medium,
fetal bovine serum, penicillin-streptomycin, L-glutamine and the
IL-8 ELISA kit were obtained from Life Technologies Corp.
(Carlsbad, CA, USA). Suramin sodium (non-selective P2 receptor
antagonist) was obtained from Wako Pure Chemical Industries (Osaka,
Japan). Pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid)
tetrasodium salt hydrate (PPADS, another non-selective P2 receptor
antagonist) was obtained from Sigma-Aldrich Japan (Tokyo, Japan).
U0126 [extracellular signal-regulated kinase 1/2 (ERK1/2)
inhibitor] and SB203580 [p38 mitogen-activated protein kinase
(MAPK) inhibitor] were obtained from Calbiochem (Darmstadt,
Germany). MRS2578 (P2Y6-specific antagonist) was
obtained from Tocris Bioscience (Ellisville, MO, USA).
Cell culture
The human colon carcinoma cell line, Caco-2, was
obtained from RIKEN BioResource Center (Ibaraki, Japan). The Caco-2
cells were grown in minimal essential medium (MEM) containing 20%
heat-inactivated fetal bovine serum, 100 μg/ml streptomycin,
100 μg/ml penicillin and 2 mM L-glutamine. The Caco-2 cells
were incubated with 50 μg/ml HNP-1 with or without 100
μM suramin, 100 μM PPADS or 10 μM MRS2578 for
24 h. The human colon cancer cell line, HT-29, was obtained from DS
Pharma Biomedical Co., Ltd. (Osaka, Japan). The HT-29 cells were
grown in McCoy's 5A medium containing 10% heat-inactivated fetal
bovine serum, 100 μg/ml streptomycin, 100 μg/ml
penicillin and 2 mM L-glutamine. The HT-29 cells were incubated
with various concentrations of HNP-1 (0–50 μg/ml), or with
50 μg/ml HNP-1 with or without 100 μM suramin, 100
μM PPADS, 10 μM MRS2578, 1 or 5 μM U0126, or 1
or 5 μM SB203580 for 24 h. For western blot analysis, the
HT-29 cells were incubated with 50 μg/ml HNP-1 for 30 min.
Both cell lines were maintained in a humidified 5% CO2
incubator at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using Isogen
(Nippon Gene, Co., Ltd., Toyama, Japan) according to the
manufacturer's instructions. The RNA was reverse transcribed using
the PrimeScript RT reagent kit (Takara Bio, Otsu, Japan). The
synthesized cDNA was amplified using SYBR Premix Ex Taq II (Takara
Bio) and analyzed using the StepOnePlus Real-Time PCR system and
StepOne Software version 2.0 (Applied Biosystems, Foster City, CA,
USA). The primers for IL-8 (Primer set ID: HA032483),
P2Y2 (HA086668) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) (HA067812) were purchased from Takara Bio.
The cycling conditions were as follows: one cycle at 95°C for 30
sec followed by 35 cycles each at 95°C for 5 sec and 60°C for 34
sec. To normalize the amount of total RNA present in each reaction,
the GAPDH gene was used as an internal standard.
RNA silencing of the P2Y2
receptor
Predesigned short interfering RNA (siRNA) specific
for human P2Y2 (Stealth RNAi, siRNA ID: HSS143207) and
the negative control (Stealth RNAi siRNA Negative Control),
Lipofectamine RNAiMAX transfection reagent and Opti-MEM were
purchased from Life Technologies Corp. The siRNA was mixed with
Lipofectamine RNAiMAX in Opti-MEM and allowed to form complexes for
20 min at room temperature. The complexes were then added to 50%
confluent HT-29 cells.
Western blot analysis
Equal amounts of cell lysates from the HT-29 cells
were run on 10% sodium dodecylsulfate polyacrylamide gels and
electroblotted onto polyvinylidene fluoride membranes. After
blocking overnight at 4°C with 5% non-fat milk, the blots were
probed with primary antibodies for 1 h at room temperature.
Polyclonal rabbit antibodies against phosphorylated ERK1/2
(p-ERK1/2; 9101) and phosphorylated c-jun N-terminal kinase (p-JNK;
9251), as well as monoclonal rabbit antibody against phosphorylated
p38 MAPK (p-p38 MAPK; 4511) were purchased from Cell Signaling
Technology (Danvers, MA, USA). Monoclonal mouse antibody against
β-actin (A5441) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). After incubating the membrane with the appropriate
peroxidase-conjugated secondary antibodies (MP Biomedicals, Santa
Ana, CA, USA) for 1 h at room temperature, the reactivity was
visualized using an electro-generated chemiluminescence detection
kit (GE Healthcare Biosciences, Tokyo, Japan).
