Introduction
Regucalcin was discovered in 1978 as a novel
calcium-regulatory protein (1–4),
and has been demonstrated to play a multifunctional role in the
regulation of various types of cells and tissues (5–7).
The regucalcin gene (rgn) is localized on the X chromosome
and has been identified in over 15 species in vertebrates and
invertebrates (7–11). Regucalcin gene expression is
regulated by various transcription factors, including activator
protein-1 (AP-1), nuclear factor I-A1 (NF1-A1), regucalcin gene
promoter region-related protein (RGPR-p117) and β-catenin, which
are modulated through intracellular signaling factors related to
the phosphorylation and dephosphorylation of various proteins in
the cytoplasm and nucleus in vitro (11). Regucalcin is expressed in various
types of cells and tissues. Regucalcin gene expression is regulated
by various hormonal factors (11,12).
Regucalcin, which is present in the cytoplasm, is
translocated to the nucleus in various cell types dependent on the
activation of calcium signaling (13). Regucalcin plays a role in the
maintenance of intracellular calcium homeostasis, the inhibition of
various protein kinases, protein phosphatases and protein synthesis
in the cytoplasm and nucleus, as well as in the nuclear gene
expression and DNA and RNA syntheses in various cell types
(5–7,13).
Moreover, regucalcin has been shown to suppress cell proliferation
and apoptotic cell death, which is mediated through various
signaling factors (14,15). Regucalcin has been suggested to
play a physiological role in maintaining cell homeostasis as a
regulatory protein in intracellular signaling systems (14,15).
Regucalcin has been demonstrated to play a
pathophysiological role in metabolic disorders and diseases
(16–19). Of note, regucalcin has also been
shown to be involved in carcinogenesis (19). The gene and protein expression of
regucalcin has been found to be suppressed in various types of
tumor tissue in mammalian models and human subjects in vivo
(19,20). It has also been shown that
regucalcin gene expressionis downregulated during the development
of carcinogenesis (14,19). The overexpression of endogenous
regucalcin has been shown to suppress the proliferation of cloned
rat hepatoma H4-II-E cells in vitro, in which regucalcin
gene expression is downregulated (21).
Moreover, regucalcin has been suggested to play a
role as a suppressor protein in human carcinogenesis (19,20). The present study was undertaken in
an effort to determine whether exogenous regucalcin exerts a
suppressive effect on the proliferation of pancreatic cancer cells
in vitro. We found that exogenous regucalcin suppressed the
in vitro proliferation of pancreatic cancer MIA PaCa-2
cells, which are resistant to radiation therapy; however,
regucalcin did not have an effect on apoptotic cell death.
Materials and methods
Materials (reagents)
Dulbecco's modified Eagle's medium (DMEM) with 4.5
g/l glucose, L-glutamine and sodium pyruvate and antibiotics
(penicillin and streptomycin) were purchased from Invitrogen Corp.
(Carlsbad, CA, USA). Fetal bovine serum (FBS) was from HyClone
(Logan, UT, USA). Tumor necrosis factor-α (TNF-α) was from R&D
Systems (Minneapolis, MN, USA). PD98059 [an extracellular
signal-regulated kinase (ERK) inhibitor], staurosporine (an
inhibitor of protein kinase C), Bay K 8644 (an agonist of
Ca2+ influx in cells), wortmannin [an inhibitor of
phosphatidylinositol 3-kinase (PI3K)] or 5,6-dichlor
o-1-β-D-ribofuranosylbenzimidazole (DRB; an inhibitor of
transcriptional activity with RNA polymerase II inhibition) and all
other reagents were purchased from Sigma-Aldrich (St. Louis, MO,
USA) unless otherwise specified. Gemcitabine was obtained from
Hospira, Inc. (Lake Forest, IL, USA), and it was diluted in
phosphate-buffered saline (PBS). All procedure and protocols for
the use of the rat livers were approved by the Institutional Animal
Care and Use Committee at Emory University.
Regucalcin
Regucalcin was isolated from rat liver cytosol, as
previously described (1). The
livers were perfused with Tris-HCl buffer (pH 7.4), containing 100
mM Tris, 120 mM NaCl, 4 mM KCl, cooled to 4°C. The livers were then
removed, cut into small sections, suspended 1:4 (w/v) in Tris-HCl
buffer (pH 7.4) and homogenized in a Potter-Elvehjem homogenizer
with a Teflon pestle, as previously described (1). The homogenate was spun at 5,500 × g
in a refrigerated centrifuge for 10 min, and the supernatant was
spun at 105,00 × g for 60 min. The resulting supernatant was
isolated to electorophoretic homogeneity by gel filtration on
Sephadex G-75 and G-50, followed by ion-exchange chromatography on
diethylaminoethyl (DEAE)-cellulose, as previously described
(1). The purity of the isolated
regucalcin was confirmed using SDS-gel electrophoresis and western
blot analysis.
Pancreatic cancer cells
For our experiments, we used pancreatic cancer MIA
PaCa-2 cells, Pt45P1 cells with a high expression of tissue factor
(high TF) or Pt45P1 cells with a high expression of alternatively
spliced variants of tissue factor (asTF) (22,23). These human pancreatic cancer cell
lines were obtained from the American Type Culture Collection
(Rockville, MD, USA).
