Introduction
In living systems, melanin offers many physiological
functions. It can protect the human skin from damage, for example
by UV irradiation (1). It also
serves as the determinant of hair color. Melanogenesis is a complex
process that results in the synthesis of melanin pigment. It occurs
within specialized intracellular organelles known as melanosomes in
melanocytes. UV irradiation increases transport of melanosomes from
melanocytes to keratinocytes (2).
Melanosomes in keratinocytes play protective roles from UV
irradiation by scattering incoming light and UV radiation-generated
free radicals in cells (3).
In melanocytes and melanoma cells, melanin synthesis
is controlled by a cascade of enzymatic reactions. Melanin
synthesis is regulated by melanogenic enzymes such as tyrosinase,
tyrosinase-related protein-1 (TRP-1), and TRP-2 (4). First, tyrosinase converts tyrosine
to 3,4-dihydroxyphenyl-alanine (DOPA). Second, tyrosinase catalyzes
the oxidation of DOPA to DOPAquinone (5). DOPAquinone is then converted to
DOPAchrome through auto-oxidation. TRP-2, which functions as
DOPAchrome tautomerase, catalyzes the transition of DOPAchrome to
5,6-dihydroxyindole-2-carboxylic acid (DHICA) (6). Subsequently, TRP-1 oxidizes DHICA to
a carboxylated indole-quinone (7)
to form brown-black pigment (eumelanin). Thus, tyrosinase, TRP-1
and TRP-2 have been recognized as three critical regulators in
melanin biosynthesis.
As an essential factor for melanocyte growth and
differentiation, microphthalmia-associated transcription factor
(MITF) has been previously studied in melano genesis. MITF is
believed to activate the tyrosinase, TRP-1, and TRP-2 promoters by
binding to the M- or E-box consensus motif (8,9).
Several signaling pathways have been identified to
play positive or negative roles in melanogenesis. For instance, PKA
activated by cyclic AMP (cAMP) translocates to the nucleus where it
phosphorylates the cAMP responsive element-binding protein (CREB).
The phosphorylated CREB then binds to the CRE site on the MITF
promoter, interacts with CREB binding protein (CBP) to increase the
expression of MITF, and eventually causes melanogenesis (10,11). However, the ERK and Akt signaling
pathways have been shown to negatively regulate melanogenesis in
melanocytes (12,13). Inhibition of ERK and PI3K/Akt can
cause the stimulation of melanogenesis (14,15).
Exposure to the sun occurs with deliberate tanning
for cosmetics purposes. However, UV-induced tanning may cause skin
cancer, make skin age and wrinkle more rapidly, mutate DNA, and
impair the immune system (16–18). Thus, a highly photoprotective tan
that does not require skin damage may be highly beneficial.
Vitiligo is a condition that causes depigmentation
of parts of the skin. It occurs when melanocytes die or are unable
to function. The exact cause of vitiligo remains unknown, although
studies suggest that it may arise from autoimmune, genetic,
oxidative stress, or viral causes (19,20). Exposing the skin to UVB light from
UVB lamps is the most common treatment for vitiligo (21). However, due to the higher risks of
skin cancer by UVB, the usage of UVB for the treatment of vitiligo
should be carefully applied. Thus, the development of new methods
of treatment and new compounds exhibiting strong melanogenesis
effects and fewer side effects is crucial.
Melia azedarach (MA) is a species of a
deciduous tree that is native to Korea, China and Southeast Asia.
It has been used in folk medicine for the treatment of several
diseases. Many constituents including limonoids, triterpenoids, and
steroids have been isolated from various parts of MA (22,23). In the present study, we
investigated the effect of an ethanol extract of MA on melanin
synthesis and the underlying molecular mechanisms in B16F10
cells.
Materials and methods
Reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT), forskolin and L-DOPA, were purchased
from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against
tyrosinase, TRP-1, TRP-2 and β-actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Preparation of the MA extract
MA collected from Korea was cut into 5-cm sections,
dried in the dark at room temperature, and stored in a dark and
cold room until needed. The dried plant material was then extracted
with 70% (v/v) ethanol (5 times as much as the weight of the dried
material) for 72 h at 25°C. The plant extract was passed through
0.45-μm filter paper and then evaporated at 60°C, after
which the viscous residue was lyophilized to yield the product. In
this experiment, DMSO was used to dissolve the product for making
stock.
Cell culture
B16F10 mouse melanoma cells (from the Korean Cell
Line Bank, Seoul, Korea) were cultured in phenol red-free
Dulbecco’s modified Eagle’s medium (DMEM), supplemented with
glutamine (2 mmol/l), penicillin (400 U/ml), streptomycin (50 g/l),
and 10% FBS at 37°C in a humidified atmosphere containing 5%
CO2.
Cell viability
An MTT assay was performed to examine cell viability
following MA extract treatment. B16F10 cells were incubated
overnight with DMEM (phenol red-free) containing 10% FBS. The cells
were then treated with different concentrations of the MA extract
for 24 h. Following treatment, MTT (dissolved in PBS to 0.5 g/l)
was added. The cells were then incubated at 37°C for an additional
4 h, the supernatant was removed and DMSO was added to dissolve the
formazan crystals. Optical absorbance was determined at 570 nm with
a microplate spectrophotometer from Molecular Devices, Inc.
(Sunnyvale, CA, USA).
Measurement of melanin content
B16F10 cells were incubated overnight with DMEM
(phenol red-free) containing 10% FBS. The cells were treated with
the MA extract or forskolin at different time points. After
incubation, the cells were collected and washed twice with PBS.
Centrifugation (Centrifuge 5424R; Eppendorf, Hamburg, Germany) at
15,000 x g for 15 min was performed and the melanin pellets were
dissolved in 1 N NaOH containing 20% DMSO for 30 min at 95°C. The
mixed homogenate (100 μl) was placed in a 96-well microplate
and optical densities (ODs) were measured at 405 nm. The protein
concentration of each sample was determined using the Bradford
assay (Bio-Rad, Hercules, CA, USA). Relative melanin production was
calculated by normalizing the OD values with the protein
concentrations (absorbance/μg protein).