Statistical analysis
All experiments were repeated three times with cells
at different passage numbers. Statistical analysis was performed
using Tukey's honest significant difference method with SPSS 15.0J
software (SPSS, Inc., Chicago, IL, USA) A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
HNP-1 upregulates IL-8 expression partly
through P2Y6 receptors in Caco-2 cells
We first investigated whether HNP-1 increases IL-8
expression in intestinal epithelial cells by using Caco-2 cells
that possess mRNA for several P2 receptor subtypes, including
P2Y6 (22,23). Incubation of the Caco-2 cells with
50 μg/ml HNP-1 significantly increased the mRNA expression
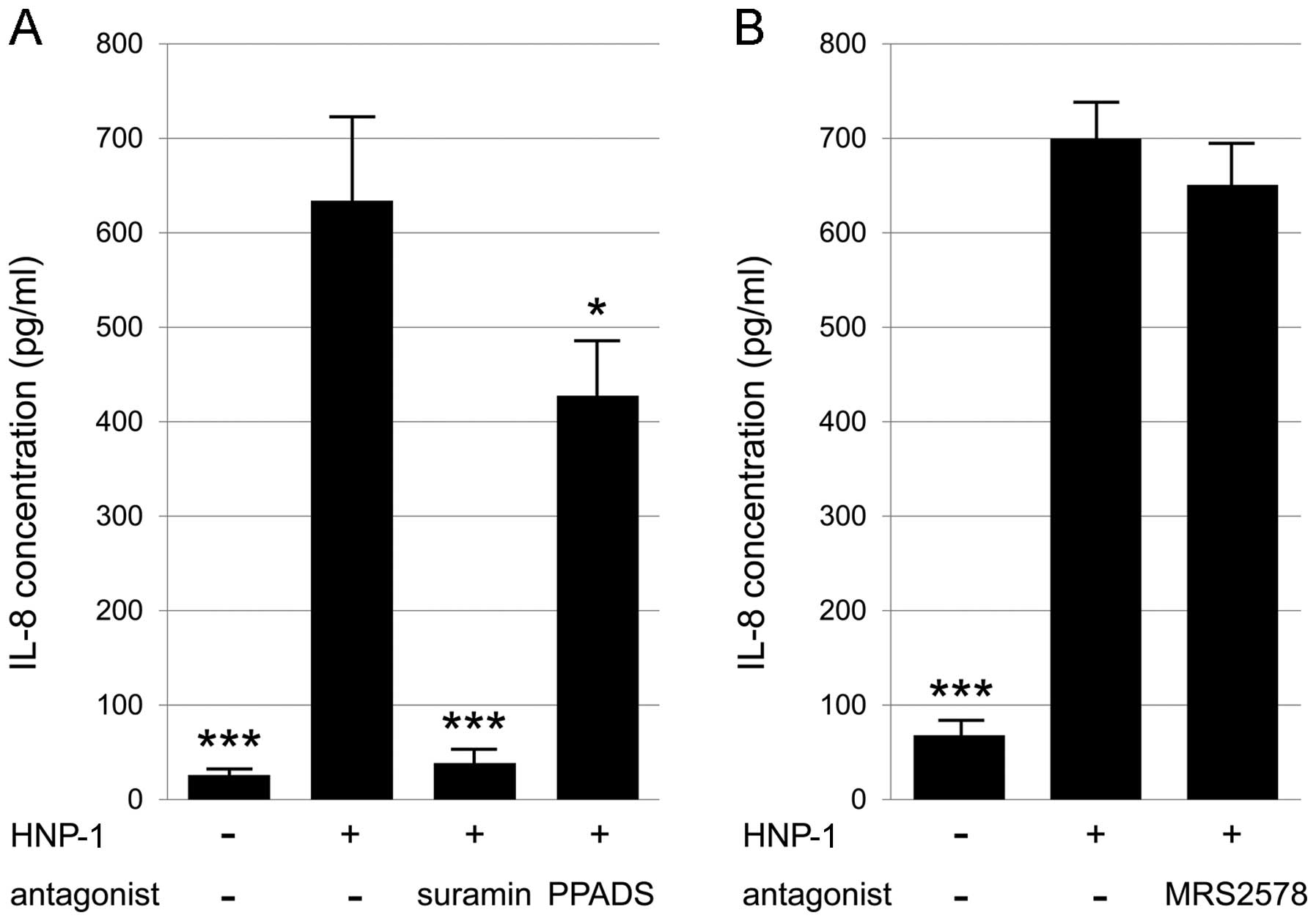
of IL-8 (Fig. 1A). To determine
the involvement of P2 receptors in the HNP-1-induced expression of
IL-8, the Caco-2 cells were treated with two non-selective P2
receptor antagonists, suramin and PPADS. Both antagonists
significantly blocked the HNP-1-induced expression of IL-8
(Fig. 1A). In addition, treatment
with the P2Y6-specific antagonist, MRS2578,
significantly decreased the expression of IL-8 (Fig. 1B). These data suggest that HNP-1
induces IL-8 expression through the P2Y6 signaling
pathway in intestinal epithelial cells. However, MRS2578 only
caused a partial reduction (37%) in IL-8 expression (Fig. 1B), suggesting that P2 receptors
other than P2Y6 are involved in the HNP-1-induced IL-8
expression.