Cell proliferation
Pancreatic cancer MIA PaCa-2, Pt45P1 (high TF) or
Pt45P1 (asTF) cells (1×105/ml/well) were cultured using
a 24-well plate in DMEM containing 10% FBS and 1%
penicillin/streptomycin (P/S) in the presence or absence of
regucalcin (0.01, 0.1, 0.5, 1 or 10 nM) for 1, 2, 3 and 7 days, as
previously described (21). In
separate experiments, the cells (1×105/ml/well) were
cultured in DMEM containing 10% FBS and 1% P/S in the presence of
TNF-α (1 ng/ml), Bay K 8644 (1 μM), PD98059 (1 μM),
staurosporine (0.1 μM), wortmannin (1 μM) or DRB (1
μM) for 3 days. Following culture, the cells were detached
from each culture dish to determine the cell number.
Apoptotic cell death
The pancreatic cancer MIA PaCa-2 or Pt45P1 (high TF)
cells (1×105/ml/well) were cultured in a 24-well plate
in DMEM containing 10% FBS and 1% P/S in the absence of regucalcin
for 7 days until they reached confluency (85–95%). Subsequently,
the cells were cultured in the presence or absence of regucalcin
(0.1, 1 or 10 nM) with or without gemcitabine (10–1,000 nM) for 7
days, as previously described (15). Following culture, the cells were
detached from each culture dish to determine the cell number.
Cell counting
Following trypsinization of each of the culture
dishes using 0.2% trypsin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C, the
detached cells from each dish were collected following
centrifugation. The cells were resuspended in PBS solution and then
stained with eosin. The cell numbers were counted under a
microscope using a hemocytometer (Sigma-Aldrich). For each dish,
the average of two countings was used. Cell numbers are presented
as the number of cells per well in each plate.
Statistical analysis
Statistical significance was determined using
GraphPad InStat software version 3 for Windows XP (GraphPad
Software, Inc., La Jolla, CA, USA). Multiple comparisons were
performed by one-way analysis of variance (ANOVA) with the
Tukey-Kramer multiple comparisons post-hoc test for parametric
data. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
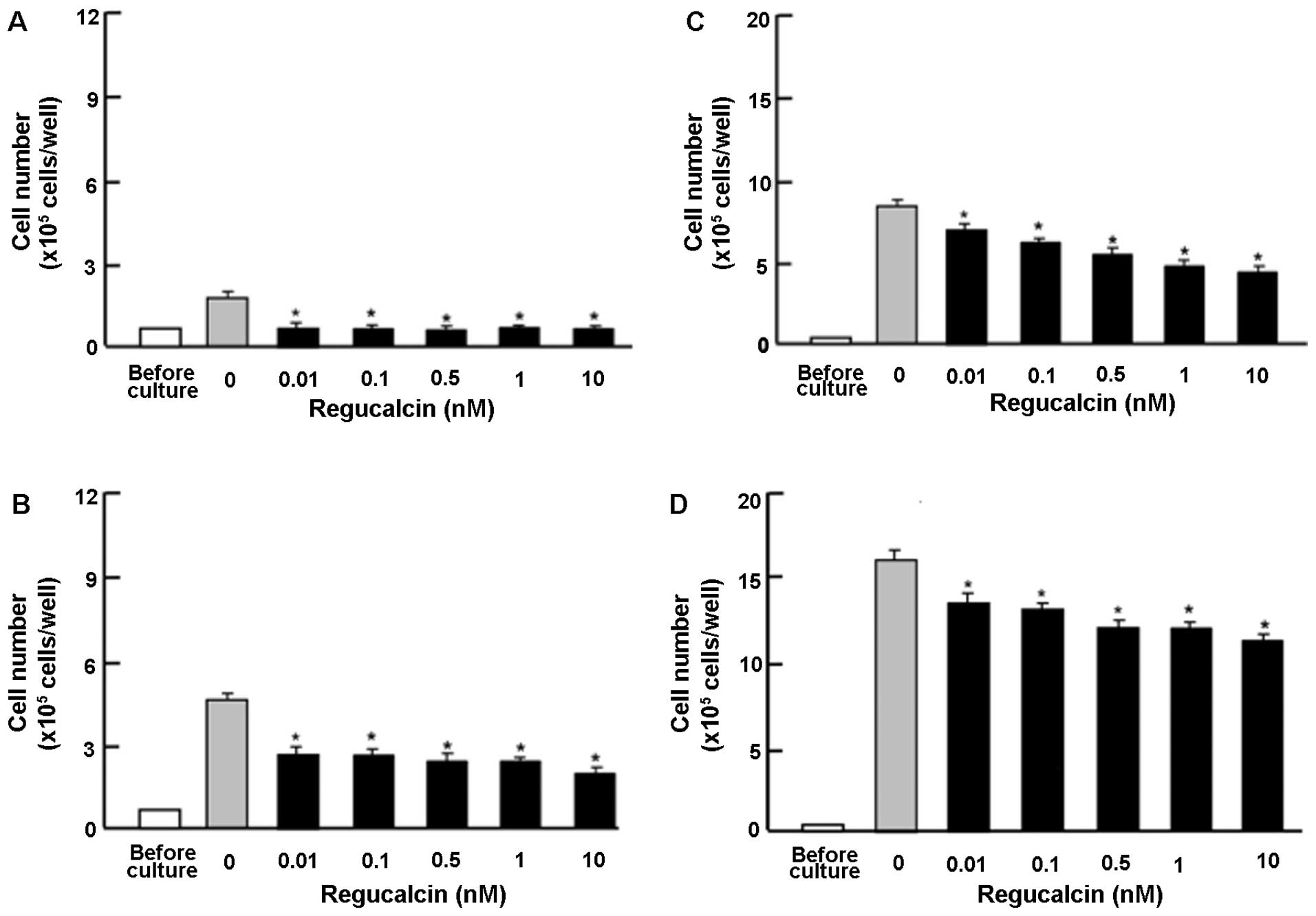
To determine the effects of exogenous regucalcin on
the proliferation of human pancreatic cancer cells, we used MIA
PaCa-2 cells, which are resistant to radiation. The MIA PaCa-2
cells were cultured in the presense of exogenous regucalcin
(0.01–10 nM) for 1–7 days (Fig.