Measurement of tyrosinase activity
B16F10 cells were incubated with the MA extract and
forskolin at the indicated time points. Cell were then washed with
ice-cold PBS, and lysed with PBS containing 1% Triton X-100. After
centrifugation at 15,000 × g for 15 min, the supernatants were
collected. The amount of each cell lysate was adjusted with lysis
buffer to yield the same protein concentra tion. Then, 90 μl
of each lysate and 10 μl of 10 mmol/l L-DOPA were added in
the well of a 96-well plate. The control wells contained 90
μl of lysis buffer and 10 μl of 10 mmol/l L-DOPA.
Absorbance was measured at 475 nm after the wells were incubated at
37°C for 30 min.
Western blot analysis
For tyrosinase, TRP-1, TRP-2 and actin protein
expression analysis, B16F10 cells were treated with MA extract, and
collected at 2 and 4 h. B16F10 cells were then lysed in cell lysis
buffer [20 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1 mmol/l
EDTA, 1 mmol/l EGTA, 1% Triton X-100, 2.5 mmol/l sodium
pyrophosphate, 1 mmol/l β-glycerophosphate, 1 mmol/l
Na3VO4, 1 mmol/l dithiothreitol, 0.01 g/l
leupeptin, and 1 mmol/l PMSF]. Total protein (30 μg) in
sample buffer was loaded in 10% SDS-polyacrylamide gels. Separated
proteins were transferred onto PVDF membranes (Roche, Mannheim,
Germany), which were then saturated with 5% dry milk in
Tris-buffered saline containing 0.4% Tween-20. The membranes were
incubated with the appropriate primary antibodies against
tyrosinase, TRP-1, TRP-2 and actin, followed by incubation with
horseradish peroxidase-conjugated secondary antibodies. Blotting
proteins were visualized by enhanced chemiluminescence (Amersham
Bioscience, Buckinghamshire, UK).
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated using TRIzol (Invitrogen,
Carlsbad, CA, USA) and 1 μg of total RNA was converted to
cDNA using a First Strand cDNA Synthesis kit (MBI Fermentas,
Vilnius, Lithuania) according to the manufacturer’s instructions.
Quantification of TRP-1 and endogenous reference GAPDH cDNA was