HNP-1 significantly increases IL-8
production through P2 receptors in P2Y6-negative HT-29
cells
To determine the non-P2Y6-mediated
mechanisms underlying the HNP-1 induction of IL-8, we used HT-29
cells in the subsequent experiments, since HT-29 cells have no, or
very low levels of P2Y6 mRNA expression (24). Exposure of the HT-29 cells to
HNP-1 significantly increased IL-8 mRNA expression in a
dose-dependent manner (Fig. 2A).
Consistent with the induction of IL-8 expression, the release of
IL-8 protein by the HT-29 cells was significantly enhanced by HNP-1
(Fig. 2B). This increase was
effectively blocked by suramin and slightly, although significantly
by PPADS (Fig. 3A), indicating
the involvement of P2 receptors in the HNP-1-induced production of
IL-8 by HT-29 cells, despite the absence of P2Y6.
Treatment of the HT-29 cells with MRS2578 had no effect on the
expression of IL-8, as was expected (Fig. 3B).
P2 receptors, other than P2Y2
and P2Y6 subtypes are involved in the HNP-1-induced
production of IL-8 by HT-29 cells
In addition to P2Y6 receptors, the
P2Y2 and P2X7 receptors are involved in the
production of IL-8 by epithelial cells. The activation of
P2Y2 and P2X7 induces the release of IL-8 in
renal epithelial cells and bronchial epithelial cells, respectively
(25,26). HT-29 cells express the receptor
for P2Y2 (24,27) but not the one for P2X7
(28). Although P2Y2
was not antagonized by PPADS, the involvement of P2Y2 in
the HNP-1-induced production of IL-8 could not be excluded, as the
inhibitory effects of PPADS on IL-8 production were much weaker
than those of suramin. Therefore, we investigated the possibility
that P2Y2 is the receptor primarily responsible for the
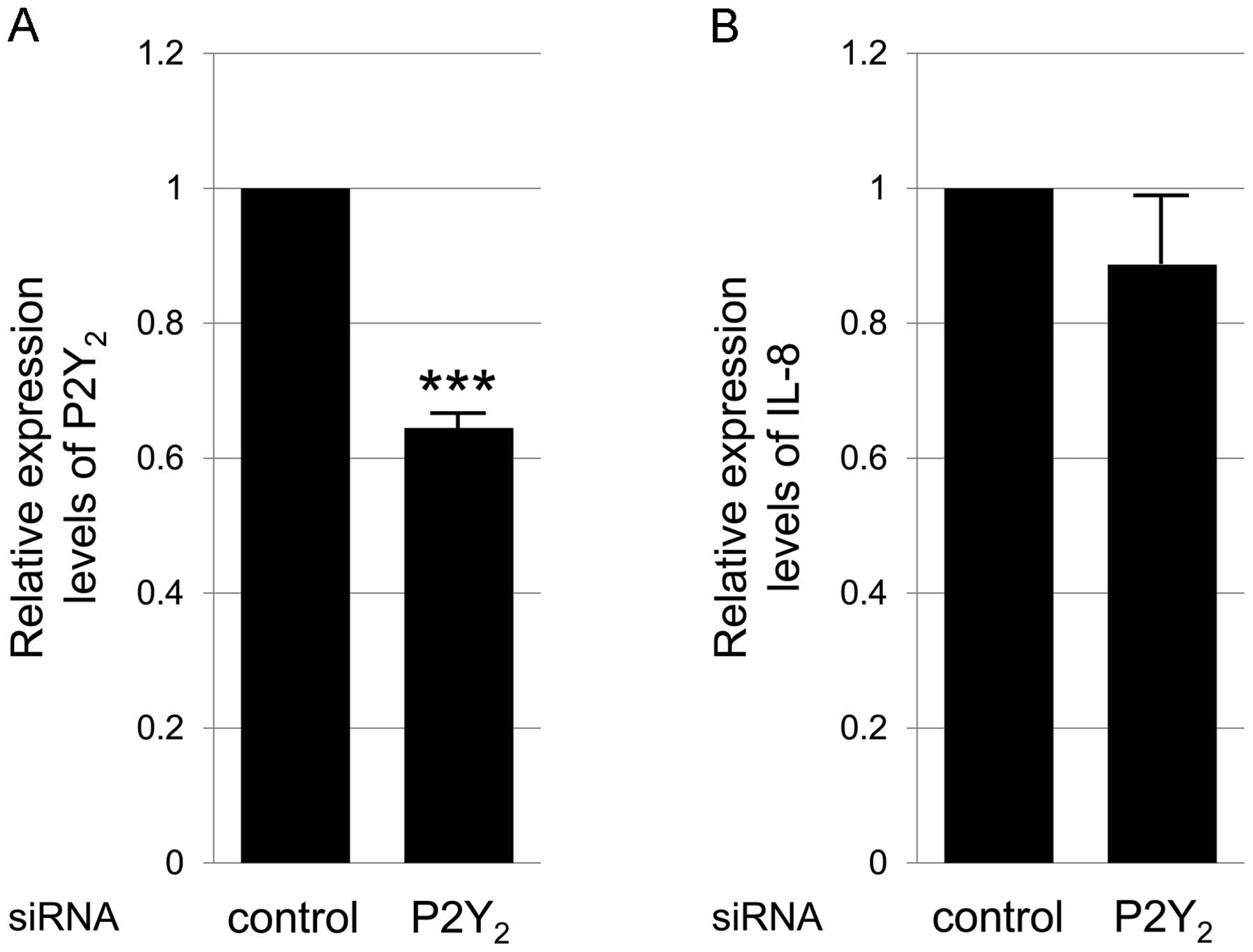
HNP-1-induced production of IL-8 in HT-29 cells. As definitive
antagonists of P2Y2 are not currently available, we
applied P2Y2 siRNA and analyzed IL-8 expression
following treatment with HNP-1. The silencing of P2Y2
decreased the mRNA expression level of P2Y2 by 40%,