1). The cell numbers increased in a time-dependent manner
(Fig. 1). The addition of
exogenous regucalcin diminished the increase in cell number
(Fig. 1), thus suggesting that
cell proliferation was is suppressed by physiological
concentrations of serum regucalcin (24).
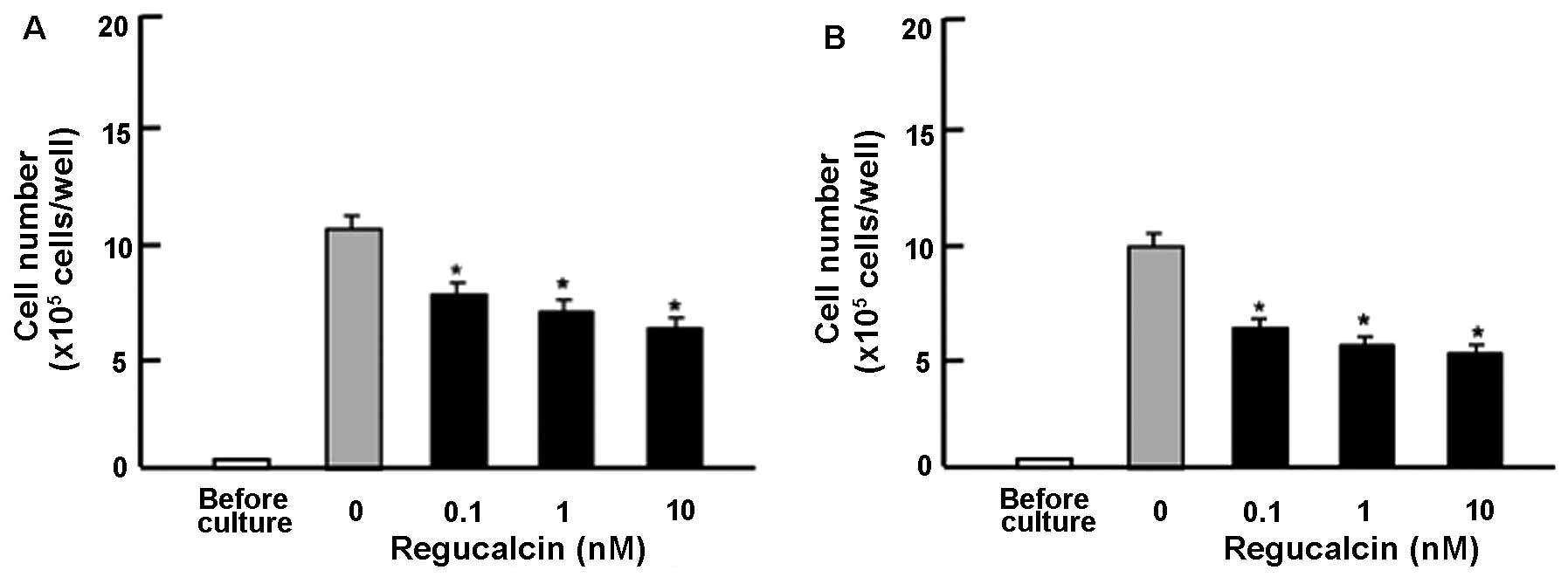
Subsequently, in order to determine the suppressive
effects of exogenous regucalcin on the proliferation of other human
pancreatic cancer cells, we used Pt45P1 cells, which highly
expressed tissue factor (high TF; Fig. 2A) or which highly expressed
alternatively spliced variants of tissue factor (asTF; Fig. 2B) in vitro. These cells
were cultured for 7 days in the presence or absence of regucalcin
(0.1, 1 or 10 nM). The addition of exogenous regucalcin had a
suppressive effect on the Pt45P1 cells (high TF and asTF;Fig. 2).