performed using a 7500 Real-time PCR system (Applied Biosystems,
Foster City, CA, USA). The SYBR Premix Ex Taq™ (Takara Bio Inc.,
Shiga, Japan) was used in all the samples and reactions were
carried out in a 10 μl final reaction volume. TRP-1 and
GAPDH primer sequences were designed as follows: mTRP-1 forward,
5′-GCT GCA GGA GCC TTC TTT CTC-3′ and reverse, 5′-AAG ACG CTG CAC
TGC TGG TCT-3′; mGAPDH forward, 5′-GAT GCC CCC ATG TTT GTG-3′ and
reverse, 5′-ACA ACC TGG TCC TCA GTG-3′. The PCR cycling conditions
were 50°C for 2 min and 95°C for 2 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min. Data were analyzed using the
2[−ΔΔC(T)] method (24). Data
were presented as the means ± SD normalized to GAPDH and relative
to the control sample. The experiments were carried out in
triplicate.
Statistical analysis
Statistical significance was performed using the
Student’s t-test. The results are presented as the means ± SD. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
MA extract does not a cause cytotoxic
effect on B16F10 cells
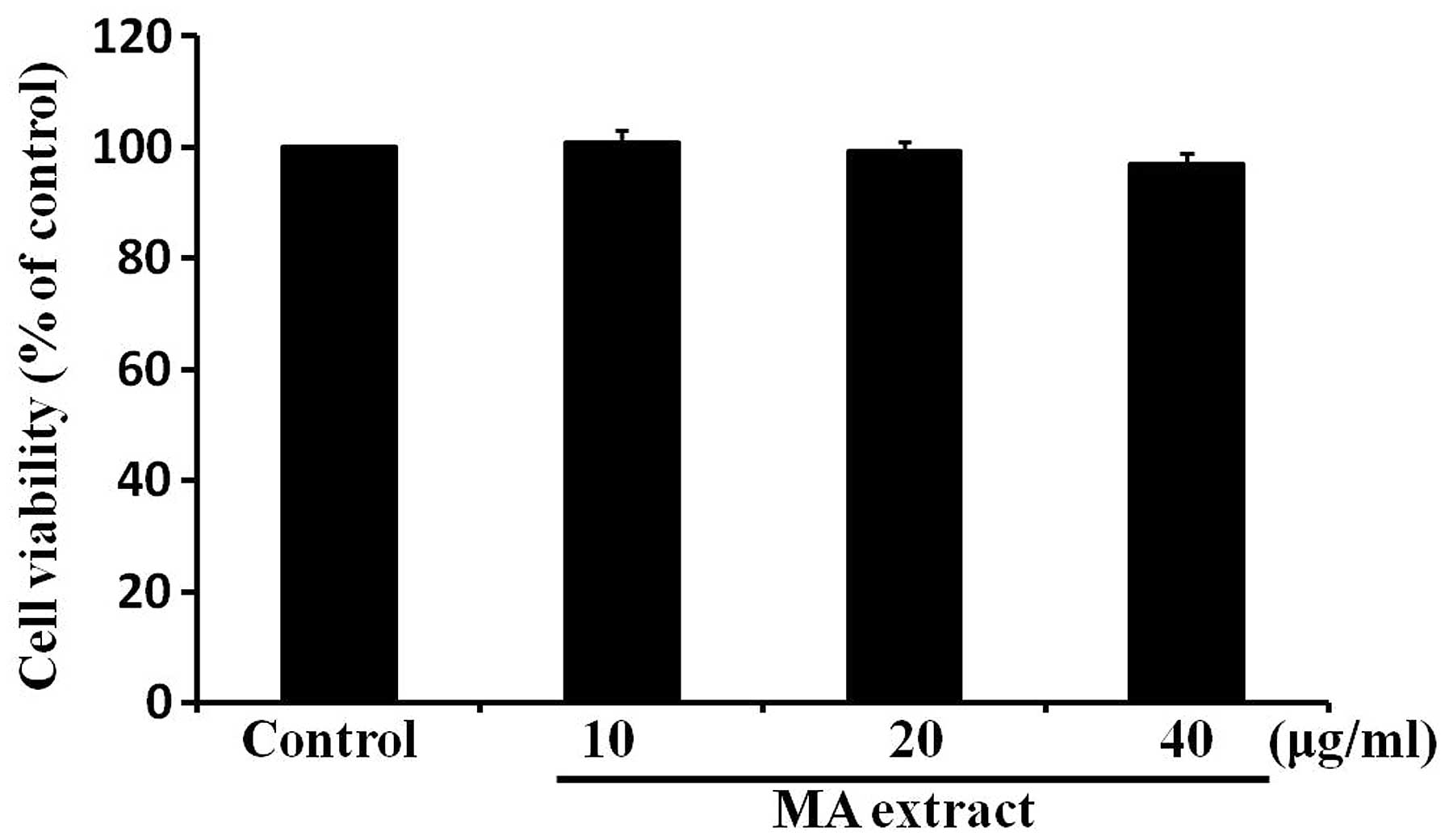
To determine whether the MA extract has a cytotoxic
effect on B16F10 cells, we treated B16F10 cells with the MA extract
for 24 h at various concentrations, ranging from 10 to 40
μg/ml. Cell viability was determined by the MTT assay. The
result showed that the MA extract had no significant effect on cell
viability (Fig. 1). This result
indicated that the MA extract was not cytotoxic to B16F10 cells at
the concentrations used in the present study.
MA extract induces melanogenesis in
B16F10 cells
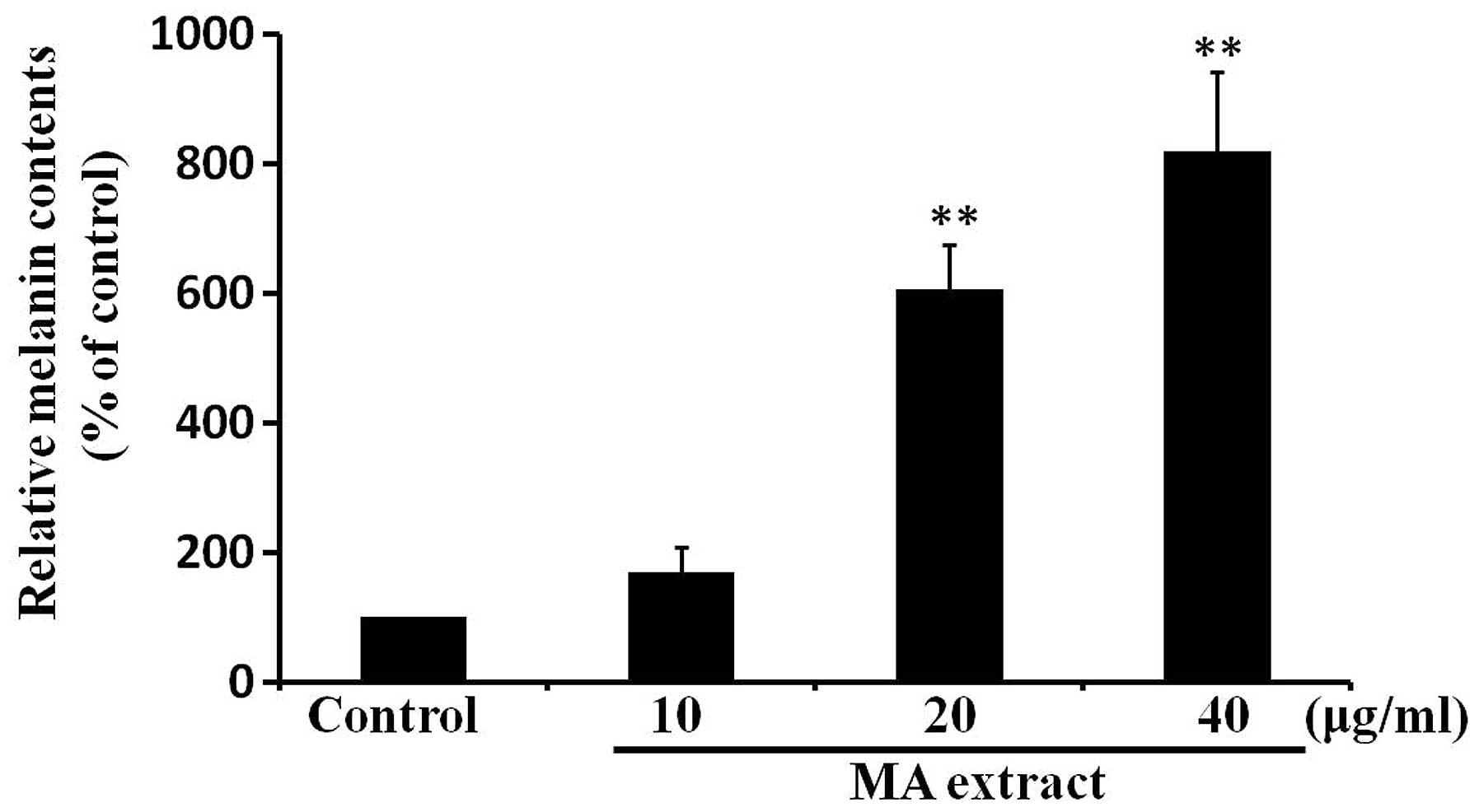
To assess the effects of the MA extract on
melanogenesis, we measured intracellular melanin contents at 24 h
after the treatment of B16F10 cells with the MA extract (10, 20 and
40 μg/ml). We found that melanin contents were significantly
increased in a dose-dependent manner by the MA extract treatment
(Fig. 2). Taken together, these
results indicated that the MA extract induces melanogenesis in
B16F10 cells.