which was a significant decrease (Fig. 4A); however, the expression of IL-8
following treatment with HNP-1 was not altered (Fig. 4B). These results indicate that P2
receptors, other than the P2Y2 and P2Y6
subtypes are involved in the HNP-1-induced production of IL-8 by
HT-29 cells.
HNP-1-induced production of IL-8 by HT-29
cells is dependent on ERK1/2 activation
In the Caco-2/15 cells, the increased production of
IL-8 downstream of P2Y6 activation is dependent on the
ERK1/2 signaling pathway (29).
ERK1/2 activation is involved in the HNP-induced production of IL-8
in lung epithelial cells and monocytes, whereas p38 MAPK activation
is required for IL-8 production only in monocytes (13). Moreover, it was recently reported
that the HNP-1-induced production of IL-8 in rheumatoid
fibroblast-like synoviocytes is regulated by the JNK and ERK
signaling pathways (16). Thus,
we sought to determine which MAPK signaling pathways are involved
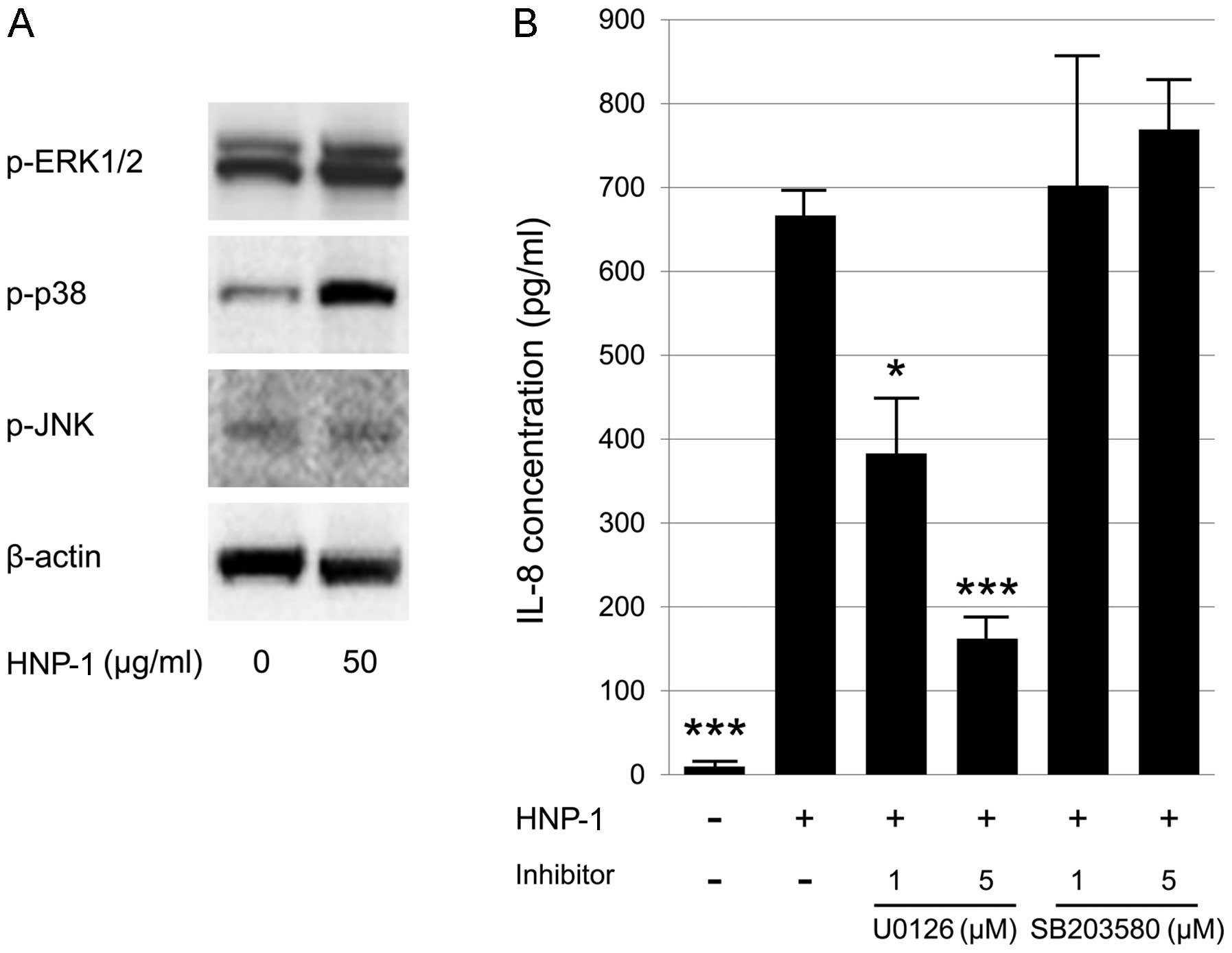
in the HNP-1-induced production of IL-8 by HT-29 cells. Using
western blot analysis, we found that HNP-1 induced the
phosphorylation of ERK1/2 and p38 MAPK, while no significant
changes were observed in JNK activity in the HT-29 cells (Fig. 5A). To identify the relevant
signaling pathway involved in the HNP-1-induced production of IL-8,
we treated the HT-29 cells with specific inhibitors of ERK1/2
(U0126) and p38 MAPK (SB203580). Treatment with U0126 significantly
reduced the HNP-1-induced production of IL-8; however, the addition
of SB203580 did not have any significant inhibitory effects on IL-8
production (Fig. 5B). These
results suggest that the HNP-1-induced production of IL-8 is
dependent on ERK1/2 activation in intestinal epithelial cells.
Discussion
In the present study, to the best of our knowledge,
we demonstrate for the first time the induction of IL-8 by HNP-1 in
intestinal epithelial cells. Our results suggest that HNP-1
released from infiltrated neutrophils induces IL-8 production by