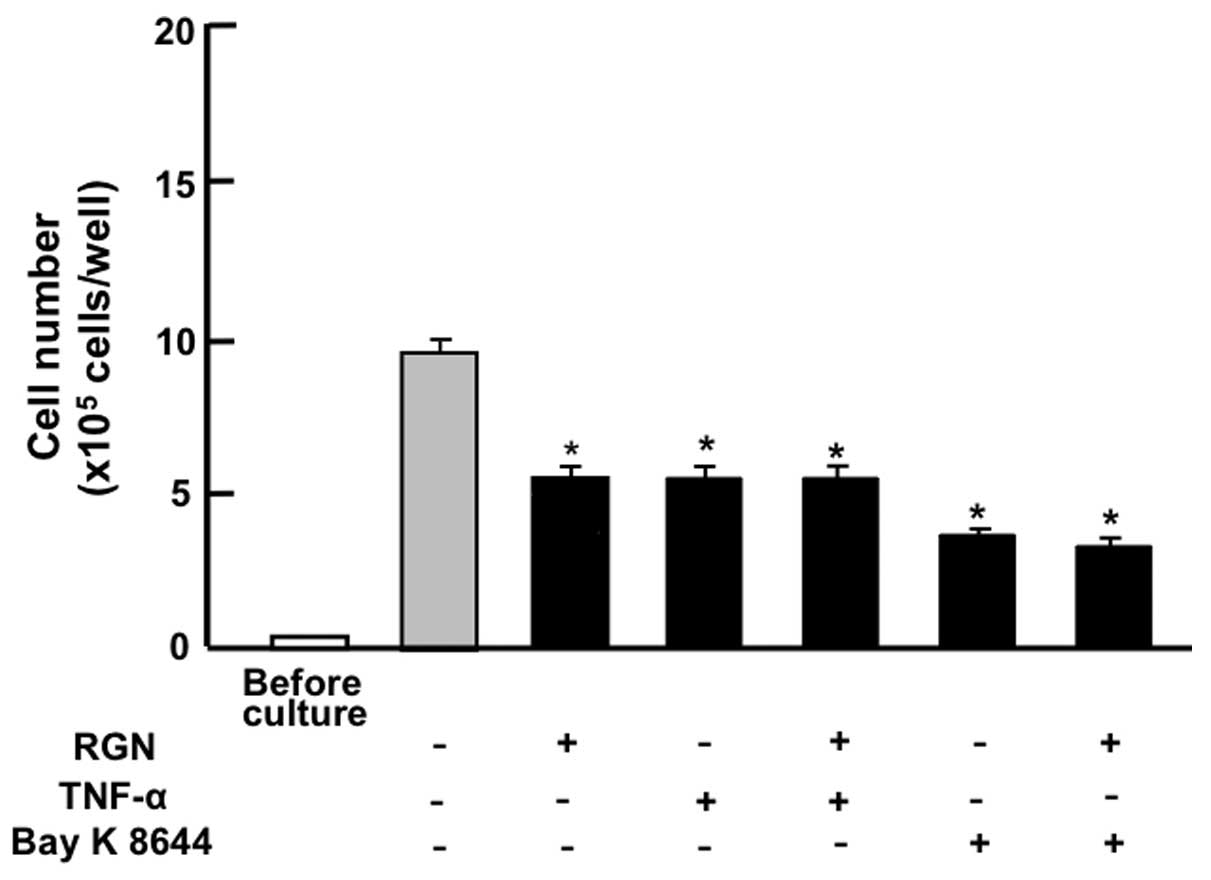
The suppressive effects of exogenous regucalcin on
the proliferation of the pancreatic cancer MIA PaCa-2 cells were
compared with the effects of other factors that have been shown to
decrease cell proliferation. As shown in Fig. 3, the suppressive effects of
exogenous regucalcin (1 nM) on the proliferation of MIA PaCa-2
cells were not enhanced in the presence of TNF-α (1 ng/ml), an
enhancer of nuclear factor-κB (NF-κB) signaling (25), or in the presence of Bay K 8644 (1