MA extract induces melanogenesis as early
as at 4 h after treatment in B16F10 cells
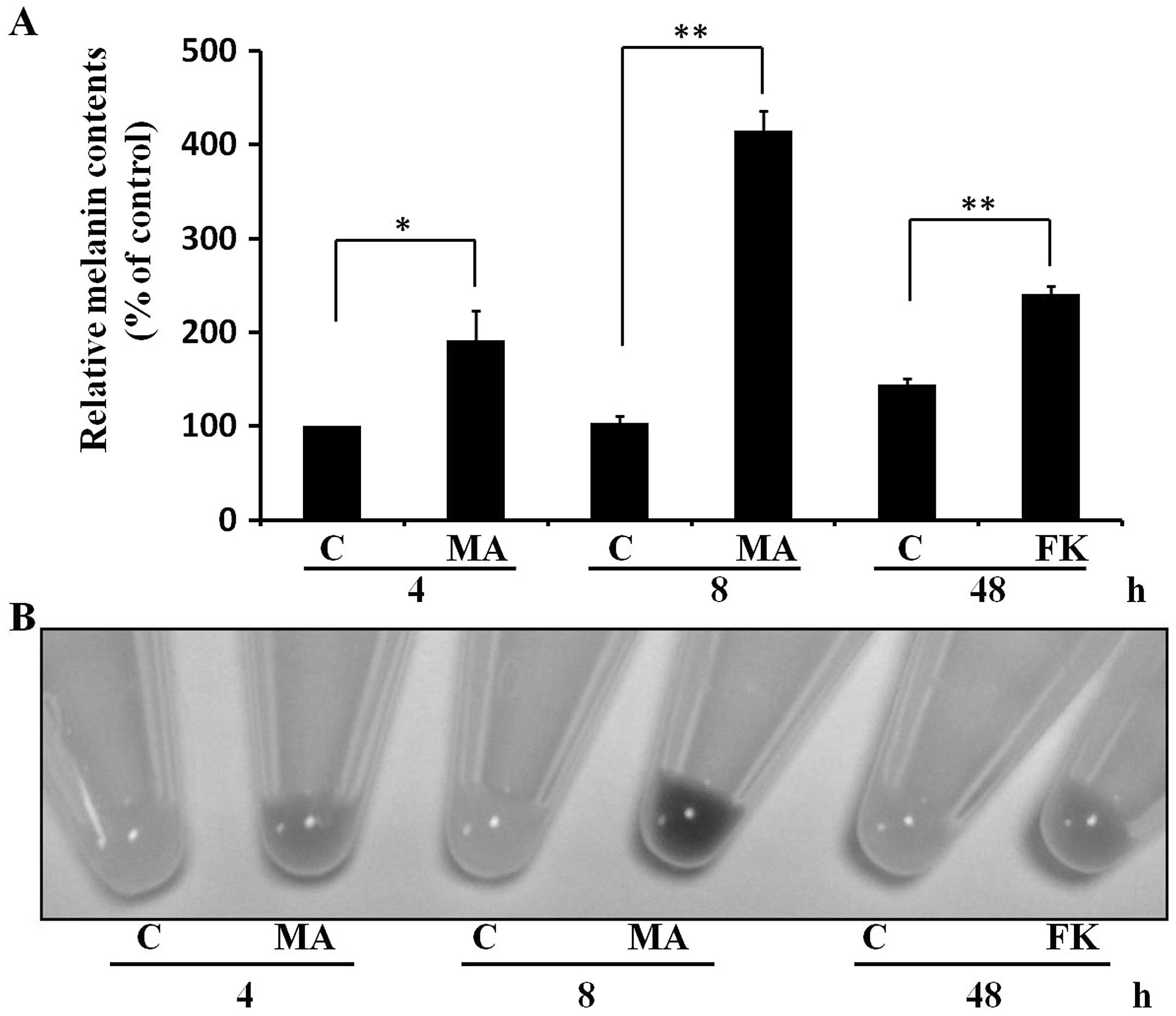
Since we found that the MA induced melanogenesis at
24 h after treatment, we determined whether MA can induce
melanogenesis at early time points <24 h in B16F10 cells. To
compare the melanogenic response ability, we treated cells with the
well-known melanogenesis inducer, forskolin (20 μM). Of
note, we found that the MA extract (20 μg/ml) induced
melanogenesis as early as at 4 h, and the melanogenic effect was
more obvious at 8 h (Fig. 3). We
also observed that forskolin (20 μM) induced melanogenesis
at 48 h (Fig. 3) but not at
<24 h (data not shown). Thus, the data indicated that the MA
extract induced melanogenesis rapidly in B16F10 cells.