the intestinal mucosa. As a result of the increase in IL-8
expression, neutrophils are recruited to the site of inflammation,
where they contribute to the extended tissue damage observed in
patients with IBD.
HNP-1 induced IL-8 expression partly through
P2Y6 in Caco-2 cells. The involvement of P2Y6
in IBD has previously been suggested. P2Y6 is highly
expressed in T-cells infiltrating active IBD (30). The mRNA expression levels of the
P2Y6 and P2Y2 receptors have been shown to be
upregulated in the colonic epithelium of patients with IBD and
DSS-treated mice (29). The
activation of P2Y6 by its natural ligand, UDP,
stimulates the sustained NaCl secretion in rat colonic enterocytes
(31). In addition to
P2Y6, we considered the involvement of other P2
receptors in HNP-1-induced IL-8 expression. Thus, we used HT-29
cells, which do not express P2Y6, in order to
investigate non-P2Y6-mediated mechanisms. As was the
case with the Caco-2 cells, HNP-1 significantly induced IL-8
production in the HT-29 cells, and this production was suppressed
by suramin and PPADS. We hypothesized that P2Y2 is
responsible for the HNP-1-induced production of IL-8 in HT-29
cells, as the P2Y2-mediated release of IL-8 by other
epithelial cell lines has been previously reported (25), and the inhibitory effects on IL-8
production by PPADS were much weaker than those exerted by suramin.
However, the silencing of P2Y2 had no effect on the
induction of IL-8. It has been reported that P2 receptors expressed
by HT-29 cells are those for P2Y1, P2Y2,
P2Y4 and P2Y11 (24,27,32). The selective P2Y1
antagonist, MRS2179, did not exert any significant inhibitory
effects on the HNP-1-induced production of IL-8 by HT-29 cells
(data not shown). The involvement of P2Y4 is unlikely as
its receptor is insensitive to suramin. Since PPADS is completely
inactive at the human P2Y11 receptor (33), this also does not appear to be a
dominant pathway involved in the HNP-1-induced production of IL-8.