μM), an agonist of Ca2+ influx in cells (26).
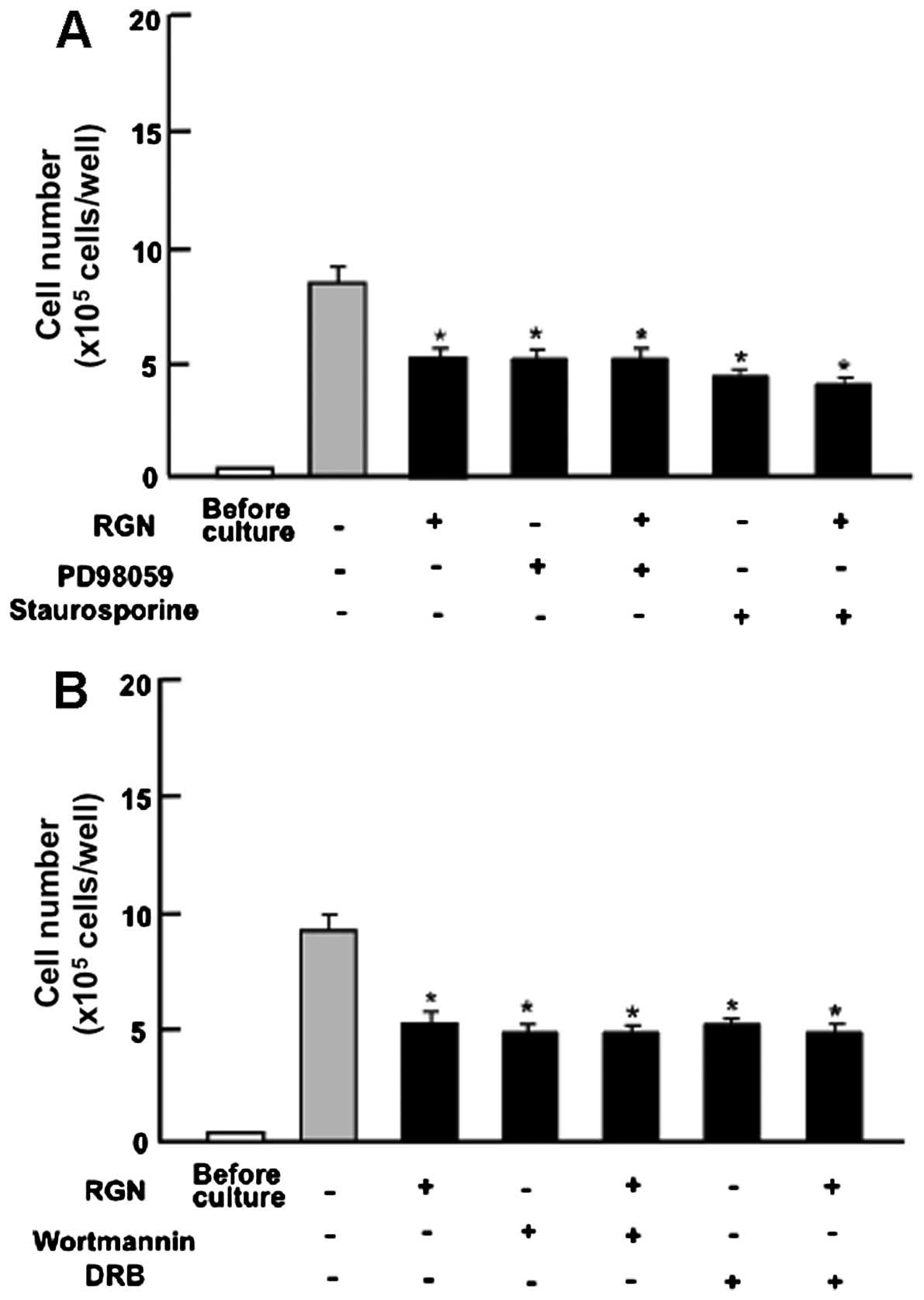
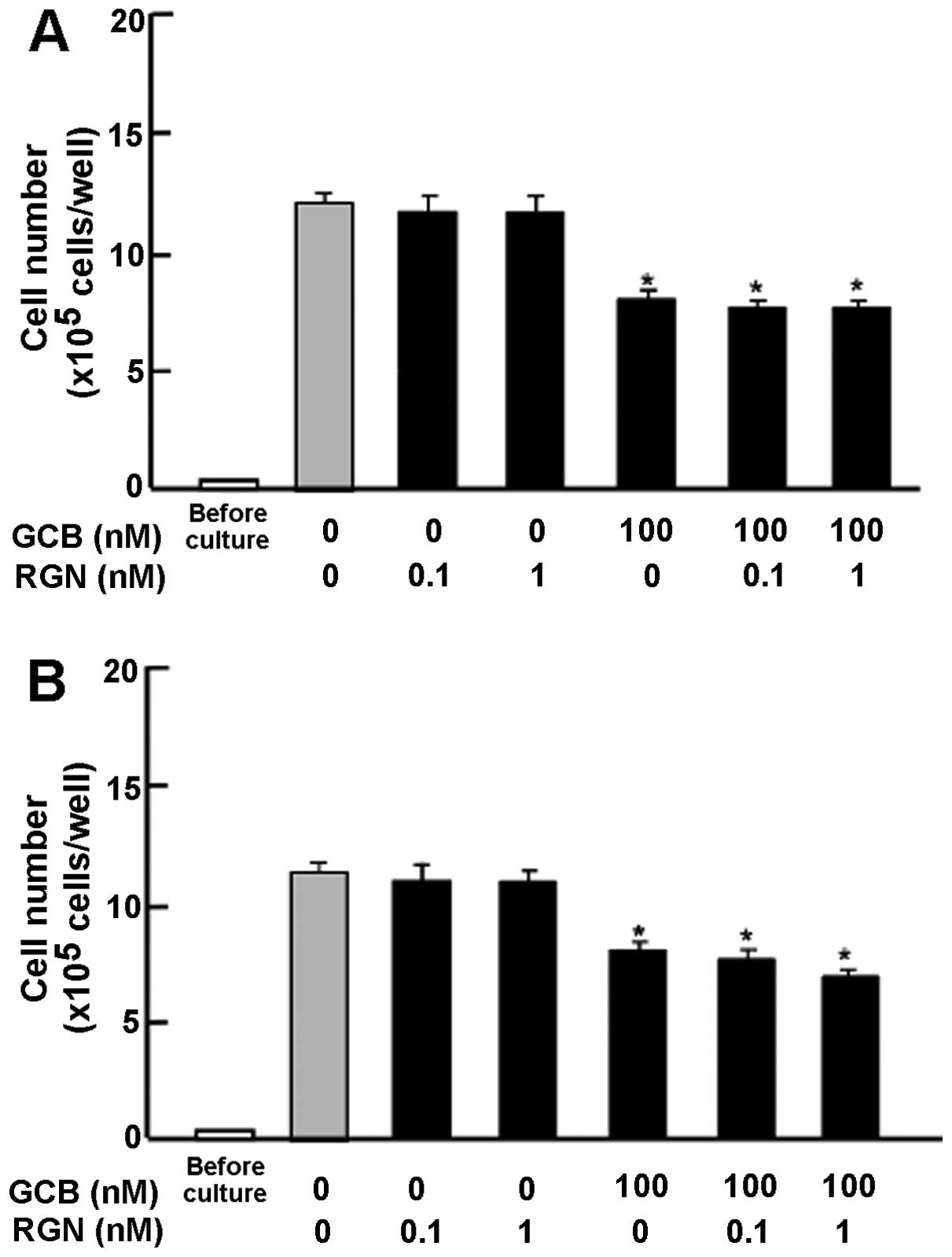
Subsequently, we determined whether the suppressive
effects of exogenous regucalcin on cell proliferation involve
intracellular signaling pathways. The results revealed that the
suppressive effects of exogenous regucalcin on cell proliferation
were not enhanced in the presence of PD98059 (1 μM), an ERK
inhibitor (27), or staurosporine
(0.1 μM), an inhibitor of protein kinase C (28) (Fig.