MA extract does not affect tyrosinase
activity in B16F10 cells
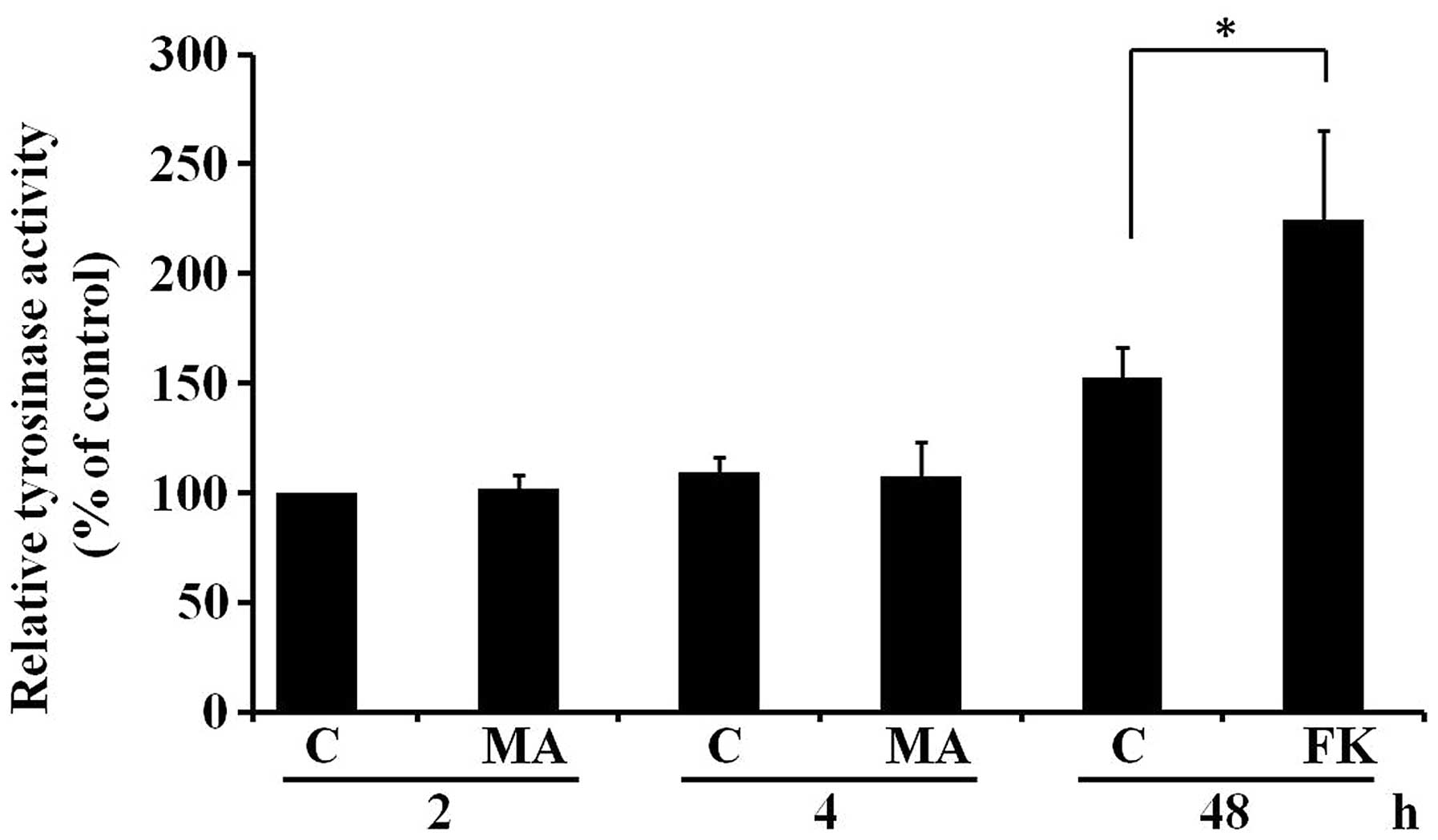
To investigate possible mechanisms responsible for
the increase in melanogenesis after 4 h of treatment with the MA
extract in B16F10 cells, the effect of the MA extract on tyrosinase
activity was examined. We treated B16F10 cells with the MA extract
at 20 μg/ml for 2 and 4 h. We then measured intracellular
tyrosinase activities. Forskolin, which is known to induce
intracellular tyrosinase activity, was used as a positive control.
Fig. 4 shows that the MA extract
had no effect on intracellular tyrosinase activity at 2 and 4 h.
Thus, the results suggested that the MA extract induced
melanogenesis at 4 h in B16F10 cells without affecting
intracellular tyrosinase activity.