Further studies on the identification of P2 receptors involved in
the HNP-induced production of IL-8 are required in order to better
understand the role of HNPs in intestinal epithelial cells.
Three MAPK pathways, the ERK1/2, JNK and p38 MAPK
cascades, contribute to the downstream activation of transcription
factors, including nuclear factor-κB (NF-κB) and activator
protein-1 (AP-1), both of which upregulate IL-8 transcription. The
involvement of particular MAPK signaling pathways in the induction
of IL-8 is dependent on the cell type and the stimulus (34). A previous study demonstrated that
the ERK1/2 and p38 MAPK pathways contribute to the secretion of
IL-8 by HT-29 cells in response to tumor necrosis factor (TNF)-α
(35). Our results revealed that
HNP-1 activated ERK1/2 and p38 MAPK in HT-29 cells. On the other
hand, the HNP-1-induced production of IL-8 was inhibited by the
blockade of ERK1/2 activation, but not by that of p38 MAPK,
indicating that ERK1/2 plays a pivotal role in IL-8 production.
Notably, P2Y6 receptor activation by UDP has been shown
to increase IL-8 production by Caco-2/15 cells through a mechanism
that is ERK1/2-dependent, but p38 MAPK-independent (29). Therefore, the HNP-1-induced
production of IL-8 appears to occur through the ERK1/2-dependent
signaling pathway in intestinal epithelial cells regardless of the
expression of P2Y6.
It has been shown that ERK1/2, p38 MAPK and JNK are
activated in the inflamed colonic mucosa of patients with IBD
(36). Mesalamine, a drug
effective in the treatment of IBD, has been shown to inhibit the
TNF-α-induced activation of ERK1/2 (37). A recent study using gene
expression profiling confirmed that the ERK/MAPK pathway is
regulated by mesalamine (38).
Another study demonstrated that the release of IL-8 triggered by
mucosal E. Coli isolated from IBD is mediated by the ERK1/2
and p38 MAPK pathways and inhibited by mesalamine, but not by
hydrocortisone (39). Hence, the
reduction in the HNP-induced production of IL-8 by the inhibition
of ERK1/2 may be part of the mechanism of action of mesalamine in
the treatment of IBD.
In conclusion, in the present study, we demonstrate
that the HNP-1-induced production of IL-8 in intestinal epithelial
cells is dependent, not only on P2Y6, but also on P2
receptors other than P2Y6. Moreover, we reveal that the
activation of the ERK1/2 pathway is required for the HNP-1-induced
production of IL-8 by intestinal epithelial cells. HNPs released by
infiltrating neutrophils in the UC intestine may stimulate
additional neutrophil accumulation by inducing IL-8. The findings
of the present study may lead to the development of novel
therapeutic strategies to reduce HNP-induced intestinal
inflammation.
References
|
1
|
Asakura K, Nishiwaki Y, Inoue N, Hibi T,
Watanabe M and Takebayashi T: Prevalence of ulcerative colitis and
Crohn's disease in Japan. J Gastroenterol. 44:659–665. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brazil JC, Louis NA and Parkos CA: The
role of polymorpho-nuclear leukocyte trafficking in the
perpetuation of inflammation during inflammatory bowel disease.
Inflamm Bowel Dis. 19:1556–1565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanmura S, Uto H, Numata M, Hashimoto S,
Moriuchi A, Fujita H, Oketani M, Ido A, Kodama M, Ohi H and
Tsubouchi H: Human neutrophil peptides 1–3 are useful biomarkers in
patients with active ulcerative colitis. Inflamm Bowel Dis.
15:909–917. 2009. View Article : Google Scholar
|
|
5
|
Hashimoto S, Uto H, Kanmura S, Sakiyama T,
Oku M, Iwashita Y, Ibusuki R, Sasaki F, Ibusuki K, Takami Y,
Moriuchi A, Oketani M, Ido A and Tsubouchi H: Human neutrophil
peptide-1 aggravates dextran sulfate sodium-induced colitis.