4A). Moreover, the suppressive effects of regucalcin on cell
proliferation were not enhanced in the presence of wortmannin (1
μM), an inhibitor of PI3K (29), or DRB (1 μM), an inhibitor
of transcriptional activity with RNA polymerase II inhibition
(30) (Fig. 4B).
Moreover, the effects of exogenous regucalcin were
compared with those of gemcitabine, an antitumor agent that induces
nuclear DNA damage (31). The
suppressive effects of regucalcin on the proliferation of
pancreatic cancer cells were examined in the presence of
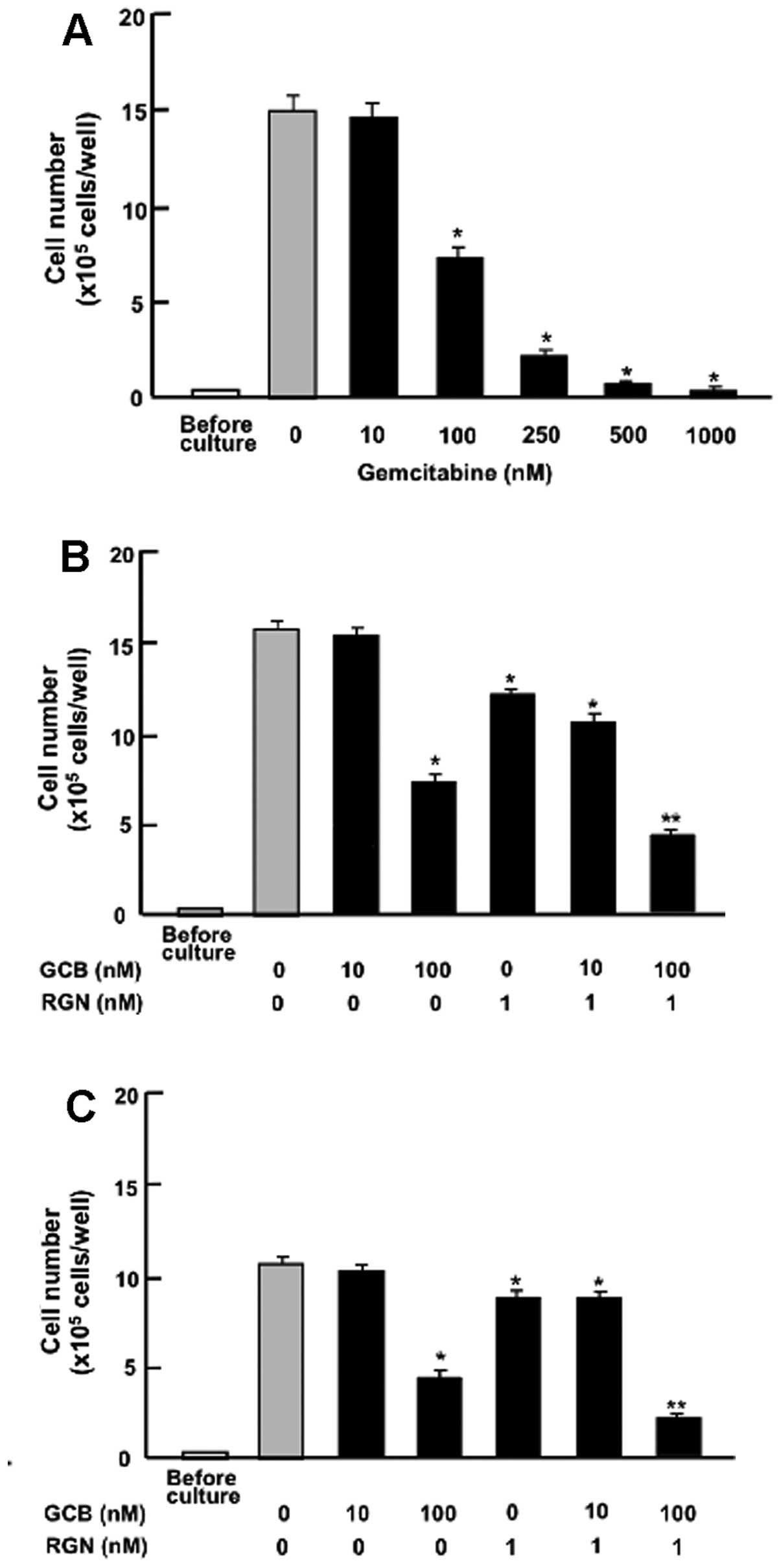
gemcitabine. Culture with gemcitabine (100–1,000 nM) suppressed the
proliferation of the MIA PaCa-2 cells (Fig. 5A). The suppressive effects of
regucalcin (1 nM) on the proliferation of the MIA PaCa-2 cells were
also observed in the presence of low concentrations of gemcitabine
(10 nM), which did not have a significant effect on cell
proliferation (Fig. 5B). However,
the suppressive effects of regucalcin (1 nM) on cell proliferation
were significantly enhanced in the presence of higher/high
concentrations of gemcitabine (100 nM) that had a suppressive
effect on cell proliferation (Fig.
5B). A similar effect was also produced by treatment with 1 nM
regucalcin in combination with 100 nM gemcitabine in the Pt45P1
(high TF) cells (Fig. 5C).