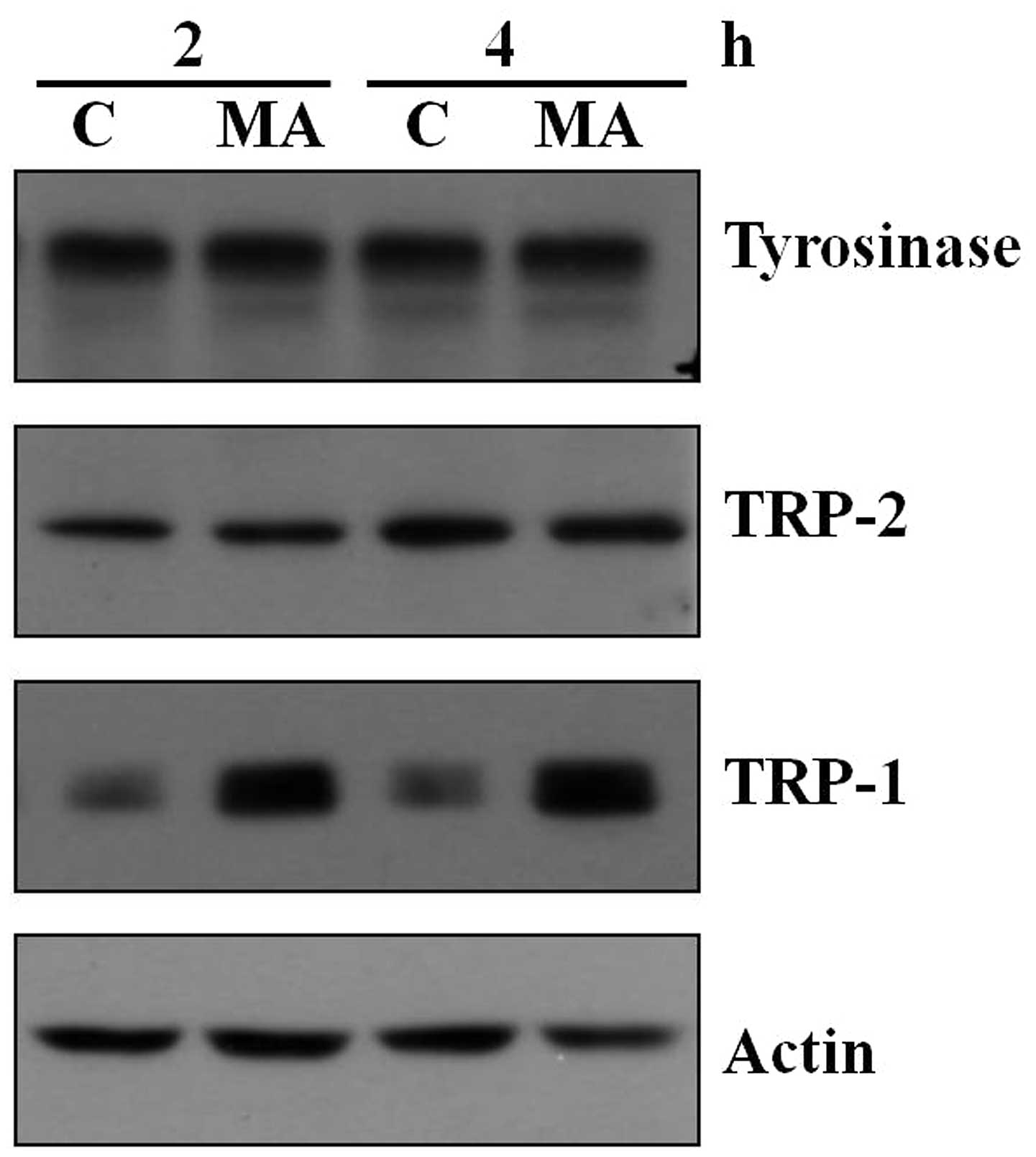
MA extract increases TRP-1 protein
expression in B16F10 cells
Tyrosinase, TRP-1 and TRP-2 are known as three
critical regulators in melanin biosynthesis. Since the MA extract
did not affect tyrosinase activity in B16F10 cells, we examined the
protein expression of the three factors after the MA extract
treatment by western blotting. We found that treatment of the MA
extract did not affect tyrosinase and TRP-2 protein expression in
samples collected at 2 and 4 h (Fig.
5). However, the MA extract obviously increased TRP-1 protein
expression at the two time points (Fig. 5).
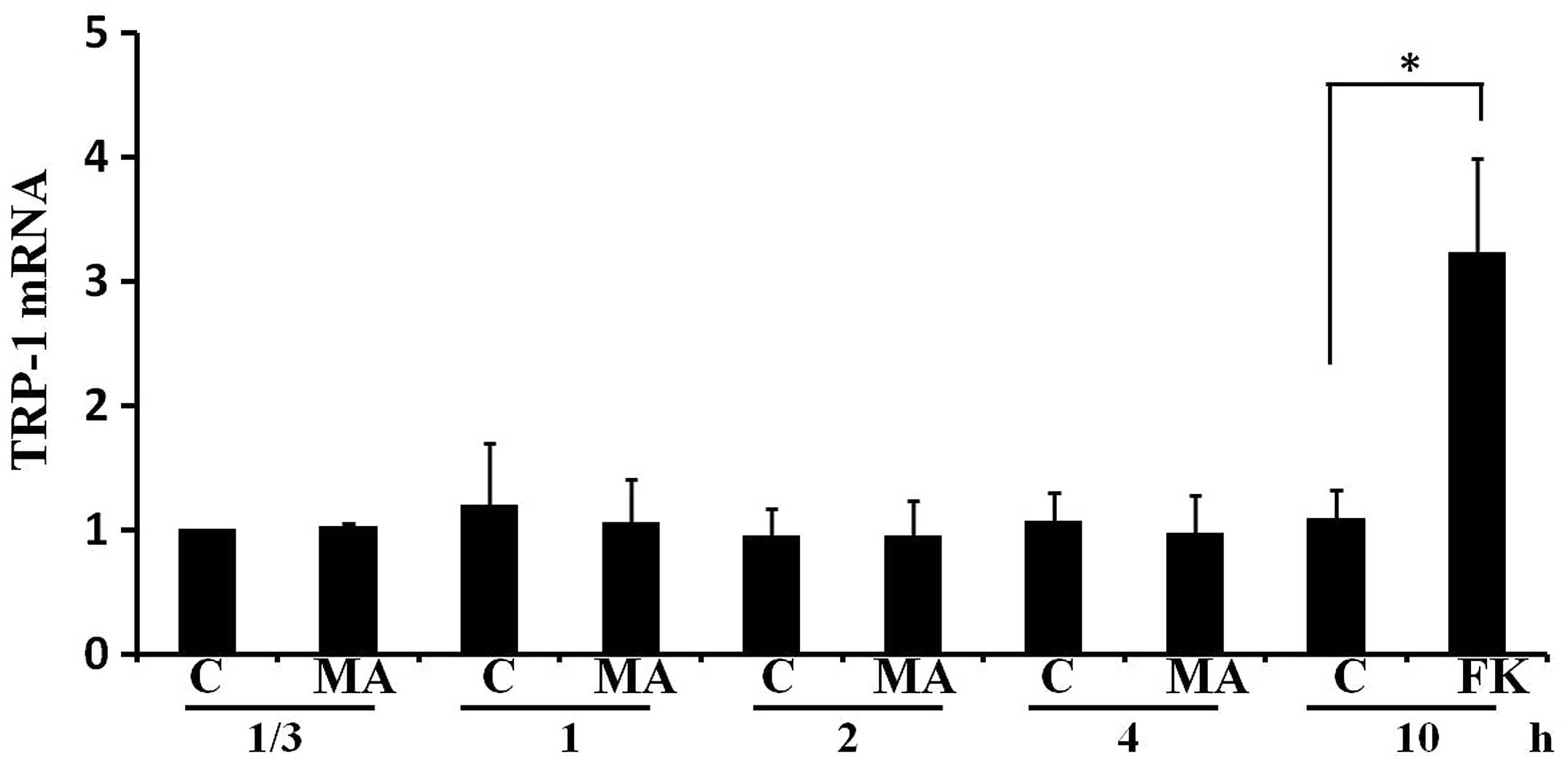
MA extract does not increase TRP-1 mRNA
levels in B16F10 cells
We examined the effect of the MA extract on the mRNA
level of TRP-1 in B16F10 cells. Fig.