Inflamm Bowel Dis. 18:667–675. 2012. View Article : Google Scholar
|
|
6
|
Territo MC, Ganz T, Selsted ME and Lehrer
R: Monocyte-chemotactic activity of defensins from human
neutrophils. J Clin Invest. 84:2017–2020. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chertov O, Michiel DF, Xu L, Wang JM, Tani
K, Murphy WJ, Longo DL, Taub DD and Oppenheim JJ: Identification of
defensin-1, defensin-2, and CAP37/azurocidin as T-cell
chemoattractant proteins released from interleukin-8-stimulated
neutrophils. J Biol Chem. 271:2935–2940. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang D, Chen Q, Chertov O and Oppenheim
JJ: Human neutrophil defensins selectively chemoattract naive T and
immature dendritic cells. J Leukoc Biol. 68:9–14. 2000.PubMed/NCBI
|
|
9
|
van Wetering S, Mannesse-Lazeroms SP, van
Sterkenburg MA and Hiemstra PS: Neutrophil defensins stimulate the
release of cytokines by airway epithelial cells: modulation by
dexamethasone. Inflamm Res. 51:8–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khine AA, Del Sorbo L, Vaschetto R, Voglis
S, Tullis E, Slutsky AS, Downey GP and Zhang H: Human neutrophil
peptides induce interleukin-8 production through the
P2Y6 signaling pathway. Blood. 107:2936–2942. 2006.
View Article : Google Scholar
|
|
11
|
Sakamoto N, Mukae H, Fujii T, Ishii H,
Yoshioka S, Kakugawa T, Sugiyama K, Mizuta Y, Kadota J, Nakazato M
and Kohno S: Differential effects of alpha- and beta-defensin on
cytokine production by cultured human bronchial epithelial cells.
Am J Physiol Lung Cell Mol Physiol. 288:L508–L513. 2005. View Article : Google Scholar
|
|
12
|
Liu CY, Lin HC, Yu CT, Lin SM, Lee KY,
Chen HC, Chou CL, Huang CD, Chou PC, Liu WT, Wang CH and Kuo HP:
The concentration-dependent chemokine release and pro-apoptotic
effects of neutrophil-derived alpha-defensin-1 on human bronchial
and alveolar epithelial cells. Life Sci. 80:749–758. 2007.
View Article : Google Scholar
|
|
13
|
Syeda F, Liu HY, Tullis E, Liu M, Slutsky
AS and Zhang H: Differential signaling mechanisms of HNP-induced
IL-8 production in human lung epithelial cells and monocytes. J
Cell Physiol. 214:820–827. 2008. View Article : Google Scholar
|
|
14
|
Amenomori M, Mukae H, Ishimatsu Y,
Sakamoto N, Kakugawa T, Hara A, Hara S, Fujita H, Ishimoto H,
Hayashi T and Kohno S: Differential effects of human neutrophil
peptide-1 on growth factor and interleukin-8 production by human
lung fibroblasts and epithelial cells. Exp Lung Res. 36:411–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Zhu HY and Beuerman RW: Stimulation
of specific cytokines in human conjunctival epithelial cells by
defensins HNP1, HBD2, and HBD3. Invest Ophthalmol Vis Sci.
50:644–653. 2009. View Article : Google Scholar
|
|
16
|
Ahn JK, Huang B, Bae EK, Park EJ, Hwang
JW, Lee J, Koh EM and Cha HS: The role of α-defensin-1 and related
signal transduction mechanisms in the production of IL-6, IL-8 and
MMPs in rheumatoid fibroblast-like synoviocytes. Rheumatology
(Oxford). 52:1368–1376. 2013. View Article : Google Scholar
|
|
17
|
Koch AE, Polverini PJ, Kunkel SL, Harlow
LA, DiPietro LA, Elner VM, Elner SG and Strieter RM: Interleukin-8
as a macrophage-derived mediator of angiogenesis. Science.
258:1798–1801. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitsuyama K, Toyonaga A, Sasaki E,
Watanabe K, Tateishi H, Nishiyama T, Saiki T, Ikeda H, Tsuruta O
and Tanikawa K: IL-8 as an important chemoattractant for
neutrophils in ulcerative colitis and Crohn’s disease. Clin Exp
Immunol. 96:432–436. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazzucchelli L, Hauser C, Zgraggen K,
Wagner H, Hess M, Laissue JA and Mueller C: Expression of
interleukin-8 gene in inflammatory bowel disease is related to the
histological grade of active inflammation. Am J Pathol.
144:997–1007. 1994.PubMed/NCBI
|
|
20
|
Rodríguez-Perálvarez ML, García-Sánchez V,
Villar-Pastor CM, González R, Iglesias-Flores E, Muntane J and
Gómez-Camacho F: Role of serum cytokine profile in ulcerative
colitis assessment. Inflamm Bowel Dis. 18:1864–1871. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kolachala VL, Bajaj R, Chalasani M and
Sitaraman SV: Purinergic receptors in gastrointestinal
inflammation. Am J Physiol Gastrointest Liver Physiol.