The effects of regucalcin on apoptotic cell death in
the pancreatic cancer MIA PaCa-2 cells were also determined. The
cells were cultured for 7 days until reaching confluency, and the
cells were then cultured for an additional 3 days (Fig. 6A) or 7 days (Fig. 6B) in the presence of regucalcin
(0.1 or 1 nM) with or without gemcitabine (100 nM). The addition of
exogenous regucalcin did not cause apoptotic cell death, whereas
culture with gemcitabine for 3 or 7 days caused apoptotic cell
death (Fig. 6). This effect was
not significantly affected in the presence of regucalcin (Fig. 6).
Discussion
Previous studies have demonstrated that regucalcin
plays a potential role as a suppressor of cell proliferation and
carcinogenesis (14,19). Regucalcin gene expression has been
found to be downregulated in the tumor tissues of human subjects
(20) and human cancer cells
(18,32). The present study demonstrated that
the proliferation of human pancreatic cancer MIA PaCa-2 and Pt45P1
(high TF and asTF) cells was suppressed by the addition of
exogenous regucalcin at physiological concentrations (24), and that regucalcin did not have an
effect on apoptotic cell death in vitro. To the best of our
knowledge, this is the first time that regucalcin was shown to play
a critical role in the suppression of human pancreatic cancer cell
proliferation.
The overexpression of endogenous regucalcin has been
shown to suppress the proliferation of cloned rat hepatoma H4-II-E
cells in vitro (14,18,21). The overexpression of endogenous
regucalcin has been demonstrated to cause G1 and G2/M phase cell
cycle arrest in rat hepatoma H4-II-E cells (33) and in rat normal kidney NRK52E
cells (34). The suppressive
effects of endogenous regucalcin on cell proliferation are mediated
through the suppression of the activities of Ca2+
signaling-dependent protein kinases, protein phosphatases and PI3K,
which are involved in various signaling pathways (14,18). The overexpression of endogenous
regucalcin has been shown to suppress c-myc, Ha-ras,
c-jun and chk2 mRNA expression or enhance p53
and Rb mRNA expression (14,19,35,36). Moreover, regucalcin has been found
to suppress cytoplasmic protein synthesis and nuclear DNA and RNA
synthesis (13,14). Thus, endogenous regucalcin exerts
suppressive effects on cell proliferation through multifunctional
pathways in rat normal and cancer cells.
In addition, regucalcin has been shown to bind to
the plasma membranes of rat liver in vitro (37). It is possible that exogenous
regucalcin may bind to the plasma membranes of human pancreatic
cancer MIA PaCa-2 cells and may thus regulate the intracellular
signaling pathways that suppress cell proliferation. Our results
revealed that the suppressive effects of regucalcin on the
proliferation of pancreatic cancer MIA PaCa-2 cells were not
enhanced either in the presence of TNF-α, an enhancer of NF-κB
signaling (25), Bay K 8644, an
agonist of Ca2+ entry in cells (26), PD98059, an ERK inhibitor (27), staurosporine, an inhibitor of
calcium-dependent protein kinase C (28), or in the presence of wortmannin,
an inhibitor of PI3K (29). Thus,
the suppressive effects of exogenous regucalcin on the
proliferation of pancreatic cancer MIA PaCa-2 cells were not
modulated in the presence of various inhibitors that regulate
intracellular signaling pathways related to cell proliferation
in vitro. These findings support the view that the
suppressive effects of exogenous regucalcin on cell proliferation
are mediated through the inhibition of various intracellular
signaling pathways (including NF-κB, calcium, ERK, protein kinase
C, and PI3K) that are related to the proliferation of human
pancreatic cancer MIA PaCa-2 cells.
Moreover, the results of this study demonstrated
that the suppressive effects of regucalcin on cell proliferation
were not enhanced in the presence of DRB, an inhibitor of
transcriptional activity with RNA polymerase II inhibition
(30). Intracellular signals for
exogenous regucalcin, which are bound receptors on the plasma
membranes of pancreatic cancer cells, may be transmitted into the
nucleus to suppress transcriptional regulation and regulate the
nuclear function of human pancreatic cancer MIA PaCa-2 cells.
However, the suppressive effects of exogenous
regucalcin on the proliferation of MIA PaCa-2 cells were enhanced
in the presence of gemcitabine, an antitumor agent that induces
nuclear DNA damage (32).
Exogenous regucalcin did not induce apoptotic cell death in human
pancreatic cancer MIA PaCa-2 cells in vitro, supporting the
view that regucalcin does not have a promoting effect on apoptosis.
Thus, the suppressive effects of exogenous regucalcin on the
proliferation of human pancreatic cancer MIA PaCa-2 cells were
independent of the induction of apoptosis. Exogenous regucalcin did
not enhance the effects of gemcitabine on the induction of
apoptosis. The mode of action of exogenous regucalcin in
suppressing cell proliferation may differ from that of gemcitabine.
However, the combination of exogenous regucalcin and gemcitabine
may be a useful tool in enhancing the antitumor effects on human
pancreatic cancer cells.
In conclusion, in this study, we demonstrated that
exogenous regucalcin had a significant suppressive effect on the
proliferation of human pancreatic cancer MIA PaCa-2 cells in
vitro, suggesting a critical role for regucalcin as a novel
cytokine that suppresses cell proliferation.
References
|
1
|
Yamaguchi M and Yamamoto T: Purification
of calcium binding substance from soluble fraction of normal rat
liver. Chem Pharma Bull (Tokyo). 26:1915–1918. 1978. View Article : Google Scholar
|
|
2
|
Yamaguchi M and Sakurai T: Inhibitory
effect of calcium-binding protein regucalcin on
Ca2+-activated DNA fragmentation in rat liver nuclei.