6 shows that the MA extract did not affect TRP-1 mRNA level in
samples collected at 1/3, 1, 2 or 4 h. As a positive control,
forskolin significantly increased the TRP-1 mRNA level at 10 h. The
results suggested that the MA extract increased TRP-1 protein
expression without affecting its mRNA level.
Discussion
Several compounds and plant extracts are capable of
increasing melanogenesis in melanocytes and melanoma cells. It has
been shown that human placental lipid (25), sesamin (26), quercetin (27), and extracts from Erica
multiflora (28) and
Pyrostegia venusta (29)
can increase melanogenesis in vitro. Thus, these compounds
and extracts can be considered as candidates for developing tanning
cosmetics and medicines for vitiligo.
Many constituents derived from MA have been
identified and studied. A limonoid isolated from MA has been
reported to possess anti-feeding and insecticidal activities
(30). Moreover, several other
constituents from MA possess anti-herpetic (31), anti-angiogenic (32) and anticancer (33) properties. However, despite
previous findings, the effect of MA on melanogenesis has not been
reported. To identify new agents for developing tanning cosmetics
and treating hypopigmentation disorders such as vitiligo, we
investigated the effect of an extract from MA on melanogenesis in
B16F10 cells.
The effects of the MA extract on melanogenesis were
assessed using melanin content assay, intracellular tyrosinase
activity assay, Western blotting, and PCR. First, we showed that
the MA extract increased melanin synthesis in a
concentration-dependent manner without cytotoxicity (10–40
μg/ml) in B16F10 cells. Second, we found that melanogenesis
of B16F10 cells by the MA extract (20 μg/ml) was rapid and
that the pellet color of cells was enhanced within 4 h after
treatment. To compare the melanogenic effect of MA with other
agents, we used the well-known melanogenic inducer forskolin at a
concentration (20 μM) that is higher than the concentrations
(5 or 10 μM) usually used in other groups (34,35). After incubation with the MA
extract (20 μg/ml) for 8 h, the pellet color was darker than
that incubated with forskolin (20 μM) for 48 h (Fig. 3B). Since melanogenesis was
observed within 4 h after the MA extract treatment, we focused on
the mechanism that the MA extract induced melanogenesis within 4 h.
Tyrosinase is an important enzyme involved in melanogenesis
(5). We examined the activity and
protein expression of tyrosinase. The MA extract did not affect
tyrosinase activity within 4 h. Additionally, the MA extract did
not affect tyrosinase protein expression. Tyrosinase is not the
only important enzyme involved in melanogenesis, with TRP-1 and
TRP-2 also playing essential roles (6,7).
We observed that the protein expression of TRP-2 was not altered
but that of TRP-1 was obviously increased after the MA extract
treatment (Fig. 5). The function
of TRP-1 in melanogenesis seems to be distinct with tyrosinase and
TRP-2. The specific melanogenic function of TRP1 is the oxidation
of DHICA to a carboxylated indole-quinone at the downstream point
in the melanin biosynthetic pathway. TRP-1 activity is essential to
further the metabolism of DHICA to a high molecular weight
pigmented biopolymer (7). Despite
the protein expression level of TRP-1 was obviously induced by the
MA extract treatment, our results have shown that the mRNA level of
TRP-1 was not affected. Thus, the overall results suggest that the
MA extract increases melanogenesis via the upregulation of TRP-1
protein expression by post-transcriptional control in B16F10
cells.
In conclusion, the present findings have shown a
rapid melanogenic effect of an ethanol extract of MA and the
underlying mechanisms involved in the process in B16F10 cells. The
results indicate that the MA extract may be useful for the
development of self-tanning cosmetics products and the treatment of
hypopigmentation disorders including vitiligo.
Acknowledgments
We would like to thank Bioland Co., Ltd. (Korea) for
preparing reagents. This study was partially supported by a grant
from the National Research Foundation of Korea (NRF) grant funded
by the Korea government (MEST) (grant no. 2011-0029819) and by a
grant of the Korean Health Technology R&D Project, Ministry of
Health and Welfare, Republic of Korea (grant no. A121851).
References
|
1
|
Eller MS, Yaar M and Gilchrest BA: DNA
damage and melanogenesis. Nature. 372:413–414. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hearing VJ: Biogenesis of pigment
granules: a sensitive way to regulate melanocyte function. J
Dermatol Sci. 37:3–14. 2005. View Article : Google Scholar
|
|
3
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
del Marmol V and Beermann F: Tyrosinase
and related proteins in mammalian pigmentation. FEBS Lett.
381:165–168. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hearing VJ and Jiménez M: Mammalian
tyrosinase - the critical regulatory control point in melanocyte
pigmentation. Int J Biochem. 19:1141–1147. 1987. View Article : Google Scholar
|
|
6
|
Yokoyama K, Yasumoto K, Suzuki H and
Shibahara S: Cloning of the human DOPAchrome
tautomerase/tyrosinase-related protein 2 gene and identification of
two regulatory regions required for its pigment cell-specific
expression. J Biol Chem. 269:27080–27087. 1994.PubMed/NCBI
|
|
7
|
Kobayashi T, Urabe K, Winder A, et al:
Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in
melanin biosynthesis. EMBO J. 13:5818–5825. 1994.PubMed/NCBI
|
|
8
|
Hemesath TJ, Price ER, Takemoto C,
Badalian T and Fisher DE: MAP kinase links the transcription factor
Microphthalmia to c-Kit signalling in melanocytes. Nature.