294:G401–G410. 2008. View Article : Google Scholar
|
|
22
|
McAlroy HL, Ahmed S, Day SM, Baines DL,
Wong HY, Yip CY, Ko WH, Wilson SM and Collett A: Multiple P2Y
receptor subtypes in the apical membranes of polarized epithelial
cells. Br J Pharmacol. 131:1651–1658. 2000. View Article : Google Scholar
|
|
23
|
Coutinho-Silva R, Stahl L, Cheung KK, de
Campos NE, de Oliveira Souza C, Ojcius DM and Burnstock G: P2X and
P2Y purinergic receptors on human intestinal epithelial carcinoma
cells: effects of extracellular nucleotides on apoptosis and cell
proliferation. Am J Physiol Gastrointest Liver Physiol.
288:G1024–G1035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bahrami F, Kukulski F, Lecka J, Tremblay
A, Pelletier J, Rockenbach L and Sévigny J: Purine-metabolizing
ectoenzymes control IL-8 production in human colon HT-29 cells.
Mediators Inflamm. 2014:8798952014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kruse R, Säve S and Persson K: Adenosine
triphosphate induced P2Y2 receptor activation induces
proinflammatory cytokine release in uroepithelial cells. J Urol.
188:2419–2425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mortaz E, Henricks PA, Kraneveld AD, Givi
ME, Garssen J and Folkerts G: Cigarette smoke induces the release
of CXCL-8 from human bronchial epithelial cells via TLRs and
induction of the inflammasome. Biochim Biophys Acta.
1812:1104–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nylund G, Nordgren S and Delbro DS:
Expression of P2Y2 purinoceptors in MCG 101 murine
sarcoma cells, and HT-29 human colon carcinoma cells. Auton
Neurosci. 112:69–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otte JM, Zdebik AE, Brand S, Chromik AM,
Strauss S, Schmitz F, Steinstraesser L and Schmidt WE: Effects of
the cathelicidin LL-37 on intestinal epithelial barrier integrity.
Regul Pept. 156:104–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grbic DM, Degagné E, Langlois C, Dupuis AA
and Gendron FP: Intestinal inflammation increases the expression of
the P2Y6 receptor on epithelial cells and the release of
CXC chemokine ligand 8 by UDP. J Immunol. 180:2659–2668. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Somers GR, Hammet FM, Trute L, Southey MC
and Venter DJ: Expression of the P2Y6 purinergic
receptor in human T cells infiltrating inflammatory bowel disease.
Lab Invest. 78:1375–1383. 1998.PubMed/NCBI
|
|
31
|
Köttgen M, Löffler T, Jacobi C, Nitschke
R, Pavenstädt H, Schreiber R, Frische S, Nielsen S and Leipziger J:
P2Y6 receptor mediates colonic NaCl secretion via
differential activation of cAMP-mediated transport. J Clin Invest.
111:371–379. 2003. View Article : Google Scholar
|
|
32
|
Delbro DS, Nylund G and Nordgren S:
Demonstration of P2Y4 purinergic receptors in the HT-29
human colon cancer cell line. Auton Autacoid Pharmacol. 25:163–166.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Communi D, Robaye B and Boeynaems JM:
Pharmacological characterization of the human P2Y11
receptor. Br J Pharmacol. 128:1199–1206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoffmann E, Dittrich-Breiholz O, Holtmann
H and Kracht M: Multiple control of interleukin-8 gene expression.
J Leukoc Biol. 72:847–855. 2002.PubMed/NCBI
|
|
35
|
Jijon HB, Panenka WJ, Madsen KL and
Parsons HG: MAP kinases contribute to IL-8 secretion by intestinal
epithelial cells via a posttranscriptional mechanism. Am J Physiol
Cell Physiol. 283:C31–C41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waetzig GH and Schreiber S: Review
article: mitogen-activated protein kinases in chronic intestinal
inflammation - targeting ancient pathways to treat modern diseases.
Aliment Pharmacol Ther. 18:17–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaiser GC, Yan F and Polk DB: Mesalamine
blocks tumor necrosis factor growth inhibition and nuclear factor
kappaB activation in mouse colonocytes. Gastroenterology.
116:602–609. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khare V, Lyakhovich A, Dammann K, Lang M,
Borgmann M, Tichy B, Pospisilova S, Luciani G, Campregher C,
Evstatiev R, Pflueger M, Hundsberger H and Gasche C: Mesalamine
modulates intercellular adhesion through inhibition of p-21
activated kinase-1. Biochem Pharmacol. 85:234–244. 2013. View Article : Google Scholar :
|
|
39
|
Subramanian S, Rhodes JM, Hart CA, Tam B,
Roberts CL, Smith SL, Corkill JE, Winstanley C, Virji M and
Campbell BJ: Characterization of epithelial IL-8 response to
inflammatory bowel disease mucosal E. coli and its inhibition by
mesalamine. Inflamm Bowel Dis. 14:162–175. 2008. View Article : Google Scholar
|