FEBS Lett. 279:281–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimokawa N and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a calcium-binding
protein regucalcin from rat liver. FEBS Lett. 327:251–255. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misawa H and Yamaguchi M: The gene of
Ca2+-binding protein regucalcin is highly conserved in
vertebrate species. Int J Mol Med. 6:191–196. 2000.PubMed/NCBI
|
|
5
|
Yamaguchi M: Role of regucalcin in calcium
signaling. Life Sci. 66:1769–1780. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (Review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
7
|
Yamaguchi M: Regucalcin and cell
regulation: role as a suppressor in signal transduction. Mol Cell
Biochem. 353:101–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimokawa N, Matsuda Y and Yamaguchi M:
Genomic cloning and chromosomal assignment of rat regucalcin gene.
Mol Cell Biochem. 151:157–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiselton DL, McDowall J, Brandau O,
Ramser J, d’Esposito F, Bhattacharya SS, Ross MT, Hardcastle AJ and
Meindl M: An integrated, functionally annotated gene map of the
DXS8026-ELK1 internal on human Xp11.3-Xp11.23: Potential hotspot
for neurogenetic disorders. Genomics. 79:560–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi M, Makino R and Shimokawa N: The
5’ end sequences and exon organization in rat regucalcin gene. Mol
Cell Biochem. 165:145–150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi M: The transcriptional
regulation of regucalcin gene expression. Mol Cell Biochem.
346:147–171. 2011. View Article : Google Scholar
|
|
12
|
Yamaguchi M: Hormonal regulation of
regucalcin gene expression: Involvement in cell metabolism. Horm
Stud. 1:12013. View Article : Google Scholar
|
|
13
|
Yamaguchi M: Role of regucalcin in cell
nuclear regulation: Involvement as a transcription factor. Cell
Tissue Res. 354:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi M: Suppressive role of
regucalcin in liver cell proliferation: Involvement in
carcinogenesis. Cell Prolif. 46:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi M: Regucalcin and metabolic
disorders: Osteoporosis and hyperlipidemia are induced in
regucalcin transgenic rats. Mol Cell Biochem. 341:119–133. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi M and Murata T: Involvement of
regucalcin in lipid metabolism and diabetes. Metabolism.
62:1045–1051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M: Regucalcin as a potential
biomarker for metabolic and neuronal diseases. Mol Cell Biochem.
391:157–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M: Involvement of regucalcin as
a suppressor protein in human carcinogenesis: insight into the gene
therapy. J Cancer Res Clin Oncol. Sep 18–2014.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murata T and Yamaguchi M: Alternatively
spliced variants of the regucalcin gene in various human normal and
tumor tissues. Int J Mol Med. 34:1141–1146. 2014.PubMed/NCBI
|
|
21
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar
|
|
22
|
Ninomiya I, Yamazaki K, Oyama K, Hayashi
H, Tajima H, Kitagawa H, Fushida S, Fujimura T and Ohta T:
Pioglitazone inhibits the proliferation and metastasis of human
pancreatic cancer cells. Oncol Lett. 8:2709–2714. 2014.PubMed/NCBI
|
|
23
|
Sipos B, Möser S, Kalthoff H, Török V,
Löhr M and Klöppel G: A comprehensive characterization of
pancreatic ductal carcinoma cell lines: Towards the establishment
of an in vitro reseach platform. Virchows Arch. 442:444–452.
2003.PubMed/NCBI
|
|
24
|
Yamaguchi M and Isogai M: Tissue
concentration of calcium-binding protein regucalcin in rats by
enzyme-linked immunoadsorbent assay. Mol Cell Biochem. 122:65–68.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee ZH, Kwack K, Kim KK, Lee SH and Kim
HH: Activation of c-Jun N-terminal kinase and activator protein 1
by receptor activator of NF-kappaB. Mol Pharmacol. 58:1536–1545.
2000.PubMed/NCBI
|
|
26
|
Cano-Abad MF, Villarroya M, García AG,
Gabilan NH and Lopez MG: Calcium entry through L-type calcium
channels causes mitochondrial disruption and chromaffin cell death.
J Biol Chem. 276:39695–39704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extracellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
28
|
Chen QW, Edvinsson and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smoth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar
|
|
29
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancere. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maia C, Santos C, Schmitt F and Socorro S:
Regucalcin is under- expressed in human breast and prostate
cancers: Effect of sex steroid hormones. J Cell Biochem.
107:667–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakagawa T, Sawada N and Yamaguchi M:
Overexpression of regucalcin suppresses cell proliferation of
cloned normal rat kidney proximal tubular epithelial NRK52E cells.
Int J Mol Med. 16:637–643. 2005.PubMed/NCBI
|
|
35
|
Tsurusaki Y and Yamaguchi M:
Overexpression of regucalcin modulates tumor-related gene
expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem.
90:619–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsurusaki Y and Yamaguchi M: Role of
regucalcin in liver nuclear function: Binding of regucalcin to
nuclear protein or DNA and modulation of tumor-related gene
expression. Int J Mol Med. 14:277–281. 2004.PubMed/NCBI
|
|
37
|
Yamaguchi M, Mori S and Kato S:
Calcium-binding protein regucalcin is an activator
(Ca2+-Mg2+)-adenosine triphosphatase in the
plasma membranes of rat liver. Chem Pharm Bull (Tokyo).
36:3532–3539. 1988. View Article : Google Scholar
|