391:298–301. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Price ER, Ding HF, Badalian T, et al:
Lineage-specific signaling in melanocytes. C-kit stimulation
recruits p300/CBP to microphthalmia. J Biol Chem. 273:17983–17986.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito H, Yasumoto K, Takeda K, Takahashi
K, Yamamoto H and Shibahara S: Microphthalmia-associated
transcription factor in the Wnt signaling pathway. Pigment Cell
Res. 16:261–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Widlund HR and Fisher DE:
Microphthalamia-associated transcription factor: a critical
regulator of pigment cell development and survival. Oncogene.
22:3035–3041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oka M, Nagai H, Ando H, et al: Regulation
of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway
in human G361 melanoma cells. J Invest Dermatol. 115:699–703. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim DS, Kim SY, Chung JH, Kim KH, Eun HC
and Park KC: Delayed ERK activation by ceramide reduces melanin
synthesis in human melanocytes. Cell Signal. 14:779–785. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buscà R, Bertolotto C, Ortonne JP and
Ballotti R: Inhibition of the phosphatidylinositol
3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell
differentiation. J Biol Chem. 271:31824–31830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Englaro W, Bertolotto C, Buscà R, et al:
Inhibition of the mitogen-activated protein kinase pathway triggers
B16 melanoma cell differentiation. J Biol Chem. 273:9966–9970.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sinha RP and Häder DP: UV-induced DNA
damage and repair: a review. Photochem Photobiol Sci. 1:225–236.
2002. View
Article : Google Scholar
|
|
18
|
Baadsgaard O: In vivo ultraviolet
irradiation of human skin results in profound perturbation of the
immune system. Relevance to ultraviolet-induced skin cancer. Arch
Dermatol. 127:99–109. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hedstrand H, Ekwall O, Olsson MJ, et al:
The transcription factors SOX9 and SOX10 are vitiligo autoantigens
in autoimmune polyendocrine syndrome type I. J Biol Chem.
276:35390–35395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seyedalinaghi SA, Karami N, Hajiabdolbaghi
M and Hosseini M: Vitiligo in a patient associated with human
immunodeficiency virus infection and repigmentation under
antiretroviral therapy. J Eur Acad Dermatol Venereol. 23:840–841.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scherschun L, Kim JJ and Lim HW:
Narrow-band ultraviolet B is a useful and well-tolerated treatment
for vitiligo. J Am Acad Dermatol. 44:999–1003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu HB, Zhang CR, Dong SH, Dong L, Wu Y
and Yue JM: Limonoids and triterpenoids from the seeds of Melia
azedarach. Chem Pharm Bull (Tokyo). 59:1003–1007. 2011. View Article : Google Scholar
|
|
23
|
Wu SB, Bao QY, Wang WX, et al: Cytotoxic
triterpenoids and steroids from the bark of Melia azedarach. Planta
Med. 77:922–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Mallick S, Singh SK, Sarkar C, Saha B and
Bhadra R: Human placental lipid induces melanogenesis by increasing
the expression of tyrosinase and its related proteins in vitro.
Pigment Cell Res. 18:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Z, Li S, Liu Y, Deng P, Huang J and
He G: Sesamin induces melanogenesis by microphthalmia-associated
transcription factor and tyrosinase up-regulation via cAMP
signaling pathway. Acta Biochim Biophys Sin (Shanghai). 43:763–770.
2011. View Article : Google Scholar
|
|
27
|
Nagata H, Takekoshi S, Takeyama R, Homma T
and Yoshiyuki Osamura R: Quercetin enhances melanogenesis by
increasing the activity and synthesis of tyrosinase in human
melanoma cells and in normal human melanocytes. Pigment Cell Res.
17:66–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Villareal MO, Han J, Matsuyama K, et al:
Lupenone from Erica multiflora leaf extract stimulates
melanogenesis in B16 murine melanoma cells through the inhibition
of ERK1/2 activation. Planta Med. 79:236–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moreira CG, Horinouchi CD, Souza-Filho CS,
et al: Hyperpigmentant activity of leaves and flowers extracts of
Pyrostegia venusta on murine B16F10 melanoma. J Ethnopharmacol.
141:1005–1011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carpinella MC, Defago MT, Valladares G and
Palacios SM: Antifeedant and insecticide properties of a limonoid
from Melia azedarach (Meliaceae) with potential use for pest
management. J Agric Food Chem. 51:369–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pifarré MP, Berra A, Coto CE and Alché LE:
Therapeutic action of meliacine, a plant-derived antiviral, on
HSV-induced ocular disease in mice. Exp Eye Res. 75:327–334. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bueno CA, Lombardi MG, Sales ME and Alché
LE: A natural antiviral and immunomodulatory compound with
antiangiogenic properties. Microvasc Res. 84:235–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kikuchi T, Pan X, Ishii K, et al:
Cytotoxic and apoptosis-inducing activities of
12-O-Acetylazedarachin B from the fruits of Melia azedarach in
human cancer cell lines. Biol Pharm Bull. 36:135–139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koo JH, Rhee KS, Koh HW, Jang HY, Park BH
and Park JW: Guggulsterone inhibits melanogenesis in B16 murine
melanoma cells by downregulating tyrosinase expression. Int J Mol
Med. 30:974–978. 2012.PubMed/NCBI
|
|
35
|
Jeon S, Kim NH, Koo BS, Lee HJ and Lee AY:
Bee venom stimulates human melanocyte proliferation, melanogenesis,
dendricity and migration. Exp Mol Med. 39:603–613. 2007. View Article : Google Scholar : PubMed/NCBI
|